Highlights
-
•
Radiation-induced contrast enhancements (RICE) may develop after radiotherapy.
-
•
Bevacizumab improved short-term imaging and symptoms and prolonged progression-free time.
-
•
Long-term recurrence-rates of RICE and associated mortality are high.
-
•
Repeated courses of bevacizumab showed high efficacy in long-term treatment.
Keywords: anti-VEGF antibody Bevacizumab, Blood brain barrier disruption, Chemotherapy, Radiation necrosis, Re-irradiation. stereotactic radiosurgery
Abstract
Purpose
The appearance of radiation-induced contrast enhancements (RICE) after radiotherapy for brain metastases can go along with severe neurological impairments. The aim of our analysis was to evaluate radiological changes, the course and recurrence of RICE and identify associated prognostic factors.
Methods
We retrospectively identified patients diagnosed with brain metastases, who were treated with radiotherapy and subsequently developed RICE. Patient demographic and clinical data, radiation-, cancer-, and RICE-treatment, radiological results, and oncological outcomes were reviewed in detail.
Results
A total of 95 patients with a median follow-up of 28.8 months were identified. RICE appeared after a median time of 8.0 months after first radiotherapy and 6.4 months after re-irradiation. Bevacizumab in combination with corticosteroids achieved an improvement of clinical symptoms and imaging features in 65.9% and 75.6% of cases, respectively, both significantly superior compared to treatment with corticosteroids only, and further significantly prolonged RICE-progression-free survival to a median of 5.6 months. Recurrence of RICE after initially improved or stable imaging occurred in 63.1% of cases, significantly more often in patients after re-irradiation and was associated with high mortality of 36.6% after the diagnosis of flare-up. Response of recurrence significantly depended on the applied treatment and multiple courses of bevacizumab achieved good response.
Conclusion
Our results suggest that bevacizumab in combination with corticosteroids is superior in achieving short-term imaging and symptom improvement of RICE and prolongs the progression-free time compared to corticosteroids alone. Long-term RICE flare-up rates after bevacizumab discontinuation are high, but repeated treatments achieved effective symptomatic control.
1. Introduction
Radiotherapy is the leading treatment option for patients diagnosed with brain metastases and advanced stereotactic techniques have allowed for delivery of high dose radiation in abbreviated treatment schedules. Moreover, the steep dose falloffs of such stereotactic radiosurgery (SRS) plans have allowed for sparing of surrounding critical structures including normal brain parenchyma. Despite promising local control, a significant subset of patients may develop radiation-induced contrast enhancements (RICE) with associated acute and/or long-term symptomatic radiation injury. The definitions of these vary largely in the literature with the main etiological theory favoring a distinction between reversible contrast-enhanced lesions as blood–brain barrier disruption and irreversible cerebral radiation necrosis (RN), with the latter being more often associated with severe neurologic symptoms and high morbidity.
The pathophysiological basis of cerebral radiation injury includes a complex interplay of radiogenic blood–brain barrier disorders with increased vascular leakage, tissue hypoxia, inflammatory mediators, and glial cell injury leading to late sequelae RN [1], [2]. Efforts to investigate radiation treatment risk associated risk factors have identified high cumulative doses, re-irradiation, critical lesion locations and large tumor sizes to be critical risk factors in the development of RN [3], [4], [5]. Furthermore, the use of systemic agents including chemotherapy, immunotherapy, and targeted therapies has shown complex associations with rates of RICE/RN with much data indicating an increased risk when employed in concert [6], [7], [8]. Overall, wide ranges of RN incidence rates of 4 to 34% after a median time of 4 to 11 months following RT have been reported [5], [6], [7], [9], [10], [11], [12].
Neurosurgery allows pathological confirmation, which is still considered the gold standard for distinguishing between tumor growth and necrosis. Nevertheless, invasive surgery cannot be performed in every patient due to a lesion’s location, general patient’s health, or lack of utility in management. Moreover, surgical samples may contain insufficient quantities of tissue, which may or may not represent the plurality of the lesion. Thus, guidelines advocate for an interdisciplinary multistep approach in managing these complex cases with respect not only to criteria of radiological courses and (ir)reversibility of RICE lesions, but also RT techniques and dose ranges, treated volumes, time intervals after RT, progression patterns and occurring symptoms [13]. Even though magnetic resonance (MR)-based imaging combined with advanced diffusion or perfusion sequences has the potential to add clarity to a nebulous spectrum of diagnoses, the treatment remains challenging [14], [15].
Although surgery may yield both diagnostic and therapeutic results, it might not sufficiently improve cognitive functions. Different drug treatment options exist and include anti-edematous corticosteroids in combination with bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), used with a median dosage of 7.5 mg/kg in 4 cycles at a time interval of 2 weeks. Bevacizumab has been shown to improve neurological outcome in both a prospective double-blind randomized study of 14 patients of Levin et al. and in a number of retrospective trials [16], [17].
Further, studies of bevacizumab for newly diagnosed glioblastoma reported lower rates of pseudoprogression after RT of 2.2% in the bevacizumab group compared to 9.3% in the placebo group [18].
Nonetheless, RICE structural changes might be irreversible and discontinuation of RICE-specific treatment can lead to severe RN and RN-recurrences [19], [20]. Further, with the rise of simultaneous application of systemic immuno- or targeted therapies in the modern era, data for the influence on RICE/RN development and treatment is limited.
Due to the severe RT-induced side effects and neurologic impairment of RN, an improved understanding of the treatment and course of changes as well as the identification of risk factors is critical. As such, we aimed to analyze the long-term outcomes of RICE/RN treatment strategies and influencing patient and dosimetric factors and, moreover, to explore risk of recurrence in this setting.
