Abstract
Sworder et al.1 developed an integrated simultaneous tumor and effector profiling (STEP) approach to study resistance mechanisms to CD19-CAR T cell therapy in large B-cell lymphomas. Their study provides novel biological insights and paves the way for future interventions.
Sworder et al.1 developed an integrated simultaneous tumor and effector profiling (STEP) approach to study resistance mechanisms to CD19-CAR T cell therapy in large B-cell lymphomas. Their study provides novel biological insights and paves the way for future interventions.
Main text
Disease-free outcomes for adult patients with relapsed/refractory large B cell lymphoma (r/r LBCL) have improved with the advent of CD19-redirected chimeric antigen receptor (CD19-CAR) T cell therapy. However, after treatment approximately half of the patients will experience disease relapse, and a subset of patients have no disease response to CD19-CAR T cell therapy at all.2 Mechanisms of resistance are likely multifactorial and relate to tumor cells and the microenvironment, CAR T cells, and native non-engineered immune cells. While many potential mechanisms of resistance to CD19-CAR T cell therapies have been described,3,4,5 comprehensive analyses that include tumor cells as well as CAR T cells are in its infancy.
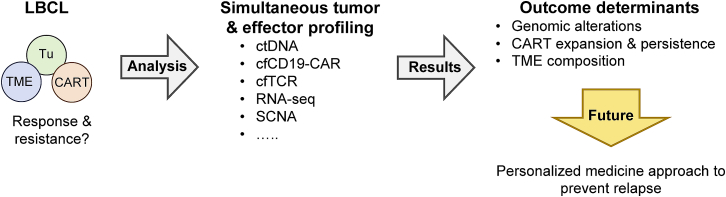
In the article “Determinants of resistance to engineered T cell therapies targeting CD19 in large B cell lymphomas,” recently published in Cancer Cell, Sworder et al.1 developed a comprehensive approach for simultaneous tumor and effector profiling (STEP) to characterize the different cellular compartments involved in treatment response and/or development of resistance (Figure 1). They profiled over 700 longitudinal peripheral blood samples from two independent cohorts (discovery, n = 65; validation, n = 73) of patients with lymphoma, who had received the FDA-approved CD19-CAR T cell product axicabtagene ciloleucel (axi-cel). Analyses included measuring levels of (1) circulating tumor DNA (ctDNA), (2) cell-free CD19-CAR retroviral fragments (cfCD19-CAR), and (3) cell-free T cell receptor (cfTCR) rearrangements. In addition, bulk RNA sequencing (RNA-seq), genome-wide somatic copy number alteration (SCNA) analyses, and other analyses including CIBERSORTx were performed on tumor tissue pre-treatment and at time of relapse.
Figure 1.
Simultaneous tumor and effector profiling to elucidate determinants of response and resistance post CD19-CAR T cell therapy in lymphoma
Simultaneous tumor and effector profiling (STEP) includes analysis of immune cells (circulating tumor DNA [ctDNA], cell-free CD19-CAR retroviral fragments [cfCD19-CAR], cell free T-cell receptor [cfTCR] rearrangements), tumor cells (bulk RNA-seq, SCNA), and the TME (e.g., CIBERSORTx). For details see text. CART: CD19-CAR T cells; LBCL: large B cell lymphoma; SCNA: somatic copy number alteration; TME: tumor microenvironment; Tu: tumor cells.
