Abstract
BACKGROUND
Periodontitis during pregnancy is associated with an increased risk of preterm birth (<37 weeks of gestation) or low birthweight (<2500 g) offspring. Beyond periodontal disease, the risk of preterm birth varies both by previous history of preterm birth and in association with social determinants prevalent among vulnerable and marginalized populations. This study hypothesized that the timing of periodontal treatment during pregnancy and/or social vulnerability measures modified the response to dental scaling and root planing for the treatment of periodontitis and prevention of preterm birth.
OBJECTIVE
This study aimed to determine the association of timing of dental scaling and root planing for gravidae with a diagnosed periodontal disease on the rates of preterm birth or low birthweight offspring among subgroups or strata of gravidae as part of the Maternal Oral Therapy to Reduce Obstetric Risk randomized controlled trial. All participants in the study had clinically diagnosed periodontal disease and differed by the timing of the periodontal treatment (dental scaling and root planing at <24 weeks [per protocol] or after delivery) or by baseline characteristics. Although all participants met the well-accepted clinical criteria for periodontitis, not all participants acknowledged a priori that they had periodontal disease.
STUDY DESIGN
This was a per-protocol analysis of data from 1455 participants of the Maternal Oral Therapy to Reduce Obstetric Risk trial evaluating dental scaling and root planing on the risk of preterm birth or low birthweight offspring. Adjusted multiple logistic regression to control for confounders was used to estimate associations comparing the timing of periodontal treatment in pregnancy to receiving treatment after pregnancy (referent control) on rates of preterm birth or low birthweight among subgroups of gravidae with known periodontal disease. Study analyses were stratified, and the associations with the following characteristics—body mass index, self-described race and ethnicity, household income, maternal education, recency of immigration, and self-acknowledgment of poor oral health, were explored.
RESULTS
Dental scaling and root planing during the second or third trimester of pregnancy were associated with an increased adjusted odds ratio of preterm birth among those at the lower body mass index strata (18.5 to <25.0 kg/m2) (adjusted odds ratio, 2.21; 95% confidence interval, 1.07–4.98), but not among individuals who were overweight (body mass index of 25.0 to <30.0 kg/m2; adjusted odds ratio, 0.68; 95% confidence interval, 0.29–1.59) or obese (body mass index of ≥30 kg/m2; adjusted odds ratio, 1.26; 95% confidence interval, 0.65–2.49). There was no significant difference in pregnancy outcomes related to the other evaluated variables: self-described race and ethnicity, household income, maternal education, immigration status, or self-acknowledgment of poor oral health.
CONCLUSION
In this per-protocol analysis of the Maternal Oral Therapy to Reduce Obstetric Risk trial, dental scaling and root planing had no preventive benefit against adverse obstetrical outcomes and were associated with increased odds of preterm birth among individuals at lower body mass index strata. There was no significant difference in the occurrence of preterm birth or low birthweight after dental scaling and root planing periodontitis treatment concerning other analyzed social determinants of preterm birth.
Key words: low birthweight, maternal health, newborn health, periodontitis, prematurity
AJOG Global Reports at a Glance.
Why was this study conducted?
This study aimed to determine whether nonsurgical periodontal therapy performed during pregnancy compared with that after pregnancy among gravidae with periodontitis led to disparate pregnancy outcomes among subgroups of gravidae with differing baseline characteristics.
Key findings
Gravidae who received nonsurgical periodontal therapy during pregnancy with prepregnant body mass indices (BMI) of 18.5 to <25.0 kg/m2 had higher rates of preterm birth (PTB) than those who received treatment after pregnancy.
What does this add to what is known?
Dental scaling and root planing had no preventive benefit against adverse obstetrical outcomes and were associated with increased odds of PTB among individuals at lower BMI strata.
Introduction
Complications of prematurity are a leading cause of death for all children under the age of 5 years.1, 2, 3 Studies have demonstrated an association between maternal periodontal disease and increased risk of preterm birth (PTB; <37 weeks of gestation) and low birthweight (LBW; <2500 g) neonates.4, 5, 6, 7, 8, 9, 10, 11 Researchers have explored whether treatment of periodontal disease during pregnancy reduces the rates of PTB. Although some initial single-center randomized clinical trials evaluating periodontal treatment during pregnancy reported a potential reduction in the rate of PTB, large multicenter randomized controlled trials have not demonstrated any difference.12, 13, 14, 15, 16
The risk of PTB is not uniform across all pregnant individuals, with lower socioeconomic status and certain sociodemographic and sociocultural characteristics (eg, living under the federal poverty level, immigration status, and self-described ethnicity) being closely related to a higher incidence of PTB.17, 18, 19, 20, 21, 22, 23, 24 Furthermore, differences in prepregnancy body mass index (BMI) are associated with differing risks of PTB with individuals of lower prepregnant BMI having higher rates of PTB.25, 26, 27 Social determinants of health are integrally related to access to dental care, inclusive of timely diagnosis and treatment of poor oral health and periodontitis.28
Several large multicenter randomized trials evaluated the obstetrical effects of nonsurgical periodontal therapy during pregnancy compared with that after pregnancy within the overall population.12, 13, 14, 15, 16 It is uncertain whether the obstetrical effect of periodontal treatment during pregnancy remains the same across subpopulations of gravidae documented as being at higher risk of adverse pregnancy outcomes. Moreover, the ideal timing of periodontal treatment during pregnancy remains uncertain.