2. Materials and methods
In this single institutional retrospective analysis, we reviewed all cases of patients with brain metastases of different cancer types, who developed RICE after treatment with radiotherapy. Ethics approval for the study and a waiver of written informed consent was granted by the Heidelberg University Ethics Review Board (S-494/2021). The analysis was performed following institutional guidelines and the Declaration of Helsinki of 1975 in its most recent version. Patient confidentiality was maintained by anonymizing patient data to remove any identifying information.
2.1. Radiotherapy and RICE-specific treatment
Patient demographic, radiation-, cancer- and RICE/RN-specific treatment, radiology as well as toxicity and oncological outcomes were reviewed. The RICE-specific treatments were determined and corticosteroid doses were calculated to dexamethasone-equivalents. Patients were grouped into three RICE-specific treatment groups: “No therapy”, “corticosteroids only” and “bevacizumab”. In the latter group of bevacizumab treatment, patients with and without a combination of bevacizumab with corticosteroids were included. The application of systemic drug treatments was defined as “combined” when given within two months before/after RT, while “simultaneous” was reserved to a shorter interval below one week.
All patients were treated with RT for brain metastases with either whole-brain RT (WBRT) or single or hypofractionated stereotactic radiosurgery. Correlations of treatment RT plans confirmed RICE localization to the previously treated lesion. Treatment technique, total dose, and fractionation was specified according to lesion number, size, and location in accordance with international guidelines and tissue constraints [21], [22]. Whole-brain radiotherapy was delivered using three-dimensional conformal RT with two laterally opposing fields using a conventional linear accelerator. For stereotactic radiosurgery either a conventional linear accelerator with micro-multileaf-collimator (Siemens Mevatron, Erlangen, Germany or Elekta Versa, Stockholm, Sweden) and a planning target volume (PTV) of 3 mm, or a frameless image-guided Cyberknife Robotic Radiosurgery (Accuray Incorporated, Sunnyvale, CA) with a planning margin of 1 mm was utilized. Prior to radiosurgery, pre-medication with corticosteroid (oral dexamethasone 8 mg before SRS, in the evening after SRS and in the morning 1 day after SRS) was administered. Data for stereotactic irradiated gross tumor and PTV, prescribed isodoses, dose conformity index and PTV-coverage were evaluated. The BED and EQD2 were calculated for each lesion and described as cumulative values in cases of re-irradiation. We assumed an α/β ratio of 2 Gy for normal brain tissue. For calculation of the biologically effective dose (BED) the linear-quadratic model was used: Biologically effective dose (Gy) = fraction dose × fraction number × (1 + fraction dose/α/β).
2.2. Imaging review
All available post-treatment brain scans of MR images were reviewed and included at least contrast-enhanced T1-weighted post-gadolinium MRI scans as well as T2-weighted imaging. Whenever available, additional information of advanced MR techniques such as diffusion or perfusion images were also assessed for diagnosis of RICE. Diagnosis of RICE was determined on the basis of radiology results and consensus in institutional interdisciplinary tumor conferences with radiologists, radiation oncologists, medical oncologists and neurosurgeons. Imaging review included largest extents of T2-weighted fluid-attenuated inversion recovery (FLAIR) signal changes (representing edema) surrounding the lesion and of contrast-enhanced T1-weighted post-gadolinium MRI scans were documented. An increased diameter of ≥ 20% was defined as progression, a decrease of ≥ 20% was defined as regression. Locations in lobe, sites and distance to the ventricular system and terminal cortical or deep zones were evaluated.
2.3. Toxicity and oncologic follow-up
For each patient, follow-up visits with clinical data and toxicities, oncologic- and RICE-specific treatment, referring physician notes and radiological imaging were reviewed. Clinical outcome included overall survival (OS) and time to distant cerebral progression. Overall survival was defined from the time of first RT until last contact or date of death, while time to distant cerebral progression was defined from the time of first RT until any cerebral progression apart from the RN lesion. Acute (≤90 days) and late (>90 days) toxicity were graded according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0). For a better overview of the results, nervous system disorders according to CTCAE terms were partly summarized as neurological impairments. An improvement or deterioration of symptoms during treatment and follow-up was defined according to changes in the CTCAE grading.
2.4. Statistical analysis
Kaplan–Meier analyses were utilized to calculate survival and the log-rank test or Cox regression to further compare subgroups, using a p-value of <0.05 as statistically significant. To assess the influence of cofactors, uni- and multivariate Cox-proportional hazards ratios (HR) with a 95% confidence interval (95%CI) were applied. Data was compared using the Mann–Whitney–U tests or Pearson Chi-Square tests for continuous or categorical data. The IBM statistical software SPSS was used for statistical calculations (version 28, Armonk, NY, USA).
3. Results
During August 2010 and December 2021 approximately 2700 patients were treated with whole-brain radiotherapy and approximately 630 with single fraction or hypofractionated stereotactic radiotherapy as treatment for brain metastases at our institution. A total of 109 patients developed RICE after intracranial radiotherapy of which fourteen patients were excluded for incomplete data (n = 1), diagnostic uncertainty (n = 3) and pathological confirmed malignancy in surgery (n = 10).
3.1. Patient and treatment characteristics
The remaining 95 patients had a median age of 6o years and were included in this analysis. Eight patients (8.4%) had histologically confirmed RN, while 87 (91.6%) had imaging-based diagnosis of RICE, which was reliable throughout the follow-up period.
Most common primary tumor entity was lung cancer (n = 50, 52.6%), followed by breast cancer (n = 16; 16.8%), melanoma (n = 12, 12.6%), renal cell carcinoma (n = 5, 5.3%), gastrointestinal tumors (n = 4, 4.2%), and other (n = 8: thyroid carcinoma = 2, thymoma = 1, leiomyosarcoma = 1, osteosarcoma = 1, bladder = 1, germ cell tumor = 1, cancer of unknown primary = 1).
