Abstract
The (+)-catechin transglucosylating activities of several glucosyltransferases (GTFs) from the genus Streptococcus were compared. For this purpose, a mixture of four GTFs from Streptococcus sobrinus SL-1 and recombinant GTF-B and GTF-D from Streptococcus mutans GS-5 expressed in Escherichia coli were studied. It was shown that after removal of α-glucosidase activity, GTF-D transglucosylated catechin with the highest efficiency. A maximal yield (expressed as the ratio of moles of glucoside formed to moles of catechin initially added) of 90% was observed with 10 mM catechin and 100 mM sucrose (Km, 13 mM) in 125 mM potassium phosphate, pH 6.0, at 37°C. 1H and 13C nuclear magnetic resonance spectroscopy revealed the structures of two catechin glucosides, (+)-catechin-4′-O-α-d-glucopyranoside and (+)-catechin-4′,7-O-α-di-d-glucopyranoside. Fructose accumulation during glucosyl transfer from sucrose to the acceptor competitively inhibited catechin transglucosylation (Ki, 9.3 mM), whereas glucose did not inhibit catechin transglucosylation. The addition of yeasts was studied in order to minimize fructose inhibition by means of fructose removal. For this purpose, the yeasts Pichia pastoris and the mutant Saccharomyces cerevisiae T2-3D were selected because of their inabilities to utilize sucrose. Addition of P. pastoris or S. cerevisiae T2-3D to the standard reaction mixture resulted in a twofold increase in the duration of the maximum GTF-D transglucosylation rate. The addition of the yeasts also stimulated sucrose utilization by GTF-D.
Many compounds with interesting physiological or organoleptic properties occur in nature as glycosides. Therefore, methods of glycosylating compounds which would otherwise be too volatile or have low solubility in aqueous systems are of interest to the pharmaceutical, cosmetics, and food industries.
There are two distinct methods of enzymatic glycosylation: reversed hydrolysis and transglycosylation. Because of the hydrolytic activity of the glycosidases catalyzing reversed hydrolysis, final glycoside yields are generally low. Use of high substrate concentrations or heterogeneous catalysis in organic solvents with low water activity may result in a reaction equilibrium shift toward reasonable glycoside synthesis (34). These complex reaction conditions can be avoided by using enzymes with transglycosylating activity and which cannot hydrolyze the glycosides formed.
Here we describe in detail the transglucosylating activity of glucosyltransferase-D (GTF-D) (EC 2.4.1.5) from Streptococcus mutans GS-5 toward the model acceptor compound (+)-catechin. Catechin is a polyphenol with a broad range of functions in medicinal and food applications (15). Most studies on transglycosylation have focused on saccharides as acceptor molecules, resulting in the formation of oligosaccharides (27). However, there have also been reports of various enzymes capable of transglycosylating (phenolic) alcohols: Bacillus subtilis X-23 α-amylase (EC 3.2.1.1) (23–25), Bacillus macerans cyclodextrin glucanotransferase (EC 2.4.1.19) (8), and Leuconostoc mesenteroides sucrose phosphorylase (EC 2.4.1.7) (15–18) and dextransucrase (EC 2.4.1.5) (27).
GTFs from the cariogenic streptococci are believed to play an important role in the formation of dental caries due to the production of glucans from dietary sucrose (12, 19). The extracellular, constitutively produced GTFs are classified according to the primer requirement to start glucan synthesis and to the type of glucan formed. The two main categories of glucans are the water-insoluble glucans, predominantly containing α-1,3-linked glucose, and the soluble glucans, rich in α-1,6-linked glucose (27). Extracellular streptococcal fluids regularly contain more than one enzymatic activity, often occurring in high-molecular-weight aggregates. Genes encoding various GTFs have been isolated. S. mutans expresses four different genes, gtfA, gtfB, gtfC, and gtfD (1, 13, 14, 26). The latter gene encodes GTF-D, which produces water-soluble glucan in a primer-stimulated manner (14). Other Streptococcus species, such as S. sobrinus and S. downei, also contain multiple gtf genes encoding distinct GTFs (11, 36).
For GTFs closely related to L. mesenteroides dextransucrase, synthesis of glucan is proposed to proceed according to a two-site insertion mechanism, resulting in addition of glucose to the reducing end of the glucan (27, 29, 31). Leucrose (5-O-α-d-glucopyranosyl-d-fructose) and glucose (arising from an acceptor reaction with water) are formed as minor products. Besides the transfer of glucose from sucrose to glucan, other carbohydrates, such as monosaccharides (d-mannose, d-galactose), disaccharides (maltose, lactose), or oligosaccharides (maltotriose), can act as acceptors for transglucosylation by dextransucrase, resulting in a homologous series of oligosaccharide products (27, 28).
In this study, we compared the transglucosylating activities of several S. mutans and S. sobrinus GTFs toward (+)-catechin as a model acceptor. The highly efficient catechin transglucosylation by GTF-D and especially the influence of fructose on GTF-D transglucosylating activity are described in detail.
MATERIALS AND METHODS
Growth conditions of S. sobrinus SL-1 and partial purification of GTF-T.
S. sobrinus SL-1 was kindly provided by R. R. B. Russell (Department of Oral Biology, University of Newcastle) and stored in glycerol (45%, vol/vol) at −80°C. A single colony of S. sobrinus SL-1 grown on an MRS plate at 37°C was used to inoculate 25 ml of MRS medium supplemented with cysteine (0.5 g/liter) and was grown statically at 37°C. Overnight cultures (1 ml) were used to inoculate 250 ml of modified Terleckyj et al. (32) medium and were incubated statically at 37°C. After reaching the stationary phase at an optical density at 600 nm (OD600) of 1.5 to 2.0, the culture supernatant was obtained by centrifugation (16,300 × g, 15 min, 4°C). A mixture of four glucosyltransferases (GTF-T) was partially purified and concentrated by ammonium sulfate precipitation (7). The supernatant was brought to 55% (326 g/liter) saturation with ammonium sulfate. The precipitate was collected by centrifugation (39,100 × g, 15 min, 4°C), dissolved in potassium phosphate buffer (25 mM, pH 6.0), and dialyzed against the same buffer.
