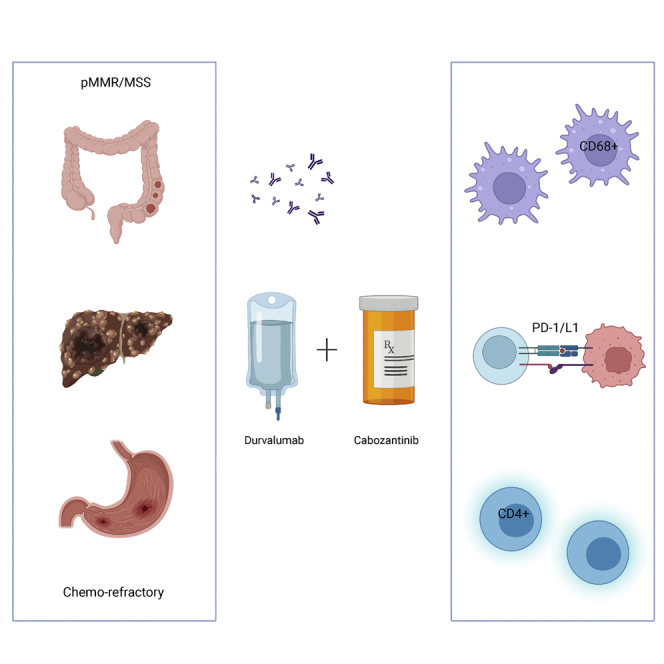
Summary
This is the phase Ib part of the phase I/II CAMILLA trial evaluating cabozantinib plus durvalumab in advanced chemo-refractory proficient mismatch repair or microsatellite stable (pMMR/MSS) gastrointestinal malignancies including gastric/gastroesophageal junction/esophageal (G/GEJ/E) adenocarcinoma, colorectal cancer (CRC), and hepatocellular carcinoma (HCC). Thirty-five patients are enrolled. There are no observed dose-limiting toxicities during dose escalation. The overall grade 3/4 treatment-related adverse event rate is 34%. Among evaluable patients (n = 30), the objective response rate (ORR) is 30%, disease control rate (DCR) 83.3%, 6-month progression-free survival (PFS) 36.7%, median PFS 4.5 months, and median overall survival (OS) 8.7 months. Responses are seen in 4 of 17, 3 of 10, and 2 of 3 patients with CRC, G/GEJ/E adenocarcinoma, and HCC, respectively. Participants with a PD-L1 combined positive score (CPS) ≥5 have numerically higher ORR, PFS, and OS. Cabozantinib plus durvalumab demonstrates a tolerable safety profile and potential efficacy in previously treated advanced pMMR/MSS gastrointestinal malignancies.
Keywords: cabozantinib, durvalumab, gastroesophageal cancer, colorectal cancer, hepatocellular carcinoma
Graphical abstract
Highlights
-
•
Cabozantinib plus durvalumab has anti-tumor activity and potential synergy
-
•
Toxicity is manageable and consistent with prior studies
-
•
PD-L1 CPS of 5 or more is predictive of response
-
•
Higher CD4 and lower CD68 infiltration correlate with longer progression-free survival
Saeed et al. show that cabozantinib plus durvalumab is a safe, active, and potentially synergistic regimen in patients with advanced gastrointestinal malignancies with proficient mismatch repair or microsatellite stable tumors including colorectal cancer, who usually do not respond to immune checkpoint inhibitors. This regimen is being evaluated in larger, later phase trials.
Introduction
Gastrointestinal (GI) malignancies account for about 18% of all new cancer cases in the United States, among which the most common tumors are colorectal cancer (CRC), hepatocellular cancer (HCC), and gastric/gastroesophageal junction/esophageal (G/GEJ/E) adenocarcinoma.1 Despite rapid advances in systemic treatment in advanced GI malignancies, prognoses of patients remain poor, as the median overall survival (OS) of patients with metastatic or advanced disease are approximately 2 years for HCC and CRC and 1 year for G/GEJ/E adenocarcinoma.1,2,3 Thus, there is an area of unmet need for systemic treatment in advanced GI malignancies that warrants further investigation.
Immune checkpoint inhibitors (ICIs) that target PD-1 or PD-L1 have changed the paradigm of systemic therapy in advanced solid tumors including in GI malignancies. Anti-PD-1/PD-L1 agents have demonstrated durable responses in patients with metastatic G/GEJ/E adenocarcinoma and HCC.4,5,6 However, therapeutic benefit is limited to a minority of patients with G/GEJC and HCC. Furthermore, most patients with metastatic CRC who harbor proficient mismatch repair or microsatellite stable (pMMR/MSS) tumors do not respond to ICI therapy.7,8 Thus, unique approaches of potentiating or enhancing ICI therapies are needed. To this end, therapeutic combinations that incorporate ICI with targeted therapies is emerging as a promising option and is currently the most intense area of research.
Cabozantinib is an orally bioavailable, multi-tyrosine kinase inhibitor with activities against the tyrosine kinases VEGFR2, MET/HGF, AXL, MER, and TYRO3. It has broad single-agent activity across various solid tumors including metastatic CRC and HCC and has demonstrated potential anti-tumor activity in pre-clinical models of G/GEJ adenocarcinoma.9,10 Moreover, the immunomodulatory effects of cabozantinib have been established in pre-clinical and clinical settings, which demonstrate its ability to counteract tumor-mediated immune suppression and potentiate innate and adaptive anti-tumor immune responses.11,12,13,14,15 Thus, cabozantinib represents an attractive therapeutic partner to combine with ICI agents for the treatment of advanced GI malignancies.