The Maternal Oral Therapy to Reduce Obstetric Risk (MOTOR) trial was a randomized clinical trial of 1806 pregnant women with known periodontal disease that evaluated the effect of nonsurgical periodontal therapy (dental scaling and root planing) during pregnancy (intervention) on the rates of PTB or LBW offspring compared with the same treatment provided after pregnancy (control).29 Although the original intention-to-treat analysis found no difference in obstetrical outcomes between groups, we aimed to perform a per-protocol analysis of the MOTOR trial to assess strict adherence to the treatment protocol and evaluate the effect of periodontal treatment on the rates of PTB or LBW offspring among subgroups of gravidae with differing socioeconomic, sociodemographic, and sociocultural characteristics. In addition, we evaluated whether timing (protocol adherence at <24 or ≥24 weeks of gestation or after delivery) of periodontal therapy was associated with differential obstetrical outcomes. We hypothesized that dental scaling and root planing would lead to variable pregnancy outcomes, such as PTB or LBW offspring, that would vary by measures of social vulnerability in a per-protocol (adherence) analysis.
Materials and Methods
The MOTOR trial was conducted at Duke University Medical Center (NC), the University of Alabama at Birmingham Medical Center (AL), and the University of Texas Health Science Center at San Antonio (TX). Gravidae were enrolled if they had at least 20 teeth with at least 3 periodontal sites with at least 3 mm of attachment loss and could complete periodontal treatment before 23 6/7 weeks of gestation. Gravidae were excluded if they had multiple pregnancy, human immunodeficiency virus infection, acquired immunodeficiency syndrome, known autoimmune disease, prepregnant diabetes mellitus, or need for antibiotic prophylaxis for periodontal treatment or probing. Before randomization, participants could receive dental care to reduce the likelihood of an acute infectious event during pregnancy. Eligible participants were randomized to receive dental scaling and root planing at <24 weeks of gestation (intervention) or delayed until after delivery (control), although some received treatment during pregnancy and at ≥24 weeks of gestation off protocol. Study participants could receive up to 4 periodontal treatment sessions. The control group received the same periodontal therapy but performed after delivery. All participants provided informed consent. Institutional review board (IRB) approval was obtained at each site before the study implementation. This secondary analysis was approved by the University of Washington IRB (STUDY00012325; January 20, 2021).
We conducted a per-protocol secondary analysis of the original MOTOR trial to evaluate the extent to which performing nonsurgical periodontal therapy during pregnancy among gravidae at potential baseline higher risk of PTB led to an effect modification on the primary outcomes—rates of PTB or LBW newborns. We evaluated whether the association between periodontal treatment and birth outcomes was modified by any of the following sociodemographic, sociocultural, socioeconomic, or health variables: maternal education level (high school or lower or above high school), income (<$20,000 or ≥$20,000), self-described ethnicity (White non-Hispanic, Hispanic, or Black non-Hispanic), time living in the United States (born in the United States, lived <3 years in the United States, or lived ≥3 years in the United States), or prepregnant BMI (normal, 18.5 to <25.0 kg/m2; overweight, 25.0 to <30.0 kg/m2; obese, ≥30.0 kg/m2), or self-acknowledgment of poor dental health at baseline, even though all participants had been evaluated and objectively diagnosed with periodontitis based on dental parameters (eg, clinical attachment loss).
The MOTOR trial enrolled gravidae who would receive periodontal treatment during pregnancy before 24 weeks of gestation. However, some gravidae were not adherent to the protocol and received initial treatment after 24 weeks of gestation, which has the potential to bias the results given a later gestational age of intervention with unknown effects on pregnancy outcomes. Thus, we sought to determine whether the timing of periodontal treatment either before or after 24 weeks of gestation modified the association with adverse pregnancy outcomes, specifically PTB or LBW offspring. Finally, we performed a sensitivity analysis evaluating whether improvement in periodontal health status during the trial resulted in differential effects on birth outcomes.
Statistical analyses
We summarized continuous variables using mean and standard deviations and categorical variables using counts and percentages. The primary outcomes were adverse birth outcomes—either LBW, PTB, or both as a composite outcome. The exposure of interest was the time of treatment, approached in 2 ways. We compared gravidae treated during pregnancy with those treated after delivery. We classified gravidae treated during pregnancy as treated at <24 and ≥24 weeks of gestation. We used a multivariable logistic regression analysis to estimate the adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for the effect of time of treatment on pregnancy outcomes and for the same subgroups. Separate models were fitted for the composite outcome of PTB or LBW and for each of them independently. The models were adjusted for age, marital status, years lived in the United States, race and ethnicity, and self-acknowledged poor dental health status, provided that a variable would be excluded as a covariate when its effect modification is evaluated. The latter was of particular interest, as all gravidae enrolled in the study met accepted clinical criteria for periodontal disease, but not all women self-acknowledged that diagnosis or its relation to “poor dental health.” In addition, we assessed the role of treatment success on PTB or LBW. A sensitivity analysis was performed comparing birth outcomes and the distribution of key variables across participants who received the actual treatment and who did not. The analysis was performed using R (version 4.1), and a P value of <.05 was used to determine statistical significance.
Results
Through a per-protocol analysis of the MOTOR trial, 73 of 903 participants randomized to receive nonsurgical periodontal therapy during pregnancy (intervention) had missing information related to the timing of their periodontal treatments and were excluded. Of note, 4 of the remaining 830 participants received treatment after pregnancy, and therefore, these 4 participants were analyzed as part of the control group in this per-protocol adherence analysis. Of the 903 participants randomized to the control group, 278 had missing information related to the timing of their periodontal treatment and, therefore, were excluded from the analysis as we could not confirm if the treatment occurred during pregnancy or after. Of the remaining 625 participants, 2 received periodontal treatment before 24 weeks of gestation, and 1 received periodontal treatment between 24 weeks of gestation and during delivery. Therefore, these 3 participants were analyzed as part of the intervention group in this per-protocol adherence analysis. Moreover, by protocol adherence, 829 total study participants received periodontal treatment during pregnancy, and 626 received treatment after pregnancy (Figure 1).