Cultivation of Escherichia coli expressing recombinant GTF-B and GTF-D and preparation of cell extracts.
Escherichia coli containing S. mutans GS-5 gtfB(pYNB13) (33) and E. coli containing S. mutans GS-5 gtfD (pYND72) (30) were kindly provided by H. K. Kuramitsu (Department of Oral Biology, State University of New York at Buffalo). The E. coli strains were stored in glycerol (14%, vol/vol) at −80°C. Both strains were grown aerobically at 30°C in TY medium (1% NaCl, 3.2% tryptone, and 2% yeast extract) with 0.2 mM isopropyl-β-d-thiogalactoside. pYNB13 was maintained in medium supplemented with 50 μg of ampicillin per ml. pYND72 was maintained in medium supplemented with 68 μg of chloramphenicol per ml. Overnight cultures were harvested by centrifugation (16,300 × g, 15 min, 4°C). The cells were washed with Tris-hydrochloride buffer (20 mM, pH 7.5) and resuspended in the same buffer at a concentration of 0.2 ± 0.1 g of wet biomass/ml. After sonication (Sonifier 250; Branson [duty cycle, 30%; output control, 3]), 0.1 mM phenylmethylsulfonyl fluoride and 0.5 mg of DNase were added to the lysate. After centrifugation (39,100 × g, 15 min, 4°C), the supernatant was dialyzed against Tris-hydrochloride buffer (20 mM, pH 7.5) and used as a crude enzyme solution.
Partial purification of GTF-B.
GTF-B produced with E. coli was partially purified by ammonium sulfate precipitation. The crude enzyme solution was brought to 10% (50 g/liter) saturation with ammonium sulfate. The supernatant with GTF activity obtained after centrifugation (39,100 × g, 15 min, 4°C) was brought to 40% (169 g/liter) saturation and allowed to stand for 30 min. After centrifugation (39,100 × g, 15 min, 4°C), the precipitate was dissolved in potassium phosphate buffer (25 mM, pH 6.0) and dialyzed against the same buffer.
Partial purification of GTF-D.
GTF-D was initially partially purified by applying the cell extract on a DEAE-Sepharose CL-6B (Pharmacia) column (2.5 by 30 cm) previously equilibrated with Tris-hydrochloride buffer (20 mM, pH 7.5). Proteins were eluted by using a linear gradient of 0 to 0.7 M NaCl in the same buffer. Fractions containing GTF activity were concentrated by ultrafiltration (YM30; Amicon Corporation). The concentrated solution was dialyzed against potassium phosphate buffer (25 mM, pH 6.0). Under the same experimental conditions, GTF-D was also purified with a DE-52 cellulose anion-exchange column (Whatman).
α-Glucosidase activity.
Enzyme solutions were incubated with p-nitrophenyl-α-d-glucopyranoside (10 mM) and potassium phosphate buffer (125 mM, pH 6.0) in a volume of 1 ml. α-Glucosidase activity present in the crude enzyme solution was measured by monitoring the increase of p-nitrophenolate at 405 nm at 30°C. The molar extinction coefficient for p-nitrophenolate at pH 6.0 was 2,300 M−1 cm−1.
Glucosyltransferase transglucosylating activity.
GTF transglucosylating activity was quantified by two different methods: measurement of the formation of reducing sugars (RS activity) and of catechin glucosides (CG activity).
RS activity was measured by the dinitrosalicylic acid (DNS) method (21) or by high-performance liquid chromatography–refractive index (HPLC-RI) analysis. With the DNS method, samples of the reaction mixture were centrifuged (13,000 × g, 5 min) and 100 μl of the supernatant was mixed with 100 μl of DNS solution. The mixture was heated at 100°C for 5 min. After cooling, 1 ml of water was added and the absorption at 575 nm was measured spectrophotometrically. Glucose was used as a standard. Quantification of the amount of reducing sugars by HPLC-RI analysis was achieved with a Rezex RCM-Monosaccharide column (300 by 7.8 mm) (Phenomenex) with RI detection. Ultrapure water was used as mobile phase at a constant flow rate of 1 ml/min at 65°C.
One unit of RS activity was defined as the amount of enzyme which caused the release of 1 μmol of reducing sugar (expressed as glucose equivalent) per min in 125 mM potassium phosphate buffer, pH 6.0, with 60 mM sucrose at 37°C.
CG activity was measured with HPLC-UV. Catechin and catechin glucoside concentrations were measured on a C18 reversed-phase column (200 by 3 mm) (ChromPack). As mobile phase, a 30:70 (vol/vol) mixture of methanol and water (adjusted to pH 2.5 with orthophosphoric acid) was used at a constant flow rate of 0.5 ml/min at 25°C. Catechin and catechin glucosides were detected spectrophotometrically at 260 nm.
One unit of CG activity was defined as the amount of enzyme which catalyzes the formation of 1 μmol of catechin glucoside per min in 125 mM potassium phosphate buffer, pH 6.0, with 60 mM sucrose and 10 mM catechin at 37°C.
Catechin transglucosylation conditions.
Catechin transglucosylation studies were performed with a reaction solution containing potassium phosphate buffer (125 mM, pH 6.0), sucrose, (+)-catechin (Sigma), and water. After addition of GTF, the reaction mixture was incubated statically at 37°C.
Samples for HPLC analysis were prepared by mixing 50 μl of reaction aliquots with 450 μl of water (adjusted to pH 2 with HCl). After centrifugation (13,000 × g, 5 min), 400 μl of supernatant was used for analysis.
Separation of catechin transfer products.
GTF-D catechin transfer products for structure elucidation were separated with a two-step approach. First, 200 μl of reaction mixture was put on a silica column (10 by 1.5 cm; Silica Gel 60 [Merck]; particle size, 0.063 to 0.200 mm). Two fractions (A and B) were collected by eluting the silica column with 23 ml of 50% ethanol-H2O (vol/vol) and 18 ml of 50% ethanol-H2O (vol/vol) with 5% acetic acid (vol/vol). Both fractions were concentrated by vacuum distillation and dissolved in 125 mM potassium phosphate, pH 6.0. Further purification of catechin transfer products was done by collecting the individual peaks with the HPLC-UV method described above.
NMR spectroscopic analysis.