Recently, both early and late phase clinical trials have studied anti-PD-1/PD-L1-based cabozantinib combinations in various advanced solid tumors.16,17,18,19,20,21 In several clinical trials, cabozantinib has been combined with nivolumab (anti-PD-1) with or without ipilimumab (anti-CTLA-4) and with pembrolizumab (anti-PD-1) in metastatic renal cell cancer, urothelial cancer, and HCC and demonstrated an acceptable safety profile and promising efficacy.20,21,22 Moreover, cabozantinib plus atezolizumab (anti-PD-L1) has been studied in metastatic renal cell carcinoma, urothelial cancer, and non-small cell lung carcinoma with encouraging safety and efficacy results.16,17,18,19 Thus, we have conducted a phase I/II trial to evaluate the safety and potential efficacy of cabozantinib with durvalumab (anti-PD-L1) in patients with advanced GI cancers.
Results
Study population and treatment
Overall, 35 patients (14 female, 21 male) were enrolled (Table 1). The patient population was predominantly White (89%) and had a median age of 53 (range: 27–79). Of these patients, 58% had CRC (20/35), 29% had G/GEJ/E adenocarcinoma (10/35), and 14% had HCC (5/35). All patients were confirmed to have pMMR/MSS tumors. Most patients had an ECOG status of 1 (77%). The median number of prior systemic therapies was 2 (range: 0–3) (Table 1). Five patients were not evaluable for dose-limiting toxicities (DLTs) or disease response: three patients in the dose-escalation phase were not evaluable due to missing more than 30% of DLT window doses, which were not related to DLTs, and two patients in the dose-expansion phase were not evaluable due to withdrawal from the study with non-completion of at least a 14-day study drug exposure.
Table 1.
Baseline patient characteristics
| Baseline characteristics | Overall (n = 35) |
|---|---|
| Gender, n (%) | |
| Female | 14 (40) |
| Male | 21 (60) |
| Median age, years (range) | 53 (27–79) |
| >60 (%) | 22 (63) |
| <60 (%) | 13 (37) |
| ECOG PS, n (%) | |
| 0 | 8 (23) |
| 1 | 27 (77) |
| Race, n (%) | |
| White | 31 (89) |
| African American | 2 (6) |
| Hispanic | 1 (3) |
| Asian | 1 (3) |
| Tumor types, n (%) | |
| GEA | 10 (29) |
| CRC | 20 (58) |
| HCC | 5 (14) |
| Prior lines of therapy | |
| Median | 2 |
| 0 | 4 (11) |
| 1 | 7 (20) |
| 2 | 11 (31) |
| 3 | 13 (37) |
| MMR status, n (%) | |
| MMR proficient | 35 (100) |
| Patients with liver metastasis, n (%) | |
| GEA | 4 (40) |
| CRC | 15 (75) |
Safety and toxicity
During dose escalation (part 1), no DLTs were observed when cabozantinib was escalated from 20 to 60 mg daily. Dose interruptions due to adverse events for durvalumab were required in 14 out of 35 patients and for cabozantinib in 14 out of 35 patients (Table 2). Discontinuation due to treatment-related adverse events (TRAEs) associated with durvalumab was necessary in 1 patient with recurrent immunotherapy-related colitis post durvalumab rechallenge. Two patients required permanent discontinuation of cabozantinib, one due to esophageal perforation and the other patient due to recurrent bleeding from known esophageal varices. Overall, 11 out of 14 patients receiving cabozantinib 60 mg daily required dose reduction to 40 mg daily after the second cycle. The dose reductions were all attributable to accumulative fatigue, anorexia, and associated weight loss. However, no DLTs were reported within the 28-day DLT window. Grade 3 or 4 TRAEs occurred in 34% of patients. The most common any-grade TRAEs were fatigue, hyperthyroidism or hypothyroidism, nausea, anorexia, weight loss, ALT and AST elevations, diarrhea, and palmar-plantar erythrodysesthesia syndrome. The most common serious AEs were thromboembolic events, fatigue, weight loss, and abdominal pain (Tables 3 and S4).
Table 2.
Summary of TRAEs
|
Parameter, n (%) |
Cabozantinib (Cabo) + durvalumab (Durva) |
|---|---|
| Overall (n = 35) | |
| TRAEs (number of events) | 313 |
| TRAEs ≥ grade 3 (%) | 32 (10) |
| TRAEs ≥ grade 3 (number of patients) (%) | 12 (34) |
| Grade 3 | 9 (26) |
| ∗Immunotherapy-related events | ∗4 (11) |
| Grade 4 | 3 (9) |
| ∗Immunotherapy-related events | ∗1 (3) |
| Grade 5 | 0 (0) |
| Dose modifications | |
| Durva dose interruptions due to AEs | 14 – Durva treatment delay/hold |
| Cabo dose interruptions or reductions due to AEs | 14 – Cabo treatment delay/hold |
| Discontinuation of Cabo or Durva due to AEs | 1 – Durva treatment discontinuation 2 – Cabo treatment discontinuation |
Table 3.