Figure 1.
CONSORT diagram
Treated during pregnancy included 3 participants who were initially assigned to the control group (treatment after delivery). Treated after delivery included 4 participants who were initially assigned to the intervention group (treatment during pregnancy).
CONSORT, Consolidated Standards of Reporting Trials; MOTOR, Maternal Oral Therapy to Reduce Obstetric Risk
Valentine. Nonsurgical periodontal therapy and newborn outcomes. Am J Obstet Gynecol Glob Rep 2023.
Assessing nonsurgical periodontal treatment during pregnancy compared to after pregnancy
There were statistically significant differences between treatment adherence groups (Table). Participants who were treated during pregnancy (intervention) were of older maternal age, were not born in the United States, had less access to medical insurance, and had higher income compared with participants who were missing treatment information or those who had postpartum treatment (control). The variables that were statistically different between groups were controlled for in logistic regression models.
Table.
Demographics and characteristics of gravidae
| Characteristic | Overall (N=1806) | Missing after delivery (n=278) | Missing before delivery (n=73) | Treated after delivery (n=626) | Treated before delivery (n=829) |
|---|---|---|---|---|---|
| Age, y | n=1805 | n=277 | n=73 | n=626 | n=829 |
| mean±SD, y | 25.3±5.5 | 24.3±5.3 | 24.2±4.9 | 25.8±5.5 | 25.4±5.5 |
| Years lived in the United States, n (%) | n=1802 | n=276 | n=73 | n=624 | n=829 |
| Born in the United States | 872 (48.0) | 166 (60.0) | 41 (56.0) | 256 (41.0) | 409 (49.0) |
| <3 | 270 (15.0) | 41 (15.0) | 12 (16.0) | 91 (15.0) | 126 (15.0) |
| ≥3 (but not born in the United States) | 660 (37.0) | 69 (25.0) | 20 (27.0) | 277 (44.0) | 294 (35.0) |
| Educational status, n (%) | n=1648 | n=262 | n=70 | n=564 | n=752 |
| None or elementary | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Junior high (7–8) | 146 (8.9) | 11 (4.2) | 3 (4.3) | 51 (9.0) | 81 (11.0) |
| High school (9–12) | 1086 (66.0) | 183 (70.0) | 53 (76.0) | 376 (67.0) | 474 (63.0) |
| College (13–16) | 361 (22.0) | 62 (24.0) | 14 (20.0) | 116 (21.0) | 169 (22.0) |
| Graduate (≥17) | 55 (3.3) | 6 (2.3) | 0 (0) | 21 (3.7) | 28 (3.7) |
| Marital status, n (%) | n=1805 | n=277 | n=73 | n=626 | n=829 |
| Divorced, widowed, or separated | 72 (4.0) | 16 (5.8) | 2 (2.7) | 20 (3.2) | 34 (4.1) |
| Married or partnered | 839 (46.0) | 93 (34.0) | 22 (30.0) | 341 (54.0) | 383 (46.0) |
| Single | 894 (50.0) | 168 (61.0) | 49 (67.0) | 265 (42.0) | 412 (50.0) |
| Race and ethnicity, n (%) | n=1797 | n=277 | n=73 | n=624 | n=823 |
| White non-Hispanic | 163 (9.1) | 17 (6.1) | 3 (4.1) | 58 (9.3) | 85 (10.0) |
| Black non-Hispanic | 671 (37.0) | 149 (54.0) | 36 (49.0) | 180 (29.0) | 306 (37.0) |
| Hispanic | 938 (52.0) | 105 (38.0) | 33 (45.0) | 376 (60.0) | 424 (52.0) |
| Others | 25 (1.4) | 6 (2.2) | 1 (1.4) | 10 (1.6) | 8 (1.0) |
| Medical insurance, n (%) | n=1763 | n=273 | n=71 | n=607 | n=812 |
| Medicaid | 1103 (63.0) | 196 (72.0) | 50 (70.0) | 356 (59.0) | 501 (62.0) |
| No insurance | 523 (30.0) | 64 (23.0) | 17 (24.0) | 199 (33.0) | 243 (30.0) |
| Private insurance | 137 (7.8) | 13 (4.8) | 4 (5.6) | 52 (8.6) | 68 (8.4) |
| Income, n (%) | n=1002 | n=169 | n=40 | n=328 | n=465 |