1H and 13C nuclear magnetic resonance (NMR) spectra were obtained with a 200 MHz AC200 spectrometer at room temperature. All nuclear Overhauser effect (NOE)-difference and two-dimensional NMR spectra measured were collected with a 400 MHz Bruker DPX 400 spectrometer.
CO2 measurements.
CO2 concentrations were determined by analyzing 100-μl gas phase samples on a Hewlett Packard HP 6890 gas chromatography system.
Growth conditions of Saccharomyces cerevisiae T2-3D and Pichia pastoris.
The mutant yeast S. cerevisiae T2-3D (35) was kindly provided by J. T. Pronk (Department of Microbiology, University of Delft). S. cerevisiae T2-3D was stored in glycerol (20%, wt/vol) at −80°C. P. pastoris (CBS 704) was stored in glycerol (50%, vol/vol) at −80°C. Both yeasts were grown aerobically in 1 liter of mineral salts medium with glucose (2%, wt/vol) and yeast extract (Difco) (0.2%, wt/vol). P. pastoris and S. cerevisiae were harvested by centrifugation (39,100 × g, 15 min, 4°C) at OD660s of 10 and 15, respectively. After washing of the cells, P. pastoris and S. cerevisiae were stored (−20°C) at concentrations of, respectively, 66 and 70 mg (dry weight) ml−1.
RESULTS
Partial purification of GTFs.
The culture supernatant from S. sobrinus SL-1 was harvested during the stationary phase. The initial RS activity of the mixture of four glucosyltransferases (GTF-T), 0.16 U/ml (DNS method), which was comparable with other reported activities for S. mutans strains (9, 10), was purified 13-fold by ammonium sulfate precipitation with 83% recovery (0.86 U/mg). After dialysis, no α-glucosidase activity was detected. This GTF-T enzyme preparation was used in the initial experiments, as shown in Table 1.
TABLE 1.
Comparison of transglucosylation activities of S. sobrinus SL-1 GTF-T, S. mutans GS-5 GTF-B, and S. mutans GS-5 GTF-D
| Enzyme | RS activity (U/ml)a
|
CG activity (U/ml)b | |
|---|---|---|---|
| Without (+)-catechin | With 5 mM (+)-catechin | ||
| GTF-T | 0.3 | 0.62 | 0.004 |
| GTF-B | 0.3 | 0.66 | 0.012 |
| GTF-D | 0.3 | 0.60 | 0.035 |
Reaction mixtures contained potassium phosphate buffer (125 mM, pH 6.0), sucrose (60 mM), and enzyme solution (0.3 U/ml) and were incubated for 30 min at 37°C. Analysis by the DNS method was performed.
In the presence of 5 mM (+)-catechin.
GTF-B and GTF-D, both expressed in E. coli containing the respective S. mutans GS-5 gtfB and gtfD genes (30, 33), were partially purified with the objective of removing α-glucosidase activity present in the cell extract. When the cell extract containing GTF-B was applied to a DEAE-Sepharose CL-6B column, GTF, assayed as RS activity (DNS method), could not be recovered with an NaCl gradient (20, 27). Subsequently, GTF-B was partially purified by ammonium sulfate precipitation to remove the α-glucosidase activity. The RS activity of GTF-B was purified twofold with 39% recovery. This partially purified enzyme preparation (5.4 U/ml and 0.5 U of RS activity per mg [DNS method]) was used in the initial experiments (Table 1).
Using cell extract containing GTF-D, it was possible to elute RS activity (DNS method) from the DEAE-Sepharose CL-6B column. The pooled fractions devoid of α-glucosidase activity were used in the initial experiments to compare the various GTF activities (Table 1). GTF-D was purified with 44% recovery, while the specific activity increased ninefold (9.1 U/mg). The RS active fractions (DNS method) did not elute as a sharp peak but like a smear (0.05 to 0.4 M NaCl). This could be overcome by using a DE-52 (Whatman) cellulose anion-exchange column (14). In this way, GTF-D was purified eightfold with 55% recovery (7.4 U/ml and 7.2 U of RS activity per mg [HPLC-RI method]). This GTF-D preparation was used to perform kinetics experiments and to identify catechin transfer products.
Catechin transglucosylation with different GTFs.
The partially purified GTF preparations (0.3 U/ml) were compared for their transglucosylating activities toward 5 mM catechin as a model acceptor. Both the RS activity (expressed as reducing sugars released in the presence of catechin [DNS method]) and the CG activity (expressed as glucose incorporated into catechin [HPLC method]) were determined (Table 1).
With all enzyme preparations, the addition of catechin resulted in approximately a twofold increase in the RS activity. A comparison between the CG and RS activities revealed that GTF-D had the highest efficiency for catechin transfer product formation, at 5 mM catechin. Therefore, catechin transglucosylation was further studied with this enzyme.
Characteristics of GTF-D transfer products.
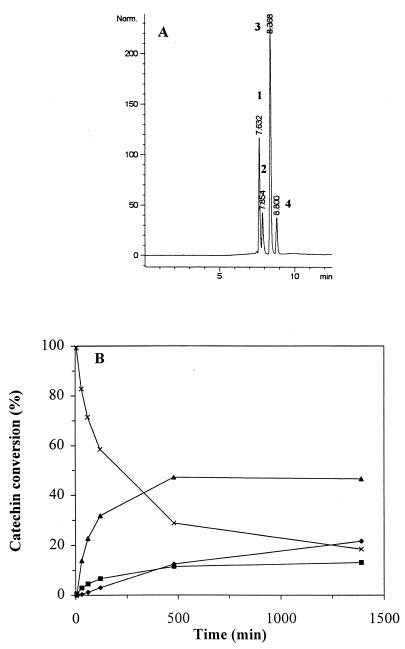
HPLC analysis showed that during the incubation of GTF-D with catechin and sucrose, three distinguishable peaks, hereafter labeled peaks 1 (tr, 7.63), 2 (tr, 7.85), and 3 (tr, 8.37) in order of increasing HPLC retention times (tr), were detected (Fig. 1A). While the total area of peaks 1, 2, and 3 increased, the catechin peak area decreased (peak 4; tr, 8.80 [Fig. 1A and B]). Catechin polymerization was not observed under these conditions. Incubation of the reaction solution with Aspergillus niger amyloglucosidase (Sigma) (EC 3.2.1.3) resulted in a decrease in the three product peaks and an increase in catechin (data not shown).