First RT treatment included stereotactic RT for 81 patients (85.3%), comprised with a median total dose of 20 Gy in one fraction (prescribed to the 70 or 80% isodose for Cyberknife or conventional linear accelerator, respectively) in 55 patients and hypofractionated treatment in 26 patients, which included the most common concepts with a total dose of 35 Gy in seven fractions in a postoperative setting in 11 patients and a total dose of 30 Gy in six fractions in 10 patients. The remaining 14 patients (14.7%) received WBRT with a median dose of 30 Gy in 10 fractions, followed by stereotactic re-irradiation concepts for oncologic progression. No patient received WBRT only before the development of RICE.
Within the whole cohort, a total of 27 patients received re-irradiation treatment with salvage WBRT (n = 6, 22.2%, median dose 30 Gy in 10 fractions), single fraction radiosurgery (n = 16, 59.3%, median dose 18 Gy) and fractionated radiosurgery (n = 5, 18.5%, median dose 30 Gy in 6 fractions) for the same lesion with a cumulative median BED of 282.5 (range: 162.5 – 440) Gy. Patient and treatment characteristics are presented in Table 1.
Table 1.
Patient and treatment characteristics.
| Characteristic | Value (range or percentage) |
|---|---|
| Median age at first RT; years (range) | 60 (23–80) |
| Median age at diagnosis of RICE; years (range) | 62 (26–82) |
| Gender | |
| male vs female | 46 (48.4%) vs 49 (51.6%) |
| Median Karnofsky Performance Score prior to first RT; % | 80 (60–100) |
| Primary cancer | |
|
50 (52.6%) |
|
16 (16.8%) |
|
12 (12.6%) |
|
5 (5.3%) |
|
4 (4.2%) |
|
8 (8.4%) |
| First RT treatment* | |
|
68 (71.6%) vs 27 (28.4%) |
|
180 (60–220) |
|
90 (30–110) |
|
3.5 (0.2–61.7) |
|
2.1 (0.2–36.8) |
|
1.1 (1–1.9) |
|
99.4 (97–100) |
| Re-irradiation treatment*; number (%) | 27 (28.4%) |
|
180 (75.0–220) |
|
90 (37.5–110) |
|
1.1 (0.1–70.4) |
|
0.7 (0.1–16.7) |
|
1.1 (1.1–2.8) |
|
98.8 (98.2–100) |
| Application of systemic therapy | |
|
65 (68.4%) |
|
61 (64.2%) |
|
10 (16.4%) |
|
30 (49.2%) |
|
19 (31.1%) |
|
2 (3.3%) |
BED: biological effective dose, EQD2: Equivalent dose in 2 Gy, RT: radiotherapy, WBRT: whole-brain radiotherapy, * doses always refer to the prescribed isodoses.
3.2. RICE-diagnosis and -specific treatment
RICE appeared after a median time of 8.0 (range: 1.3 – 48.4) months in patients with only one course of RT treatment and after a median of 6.4 (range: 0.6 – 38.2) months in patients after re-irradiation. Detailed characteristics are presented in Table 2.
Table 2.
RICE characteristics.
| Characteristic | Value (range or percentage) |
|---|---|
| Median time to diagnosis of RICE | |
| after single course RT; months | 8.0 (1.3–48.4) |
| after re-irradiation; months | 6.4 (0.6–38.2) |
| Symptoms at diagnosis of RICE | |
| yes vs no | 64 (67.4%) vs 31 (32.6%) |
| RICE-specific treatment | |
| no therapy | 17 (17.9%) |
| corticosteroids only | 37 (38.9%) |
| bevacizumab + corticosteroids | 38 (40.0%) |
| bevacizumab only | 3 (3.2%) |
| median dexamethasone dose per day; mg | 16 (1.6–32) |
| median cycles of bevacizumab | 4 (1–5) |
| Location of RICE | |
| right/left/center | 42 (44.2%)/48 (50.5%)/5 (5.3%) |
| near ventricular system (<1cm) | 42 (44.2%) |
| near cerebral falx (<1cm) | 28 (29.5%) |
| near skullcap (<1cm) | 47 (49.5%) |
| Lobe | |
| brainstem | 4 (4.2%) |
| frontal | 31 (32.6%) |
| parietal | 17 (17.9%) |
| temporal | 17 (17.9%) |
| occipital | 18 (18.9%) |
| cerebellum | 8 (8.4%) |
| RICE | |
| intracranial bleeding | 20 (21.1%) |
| midline shift | 17 (17.9%) |
| Response to treatment | symptomatic/imaging |
| No therapy | 17 |
| Progression | 10 (58.8%)/12 (70.6%) |
| Stable | 7 (41.2%)/5 (29.4%) |
| Remission | 0 (0%)/0 (0%) |
| Corticosteroids only | 37 |
| Progression | 10 (27.0%)/10 (27.0%) |
| Stable | 12 (32.4%)/11 (29.7%) |
| Remission | 15 (40.5%)/16 (43.2%) |
| Bevacizumab +/- corticosteroids | 41 |
| Progression | 3 (7.3%)/3 (7.3%) |
| Stable | 11 (26.8%)/7 (17.1%) |
| Remission | 27 (65.9%)/31 (75.6%) |
RICE: radiation-induced contrast enhancement, RT: radiotherapy.
Neurological symptoms at the time of first RICE diagnosis were recorded in 67.4% of the patients with low-grade impairments (grade 1, n = 20, 31.3%), grade = 2 (n = 22, 34.4%) and higher-grade symptoms with grade 3 (n = 19, 29.7% including: n = 7 seizures, n = 7 motoric deficits, n = 2 cognitive disturbance, n = 1 somnolence, n = 1 dizziness, n = 1 dysfunction optical system) and grade 4 (n = 3, 4.7%) with prolonged repetitive seizures (n = 2) and motoric deficits (n = 1).
Seventeen patients (17.9%) received no anti-inflammatory therapy, but close follow-up supervision, 37 (38.9%) patients received a dexamethasone pulse and maintenance therapy, 38 (40.0%) received a combination of dexamethasone and bevacizumab, and 3 patients (3.2%) received bevacizumab only.