Most common TRAEs by preferred term and grade
|
Preferred term, n (%) |
Cabo + Durva (n = 35) |
||||||
|---|---|---|---|---|---|---|---|
| Any grade (%) | G1 | G2 | Grade ≥ 3 (%) | G3 | G4 | G5 | |
| Fatigue | 21 (60) | 12 | 9 | 2 (6) | 2 | 0 | 0 |
| Hyperthyroidism | 20 (57) | 20 | 0 | 1 (3) | 1 | 0 | 0 |
| Nausea | 18 (51) | 17 | 1 | 1 (3) | 1 | 0 | 0 |
| Anorexia | 14 (40) | 10 | 4 | 0 (0) | 0 | 0 | 0 |
| Alanine aminotransferase increased | 13 (36) | 9 | 4 | 1 (3) | 1 | 0 | 0 |
| Diarrhea | 13 (36) | 10 | 3 | 0 (0) | 0 | 0 | 0 |
| Aspartate aminotransferase increased | 12 (34) | 9 | 3 | 0 (0) | 0 | 0 | 0 |
| Hypothyroidism | 10 (29) | 9 | 1 | 0 (0) | 0 | 0 | 0 |
| Weight Loss | 10 (29) | 1 | 9 | 2 (6) | 2 | 0 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 9 (26) | 3 | 6 | 1 (3) | 1 | 0 | 0 |
Efficacy
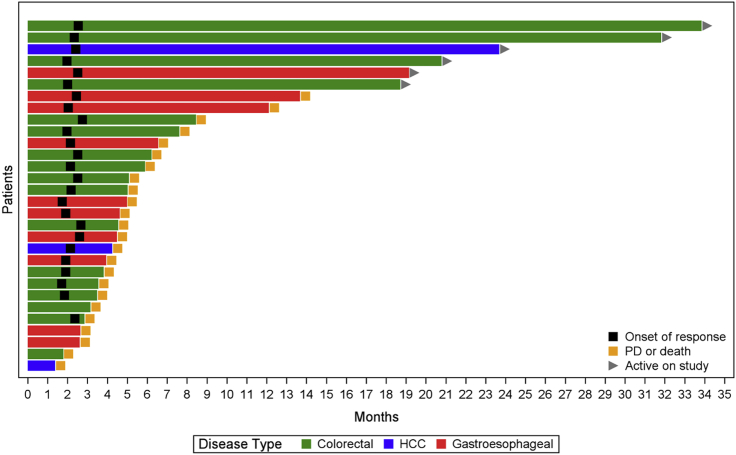
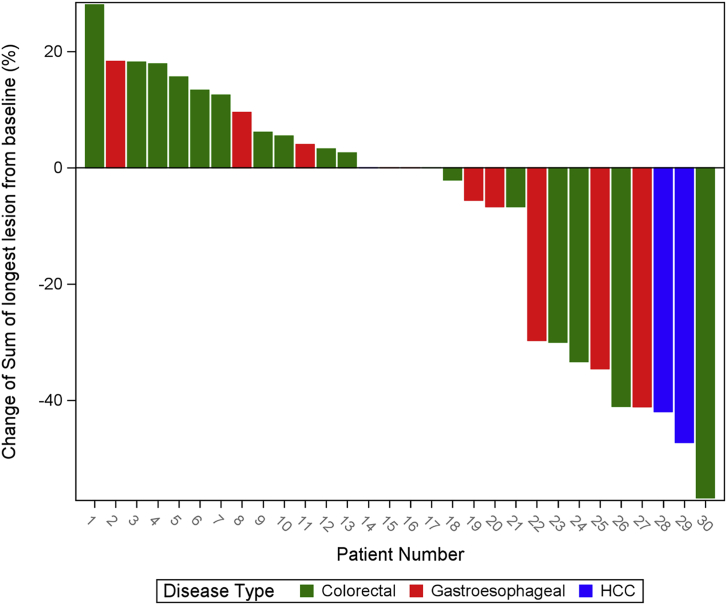
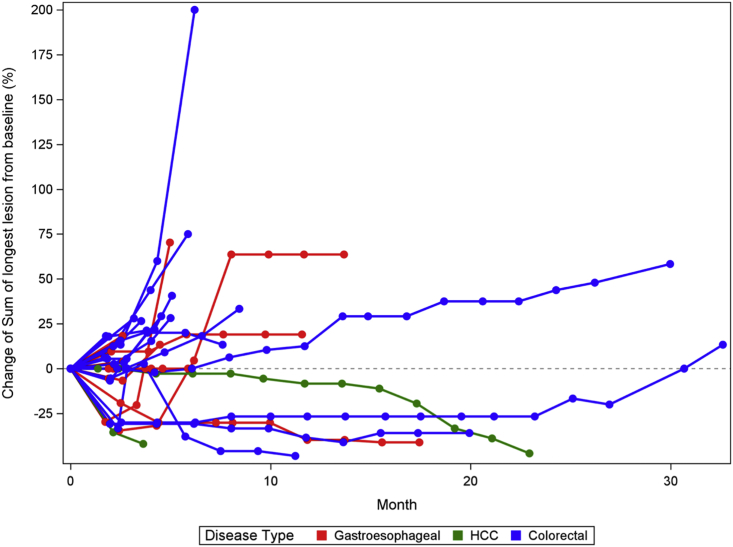
In total, 30 patients were eligible for efficacy assessment. Among them, 17 patients had CRC (57%), 10 patients had G/GEJ/E adenocarcinoma (33%), and 3 patients had HCC (10%). Overall, the objective response rate (ORR) was 30% (9/30); disease control rate (DCR) was 83.33% (25/30) (90% confidence interval [CI], 68.1%–93.19%); median progression-free survival (PFS) was 4.5 months (90% CI, 3.7–6.2 months); 6-month PFS was 36.67% (11/30) (90% CI, 22.11%–53.31%); and median OS was 8.7 months (90% CI, 7.2–19.7 months) (Figures 1, 2, and 3; Tables 4 and S1). Of the 12 patients with a PD-L1 combined positive score (CPS) ≥5, ORR was 41.67% (5/12) (90% CI, 18.1%–68.48%); DCR was 91.67% (11/12) (90% CI, 66.13%–99.57%); median PFS was 6.1 months (90% CI, 3.7–not reached [NR] months); 6-month PFS was 50% (6/12) (90% CI, 24.53%–75.47%); and median OS was 14.4 months (6.1–NR months) (Tables 4 and S3). In patients with G/GEJ/E adenocarcinoma (n = 10), the ORR and DCR were 30% and 80% and mPFS and mOS were 4.6 and 7.2 months respectively. In patients with CRC (n = 17), the ORR and DCR were 23.5% and 88.2% and the median PFS (mPFS) and mOS were 4.6 and 9.6 months, respectively (Table S2). Among patients with HCC (n = 3), 2 patients had an objective response.
Figure 1.
Swimmer plot showing the tumor response with duration by tumor type and month
Figure 2.