| <$20,000 | 704 (70.0) | 121 (72.0) | 36 (90.0) | 213 (65.0) | 334 (72.0) |
| ≥$20,000 | 298 (30.0) | 48 (28.0) | 4 (10.0) | 115 (35.0) | 131 (28.0) |
| Public assistance, n (%) | n=1796 | n=276 | n=73 | n=623 | n=824 |
| Enrolled, n (%) | 1249 (70.0) | 207 (75.0) | 51 (70.0) | 431 (69.0) | 560 (68.0) |
| Occupation, n (%) | n=1803 | n=277 | n=73 | n=626 | n=276 |
| Employed full time | 309 (17.0) | 39 (14.0) | 14 (19.0) | 103 (16.0) | 153 (19.0) |
| Employed part time | 240 (13.0) | 42 (15.0) | 8 (11.0) | 81 (13.0) | 109 (13.0) |
| Home maker | 647 (36.0) | 66 (24.0) | 22 (30.0) | 260 (42.0) | 299 (36.0) |
| Student | 113 (6.3) | 26 (9.4) | 3 (4.1) | 27 (4.3) | 57 (6.9) |
| Unemployed | 494 (27.0) | 104 (38.0) | 26 (36.0) | 155 (25.0) | 209 (25.0) |
| Self-described dental health, n (%) | n=1806 | n=278 | n=73 | n=626 | n=829 |
| Unhealthy | 1374 (76.0) | 217 (78.0) | 48 (66.0) | 469 (75.0) | 640 (77.0) |
| STI or HIV, n (%) | n=661 | n=97 | n=25 | n=226 | n=313 |
| No | 661 (100.0) | 97 (100.0) | 25 (100.0) | 226 (100.0) | 313 (100.0) |
| Antibiotics during pregnancy, n (%) | n=1123 | n=162 | n=47 | n=389 | n=525 |
| No | 1123 (100.0) | 162 (100.0) | 47 (100.0) | 389 (100.0) | 525 (100.0) |
| BMI, n (%) | n=1734 | n=267 | n=72 | n=599 | n=796 |
| 18.5 to <25.0 kg/m2 | 631 (36.0) | 94 (35.0) | 22 (31.0) | 209 (35.0) | 306 (38.0) |
| 25.0 to 29.9 kg/m2 | 500 (29.0) | 84 (31.0) | 18 (25.0) | 176 (29.0) | 222 (28.0) |
| ≥30.0 kg/m2 | 603 (35.0) | 89 (33.0) | 32 (44.0) | 214 (36.0) | 268 (34.0) |
BMI, body mass index; SD, standard deviation; STI, sexually transmitted infection.
Valentine. Nonsurgical periodontal therapy and newborn outcomes. Am J Obstet Gynecol Glob Rep 2023.
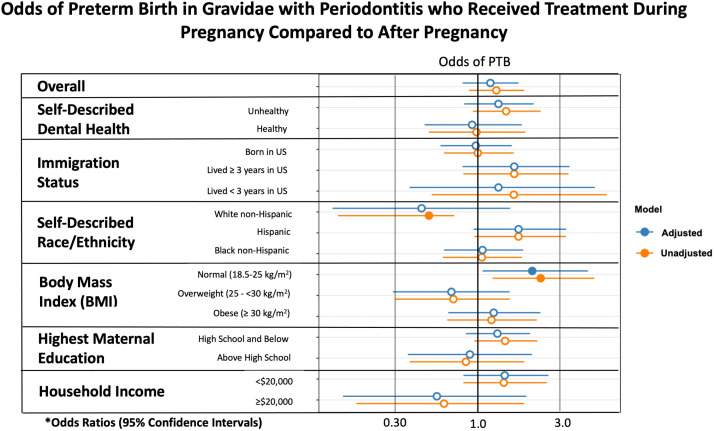
When evaluating nonsurgical periodontal treatment during pregnancy (intervention) compared with that after pregnancy (control) through a per-protocol analysis, gravidae with a prepregnant BMI of 18.5 to <25.0 kg/m2 had significantly higher odds of PTB (13.2% vs 5.7%; aOR, 2.21; 95% CI, 1.07–4.98). Among participants who were classified as overweight (BMI, 25.0 to <30.0 kg/m2; 6.7% vs 9.4%; aOR, 0.68; 95% CI, 0.29–1.59) or obese (BMI, ≥30 kg/m2; 11.0% vs 9.2%, aOR, 1.26; 95% CI, 0.65–2.49) or among the other subgroups analyzed, no significant difference was seen between the intervention and control groups (Figure 2).
Figure 2.
Odds of PTB in gravidae receiving treatment during pregnancy
The asterisk represents adjusted odds ratios were adjusted for maternal age, marital status, years lived in the United States, self-described race or ethnicity, and self-described dental health if not the variable under analysis (eg, self-described dental health–adjusted analysis included adjustment for maternal age, marital status, years lived in the United States, and self-described race or ethnicity).
BMI, body mass index; PTB, preterm birth.
Valentine. Nonsurgical periodontal therapy and newborn outcomes. Am J Obstet Gynecol Glob Rep 2023.
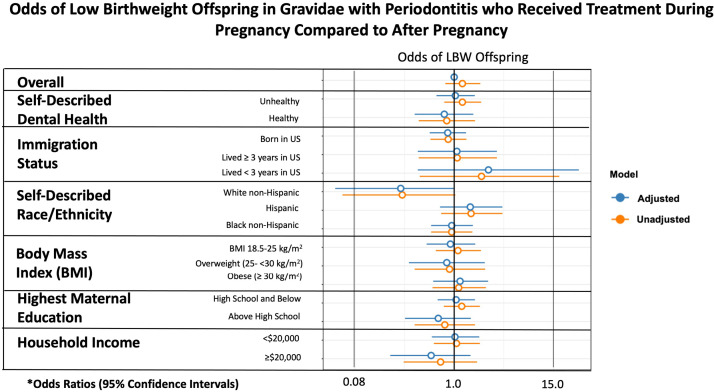
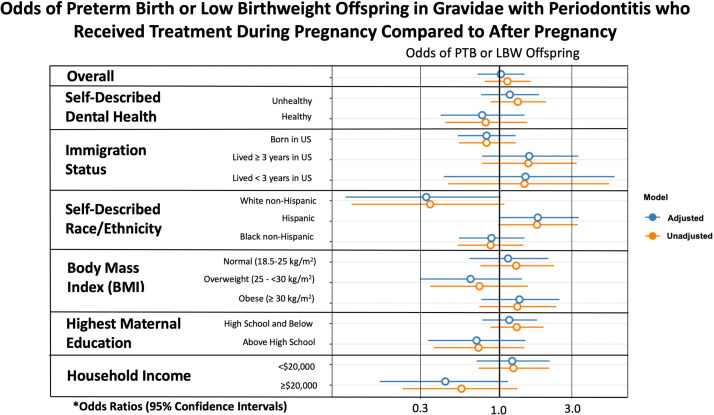
When assessing only LBW as an outcome, there was no statistically significant difference based on subgroup analysis (Supplemental Figure). Moreover, there was no significant difference found when evaluating the composite outcome of PTB or LBW based on subgroup analysis (Figure 3).