Patients in the three RICE-specific treatment groups were well balanced concerning age, but significantly more asymptomatic patients were in the “no therapy” group (p = 0.005).
RICE-specific therapy started after a median time of 5.5 days and an early start of therapy after diagnosis was superior to achieve an improvement of clinical symptoms (p = 0.014) but not for imaging outcome (p = 0.263).
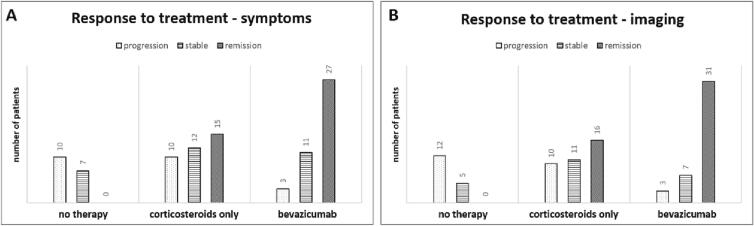
An improvement of clinical symptoms and imaging features was documented in 65.9% and 75.6% of the patients with bevacizumab (+/-corticosteroids) treatments, in 40.5% and 43.2% for corticosteroid only application and in 0% of patients with no therapy, respectively (Fig. 1). Median change of symptoms in the “no therapy” group was a deterioration of 1 CTCAE grade (range: +1 to + 4), of 0 CTCAE grade (range: −3 to + 2) in the “corticosteroids only” group and an improvement of 1 CTCAE grade (range: −3 to + 1) in the patients that received bevacizumab(+/-corticosteroids).
Fig. 1.
Overall response to treatment for symptoms (A) and imaging results (B) depending on the applied treatment.
Baseline values of the largest RN extent of T1- and T2-weighted imaging were significantly different between the groups with “no therapy” and “bevacizumab(+/-corticosteroids)” (T1: p = 0.014 and T2: p < 0.001) with higher values in the bevacizumab-group.
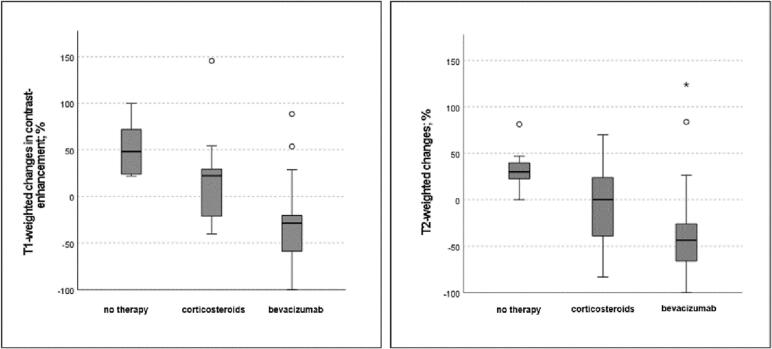
With bevacizumab there was a median decrease in the largest extent of T1-weighted contrast enhancement of −28.7%, while patients with corticosteroids or “no therapy” had a significantly inferior outcome with a median increase of + 22.2% and + 48.1%, respectively. The T2-weighted imaging showed a median diameter decrease of −43.7% for patients treated with bevacizumab and a median increase of + 2.9% and + 30.2% for corticosteroid and “no therapy” groups (Fig. 2). Bevacizumab(+/-corticosteroids) compared to treatments with corticosteroids only was significantly superior in terms of imaging response of T1- (p = 0.001) and T2-outcome (p = 0.015) and symptom relief (p = 0.025).
Fig. 2.
Boxplots of changes of the largest extent of T1-weighted contrast enhancements and T2-weighted signal changes depending on treatment type with median, first and third quartiles; outliners (° and *) were not included in the boxplot.
After the start of RICE-specific therapy there was a stabilization in imaging after a median time of 41.5 (8–155) days for the corticosteroid-group, while the improved decrease and time to best imaging response was significantly (p < 0.001) longer for the bevacizumab(+/-corticosteroids) group with a median time of 125.0 (34–386) days.
Median dose of corticosteroid treatment was 16 mg dexamethasone-equivalent per day (range: 1.6–32.0 mg) without any significant correlation of the utilized dose to RICE-outcome. Only six patients had a dexamethasone dose of below 8 mg. For a subgroup of 31 patients, data for the time interval from the start of corticosteroid treatment until the last day of application was available and significantly correlated to a worse symptomatic (p = 0.022) and imaging (p = 0.011) outcome in patients with a shortened corticosteroid reduction interval of below 30 days. Twelve patients (32.4%) had higher grade (≥3°) adverse side effects related to corticosteroid application (n = 6 psychiatric, n = 4 metabolic, n = 2 acute diabetic decompensation).
Median therapy with bevacizumab was 4 cycles (range: 1 – 5). An application of below 3 or less (n = 12) cycles lead to a significant inferior symptom (p = 0.022) and imaging outcome (p = 0.017). Four patients (9.8%) had higher grade (≥3°) side effects of bevacizumab with bleeding (n = 2, grade 4), gastrointestinal toxicity (n = 1, grade 3) and allergic reaction (n = 1, grade 3).
3.3. Flare-up
As illustrated in Fig. 3, a recurrence of RICE in patients with initially improved or stable imaging (n = 65) occurred in 41 patients (63.1%): 23 (56.1%) patients after bevacizumab therapy with a median time of 5.6 (1–25.2) months and in 18 (48.6%) patients after corticosteroid treatment after a median time of 2.9 (0.3–24.1) months. This progression-free time from start of specific therapy to flare-up was significantly (p = 0.004) longer in patients who received bevacizumab compared to patients with corticosteroids only.