FIG. 1.
Formation of GTF-D catechin transfer products. (A) HPLC chromatogram showing the three catechin transfer products (1, 2, and 3) and catechin (4) after 24 h of incubation with 10 mM catechin, 60 mM sucrose, and 37 mU of GTF-D per ml at 37°C. (B) Formation of the three catechin transfer products (peak 1 [⧫], peak 2 [■], peak 3 [▴]) and catechin (×) with 10 mM catechin, 60 mM sucrose, and 37 mU of GTF-D per ml at 37°C.
Comparison of the UV spectra between 200 and 400 nm of catechin and of the catechin transfer products revealed no significant differences. Besides absorption maxima at 220 and 277 nm, an absorption minimum at 251 nm and a shoulder at 231 were detected. Therefore, the molar extinction coefficients of catechin and the catechin transfer products were assumed to be the same.
Identification of catechin transfer products.
The exact identity of the GTF-D catechin transfer products was established by a comprehensive NMR spectroscopic study. To obtain the purified catechin transfer products, the mixture of GTF-D catechin transfer products was separated in two steps as described in Materials and Methods. After the first step (silica column chromatography), two fractions, labeled A and B, were collected. Fraction A contained peaks 1 and 3, hereafter labeled products 1 and 3. Fraction B contained peak 2, hereafter labeled product 2, and catechin. After purification, we obtained 6.7, 3.2, and 16.1 μmol of products 1, 2, and 3, respectively, from the initial 250 μmol of catechin (25-ml reaction volume containing 60 mM sucrose and 37 mU of GTF-D/ml). Product 2 was not chemically stable upon prolonged storage, so no further attempts were made to elucidate its structure.
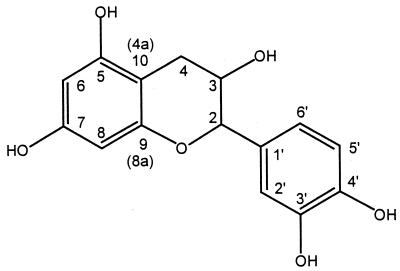
The spectroscopic structure elucidation of products 1 and 3 started with the assignment of catechin protons and carbon atoms (Fig. 2; Table 2). This assignment was based on COLOC measurements in dimethyl sulfoxide-d6 in combination with 1H and 13C (broad-band proton-decoupled and DEPT) NMR. The assignment of catechin is in line with a recent analysis (3) in which the assignment was based on HMQC, HMBC, and NOE-difference spectroscopy.
FIG. 2.
Structure and atom numbering of catechin.
TABLE 2.
Assigned NMR data for catechin and products 1 and 3
| δ Value versus tetramethyl silane (position) [difference]a
| ||
|---|---|---|
| Catechin | Product 1 | Product 3 |
| 1H NMR spectrum | ||
| 2.45 (dd, 1H, H-4a) | 2.40 (H-4a) | 2.35 (H-4a) [−0.10] |
| 2.75 (dd, 1H, H-4b) | 2.70 (H-4b) | 2.70 (H-4b) |
| 3.92 (m, 1H, H-3) | 3.89 (H-3) [−0.03] | 3.85 (H-3) [−0.07] |
| 4.58 (d, 1H, H-2) | 4.60 (H-2) [+0.02] | 4.55 (H-2) [−0.03] |
| 4.95 (d, 1H, OH) | ||
| 5.79 (d, 1H, H-8) | 6.05 (H-8) [+0.26] | 5.70 (H-8) [−0.09] |
| 5.98 (d, 1H, H-6) | 6.16 (H-6) [+0.18] | 5.90 (H-6) [−0.08] |
| 6.69 (dd, 1H, H-6′) | 6.73 (H-6′) [+0.04] | 6.72 (H-6′) [+0.03] |
| 6.78 (d, 1H, H-5′) | 7.15 (H-5′) [+0.37] | 7.14 (H-5′) [+0.36] |
| 6.82 (d, 1H, H-2′) | 6.80 (H-2′) [−0.02] | 6.79 (H-2′) [−0.03] |
| 5.18 and 5.22 (H-1" gluc) | 5.17 (H-1" gluc) | |
| 13C NMR spectrum | ||
| 27.87 (C-4) | 27.75 | 27.86 |
| 60.62, 60.75 | 60.77 | |
| 66.37 (C-3) | 66.02 | 66.30 |
| 69.87–73.73 (8×) | 70.01–73.75 (4×) | |
| 81.04 (C-2) | 80.77 | 80.70 |
| 93.93 (C-8) | 95.50* and 96.72* [+1.57] | 93.87 |
| 95.19 (C-6) | 97.97* [+2.78] | 95.26 |
| 99.13 (C-10 [4a]) | 100.30* and 102.07* | 99.03* and 100.28* |
| 114.57 (C-2′) | 114.75 | 114.83 |
| 115.16 (C-5′) | 117.31 [+2.15] | 117.25 [+2.09] |
| 118.50 (C-6′) | 118.21 | 118.30 |
| 130.67 (C-1′) | 134.51 [+3.84] | 134.70 [+4.03] |
| 144.89 (C-3′ + C-4′) | 144.72 | 144.71 |
| 147.04 [+2.15] | 147.01 [+2.12] | |
| 155.42 (C-9 [8a]) | 155.11* | 155.24* |
| 156.23 (C-5) | 156.14* | 156.25* |
| 156.51 (C-7) | 156.57* | 156.54* |
δ Values are in parts per million. Values in brackets are differences between δ values for catechin and the corresponding values for product 1 or 3. *, assignments may be interchangeable.
After the assignment of catechin, the spectra of products 1 and 3 were analyzed. These spectra were obtained with a combination of 1H, 13C, and H,C-correlated HETCOR + COLOC spectroscopy and NOE-difference spectroscopy. The 1H NMR spectra showed peaks in the range of 3.14 to 3.91 ppm, characteristic of sugar protons. Critical in the assignment of products 1 and 3 was the analysis of H-1" (proton at the anomeric sugar carbon) and H-2 peaks (Table 2). Both proton peaks were assigned unambiguously and were clearly separated from any other peaks in the spectrum. Based on the integral ratio of the peaks for H-1" and H-2 with respect to the peaks at 3.14 to 3.91 ppm, it was concluded that products 1 and 3 are sugar derivatives of catechin. For product 3, the integral ratio of H-1" to H-2 to the 3.14-to-3.91-ppm peaks was 1.00:0.80:10.91, which is indicative of a monoglucosylated catechin. For product 1, this ratio was 2.00:0.92:21.90, which is indicative of a diglucose-substituted catechin.