Figure 3.
Odds of PTB or LBW offspring in gravidae who received treatment in pregnancy
The asterisk represents adjusted odds ratios were adjusted for maternal age, marital status, years lived in the United States, self-described race or ethnicity, and self-described dental health if not the variable under analysis (eg, self-described dental health–adjusted analysis included adjustment for maternal age, marital status, years lived in the United States, and self-described race or ethnicity).
BMI, body mass index; LBW, low birthweight; PTB, preterm birth.
Valentine. Nonsurgical periodontal therapy and newborn outcomes. Am J Obstet Gynecol Glob Rep 2023.
Evaluation of nonsurgical periodontal therapy among patients with improved periodontal health status compared to those without improvement
Of note, 77% of pregnant participants still met the inclusion criteria for having periodontitis at the end of their periodontal treatments. Among only gravidae with improved periodontal status and assessing PTB and LBW outcomes of those treated during pregnancy vs those treated after pregnancy, the rates of PTB and LBW were not significantly different (13.8% vs 10.4%; aOR, 1.15; 95% CI, 0.52–2.70). Among gravidae without improvements in periodontal status, those treated during pregnancy vs those treated after pregnancy did not have significantly different rates of PTB and LBW (10.1% vs 10.4%; aOR, 0.89; 95% CI, 0.58–1.38).
Assessing timing of periodontal treatment during pregnancy prior to 24 weeks to after 24 weeks of gestation on preterm birth or low birthweight
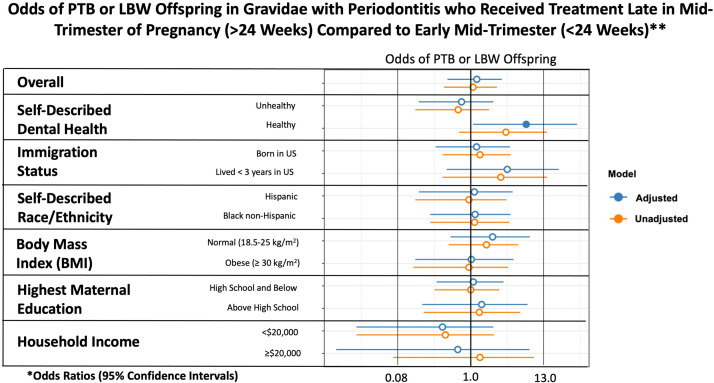
As a further exploratory analysis evaluating whether dental scaling and root planing were performed in a protocol-adherent manner (<24 weeks of gestation) vs a protocol nonadherent manner (≥24 weeks of gestation), we evaluated the outcomes among only those participants who received treatment during pregnancy (intervention group). Baseline outcomes among those who received treatment before 24 weeks of gestation, after 24 weeks of gestation, or after delivery (control) are shown in Supplemental Table. The only differences between those who had treatment performed at <24 weeks of gestation and those who had treatment performed during pregnancy but ≥24 weeks of gestation included a higher percentage of individuals who had immigrated and lived in the United States <3 years; fewer participants who were divorced, widowed, or separated; and a higher income among those that received periodontal treatment at ≥24 weeks of gestation.
There was no statistically significant difference in PTB and LBW based on immigration status, time in the United States, self-described ethnicity, maternal prepregnant BMI, maternal education status, or household income (Figure 4). The only statistically significant finding was among gravidae who had a lack of self-acknowledgment of their periodontal disease when they met the well-established clinical criteria for periodontal disease; among this subgroup, statistically significant higher rates of PTB and LBW occurred in those with initiation of periodontal therapy at ≥24 weeks of gestation compared with those who received periodontal therapy at <24 weeks of gestation (33.3% vs 13.1%; aOR, 6.67; 95% CI, 1.08–38.0).
Figure 4.
Odds of PTB or LBW offspring in gravidae who received treatment ≥24 weeks in pregnancy
The asterisk denotes adjusted odds ratios were adjusted for maternal age, marital status, years lived in the United States, self-described race or ethnicity, and self-described dental health if not the variable under analysis (eg, self-described dental health–adjusted analysis included adjustment for maternal age, marital status, years lived in the United States, and self-described race or ethnicity). Double asterisk denotes because of smaller sample sizes within the later treatment groups, several subanalyses had 0 PTB or LBW offspring, which prevented certain subgroup analyses.
BMI, body mass index; LBW, low birthweight; PTB, preterm birth.
Valentine. Nonsurgical periodontal therapy and newborn outcomes. Am J Obstet Gynecol Glob Rep 2023.