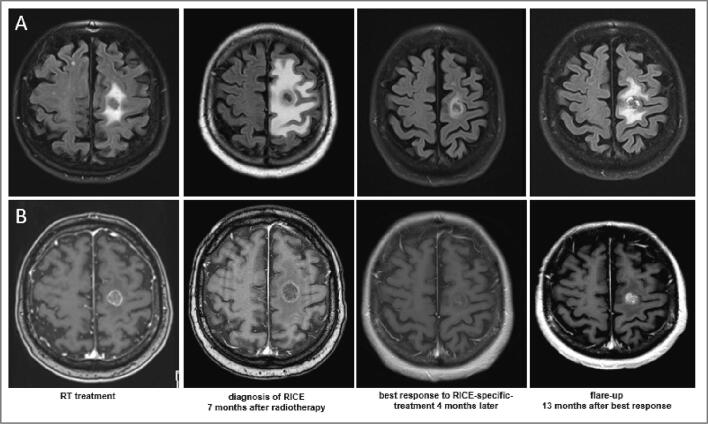
Fig. 3.
Magnetic resonance images with T2-weighted fluid-attenuated inversion recovery (FLAIR) (A) and T1-weighted contrast enhancements (B) signal changes after stereotactic radiosurgery with 18 Gy prescribed to the 70% isodose line in a single fraction and good response to treatment with bevacizumab and corticosteroids and flare-up 13 months after best response.
Flare-up treatment included no further therapy (n = 14, remission rate: 0%), dexamethasone (n = 13, remission rate: 23%), bevacizumab re-exposition (n = 11, remission rate: 90%) or surgery (n = 2, remission rate: 50%). For one patient treatment for flare-up was unknown, two patients were lost to follow up for evaluation of response.
Response of flare-up significantly depended on the utilized treatment (p < 0.001). Eleven patients received bevacizumab re-exposition after a median time of 5.4 months (2.3–15.0) with a median cumulative dose of 8 cycles. Follow-up results were available for ten of them, of which 90% had again an improvement of RICE on imaging and clinical relief. Two patients had re-recurrences and received returned 4-cycle-treatments with bevacizumab up to total of 18 and 19 cycles with improved clinical outcome after each new application.
3.4. Outcome and influencing factors
Median follow-up was 28.8 (range: 4.3 – 119.6) months. At the end of the follow-up period 35 of 95 patients were dead, 16 due to extracranial progressive oncological disease. The remaining 19 patients died of fatal RICE-complications: Four patients (n = 3 no therapy, n = 1 bevacizumab) died of neurological disorders (n = 3) and brainstem edema (n = 1) after a median time of 4.0 months after first diagnosis of RICE (range 1.6–6.8), while fifteen patients died after a median time of 2.8 (range 0.2–7.8) months after flare-up due to brain death (n = 4 patients with brainstem edema, n = 2 status epilepticus, n = 6 other neurological disorders), thromboembolic events/bleeding (n = 2) and febrile neutropenic sepsis (n = 1).
RICE lesion’s location in the lobe (brainstem, frontal, parietal, temporal, occipital or cerebellum, symptomatic p = 0.289/imaging p = 0.408), a midline shift (symptomatic p = 0.569/imaging p = 0.739), or a location near the ventricular system (<1cm) (symptomatic p = 0.243/imaging p = 0.201), near the cerebral falx (<1cm) (symptomatic p = 0.695/imaging p = 0.490) or skullcap (<1cm) (symptomatic p = 0.133/imaging p = 0.466) had no significant impact on symptomatic or imaging outcome. Patients with intracranial bleeding associated with RICE (p = 0.035) had a higher likelihood of symptom relief during follow up, without any impact on imaging outcome (p = 0.276).
Age at RICE-diagnosis, dosimetric evaluation of maximum dose, PTV and GTV volume, treatment concept (definitive or postoperative), conformity index, and dose fractionation as well as BED and EQD2 were not significantly correlated with symptomatic or imaging improvement. Overall, a recurrence of RICE was significantly more often in patients after re-irradiation (p = 0.001).
Extracranial progressive disease at the time point of first diagnosis of RICE was associated with an inferior improvement of RICE in imaging (p = 0.029). The application of systemic therapy during RICE-treatment did not affect the outcome significantly, neither could a correlation between the type of systemic therapy (chemotherapy, immunotherapy, targeted therapies, other) and outcome be found (symptomatic p = 0.415/imaging p = 0.102). The absence of systemic therapy prior to first RT improved imaging outcomes (p = 0.029) more likely.
4. Discussion
Our study consists of a large group of patients with brain metastases, who suffered from radiation-induced contrast enhancements after RT and demonstrated a good response to bevacizumab whether delivered initially or in a long-term salvage scenario.
In the modern era, improvements in systemic and targeted therapies as well as radiation techniques have increased the number of long-term survivors with stage IV disease. The advent of stereotactic radiosurgery for intracranial malignancies has yielded high local control even in critical locations, offers superior neurocognition outcome and sparing of surrounding healthy tissue than WBRT and enables the possibility of multiple treatment rounds [23], [24]. The occurrence of RICEs has been described after varying fractionation schedules, RT techniques and radiation types (i.e. photons or particle therapy), and various tumor histologies [25], [26], [27]. A high cumulative dose is a known risk factor increasing RICE rates [3], [4], [8]. Moreover, our analysis demonstrates a high recurrence rate of RICE flare-ups, that was significantly correlated with re-irradiation concepts. Considering the various existing definitions and etiologies of RICE, these recurrences might reflect a severe irreversible brain tissue damage, which can be classified as RN rather than blood barrier disruptions or pseudoprogressions. This makes an interdisciplinary approach for diagnostic review and a treatment in specialized centers to avoid devastating complications of RN all the more important. [13], [28].