Based on the coupling constant between glucose H-1" and H-2" (J = 3.6 Hz [data not shown]), it was concluded that both products 1 and 3 were α-glucosylated catechin molecules. In the case of a β-glucosylated catechin molecule, the coupling constant between H-1" and H-2" should be about 7 Hz.
The position of the glucose substituent of product 3 was assigned by comparison of the 1H and 13C NMR data (Table 2) with literature data. Our data were in line with data obtained (in CD3OD) for substitution at the 4′ position (6), as the δ-value for H-5′ has shifted 0.36 ppm downfield with respect to its position in catechin. This was at variance with data obtained for substitution at the 3′ position (8). Subsequently, NOE-difference 1H spectroscopy, which showed no cross-coupling on irradiation at H-2 and H-8, was performed. This indicated that no substitution at the 3 or 7 position had taken place. Clear cross-coupling with the H-1" peak (5.17 ppm) upon irradiation of H-5′ was observed. This unambiguously supported the assignment of a substitution at 4′. Therefore, product 3 was identified as catechin-4′-α-O-glucoside.
Based on the assignment of the monoglucosylated product 3, the spectra of the diglucosylated product 1 were interpreted. The H-5′ signal of product 1 has a very similar downfield shift, as was observed for this proton in product 3 (Table 2), indicative of the substitution of one glucose molecule at the 4′ position of catechin. Observation of the substantial difference of the 1H signals for H-6 and H-8 suggests that substitution of the second glucose molecule has taken place at the 5 or 7 position rather than, e.g., at the 3 or 3′ position, or that a second substitution on the glucose at the 4′ position has occurred. In combination with literature data for substitution at the 5 position (2) or 7 position (6), it became clear that simple one-dimensional 1H or 13C NMR spectroscopy could not answer this question. However, with NOE-difference 1H spectroscopy, the structure of product 1 could be safely assigned. Irradiation at H-8 gave a cross-peak with the H-1" of one glucose molecule, while irradiation at H-5′ gave cross-coupling with H-1" of the second glucose. Consequently, product 1 was identified as catechin-4′,7-O-α-di-d-glucoside.
Hydrolysis of catechin glucosides by amyloglucosidase.
The identification of product 3 as catechin-4′-O-α-d-glucoside and product 1 as catechin-4′-7-O-α-di-d-glucoside made it tempting to assume that product 2 was catechin-7-O-α-glucoside. Hydrolysis of the identified catechin glucosides by A. niger amyloglucosidase was used in an attempt to confirm this assumption.
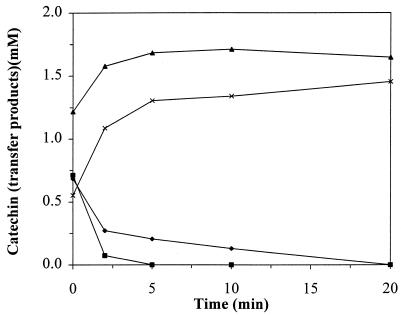
Incubation of product 1 with amyloglucosidase resulted in hydrolysis of product 1 and simultaneous accumulation of product 3, while no accumulation of product 2 was observed. Prolonged incubation with A. niger amyloglucosidase resulted in complete hydrolysis of product 3 with quantitative recovery of catechin (data not shown). Based on this experiment, the identity of product 2 as catechin-7-O-α-glucoside could not be confirmed. More insight was gained by hydrolyzing a mixture of the three different catechin glucosides by A. niger amyloglucosidase (Fig. 3). Here the direct formation of catechin due to hydrolysis of product 2 and the formation of product 3 due to hydrolysis of product 1 can be observed.
FIG. 3.
Hydrolysis of the three catechin transfer products (1 [⧫], 2 [■], 3 [▴]) and formation of catechin (×), with 140 U of A. niger amyloglucosidase per ml at 55°C.
Kinetics of GTF-D catechin transglucosylation.
Catechin transglucosylation assays were performed with 125 mM potassium phosphate buffer at pH 6.0. It appeared that under these reaction conditions the reaction rate was more or less constant during the first 60 min (Fig. 1B). Subsequently initial CG activities were determined based on the amount of catechin glucoside formed after 60 min of incubation. Variations in potassium phosphate concentration (25 to 250 mM) and pH (5.0 to 7.5) had very little effect on the initial CG activity (with 60 mM sucrose and 10 mM catechin).
The effect of the catechin concentration on initial catechin transglucosylation rates was studied at catechin concentrations varying between 1 and 20 mM with 60 mM sucrose. The reaction appeared to be first order with respect to catechin concentrations below 15 mM (60 mU/ml [CG activity]). The yield of the catechin transglucosylation reaction (expressed as the ratio of moles of catechin glucoside formed after 24 h to the amount of catechin initially added) varied from 35% at 1 mM initial catechin to 75% at an initial catechin concentration of 15 mM. Due to the low water solubility of catechin (25 mM) the Km could not be determined.
With an initial concentration of 10 mM catechin, the effect of the initial sucrose concentration on the glycoside yield was studied. The yield was 55% at a 10 mM initial sucrose concentration and increased to 90% at sucrose concentrations above 100 mM. The Km for sucrose was estimated to be 13 mM (24 mU of CG activity per ml) by plotting the experimental data in a Lineweaver-Burk plot.
Fructose inhibition of GTF-D activity.
The total catechin glucoside formation rate, as shown in Fig. 1B, gradually decreased as the reaction proceeded. The decrease in catechin concentration during the reaction was simulated, assuming first-order kinetics with respect to the catechin concentration and zero-order kinetics with respect to the sucrose concentration. Comparison of the experimentally determined data (Fig. 1B) with the simulated curve (data not shown) showed that the decrease in the reaction rate was stronger than would be expected based solely on the decrease in the catechin concentration. In other studies with glucosyltransferases, the accumulation of fructose was shown to competitively inhibit GTF enzyme activity (4). We therefore studied the inhibitory effect of fructose on catechin transglucosylation by GTF-D.