Discussion
Principal findings
Overall, in this per-protocol analysis, dental scaling and root planing performed during pregnancy did not seem to show any preventive benefit on obstetrical outcomes among subgroups with a potential higher baseline risk of PTB or LBW offspring.12, 13, 14, 15, 16 We found that gravidae with a prepregnant BMI of 18.5 to <25.0 kg/m2 who received nonsurgical periodontal therapy during pregnancy had more than a 2-fold increased odds of PTB compared with similar gravidae who delayed treatment until after pregnancy; however, there was no statistically significant association with adverse pregnancy outcomes among gravidae of higher prepregnant BMIs or among gravidae with differing social determinants of health, including immigration status, time in the United States, self-described ethnicity, maternal education status, household income, or self-described dental health.
Results
Previously published studies have reported higher odds of PTB among gravidae of lower prepregnant BMI but not of higher BMIs.26,27 The current study findings were consistent with these previous studies and further demonstrated that dental scaling and root planing performed during pregnancy may increase the odds of PTB among individuals with a BMI of 18.5 to <25.0 kg/m2. However, our findings may also be affected by selection bias that led to differences in characteristics and outcomes. For instance, the control group's rate of PTB was 5.7%, which is lower than the US national average of 10% to 12% of births. However, the US average is just that, an average that includes subgroups with a higher incidence of PTB and subgroups with a lower incidence of PTB. Overall, our per-protocol assessment of the MOTOR trial suggested that there is a significant interaction between biologic and social determinants (maternal BMI) and the risk of occurrence of PTB with periodontal disease and/or timing of nonsurgical periodontal therapy. Further teasing apart these factors will require prospective studies aimed at identifying the underlying causal mechanisms, inclusive of social determinants.
In addition, when evaluating protocol-adherent treatment (dental scaling and root planing performed at <24 weeks of gestation) vs protocol nonadherent treatment (dental scaling and root planing performed at ≥24 weeks of gestation) during pregnancy, the only significant finding was among individuals with a lack of acknowledgment of poor oral health, even though they objectively were diagnosed to have periodontal disease. Lack of acknowledgment of poor oral health is a metric of understanding one's medical conditions, and this lack of understanding among study participants was associated with both a delay in per-protocol therapy and a significantly increased risk of PTB in participants who received treatment at ≥24 weeks of gestation compared with those who were treated at <24 weeks of gestation. A possible reason for this finding is that individuals who believed there was no concern about their oral health status potentially did not complete home cleaning regimens to improve their overall periodontal health status. This analysis was exploratory, and caution should be used in its interpretation. Our findings highlighted the importance of educating and facilitating the understanding of the importance of assessing one's oral health and correlating that with objective measures and suggested that risk-based stratification based on the patient report may not reflect actual periodontal disease. Further studies are needed to confirm our findings and more rigorously evaluate whether a patient's lack of understanding of their poor oral health leads to delays in treatment and adversely affects pregnancy and offspring outcomes.
Clinical implications
The mechanism for how dental scaling and root planing may affect the rates of PTB or LBW offspring are currently unknown; however, there are 2 potential pathways: bacteremia with microbiome alterations and/or effects on localized and systemic inflammation.
Transient bacteremia occurs during dental cleaning, including dental scaling and root planing, which, for some populations, can result in systemic infection. As such, the American Heart Association recommends that individuals with known risk factors (ie, prosthetic heart valves) receive antibiotic prophylaxis before invasive dental cleaning.30,31 Studies evaluating the sparse, low biomass placental microbiome have demonstrated that the greatest degree of sharedness occurs with the oral microbiota.32, 33, 34, 35 Thus, 1 theory on how periodontal treatment during pregnancy may alter the risk of PTB is through modulation or dysregulation of other niche microbiomes during these transient bacterial seeding events, where the oral microbes serve as both the inoculum and source of hematogenous spread.
Nonsurgical periodontal treatment alters systemic markers of inflammation, and inflammation is a well-known risk factor for PTB.36, 37, 38, 39, 40 Within 1 day after dental scaling and root planing, markers of systemic inflammation (including high-sensitive C-reactive protein, interferon-, and interleukin 12p70) and baseline body temperature increase.41 Although one study demonstrated no improvement in systemic markers of inflammation up to 6 weeks after treatment, other studies have revealed decreases in biomarkers of inflammation up to 4 weeks after treatment.41, 42, 43 These data indicate that there is a transient increase in markers of systemic inflammation directly after nonsurgical periodontal therapy that later leads to likely improvements in systemic inflammation.
Research implications
Our study demonstrated that there may exist varying obstetrical outcomes related to dental scaling and root planing during pregnancy among differing subsets of gravidae, namely, those with lower BMIs. Previous studies alluding to transient increased systemic inflammation and transient bacteremia in the days after treatment provided biologic plausibility that our findings may be related to these known triggers of PTB. Innovative efforts focusing on the prevention rather than the treatment of periodontitis, thereby avoiding increased transient systemic inflammation or possible alterations in the microbiome, are of great importance. Future studies should consider the evaluation of interventions to prevent or treat periodontitis concerning both timing and baseline obstetrical risk by maternal characteristics.
Strengths and limitations
Our study has several notable strengths. The large sample size of 1455 participants provided the ability to detect differences between subgroups and increased internal validity. In addition, our per-protocol analysis ensured that study participants assigned to the treatment or control group did not have contamination between groups as we accounted for any treatment during pregnancy in the treatment group. Moreover, there were 351 of 1806 participants (19.4%) enrolled in the MOTOR trial that did not have data on the timing of periodontal treatment during or after pregnancy, preventing adjudication and evaluation of potential crossover, and the number of participants with these missing data was unbalanced between groups (73 in the treatment group and 278 in the control group). There was evidence for crossover between groups, as seen in the current per-protocol analysis. In the subset of participants without data on when they received dental scaling and root planing, we were unable to determine which participants followed the protocol and which participants did not.