Therapy for RICE and RN consist of complex multimodality approaches, in which corticosteroids, bevacizumab and surgery claim the leading concept options. anti-Inflammatory corticosteroids have traditionally been the mainstay of treatment and prolonged application of corticosteroid treatment had a positive effect on symptomatic and imaging outcome of RICE in our analysis. However, long-term use can lead to severe metabolic, cardiovascular, musculoskeletal, psychiatric, and central nervous system side effects. The use of the monoclonal antibody bevacizumab as an angiogenesis inhibitor by inhibiting vascular endothelial growth (VEGF) in common dosage concepts of 5.0–7.5 mg/kg every-two weeks was assessed in randomized and retrospective studies [16], [29], [30]. Our data demonstrate symptomatic and radiological improvements with the use of bevacizumab relative to corticosteroids only. We replicate the benefit of bevacizumab (+/-corticosteroids) in the present study, which in fact also translated in improvements for secondary recurrences of RICE. While our study showed an inferior outcome for patients with below three cycles for initial treatment, dose therapeutic ranges and treatment application intervals of bevacizumab are wide and with the proper duration remaining unclear. The adverse high-grade (≥3°) side-effects rate of RICE-specific bevacizumab treatment in our cohort was 9.8% and must be weighed against clinical benefits as these advances come at the cost of a considerable number of potentially life-threatening complications as also reported in previous studies [31]. Other analyses have shown slightly lower high-grade adverse events in about 1.8%, assuming that RNI-specific treatments can be safely applied [30], [32], [33], [34]. A low-dose bevacizumab study of Zhuang et al has shown a high efficacy of 1.0 mg/kg treatment concepts every-three weeks for at least three continuous applications [35]. Nevertheless, even though many analyses present positive effects of bevacizumab application, the results remain controversial with few studies reporting on bevacizumab resistance and still a lack of firm evidence for pharmacological and non-pharmacological interventions in the treatment of brain radiation necrosis [36], [37].
Overall, radiation brain injury can have devastating and life-threatening complications, especially in cases of RICE recurrences that can suggest RN, and prompt therapy initiation was supported by our analysis. International guidelines provide treatment strategies for initial treatment, but data for recurrences after RICE-improvement are lacking and prior studies report wide ranges of 5.9% to 76.9% in small patient cohorts with undefined treatment recommendations [13], [20], [28], [34]. Our study suggests that bevacizumab is also superior for the treatment of flare-ups compared to corticosteroids.
In our population, the administration of systemic therapy simultaneously to RICE-specific treatment was not associated with inferior outcome. However, patients with extracranial progressive disease at the time point of first diagnosis of RICE had an inferior improvement in imaging results. Prior analyses exploring the impact of blood–brain barrier penetration of substances on toxicities are quite controversial and increased rates of RICE development up to 16.9% after chemotherapy, 25.0% after targeted and 37.5% after immunotherapy have been reported [6], [38]. While simultaneous or combined application of systemic therapies might also lead to a deterioration of RICE/RNI outcomes, we were not able to detected specific high-risk substances, which might have been to the high amount of various substances, and combination therapy concepts that were applied in our cohort.
Limitations of our analysis include its retrospective nature and wide heterogenous range of applied RICE- and especially cancer-specific applied treatment concepts as well as different existing definitions of RN in published literature, which make comparisons to other studies challenging. Even though we could not find any correlation of RICE responders or non-responders to treatment concepts, the inclusion of patients with postoperative RT treatment concepts may have different underlying RICE or glial damage mechanisms after surgery than RICE after RT in a definitive setting. Further, influencing factors of applied systemic therapies or with the timing and dosage of corticosteroids for prophylaxis and treatment especially in the combination group of bevacizumab (+/-corticosteroids) might have retrospectively not fully been available and consecutive interferences with our results cannot be ruled out completely.
Radiological imaging was carefully reviewed and patients with uncertainties were excluded from the analyses, nevertheless, the existence of cases with tumor progression cannot be completely ruled out. The same applies for the analysis of causes of death due to RICE or oncologic progression; especially in palliative treatment settings, intensive diagnostic and imaging approaches and examinations are often omitted, which might have influenced the outcome of our analysis. Even though the reported high mortality rate of 36.6% in our cohort refers to deaths after prolonged treatments courses with RICE flare-ups and not initial RICE-diagnosis, it might have been overestimated. Further, analysis of the diameter changes of edema instead of volumes and contrast enhancements as well as improvement or deterioration of symptoms might depend on the extent of baseline values. These were, most likely due to the classification of the severity of symptoms and corresponding therapy recommendation in clinical routine, not well balanced in the three different treatment approach groups in our cohort and could have led to misleading results or misinterpretation of medication effects. Consequences for treatment recommendation must therefore be interpreted and generalized with caution and clinical practice.
However, data for the course of RICE and RN and the effect of re-exposition for flare-up lesions have only been reported in small numbers. Evidence is highly needed as with the increased use of targeted therapies the rates of RICE might even increase. Moreover, a better understanding and analysis of more factors than RT solely is needed; controversial results of RICE, which developed after SRS in combination with immune checkpoint inhibitors, were seen as a benign immune response reaction associated with superior OS, need to be further analyzed and differentiated from malign RICE/RN. [39] Further, future research needs to focus on improvements of diagnostic imaging for a better differentiation of RICE and RN, which still remains challenging and is to date not able to accurately differentiate between tumor progression. The additional benefit of modern imaging or artificial intelligence analyses need to be evaluated and assessed.