Waterfall plot showing the percentage of change from baseline to nadir in sums of diameters of target lesions
Figure 3.
Spider plot showing the percentage of change from baseline in sums of diameters of target lesions over time
Table 4.
Key efficacy outcomes for the overall and PD-L1 CPS ≥5 population
| Overall | PD-L1 CPS ≥5 | |
|---|---|---|
| 1. ORR | 30% (16.63%, 46.51%), (9/30) | 41.67% (18.1%, 68.48%), (5/12) |
| 2. Disease control rate | 83.33% (68.1%, 93.19%), (25/30) | 91.67% (66.13%, 99.57%), (11/12) |
| 3. Median PFS (months) | 4.5 (3.7, 6.2) | 6.1 (3.7, not estimable) |
| 4. Median OS (months) | 8.7 (7.2, 19.7) | 14.4 (6.1, not estimable) |
| 5. 6-month PFS rate | 36.67% (22.11%, 53.31%), (11/30) | 50% (24.53%, 75.47%), (6/12) |
Immune correlates
Subgroup analysis showed patients with a PD-L1 CPS of 5 or higher had significantly improved OS (log rank test, p = 0.0616) and PFS (log rank test, p = 0.0462) (Figures S2 and S3). Cox proportional hazard (PH) analysis demonstrated that a PD-L1 CPS of 5 or higher (hazard ratio [HR] 0.406, 90% CI 0.188–0.875), less tumor-associated macrophages (TAMs) (CD68+) (HR 0.368, 90% CI 0.158–0.855), and greater tumor-infiltrating CD4 T cells (CD4+) (HR 0.420, 90% CI 0.189–0.932) as well as a lower ratio of TAMs/CD4 T cells (CD68/CD4) (HR 0.319, 90% CI 0.124–0.821) were associated with improved PFS (Figure S1).
Discussion
This study demonstrates the tolerable safety profile and promising efficacy of cabozantinib plus durvalumab in patients with chemotherapy-refractory unresectable or metastatic GI malignancies including HCC, CRC, and G/GEJ/E adenocarcinoma. This study evaluates a cabozantinib-based ICI combination in patients with pMMR/MSS G/GEJ/E adenocarcinoma and CRC and highlights the regimen’s potential efficacy in this otherwise ICI-resistant patient population. Furthermore, we demonstrate that PD-L1 CPS may be a potential predictive marker for this ICI-based combination therapy in this patient population.
Cabozantinib 40 mg daily was determined to be the recommended phase 2 dose (RP2D) instead of 60 mg daily, as most of the patients receiving the 60 mg daily dose required an early dose reduction. Grade 3 or 4 TRAEs were seen in 34% of patients treated with cabozantinib plus durvalumab, which is consistent with that observed for cabozantinib plus ICI combinations in prior studies such as in combination with anti-PD-1 (47%–60%) and with anti-PD-L1 (47%–71%).17,18,19,20,21,22 Most TRAEs were manageable with dose interruptions, and almost every patient remained on treatment without discontinuation. The most common any-grade AEs and severe AEs were mainly constitutional or GI, which is consistent with the known toxicities of cabozantinib. Overall, no new safety signals were identified, and the toxicity of cabozantinib plus durvalumab was tolerable and comparable to other studies in non-GI tumors.
The efficacy in our study population composed primarily of pMMR/MSS G/GEJ/E adenocarcinoma and CRC is promising as those tumor types are well known to have poor responses to ICI monotherapy.23,24 The REGONIVO study, which was a phase I/II study conducted in Japan, showed that nivolumab plus regorafenib elicited an ORR of 36% and an mPFS of 7.9 months in patients with chemorefractory metastatic pMMR/MSS CRC. However, studies that were conducted subsequently showed that regorafenib plus nivolumab or pembrolizumab failed to replicate the anti-tumor activity in North American populations (ORR 0%–10%, mPFS 2–4.3 months).25,26,27,28 However, more recent early phase studies conducted in North American populations such as the LEAP-005 trial that evaluated lenvatinib plus pembrolizumab showed an ORR of 22%, whereas the RIN study showed the regorafenib plus nivolumab and ipilimumab elicited an ORR of 27.6% in treatment-refractory CRC, suggesting that specific combinations of ICI plus TKI are active in pMMR/MSS CRC and that exploratory tumor molecular studies are needed to identify biomarkers of response and to help with future trials development in this space.29,30 Of note, while one study demonstrated that single-agent cabozantinib has disease-stabilizing activity in patients with CRC, the depth and frequency of tumor responses were underwhelming, with only 1 out of 32 patients having achieved objective responses.10 The tumor responses elicited by cabozantinib plus durvalumab suggest synergy between these agents and demonstrate that cabozantinib may potentiate ICIs’ efficacy in these difficult-to-treat patients. The interim results of the phase II CRC cohort of the CAMILLA study presented at the GI ASCO 2022 symposium further validate the anti-tumor activity of cabozantinib plus durvalumab in these patients.31
Similarly, cabozantinib plus durvalumab also demonstrated promising efficacy in our pre-treated, advanced-stage, non-biomarker-selected G/GEJ/E adenocarcinoma population, which are also largely poorly responsive to single-agent ICI therapies.32 The REGONIVO study had enrolled 25 patients with gastric cancer in parallel with the patients with CRC and demonstrated in this population that regorafenib plus nivolumab elicited an ORR of 44% and an mPFS of 5.6 months.25 In addition, the phase II LENPEM study, which was conducted in Asia, demonstrated that lenvatinib plus pembrolizumab elicited an ORR of 69% and an mPFS of 7.1 months.33 Recently, the standard-of-care first-line systemic therapy for patients with unresectable G/GEJ/E adenocarcinoma has changed with the approval of combination chemoimmunotherapy regimens with pembrolizumab or nivolumab.34,35 These recent changes and the promising efficacy of cabozantinib plus durvalumab taken together warrant the evaluation of cabozantinib-based ICI combinations in the maintenance or second-line setting for metastatic pMMR/MSS G/GEJ/E adenocarcinoma. The phase III COSMIC-312 trial demonstrated that atezolizumab (anti-PD-L1) plus cabozantinib led to a PFS benefit over sorafenib but did not result in an OS benefit.36 Nonetheless, the recent reporting of the phase III HIMALAYA study confirmed the superior OS of durvalumab plus tremelimumab (anti-CTLA-4) over standard-of-care sorafenib in first-line settings in advanced HCC. Interestingly, in this STRIDE regimen, only a single priming dose of tremelimumab was added to durvalumab, which was effective yet associated with less toxicity.37 Based on these results, an additional phase II HCC cohort has been added to the CAMILLA trial and is enrolling patients to evaluate the triplet regimen of cabozantinib plus durvalumab plus tremelimumab.