Reaction mixtures containing 37 mU of GTF-D per ml, 10 to 150 mM sucrose, and 0 to 200 mM fructose indicated the competitive inhibition of catechin transglucosylation by fructose. With Lineweaver-Burk plots (data not shown), the Ki for fructose was calculated to be about 9.3 ± 1.0 mM. Under the same experimental conditions used to determine fructose inhibition, glucose (10 to 150 mM) showed no significant inhibition of catechin transglucosylation.
Glucose and fructose consumption by P. pastoris and S. cerevisiae T2-3D.
To overcome the inhibitory influence of fructose accumulation on catechin transglucosylation, we studied the possibility of using yeasts to remove fructose. Two yeasts, the methylotrophic yeast P. pastoris and a mutant, S. cerevisiae T2-3D, were selected. Both strains are incapable of fermenting sucrose. This was confirmed by measuring saccharide-dependent carbon dioxide formation. No additional formation of CO2 was observed after addition of sucrose in contrast to addition of either glucose or fructose. Furthermore, the sucrose concentration, monitored by HPLC-RI, did not change during incubation of P. pastoris or S. cerevisiae with sucrose.
The potential toxic and/or inhibitory influence of the polyphenol catechin on P. pastoris and S. cerevisiae was also examined. Both yeasts were incubated for 60 min with different concentrations of catechin (1 to 10 mM). The maximal CO2 formation rate was determined after addition of a pulse of fructose (20 mM). With P. pastoris there appeared to be no effect on fructose fermentation, whereas S. cerevisiae appeared to be slightly inhibited by higher catechin concentrations. Based on these experiments, P. pastoris appeared to be the most suitable strain for the selective removal of fructose during catechin transglucosylation. However, both yeasts were tested.
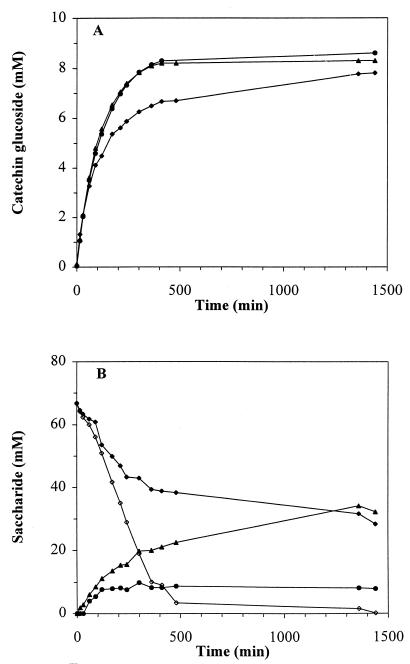
During a 24-h incubation period in the absence or presence of yeast cells (P. pastoris or S. cerevisiae), both catechin glucoside and saccharide concentrations (fructose, glucose, and sucrose) were monitored (Fig. 4). In the presence of P. pastoris or S. cerevisiae, no accumulation of significant amounts (less than 1 mM) of glucose or fructose was observed. Incubation without added yeasts showed accumulation of 29 mM fructose and 8 mM glucose after 24 h of incubation. Fructose and glucose consumption by P. pastoris (Fig. 4) or S. cerevisiae resulted in a prolongation of the maximum CG activity and an increase in the final catechin glucoside yield.
FIG. 4.
(A) Catechin glucoside formation by S. mutans GS-5 GTF-D in the absence (⧫) or presence of P. pastoris (●) (66 mg [dry weight]/ml) or S. cerevisiae T2-3D (▴) (70 mg [dry weight]/ml). (B) Saccharide concentrations (⧫, sucrose; ▴, fructose; ●, glucose) in the absence (closed symbols) and in the presence (open symbols) of P. pastoris. The saccharide profile with S. cerevisiae T2-3D was very similar to the saccharide profile with P. pastoris. The reaction solutions (5 ml) contained 125 mM potassium phosphate buffer (pH 6.0), 60 mM sucrose, 10 mM catechin, and 37 mU of GTF-D per ml.
Interestingly, monosaccharide removal also affected sucrose consumption by GTF-D. After 500 min, the sucrose was almost completely consumed in the presence of the yeasts, whereas without yeast addition more than half of the sucrose was still present.
DISCUSSION
In this study we have compared the transglucosylating activity toward catechin of several GTFs from the genus Streptococcus. The transglucosylation characteristics of S. mutans GS-5 GTF-D were studied in more detail. Although catechin has a complex structure, containing five hydroxyl groups, this compound was used as a model acceptor to allow comparison with literature data on other catechin-transglucosylating enzymes (8, 15, 22).
The different GTF preparations tested were a mixture of the four GTFs from S. sobrinus SL-1 (GTF-T) and the heterologously produced GTF-B and GTF-D from S. mutans GS-5. Note that the three GTF preparations were not purified to homogeneity.
All GTF preparations were capable of catechin transglucosylation (Table 1), although at different rates. The CG activity of GTF-D was almost three times higher than the CG activity of GTF-B and eight times higher than the CG activity of GTF-T. Considering the ratio between the RS and CG activity of the three GTF preparations, we decided to study the transglucosylation of catechin in more detail using GTF-D from S. mutans GS-5.
Besides catechin transglucosylating activity, all three GTF preparations showed an increase in the formation rate of reducing sugars in the presence of catechin. Especially in the case of GTF-T, this increase can hardly be explained by assuming that catechin is a better glucosyl acceptor than dextran, because almost no catechin glucoside formation was observed. An explanation could be that upon dextran chain elongation the glucosyl transfer rate decreases. If we assume that the presence of catechin results in premature displacement of the growing dextran chain from the active site, catechin addition would result in an enhanced rate of (short-chain) dextran formation and hence also in an increase in reducing sugar formation.