5. Conclusion
Our data demonstrate that bevacizumab (+/-corticosteroids) in patients with RICE after RT for brain metastases is effective for short-term improvement and prolongs the recurrence-free time to flare-up compared to corticosteroids alone. However, long-term recurrence-rates and associated mortality in initially responsive or stable and re-irradiated patients are high. Our results suggest that repeated courses of bevacizumab applications show high efficacy not only in imaging but also in clinical symptom relief and are superior to other treatment options. Nonetheless, the treatment of RICE requires a balanced, interdisciplinary multistep approach based on the anatomical region and symptomatology.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors declare no conflict of interest and that no funds, grants, or other support were received during the preparation of this manuscript. This research received no external funding. Outside the submitted work: EM received speaker fees from Elekta outside the submitted work. LK received speaker fees and travel reimbursement from Accuray International Sàrl., and NovoCure outside the submitted work. JD received grants from Accuray International Sàrl, Merck Serono GmbH, CRI – The Clinical Research Institute GmbH, View Ray Inc., Accuray Incorporated, RaySearch Laboratories AB, Vision RT limited, Astellas Pharma GmbH, Astra Zeneca GmbH, Solution Akademie GmbH, Ergomed PLC Surrey Research Park, Siemens Healthcare GmbH, Quintiles GmbH, NovoCure, Pharmaceutecal Research Associates GmbH, Boehringer Ingelheim Pharma GmbH Co, PTW-Freiburg Dr. Pychlau GmbH, Nanobiotix A.A. and IntraOP Medical outside the submitted work. JHR received speaker fees and travel reimbursement from ViewRay Inc., travel reimbursement from IntraOP Medical and Elekta Instrument AB, a grant from IntraOP Medical outside the submitted work.
References
- 1.Remler M.P., Marcussen W.H., Tiller-Borsich J. The late effects of radiation on the blood brain barrier. Int J Radiat Oncol Biol Phys. 1986;12:1965–1969. doi: 10.1016/0360-3016(86)90133-1. [DOI] [PubMed] [Google Scholar]
- 2.Tsao M.N., Li Y.Q., Lu G., Xu Y., Wong C.S. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999 Oct;58(10):1051–1060. doi: 10.1097/00005072-199910000-00003. PMID: 10515228. [DOI] [PubMed] [Google Scholar]
- 3.Moreau J., Khalil T., Dupic G., et al. Second course of stereotactic radiosurgery for locally recurrent brain metastases: safety and efficacy. PLoS One. 2018;13:e0195608. doi: 10.1371/journal.pone.0195608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berber T., Raturi V., Aksaray F., et al. Clinical outcome after CyberKnife® radiosurgery re-irradiation for recurrent brain metastases. Cancer Radiother. 2021;25:457–462. doi: 10.1016/j.canrad.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Kohutek Z.A., Yamada Y., Chan T.A., Brennan C.W., Tabar V., Gutin P.H., Yang T.J., Rosenblum M.K., Ballangrud Å., Young R.J., Zhang Z., Beal K. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125(1):149–156. doi: 10.1007/s11060-015-1881-3. Epub 2015 Aug 26. PMID: 26307446; PMCID: PMC4726630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colaco R.J., Martin P., Kluger H.M., Yu J.B., Chiang V.L. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125(1):17–23. doi: 10.3171/2015.6.JNS142763. Epub 2015 Nov 6 PMID: 26544782. [DOI] [PubMed] [Google Scholar]
- 7.Sneed P.K., Mendez J., Vemer-van den Hoek J.G., et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123:373–386. doi: 10.3171/2014.10.jns141610. [DOI] [PubMed] [Google Scholar]
- 8.Ruben J.D., Dally M., Bailey M., Smith R., McLean C.A., Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499–508. doi: 10.1016/j.ijrobp.2005.12.002. Epub 2006 Mar 6 PMID: 16517093. [DOI] [PubMed] [Google Scholar]
- 9.Ohtakara K., Hayashi S., Nakayama N., et al. Significance of target location relative to the depth from the brain surface and high-dose irradiated volume in the development of brain radionecrosis after micromultileaf collimator-based stereotactic radiosurgery for brain metastases. J Neurooncol. 2012;108:201–209. doi: 10.1007/s11060-012-0834-3. [DOI] [PubMed] [Google Scholar]
- 10.Blonigen B.J., Steinmetz R.D., Levin L., et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Minniti G., Clarke E., Lanzetta G., et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller J.A., Bennett E.E., Xiao R., et al. Association between radiation necrosis and tumor biology following stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol. 2016 doi: 10.1016/j.ijrobp.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Bernhardt D., König L., Grosu A., Wiestler B., Rieken S., Wick W., et al. Expert Panel of the German Society of Radiation Oncology (DEGRO). DEGRO practical guideline for central nervous system radiation necrosis part 1: classification and a multistep approach for diagnosis. Strahlenther Onkol. 2022 doi: 10.1007/s00066-022-01994-3. DOI: 10.1007/s00066-022-01994-3. Epub ahead of print. PMID: 36038669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dequesada I.M., Quisling R.G., Yachnis A., Friedman W.A. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63:898–903. doi: 10.1227/01.NEU.0000333263.31870.31. discussion 904. [DOI] [PubMed] [Google Scholar]
- 15.Shah R., Vattoth S., Jacob R., Manzil F.F., O'Malley J.P., Borghei P., Patel B.N., Curé J.K. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32(5):1343–1359. doi: 10.1148/rg.325125002. PMID: 22977022. [DOI] [PubMed] [Google Scholar]
- 16.Levin V.A., Bidaut L., Hou P., Kumar A.J., Wefel J.S., Bekele B.N., et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. Erratum. In: Int J Radiat Oncol Biol Phys. 2012 Sep 1;84(1):6. Grewal, Jai [added]. PMID: 20399573; PMCID: PMC2908725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tye K., Engelhard H.H., Slavin K.V., Nicholas M.K., Chmura S.J., Kwok Y., et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neurooncol. 2014 Apr;117(2):321–327. doi: 10.1007/s11060-014-1391-8. Epub 2014 Feb 7 PMID: 24504500. [DOI] [PubMed] [Google Scholar]