Our study demonstrates that baseline PD-L1 CPS as well as tumor CD68 and CD4 protein levels via immunohistochemistry (IHC), which represent cell surface protein markers for TAMs and tumor-infiltrating CD4 T cells, respectively, are potential predictive markers for cabozantinib plus durvalumab. Notably, enrichment of tumors with subsets of TAMs characterized in part by CD68 expression have been associated with a lower complete response rate in patients with melanoma treated with anti-PD-1 therapy and with reduced OS in patients with head and neck cancer.38 Thus, our findings warrant further evaluation of PD-L1 CPS as well as CD68 and CD4 levels and CD68/CD4 ratio as potential ICI biomarkers in patients with pMMR/MSS CRC, G/GEJC, and HCC in a larger trial population.
In conclusion, combined cabozantinib and durvalumab is fairly tolerated and has potential efficacy in treatment-refractory, unresectable, or metastatic pMMR/MSS GI malignancies. The multicohort phase II of the CAMILLA study is currently ongoing, with results anticipated soon.
Limitations of the study
There are several limitations to our study. First, given the non-randomized, single-arm nature of the study, no within-study comparisons were made of cabozantinib plus durvalumab with established therapeutic agents for each tumor types in respective treatment settings or with cabozantinib or durvalumab alone. Second, the limited number of patients enrolled across the different tumor types limits the power of the study, and thus a p value cut-off of 0.10 was used to define significance. Evaluation of cabozantinib plus durvalumab in a larger population is therefore warranted.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Formalin-fixed paraffin-embedded archival tumor specimens | This manuscript | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Cabozantinib | Exelixis (Alameda, CA, USA) | www.cabometyxhcp.com |
| Durvalumab | Astrazeneca (Cambridge, UK) | www.imfinzi.com |
| PD-L1 Rabbit mAb #13684 | Cell Signaling Technology (Danvers, MA, USA) | www.cellsignal.com; RRID:AB_2687655 |
| CD68 Rabbit mAb #76437 | Cell Signaling Technology (Danvers, MA, USA) | www.cellsignal.com; RRID:AB_2799882 |
| CD4 Rabbit mAb #48274 | Cell Signaling Technology (Danvers, MA, USA) | www.cellsignal.com |
| Deposited data | ||
| Patient data | This manuscript | N/A |
| Software and algorithms | ||
| SAS software version 9.4 | SAS institute, Cary, NC, USA | www.sas.com |
| R version 3.6.2 | The R Foundation | www.r-project.org |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Anwaar Saeed (dranwaarsaeed1@gmail.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines and local institutional review board at the participating site.
Human subjects
Patients with histologically confirmed hepatocellular carcinoma, gastric/gastroesophageal junction/esophageal adenocarcinoma, or colorectal cancer were enrolled in this study. Demographic information was provided in Table 1. All patients provided written consent prior to enrollment.
Patient eligibility
Patients 18 years or older with Eastern Cooperative Oncology Group (ECOG) performance status 0–1 with histologically confirmed advanced or locally advanced unresectable (stage IV or unresectable III) G/GEJ/E adenocarcinoma, CRC, or HCC with measurable disease per modified RECIST (mRECIST) 1.1 were eligible for enrollment. Patients must have demonstrated progression or intolerance to at least 1 prior line of standard of care systemic therapy for patients with G/GEJC, at least 2 lines of therapy for patients with CRC, and 0 to 1 line of therapy for patients with HCC. Patients with RAS wild-type CRC must have demonstrated failure to anti-EGFR therapy. Patients with HER2 positive G/GEJ/E adenocarcinoma must have demonstrated failure to anti-HER2 therapy. Patients with prior exposure to anti PD-1/L1, or other co-inhibitory receptors such as CTLA-4 were excluded from enrollment, except for the HCC cohorts, as were patients with prior treatment with cabozantinib or monoclonal antibodies or tyrosine kinase Inhibitors (TKIs) against MET or MET/HGF. Patients with a history of autoimmune disorder or immune deficiency were also excluded from the study. Eligible patients must have an accessible primary or metastatic lesion for a required baseline biopsy.
Subject allocation
The phase Ib part of this trial reported here is a single arm study with no control group.
Method details
Study design
This is an ongoing non-randomized, open-label, phase I/II clinical trial registered at clinicaltrials.gov (NCT03539822). The trial consisted of an initial phase of dose limiting toxicities (DLT) evaluation (phase I) and a subsequent dose-expansion phase to better evaluate the regimen’s safety profile at the Maximum Tolerated Dose (MTD). While the planned duration of therapy was 12 cycles per participant, treatment was continued until disease progression, unacceptable toxicity, patient desire to discontinue therapy, or whichever occurred first. The primary outcome of interest was the MTD defined as the highest dose studied for which the observed incidence of DLT is less than 33% as per Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The MTD was used to inform the RP2D. Secondary outcomes were ORR defined as the proportion of patients achieving a complete response (CR) or partial response (PR), DCR defined as the proportion of patients achieving a CR, PR or stable disease (SD), PFS defined as the duration of time from start of treatment until objective tumor progression or death, OS defined as the duration of time from start of treatment to death, and proportion of participants with adverse events. DLT was evaluated at the end of cycle 1. Patients who did not complete cycle 1 and did not meet criteria for DLT were considered unevaluable.