Based on HPLC analysis, glucosylation of catechin resulted in the formation of (at least) three catechin transfer products. Two catechin transfer products were characterized in more detail. Although catechin glucoside separation was not optimized, sufficient catechin glucoside was isolated to perform 1H and 13C NMR spectroscopy. The structures of two catechin transfer products were identified as (+)-catechin-4′-O-α-d-glucoside and (+)-catechin-4′,7-O-α-di-d-glucoside. In the literature, 4′-O-α (22) and 3′-O-α (8, 15) glucosylated catechins have been described. The catechin diglucoside characterized in this study is to our knowledge the first catechin diglucoside described. The structure of the third catechin transfer product (labeled product 2) could not be spectroscopically elucidated. However, the deglucosylation study with A. niger amyloglucosidase suggests that this compound could be 7-O-α monoglucosylated catechin. Note that the A. niger amyloglucosidase preferentially deglucosylates the 7-O-α glucose of the diglucoside. The fast hydrolysis of product 1 into product 3 and the fast hydrolysis of product 2 into catechin are therefore indicative of glucosylation of C-7 rather than C-5, C-3, or C-3′ in product 2.
In comparison with literature data concerning catechin transglucosylation (15, 22), the catechin transglucosylation efficiency of GTF-D appears to be very high. Based on the data in Table 1, a minimal glucosyltransfer efficiency of 58% can be calculated assuming that all the reducing sugars formed are fructose. In a number of studies, the transfer ratio (or yield) has been used to quantify the transglucosylating potential of enzyme preparations (15, 22). The transfer ratio is expressed as the ratio between the moles of catechin glucoside formed and moles of catechin initially added. Depending on the sucrose concentration, the transfer ratio for catechin glucosylation with GTF-D varied between 55 and 90%. Sucrose phosphorylase (15) was reported to have a similar transfer ratio, but a much higher sucrose concentration (30%, wt/vol) was used. The mixture of GTFs from S. sobrinus (22) also transglucosylates catechin, but the reported transfer ratio is lower than the transfer ratio of the partially purified GTF-D that we studied. The data reported for catechin transglucosylation with cyclodextrin glucanotransferase are difficult to compare because of the use of soluble starch as the glucosyl donor (8).
Detailed analysis of catechin glucosylation kinetics made it clear that the decrease in the catechin concentration during the reaction could not solely explain the observed decrease in GTF-D activity. We showed that fructose, which can be regarded as a side product of the transglucosylation reaction, competitively inhibits GTF-D. The observed decrease in GTF-D activity as the reaction proceeds is therefore most likely the result of a combination of the decrease in catechin concentration and fructose inhibition, although product inhibition by catechin glucosides cannot be ruled out.
To demonstrate and minimize the influence of fructose on catechin transglucosylation, the effect of fructose removal from the reaction mixture was studied. To achieve this, two yeasts, P. pastoris and S. cerevisiae T2-3D, incapable of sucrose consumption, were used. Addition of P. pastoris or S. cerevisiae to the catechin transglucosylation mixture resulted in a twofold increase of the duration of the maximal GTF-D activity, an increase of catechin glucoside production (maximum, 24%), and complete sucrose utilization (Fig. 4). The decrease in catechin glucoside formation rate after 500 min of incubation was most likely due to sucrose depletion and very low catechin concentrations. Interestingly, the removal of fructose and, hence, fructose inhibition by fermentation also resulted in complete sucrose utilization. As sucrose consumption by P. pastoris and S. cerevisiae was ruled out, the complete sucrose utilization by GTF-D is due to the removal of fructose.
The transglucosylation efficiency based on sucrose utilization by GTF-D can be very high. However, the use of yeasts to enhance the GTF-D transglucosylation rate resulted in drastically decreased efficiency of sucrose utilization. Therefore, depending on the optimization objective, yeasts should be added or omitted. It would be interesting to study whether dextran formation can also be stimulated by fructose removal, as has been shown here for catechin transglucosylation.
ACKNOWLEDGMENTS
We thank H. K. Kuramitsu, J. T. Pronk and R. R. B. Russell for their contributions to this study.
This investigation was supported by the Innovation Oriented Research Program on Catalysis of The Netherlands Ministry of Economic Affairs.
REFERENCES
- 1.Aoki H, Shiroza T, Hayakawa M, Sato S, Kuramitsu H K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986;53:587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui C B, Tezuka Y, Kikuchi T, Nakano H, Tamaoki T, Park J H. Constituents of a fern, Davallia mariesii Moore. IV. Isolation and structures of a novel norcarotane sesquiterpene glycoside, a chromone glucuronide, and two epicatechin glycosides. Chem Pharm Bull. 1992;40:2038–2040. [Google Scholar]
- 3.Davis A L, Cai Y, Davies A P, Lewis J R. 1H and 13C NMR assignments of some green tea polyphenols. Magn Reson Chem. 1996;34:887–890. [Google Scholar]
- 4.Devulapalle K S, Mooser G. Subsite specificity of the active site of glucosyltransferases from Streptococcus sobrinus. J Biol Chem. 1994;269:11967–11971. [PubMed] [Google Scholar]
- 5.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 6.Foo L Y, Karchesy J J. Polyphenolic glycosides from Douglas fir inner bark. Phytochemistry. 1989;28:1237–1240. [Google Scholar]
- 7.Fukui K, Moriyama T, Miyake Y, Mizutani K, Tanaka O. Purification and properties of glucosyltransferase responsible for water-insoluble glucan synthesis from Streptococcus mutans. Infect Immun. 1982;37:1–9. doi: 10.1128/iai.37.1.1-9.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funayama M, Nishino T, Hirota A, Murao S, Takenishi S, Nakano H. Enzymatic synthesis of (+)-catechin-α-glucoside and its effect on tyrosinase activity. Biosci Biotechnol Biochem. 1993;57:1666–1669. [Google Scholar]