- 18.Chinot O.L., Wick W., Mason W., Henriksson R., Saran F., Nishikawa R., et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. PMID: 24552318. [DOI] [PubMed] [Google Scholar]
- 19.Furuse M., Kawabata S., Kuroiwa T., Miyatake S. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neuro-Oncol. 2011;102(3):471–475. doi: 10.1007/s11060-010-0333-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang H, Yuan X, Chang JY, Song Y, Wang J, Yuan Z, et al. Exploration of the recurrence in radiation brain necrosis after bevacizumab discontinuation. Oncotarget. 7768. [DOI] [PMC free article] [PubMed]
- 21.Kocher M., Wittig A., Piroth M.D., et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO working group on stereotactic radiotherapy. Strahlenther Onkol. 2014;190:521–532. doi: 10.1007/s00066-014-0648-7. [DOI] [PubMed] [Google Scholar]
- 22.Hanna G.G., Murray L., Patel R., et al. Uk consensus on normal tissue dose constraints for stereotactic radiotherapy. Clin Oncol. 2018;30:5–14. doi: 10.1016/j.clon.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen W.C., Baal U.H., Baal J.D., et al. Efficacy and safety of stereotactic radiosurgery for brainstem metastases: a systematic review and meta-analysis. JAMA Oncol. 2021:7. doi: 10.1001/jamaoncol.2021.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown P.D., Ballman K.V., Cerhan J.H., et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichkorn T., Lischalk J.W., Sandrini E., Meixner E., Regnery S., Held T., Bauer J., Bahn E., Harrabi S., Hörner-Rieber J., Herfarth K., Debus J., König L. Iatrogenic influence on prognosis of radiation-induced contrast enhancements in patients with glioma WHO 1-3 following photon and proton radiotherapy. Radiother Oncol. 2022 doi: 10.1016/j.radonc.2022.08.025. Aug 27:S0167-8140(22)04246-3. 10.1016/j.radonc.2022.08.025. Epub ahead of print. PMID: 36041565. [DOI] [PubMed] [Google Scholar]
- 26.Rowe L.S., et al. Differentiating pseudoprogression from true progression: analysis of radiographic, biologic, and clinical clues in GBM. J Neurooncol. 2018;139:145–152. doi: 10.1007/s11060-018-2855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eichkorn T., Bauer J., Bahn E., Lischalk J.W., Meixner E., Sandrini E., et al. Radiation-induced contrast enhancement following proton radiotherapy for low-grade glioma depends on tumor characteristics and is rarer in children than adults. Radiother Oncol. 2022;172:54–64. doi: 10.1016/j.radonc.2022.05.005. Epub 2022 May 11 PMID: 35568281. [DOI] [PubMed] [Google Scholar]
- 28.Bernhardt D., König L., Grosu A.L., Rieken S., Krieg S.M., Wick W., et al. Expert Panel of the German Society of Radiation Oncology (DEGRO). DEGRO practical guideline for central nervous system radiation necrosis part 2: treatment. Strahlenther Onkol. 2022 doi: 10.1007/s00066-022-01973-8. DOI: 10.1007/s00066-022-01973-8. Epub ahead of print. PMID: 36038670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y., et al. Bevacizumab monotherapy reduces radiationinduced brain necrosis in Nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2018;101:1087–1095. doi: 10.1016/j.ijrobp.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 30.Sadraei N.H., Dahiya S., Chao S.T., Murphy E.S., Osei-Boateng K., Xie H., et al. Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol. 2015;38(3):304–310. doi: 10.1097/COC.0b013e31829c3139. PMID: 23799286. [DOI] [PubMed] [Google Scholar]
- 31.Lubelski D., Abdullah K.G., Weil R.J., Marko N.F. Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neuro-Oncol. 2013;115(3):317–322. doi: 10.1007/s11060-013-1233-0. [DOI] [PubMed] [Google Scholar]
- 32.Khan M., Zhao Z., Arooj S., Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer. 2021 doi: 10.1186/s12885-021-07889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang H., Yuan X., Zheng Y., Li X., Chang J.Y., Wang J., et al. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep. 2016;12(6):24364. doi: 10.1038/srep24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Pan L., Sheng X., Mao Y., Yao Y., Wang E., et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res. 2012;17(1):25. doi: 10.1186/2047-783X-17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang H., Zhuang H., Shi S., Wang Y. Ultra-low-dose bevacizumab for cerebral radiation necrosis: a prospective phase II clinical study. Onco Targets Ther. 2019;12:8447–8453. doi: 10.2147/OTT.S223258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Huang X., Jiang J., Hu W., Hu J., Cai J., et al. Clinical variables for prediction of the therapeutic effects of bevacizumab monotherapy in nasopharyngeal carcinoma patients with radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2018;100(3):621–629. doi: 10.1016/j.ijrobp.2017.11.023. Epub 2017 Nov 21 PMID: 29413276. [DOI] [PubMed] [Google Scholar]
- 37.Chung C., Bryant A., Brown P.D. Interventions for the treatment of brain radionecrosis after radiotherapy or radiosurgery. Cochrane Database Syst Rev. 2018;7(7):CD011492. doi: 10.1002/14651858.CD011492.pub2. PMID: 29987845; PMCID: PMC6513335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trommer M., Adams A., Celik E., Fan J., Funken D., Herter J.M., et al. Oncologic outcome and immune responses of radiotherapy with anti-PD-1 treatment for brain metastases regarding timing and benefiting subgroups. Cancers (Basel) 2022;14(5):1240. doi: 10.3390/cancers14051240. PMID: 35267546; PMCID: PMC8909717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehrer E.J., Ahluwalia M.S., Gurewitz J., Bernstein K., Kondziolka D., Niranjan A., et al. Imaging-defined necrosis after treatment with single-fraction stereotactic radiosurgery and immune checkpoint inhibitors and its potential association with improved outcomes in patients with brain metastases: an international multicenter study of 697 patients. J Neurosurg. 2022;16:1–10. doi: 10.3171/2022.7.JNS22752. PMID: 36115055. [DOI] [PubMed] [Google Scholar]