Sample size estimation
The number of patients (n = 35) enrolled in this phase 1b study was justified according to the requirements of the 3 + 3 design (number of dose increments to find the MTD) followed by the dose expansion scheme consistent with the study protocol.
Drug administration
The phase I part was conducted in the classic 3 + 3 dose escalation design where participants received durvalumab 1500 mg every four weeks intravenously plus a starting dose of cabozantinib 20 mg daily per oral and titrated up to 40mg then 60 mg daily. Cabozantinib was administered daily throughout the 28-day cycles and durvalumab was administered intravenously on day 1 of every 28-day cycles. In part 2, patients were administered durvalumab 1500 mg every 28 days intravenously plus cabozantinib at the recommended phase II dose (RP2D).
Assessments
Treatment response was evaluated every 2 cycles using the mRECIST criteria version 1.1 and patients with at least two tumor imaging scans were eligible for assessment. mRECIST criteria version 1.1 allows patients with no clinical deterioration to continue trial therapy beyond first Progressive Disease (PD) on scans to assess for possibility of pseudoprogression. PD confirmatory scan was done at least 4 weeks from the first one.
Quantification and statistical analysis
- The observed TRAEs were summarized by type and severity according to the CTCAE 5.0. The research questions concerning primary, secondary and exploratory analyses required the use of several survival analyses methods. As this is a small sample study (N ranging from 24 to 26 for most analyses), results of all statistical tests were reported using 90% confidence intervals and p-values of statistical tests were assessed at the 10% level of significance. The primary outcomes of ORR, DCR and 6-month PFS rate were reported as proportions while PFS and OS was reported using median and the corresponding 90% confidence interval. For calculation of median PFS and median OS, all alive subjects (or no progression patients) were flagged as censored on 02/18/2022. Kaplan Meier curves for both OS and PFS were generated and a comparison of PD-L1 CPS levels ≥5 vs CPS <5 was made using a weighted log-rank test. As the research interest was focused on comparing long-term survivors in the two groups, the Fleming-Harrington (0,1) weights were used for this analysis. Additionally, a Cox proportional hazards (PH) model was used to compare PD-L1 CPS levels (≥5 vs CPS <5), CD68 levels (<5% vs ≥ 5%), CD4 levels (≥10% vs < 10%) and CD68/CD4 ratio (<1 vs ≥ 1) and results were reported using a forest plot. The proportional hazards assumption was validated using two methods – the log-log survival plot and the observed vs expected survival plot – and was found to be appropriate. Likewise, all exploratory molecular subgroup analyses were conducted using the Cox PH model. Additional analyses included using the two-sample test for comparing survival rates for PD-L1 CPS levels ≥5 vs CPS <5 at the landmark times of 12 months and 24 months respectively. The median PFS and OS in these two groups was compared using the Chen test for small sample censored data to correct for the inflation in type I error emerging from the Brookmeyer and the Crowley method.3
Additional resources
This study has been registered on clinicaltrials.gov (NCT03539822).
Acknowledgments
This trial has been funded by research grants from AstraZeneca and Exelixis. The authors would like to acknowledge support from the University of Kansas (KU) Cancer Center’s Biospecimen Repository Core Facility staff for helping obtain human specimens and performing histological work. The BRCF staff was supported in part by a grant from the National Cancer Institute, i.e., the KU Cancer Center’s Cancer Center Support Grant (P30 CA168524)
Author contributions
Conceptualization, A.S.; methodology, A.S., J.D., and M.P.; software, J.D. and M.P.; validation, all authors; formal analysis, A.S., J.D., and M.P.; investigation, all authors; resources, A.S.; data curation, J.D., M.P., K.M., M.S., J.F.-B., and J.R.; writing – original draft preparation, A.S., R.P., and M.P.; writing – review and editing, all authors; visualization, J.D. and M.P.; supervision, A.S.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
A.S. reports research grants (to institution) from AstraZeneca, Bristol Myers Squibb, Merck, Clovis, Exelixis, Actuate Therapeutics, Incyte Corporation, Daiichi Sankyo, Amgen, Innovent Biologics, Dragonfly Therapeutics, KAHR Medical, and Biontech and advisory board fees from AstraZeneca, Bristol Myers Squibb, Exelixis, Pfizer, and Daiichi Sankyo. R.A.-R. reports research grants (to institution) from AstraZeneca, Bayor, Merck, Bristol Myers Squibb, Exelixis, and Eureka Therapeutics and stock ownership in Actinium Pharmaceuticals and Seagen. A.K. reports research funding (to institution) from Astellas, Tesaro, and Bavarian Nordic. J.B. reports research grants (to institution) from Astellas, Chungchun Intellicrown, Impact Therapeutics, Poseida Therapeutics, Takeda Oncology, and Genome Co. and consultant fees (to institution) from Sanofi. A.K.G. reports research funding from Predicine and VITRAC Therapeutics, is a co-founder of Sinochips Diagnostics, and serves as a scientific advisory board member to Biovica, Clara Biotech, and Sinochips Diagnostics. S.W. reports stock/other ownership in Horizon Therapeutics, Iovance Biotherapeutics, and Merus and institutional research funding from Aleon Pharma, Astellas Pharma, Bayer Health, Bristol Myers Squibb, Daiichi Sankyo, EMD Serono, Merck Serono, Nektar, Novartis, Pharmacyclics, AbbVie, Regeneron, Rogosin Institute, Sanofi, Seagen, and Sotio.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: January 25, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.100916.