The limitations of this secondary analysis of the MOTOR trial data included the lack of power to demonstrate statistically significant associations. In posthoc analyses, we determined that we had a 60% power to detect an odds ratio (OR) of <2.0 in the total sample population and a 50% power to detect an OR of <2.0 comparing participants treated at <24 vs >24 weeks. Therefore, the results of this study were exploratory. However, the results need cautious interpretation and confirmation in larger studies. Our results only suggested associations and did not infer causality. Moreover, a per-protocol analysis has the potential for attrition bias that results in confounding and could have affected our results. Although the individual randomized nature of the study helped promote equal distribution of participants with various background differences to ensure neither the control group nor the intervention group had a more or less predominance of any subpopulation, we were unable to evaluate the history of PTB as a variable of interest because of the 407 participants having missing data on this variable. Finally, we did not correct for multiple comparisons within our analyses because of the independent nature of each analysis.
Conclusion
Overall, nonsurgical periodontal therapy performed during the second or third trimester of pregnancy, as implemented through the protocol in the MOTOR trial, was not associated with any preventive benefit on obstetrical outcomes. However, periodontal treatment during pregnancy was associated with increased odds of PTB among women with a prepregnant BMI of 18.5 to <25.0 kg/m2 compared with women who had delayed treatment after pregnancy. Future studies are needed to explore potential biologic pathways linking such characteristics to obstetrical outcomes and the relationship with periodontitis.
Glossary
aOR: Adjusted odds ratio
BMI: Body mass index
CI: Confidence interval
LBW: Low birthweight newborn
MOTOR: Maternal Oral Therapy to Reduce Obstetric Risk
OR: Odds ratio
PTB: Preterm birth
Footnotes
The authors report no conflict of interest.
The original Maternal Oral Therapy to Reduce Obstetric Risk (MOTOR) trial was funded by the National Institute of Dental and Craniofacial Research (grant number U01-DE014577) and the National Center for Research Resources (grant number M01-RR000046); awarded to Dr. Steven Offenbacher. This current secondary analysis was not funded.
The MOTOR trial was registered on ClinicalTrials.gov (registration number: NCT00097656).
Individual patient consent was obtained as part of the MOTOR trial.
Cite this article as: Valentine G, Perez K, Tsegaye AT, et al. Nonsurgical periodontal treatment during pregnancy and rates of preterm birth. Am J Obstet Gynecol Glob Rep 2023;XX:x.ex–x.ex.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2023.100167.
Appendix. Supplementary materials
References
- 1.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencowe H, Cousens S, Chou D, et al. Born Too Soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 4.Vivares-Builes AM, Rangel-Rincón LJ, Botero JE, Agudelo-Suárez AA. Gaps in knowledge about the association between maternal periodontitis and adverse obstetric outcomes: an umbrella review. J Evid Based Dent Pract. 2018;18:1–27. doi: 10.1016/j.jebdp.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Manrique-Corredor EJ, Orozco-Beltran D, Lopez-Pineda A, Quesada JA, Gil-Guillen VF, Carratala-Munuera C. Maternal periodontitis and preterm birth: systematic review and meta-analysis. Community Dent Oral Epidemiol. 2019;47:243–251. doi: 10.1111/cdoe.12450. [DOI] [PubMed] [Google Scholar]
- 6.Chambrone L, Pannuti CM, Guglielmetti MR, Chambrone LA. Evidence grade associating periodontitis with preterm birth and/or low birth weight: II: a systematic review of randomized trials evaluating the effects of periodontal treatment. J Clin Periodontol. 2011;38:902–914. doi: 10.1111/j.1600-051X.2011.01761.x. [DOI] [PubMed] [Google Scholar]
- 7.Moliner-Sánchez CA, Iranzo-Cortés JE, Almerich-Silla JM, et al. Effect of per capita income on the relationship between periodontal disease during pregnancy and the risk of preterm birth and low birth weight newborn. Systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17:8015. doi: 10.3390/ijerph17218015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim AJ, Lo AJ, Pullin DA, Thornton-Johnson DS, Karimbux NY. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: a systematic review and meta-analysis of randomized controlled trials. J Periodontol. 2012;83:1508–1519. doi: 10.1902/jop.2012.110636. [DOI] [PubMed] [Google Scholar]
- 9.Boutin A, Demers S, Roberge S, Roy-Morency A, Chandad F, Bujold E. Treatment of periodontal disease and prevention of preterm birth: systematic review and meta-analysis. Am J Perinatol. 2013;30:537–544. doi: 10.1055/s-0032-1329687. [DOI] [PubMed] [Google Scholar]
- 10.Bi WG, Emami E, Luo ZC, Santamaria C, Wei SQ. Effect of periodontal treatment in pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2021;34:3259–3268. doi: 10.1080/14767058.2019.1678142. [DOI] [PubMed] [Google Scholar]
- 11.Iheozor-Ejiofor Z, Middleton P, Esposito M, Glenny AM. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD005297.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol. 2002;73:911–924. doi: 10.1902/jop.2002.73.8.911. [DOI] [PubMed] [Google Scholar]
- 13.Jeffcoat MK, Hauth JC, Geurs NC, et al. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol. 2003;74:1214–1218. doi: 10.1902/jop.2003.74.8.1214. [DOI] [PubMed] [Google Scholar]