- 9.Furuta T, Koga T, Nisizawa T, Okahashi N, Hamada S. Purification and characterization of glucosyltransferases from Streptococcus mutans 6715. J Gen Microbiol. 1985;131:285–293. doi: 10.1099/00221287-131-2-285. [DOI] [PubMed] [Google Scholar]
- 10.Furutani M, Iwaki M, Yagi T, Iida M, Horiike K, Nozaki M. A simple purification method for a glucosyltransferase complex from Streptococcus mutans OMZ 176 with a high yield. Int J Biochem. 1988;20:1327–1332. doi: 10.1016/0020-711x(88)90238-8. [DOI] [PubMed] [Google Scholar]
- 11.Giffard P M, Allen D M, Milward C P, Simpson C L, Jaques N H. Sequence of the gtfK gene of Streptococcus salivarius ATCC 25975 and evolution of the gtf genes of oral streptococci. J Gen Microbiol. 1993;139:1511–1522. doi: 10.1099/00221287-139-7-1511. [DOI] [PubMed] [Google Scholar]
- 12.Hamada S, Slade H D. Biology, immunology and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988;56:1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989;57:2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitao S, Ariga T, Matsudo T, Sekine H. The syntheses of catechin-glucosides by transglycosylation with Leuconostoc mesenteroides sucrose phosphorylase. Biosci Biotechnol Biochem. 1993;57:2010–2015. [Google Scholar]
- 16.Kitao S, Sekine H. Transglucosylation catalyzed by sucrose phosphorylase from Leuconostoc mesenteroides and production of glucosyl-xylitol. Biosci Biotechnol Biochem. 1992;56:2011–2014. doi: 10.1271/bbb.58.38. [DOI] [PubMed] [Google Scholar]
- 17.Kitao S, Sekine H. α-d-Glucosyl transfer to phenolic compounds by sucrose phosphorylase from Leuconostoc mesenteroides and production of α-arbutin. Biosci Biotechnol Biochem. 1994;58:38–42. doi: 10.1271/bbb.58.38. [DOI] [PubMed] [Google Scholar]
- 18.Kitao S, Sekine H. Synthesis of two kojic acid glucosides with sucrose phosphorylase from Leuconostoc mesenteroides. Biosci Biotechnol Biochem. 1994;58:419–420. doi: 10.1271/bbb.58.38. [DOI] [PubMed] [Google Scholar]
- 19.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Lassiter M O, Banas J A, Galperin M Y, Taylor K G, Doyle R J. Multiple glucan-binding proteins of Streptococcus sobrinus. J Bacteriol. 1996;178:1572–1577. doi: 10.1128/jb.178.6.1572-1577.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller G L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 22.Nakahara K, Kontani M, Ono H, Kodama T, Tanaka T, Ooshima T, Hamada S. Glucosyltransferase from Streptococcus sobrinus catalyzes glucosylation of catechin. Appl Environ Microbiol. 1995;61:2768–2770. doi: 10.1128/aem.61.7.2768-2770.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura T, Kometani T, Takii H, Terada Y, Okada S. Purification and some properties of α-amylase from Bacillus subtilis X-23 that glucosylates phenolic compounds such as hydroquinone. J Ferment Bioeng. 1994;78:31–36. [Google Scholar]
- 24.Nishimura T, Kometani T, Takii H, Terada Y, Okada S. Acceptor specificity in the glucosylation reaction of Bacillus subtilis X-23 α-amylase towards various phenolic compounds and the structure of kojic acid glucoside. J Ferment Bioeng. 1994;78:37–41. [Google Scholar]
- 25.Nishimura T, Kometani T, Takii H, Terada Y, Okada S. Glucosylation of caffeic acid with Bacillus subtilis X-23 α-amylase and a description of the glucosides. J Ferment Bioeng. 1995;80:18–23. [Google Scholar]
- 26.Robeson J P, Barletta R G, Curtiss R., III Expression of a Streptococcus mutans glucosyltransferase gene in Escherichia coli. J Bacteriol. 1983;153:211–221. doi: 10.1128/jb.153.1.211-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robyt J F. Mechanisms in the glucansucrase synthesis of polysaccharides and oligosaccharides from sucrose. Adv Carbohydr Chem Biochem. 1995;51:131–168. doi: 10.1016/s0065-2318(08)60193-6. [DOI] [PubMed] [Google Scholar]
- 28.Robyt J F, Eklund S H. Relative, quantitative effects of acceptors in the reaction of Leuconostoc mesenteroides B-512F dextransucrase. Carbohydr Res. 1983;121:279–286. doi: 10.1016/0008-6215(83)84024-5. [DOI] [PubMed] [Google Scholar]
- 29.Robyt J F, Walseth T F. The mechanism of acceptor reactions of Leuconostoc mesenteroides B-512F dextransucrase. Carbohydr Res. 1978;61:433–445. doi: 10.1016/s0008-6215(00)84503-6. [DOI] [PubMed] [Google Scholar]
- 30.Shimamura A, Nakano Y, Musaka H, Kuramitsu H K. Identification of amino acid residues in Streptococcus mutans glucosyltransferases influencing the structure of the glucan product. J Bacteriol. 1994;176:4845–4850. doi: 10.1128/jb.176.16.4845-4850.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su D, Robyt J F. Determination of the number of sucrose and acceptor binding sites for Leuconostoc mesenteroides B-512FM dextransucrase, and the confirmation of the two-site mechanism for dextran synthesis. Arch Biochem Biophys. 1994;308:471–476. doi: 10.1006/abbi.1994.1067. [DOI] [PubMed] [Google Scholar]
- 32.Terleckyj B, Willett N P, Shockman G D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975;11:649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsumori H, Minami T, Kuramitsu H K. Identification of essential amino acids in the Streptococcus mutans glucosyltransferases. J Bacteriol. 1997;179:3391–3396. doi: 10.1128/jb.179.11.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vic G, Biton J, Le Beller D, Michel J M, Thomas D. Enzymatic glucosylation of hydrophobic alcohols in organic medium by the reverse hydrolysis reaction using almond-β-d-glucosidase. Biotechnol Bioeng. 1995;46:109–116. doi: 10.1002/bit.260460204. [DOI] [PubMed] [Google Scholar]
- 35.Wenzel T J, van den Berg M A, Visser W, van den Berg J S, Steensma H Y. Characterization of Saccharomyces cerevisiae mutans lacking the E1α subunit of the pyruvate dehydrogenase complex. Eur J Biochem. 1992;209:697–705. doi: 10.1111/j.1432-1033.1992.tb17338.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita Y, Hanada N, Takehara T. Purification of a fourth glucosyltransferase from Streptococcus sobrinus. J Bacteriol. 1989;171:6265–6270. doi: 10.1128/jb.171.11.6265-6270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]