Supplemental information
Data and code availability
All data reported in this paper and any additional information required to reanalyze the data will be shared by the lead contact upon reasonable request (Table S5). Specifically, de-identified individual patient level data such as baseline clinical variables, treatment outcomes including treatment response and survival, and incidence and grade of adverse events for each trial participant will be available upon request. Any additional information regarding individual participants that may result in breach of patient confidentiality will not be provided.
This publication does not generate new code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA A Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biller L.H., Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 4.Sangro B., Sarobe P., Hervás-Stubbs S., Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan E., Sahin I.H. Defining the current role of immune checkpoint inhibitors in the treatment of mismatch repair-deficient/microsatellite stability-high colorectal cancer and shedding light on future approaches. Expet Rev. Gastroenterol. Hepatol. 2021;15:735–742. doi: 10.1080/17474124.2021.1886077. [DOI] [PubMed] [Google Scholar]
- 6.Joshi S.S., Badgwell B.D. Current treatment and recent progress in gastric cancer. CA A Cancer J. Clin. 2021;71:264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puccini A., Battaglin F., Iaia M.L., Lenz H.-J., Salem M.E. Overcoming resistance to anti-PD1 and anti-PD-L1 treatment in gastrointestinal malignancies. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haibe Y., Kreidieh M., El Hajj H., Khalifeh I., Mukherji D., Temraz S., Shamseddine A. Resistance mechanisms to anti-angiogenic therapies in cancer. Front. Oncol. 2020;10:221. doi: 10.3389/fonc.2020.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Alfa G.K., Meyer T., Cheng A.-L., El-Khoueiry A.B., Rimassa L., Ryoo B.-Y., Cicin I., Merle P., Chen Y., Park J.-W., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott A.J., Cohen S.J., Basu Mallick A., Dotan E., Gold P.J., Hochster H.S., Subramaniam S., Barzi A., Blatchford P.J., Messersmith W.A. A phase II study investigating cabozantinib in patients with refractory metastatic colorectal cancer (AGICC 17CRC01) J. Clin. Oncol. 2020;38:103. doi: 10.1200/JCO.2020.38.4_suppl.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwilas A.R., Ardiani A., Donahue R.N., Aftab D.T., Hodge J.W. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J. Transl. Med. 2014;12:294. doi: 10.1186/s12967-014-0294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patnaik A., Swanson K.D., Csizmadia E., Solanki A., Landon-Brace N., Gehring M.P., Helenius K., Olson B.M., Pyzer A.R., Wang L.C., et al. Cabozantinib eradicates advanced murine prostate cancer by activating antitumor innate immunity. Cancer Discov. 2017;7:750–765. doi: 10.1158/2159-8290.Cd-16-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X., Horner J.W., Paul E., Shang X., Troncoso P., Deng P., Jiang S., Chang Q., Spring D.J., Sharma P., et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543:728–732. doi: 10.1038/nature21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H., Sun S., Wang G., Lu M., Zhang X., Wei X., Gao X., Huang C., Li Z., Zheng J., Zhang Q. Tyrosine kinase inhibitor cabozantinib inhibits murine renal cancer by activating innate and adaptive immunity. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.663517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho W.J., Zhu Q., Durham J., Popovic A., Xavier S., Leatherman J., Mohan A., Mo G., Zhang S., Gross N., et al. Neoadjuvant cabozantinib and nivolumab convert locally advanced hepatocellular carcinoma into resectable disease with enhanced antitumor immunity. Nat. Can. (Que.) 2021;2:891–903. doi: 10.1038/s43018-021-00234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri T.K., Powles T., Burotto M., Escudier B., Bourlon M.T., Zurawski B., Oyervides Juárez V.M., Hsieh J.J., Basso U., Shah A.Y., et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal N., Loriot Y., McGregor B.A., Dreicer R., Dorff T.B., Maughan B.L., Kelly W.K., Pagliaro L.C., Srinivas S., Squillante C.M., et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results of cohort 6 of the COSMIC-021 study. J. Clin. Oncol. 2020;38:5564. doi: 10.1200/JCO.2020.38.15_suppl.5564. [DOI] [Google Scholar]
- 18.Neal J.W., Lim F.L., Felip E., Gentzler R.D., Patel S.B., Baranda J., Fang B., Squillante C.M., Simonelli M., Werneke S., et al. Cabozantinib in combination with atezolizumab in non-small cell lung cancer (NSCLC) patients previously treated with an immune checkpoint inhibitor: results from cohort 7 of the COSMIC-021 study. J. Clin. Oncol. 2020;38:9610. doi: 10.1200/JCO.2020.38.15_suppl.9610. [DOI] [Google Scholar]
- 19.Pal S.K., Agarwal N., Loriot Y., Suarez Rodriguez C., Singh P., Vaishampayan U.N., McIlvaine E., Curran D., Castellano D., Necchi A. Cabozantinib in combination with atezolizumab in urothelial carcinoma previously treated with platinum-containing chemotherapy: results from cohort 2 of the COSMIC-021 study. J. Clin. Oncol. 2020;38:5013. doi: 10.1200/JCO.2020.38.15_suppl.5013. [DOI] [Google Scholar]
- 20.Yau T., Zagonel V., Santoro A., Acosta-Rivera M., Choo S.P., Matilla A., He A.R., Cubillo Gracián A., El-Khoueiry A.B., Sangro B., et al. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J. Clin. Oncol. 2020;38:478. doi: 10.1200/JCO.2020.38.4_suppl.478. [DOI] [Google Scholar]