- 14.Sadatmansouri S, Sedighpoor N, Aghaloo M. Effects of periodontal treatment phase I on birth term and birth weight. J Indian Soc Pedod Prev Dent. 2006;24:23–26. doi: 10.4103/0970-4388.22831. [DOI] [PubMed] [Google Scholar]
- 15.Tarannum F, Faizuddin M. Effect of periodontal therapy on pregnancy outcome in women affected by periodontitis. J Periodontol. 2007;78:2095–2103. doi: 10.1902/jop.2007.060388. [DOI] [PubMed] [Google Scholar]
- 16.Michalowicz BS, Hodges JS, DiAngelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 17.Whitehead NS. The relationship of socioeconomic status to preterm contractions and preterm delivery. Matern Child Health J. 2012;16:1645–1656. doi: 10.1007/s10995-012-0948-4. [DOI] [PubMed] [Google Scholar]
- 18.Peacock JL, Bland JM, Anderson HR. Preterm delivery: effects of socioeconomic factors, psychological stress, smoking, alcohol, and caffeine. BMJ. 1995;311:531–535. doi: 10.1136/bmj.311.7004.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgen CS, Bjørk C, Andersen PK, Mortensen LH, Nybo Andersen AM. Socioeconomic position and the risk of preterm birth–a study within the Danish National Birth Cohort. Int J Epidemiol. 2008;37:1109–1120. doi: 10.1093/ije/dyn112. [DOI] [PubMed] [Google Scholar]
- 20.DeFranco EA, Lian M, Muglia LA, Schootman M. Area-level poverty and preterm birth risk: a population-based multilevel analysis. BMC Public Health. 2008;8:316. doi: 10.1186/1471-2458-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace ME, Mendola P, Chen Z, Hwang BS, Grantz KL. Preterm birth in the context of increasing income inequality. Matern Child Health J. 2016;20:164–171. doi: 10.1007/s10995-015-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekwo E, Moawad A. The risk for recurrence of premature births to African-American and white women. J Assoc Acad Minor Phys. 1998;9:16–21. [PubMed] [Google Scholar]
- 23.Reagan PB, Salsberry PJ. Race and ethnic differences in determinants of preterm birth in the USA: broadening the social context. Soc Sci Med. 2005;60:2217–2228. doi: 10.1016/j.socscimed.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Gemmill A, Catalano R, Casey JA, et al. Association of preterm births among US Latina Women with the 2016 presidential election. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosa JL, Guendelman S, Pearl M, Graham S, Abrams B, Kharrazi M. The association between pre-pregnancy BMI and preterm delivery in a diverse Southern California population of working women. Matern Child Health J. 2011;15:772–781. doi: 10.1007/s10995-010-0633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharifzadeh F, Kashanian M, Jouhari S, Sheikhansari N. Relationship between pre-pregnancy maternal BMI with spontaneous preterm delivery and birth weight. J Obstet Gynaecol. 2015;35:354–357. doi: 10.3109/01443615.2014.968101. [DOI] [PubMed] [Google Scholar]
- 27.Kashanian M, Sharifzadeh F, Jouhari S. Relationship between pre pregnancy maternal body mass index (BMI) with birth weight, and spontaneous preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2019;234:e19. doi: 10.3109/01443615.2014.968101. [DOI] [PubMed] [Google Scholar]
- 28.Committee Opinion No. 569: oral health care during pregnancy and through the lifespan. Obstet Gynecol. 2013;122:417–422. doi: 10.1097/01.AOG.0000433007.16843.10. [DOI] [PubMed] [Google Scholar]
- 29.Offenbacher S, Beck JD, Jared HL, et al. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstet Gynecol. 2009;114:551–559. doi: 10.1097/AOG.0b013e3181b1341f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson GE, Crowley AL. Antibiotic prophylaxis for infective endocarditis. Circulation. 2019;140:181–183. doi: 10.1161/CIRCULATIONAHA.119.041085. [DOI] [PubMed] [Google Scholar]
- 31.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 32.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentine G, Prince A, Aagaard KM. The neonatal microbiome and metagenomics: what do we know and what is the future? Neoreviews. 2019;20:e258–e271. doi: 10.1542/neo.20-5-e258. [DOI] [PubMed] [Google Scholar]
- 34.Chu DM, Valentine GC, Seferovic MD, Aagaard KM. The development of the human microbiome: why moms matter. Gastroenterol Clin North Am. 2019;48:357–375. doi: 10.1016/j.gtc.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valentine G, Chu DM, Stewart CJ, Aagaard KM. Relationships between perinatal interventions, maternal-infant microbiomes, and neonatal outcomes. Clin Perinatol. 2018;45:339–355. doi: 10.1016/j.clp.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Dm Sherer. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 39.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morozumi T, Yashima A, Gomi K, et al. Increased systemic levels of inflammatory mediators following one-stage full-mouth scaling and root planing. J Periodont Res. 2018;53:536–544. doi: 10.1111/jre.12543. [DOI] [PubMed] [Google Scholar]
- 42.Konopka L, Pietrzak A, Brzezińska-Błaszczyk E. Effect of scaling and root planing on interleukin-1β, interleukin-8 and MMP-8 levels in gingival crevicular fluid from chronic periodontitis patients. J Periodontal Res. 2012;47:681–688. doi: 10.1111/j.1600-0765.2012.01480.x. [DOI] [PubMed] [Google Scholar]
- 43.López NJ, Quintero A, Casanova PA, Ibieta CI, Baelum V, López R. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a controlled clinical trial. J Periodontol. 2012;83:267–278. doi: 10.1902/jop.2011.110227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.