- 21.Apolo A.B., Nadal R., Girardi D.M., Niglio S.A., Ley L., Cordes L.M., Steinberg S.M., Sierra Ortiz O., Cadena J., Diaz C., et al. Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J. Clin. Oncol. 2020;38:3672–3684. doi: 10.1200/JCO.20.01652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keeler M.E., Kessler E.R., Bernard B., Weisdack S., Breaker K.M., Wold M., Ertz D., Weitzenkamp D., Flaig T.W., Lam E.T. Pembrolizumab (pembro) and cabozantinib (cabo) in patients (pts) with metastatic renal cell carcinoma (mRCC): phase I results. J. Clin. Oncol. 2019;37:600. doi: 10.1200/JCO.2019.37.7_suppl.600. [DOI] [Google Scholar]
- 23.O'Neil B.H., Wallmark J.M., Lorente D., Elez E., Raimbourg J., Gomez-Roca C., Ejadi S., Piha-Paul S.A., Stein M.N., Abdul Razak A.R., et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12 doi: 10.1371/journal.pone.0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eng C., Kim T.W., Bendell J., Argilés G., Tebbutt N.C., Di Bartolomeo M., Falcone A., Fakih M., Kozloff M., Segal N.H., et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20:849–861. doi: 10.1016/s1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 25.Fukuoka S., Hara H., Takahashi N., Kojima T., Kawazoe A., Asayama M., Yoshii T., Kotani D., Tamura H., Mikamoto Y., et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603) J. Clin. Oncol. 2020;38:2053–2061. doi: 10.1200/jco.19.03296. [DOI] [PubMed] [Google Scholar]
- 26.Fakih M., Raghav K.P.S., Chang D.Z., Bendell J.C., Larson T., Cohn A.L., Huyck T.K., Cosgrove D., Fiorillo J.A., Garbo L.E., et al. Single-arm, phase 2 study of regorafenib plus nivolumab in patients with mismatch repair-proficient (pMMR)/microsatellite stable (MSS) colorectal cancer (CRC) J. Clin. Oncol. 2021;39:3560. doi: 10.1200/JCO.2021.39.15_suppl.3560. [DOI] [Google Scholar]
- 27.Barzi A., Azad N.S., Yang Y., Tsao-Wei D., Rehman R., Fakih M., Iqbal S., El-Khoueiry A.B., Millstein J., Jayachandran P., et al. Phase I/II study of regorafenib (rego) and pembrolizumab (pembro) in refractory microsatellite stable colorectal cancer (MSSCRC) J. Clin. Oncol. 2022;40:15. doi: 10.1200/JCO.2022.40.4_suppl.015. [DOI] [Google Scholar]
- 28.Kim R.D., Kovari B.P., Martinez M., Xie H., Sahin I.H., Mehta R., Strosberg J., Imanirad I., Ghayouri M., Kim Y.C., Kim D.W. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur. J. Cancer. 2022;169:93–102. doi: 10.1016/j.ejca.2022.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Fakih M.G., J.S., Lim D., Li S.M., Wang C. In Chemotherapy Resistant MSS Metastatic Colorectal Cancer (mCRC) ESMO; 2022. A phase I clinical trial of regorafenib, ipilimumab, and nivolumab (RIN) [Google Scholar]
- 30.Gomez-Roca C., Yanez E., Im S.-A., Castanon Alvarez E., Senellart H., Doherty M., García-Corbacho J., Lopez J.S., Basu B., Maurice-Dror C., et al. LEAP-005: a phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—results from the colorectal cancer cohort. J. Clin. Oncol. 2021;39:94. doi: 10.1200/JCO.2021.39.3_suppl.94. [DOI] [Google Scholar]
- 31.Saeed A., Park R., Dai J., Al-Rajabi R.M.T., Kasi A., Saeed A., Collins Z., Thompson K., Barbosa L., Mulvaney K., et al. Phase II trial of cabozantinib (Cabo) plus durvalumab (Durva) in chemotherapy refractory patients with advanced mismatch repair proficient/microsatellite stable (pMMR/MSS) colorectal cancer (CRC): CAMILLA CRC cohort results. J. Clin. Oncol. 2022;40:135. doi: 10.1200/JCO.2022.40.4_suppl.135. [DOI] [Google Scholar]
- 32.Fuchs C.S., Doi T., Jang R.W., Muro K., Satoh T., Machado M., Sun W., Jalal S.I., Shah M.A., Metges J.P., et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawazoe A., Fukuoka S., Nakamura Y., Kuboki Y., Wakabayashi M., Nomura S., Mikamoto Y., Shima H., Fujishiro N., Higuchi T., et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1057–1065. doi: 10.1016/S1470-2045(20)30271-0. [DOI] [PubMed] [Google Scholar]
- 34.Janjigian Y.Y., Shitara K., Moehler M., Garrido M., Salman P., Shen L., Wyrwicz L., Yamaguchi K., Skoczylas T., Campos Bragagnoli A., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato K., Sun J.M., Shah M.A., Enzinger P.C., Adenis A., Doi T., Kojima T., Metges J.P., Li Z., Kim S.B., et al. LBA8_PR Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: the phase 3 KEYNOTE-590 study. Ann. Oncol. 2020;31:S1192–S1193. doi: 10.1016/j.annonc.2020.08.2298. [DOI] [Google Scholar]
- 36.Kelley R.K., Rimassa L., Cheng A.-L., Kaseb A., Qin S., Zhu A.X., Chan S.L., Melkadze T., Sukeepaisarnjaroen W., Breder V., et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 37.Abou-Alfa G.K., Chan S.L., Kudo M., Lau G., Kelley R.K., Furuse J., Sukeepaisarnjaroen W., Kang Y.-K., Dao T.V., De Toni E.N., et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022;40:379. doi: 10.1200/JCO.2022.40.4_suppl.379. [DOI] [Google Scholar]
- 38.Kwak T., Wang F., Deng H., Condamine T., Kumar V., Perego M., Kossenkov A., Montaner L.J., Xu X., Xu W., et al. Distinct populations of immune-suppressive Macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper and any additional information required to reanalyze the data will be shared by the lead contact upon reasonable request (Table S5). Specifically, de-identified individual patient level data such as baseline clinical variables, treatment outcomes including treatment response and survival, and incidence and grade of adverse events for each trial participant will be available upon request. Any additional information regarding individual participants that may result in breach of patient confidentiality will not be provided.
This publication does not generate new code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.