Consistent with prior reports,6 Sworder et al.1 found higher levels of pre-treatment ctDNA were associated with worse event-free survival (EFS) post CD19-CAR T cell therapy. Measurement of cfCAR19 allowed for tracking of CAR T cells post-infusion, similar to FACS analysis. While CD19-CAR T cell levels differed between detection methods, the measurements were significantly correlated. Interestingly, while the levels of cfCD19-CAR did not differ between patients with ongoing disease response versus those with subsequent treatment failure, cfCD19-CAR fragment length did (“short” versus ”long”). This included correlation of long, but not short, cfCD19-CAR fragments with FACS analysis, suggesting that short cfCAR19 fragments likely are derived from tumor-infiltrating CAR T cells. If confirmed in other studies, this would present a significant advance since for the first time we would be able to survey the presence of CAR T cells within tumors with a ‘peripheral blood test’. cfCAR19 levels correlated with cfTCR levels early post-infusion, however this correlation weakened over time. This finding suggests different expansion and/or contraction kinetics of CAR and non-engineered T cells, differences in their biodistribution, and/or potentially the induction of endogenous lymphoma-specific T cell responses.
The authors then explored the genomic characteristics of tumor samples at baseline and post CD19-CAR T cell therapy. They characterized genomic characteristics of r/r LBCL of this study cohort with a separate treatment naive LBCL cohort and then explored baseline and emergent genomic alterations associated with therapeutic resistance. They identified diverse factors that influence outcome and observed reciprocal interactions between tumor cells, CD19-CAR T cells, and the tumor microenvironment. The mutational profile of r/r and naive LBCL overall was similar. However, there was an enrichment in alteration in several genes including TP53 and MYC, and differences in cell of origin and LymphGen subtype distribution. Mutations in several genes were significantly associated with poorer EFS, including PAX5, IRF8, TP53, and TMEM30A. Genetic alterations emerged in several genes in the setting of subsequent treatment failure, included CD19, PPM1D, TP53, and PAX5. In addition, utilizing SCNA analyses, the authors identified 9p24.1 amplifications, a gene locus that encodes the immune checkpoint molecules CD274 (PD-L1) and PDCD1LG2 (PD-L2).
Exploration of potential interaction between tumor cells and CAR T cells showed that while the tumor mutational profile was largely similar across patients, several mutations were associated with higher (TNFRSF14, BCL2, BTG) and lower (IRF4) CD19-CAR T cell expansion. Interestingly, mutations in TNFRSF14 were associated with a unique composition of immune cells in the microenvironment, suggesting that such alterations may promote immunosuppression. Furthermore, tumors with high levels of infiltrating CD19-CAR T cells at the time of disease progression exhibited gene signatures associated with increased T cell exhaustion and inflammation.
Finally, Sworder et al.1 conducted a multivariable analysis to assess the prognostic impact of tumor, CD19-CAR T cell, and non-engineered immune cell features and patient outcomes. Higher levels of ctDNA pre- and post-treatment (weeks 1 and 4) maintained clinical significance, predicting worse EFS and overall survival (OS). Additionally, higher levels of cfCAR19 at week 1 were associated with improved EFS, suggesting that early CD19-CAR T cell expansion played a role in long-term disease response. Evaluation of clinical and molecular features together in a stepwise model identified that the inclusion of ctDNA levels at week 4 with cfCAR19 levels at week 1 was prognostic for both EFS and OS. Finally, the developed STEP score when using ctDNA levels at 4 weeks post-infusion was able to be stratify patient outcome.
In conclusion, this study highlights the tremendous need for continued investigation into the multifaceted aspects that influence response to cellular therapeutics. The study presents a tour de force in which the authors implemented novel techniques to evaluate the complex tumor versus cell therapy ecosystem. This work is important as the ability to predict long-term response after CD19-CAR T cell therapy is currently lacking, and novel biomarkers and prognostic models are urgently needed to guide treatment decisions. While larger studies are needed to confirm the authors findings, measuring ctDNA and cfCD19-CAR levels could be readily developed into clinical laboratory improvement amendments (CLIA)-approved laboratory tests.
One somber finding of the study is that there is not a unifying mechanism of immune evasion to CD19-CAR T cell therapy, which is analogous to resistance mechanisms to immune checkpoint blockade.7 In this regard, one would predict that resistance mechanisms to CD19-CAR T cell products are not uniform, in particular to CD19-CAR T cell products that utilize a different costimulatory domain (CD28: axi-cel; 41BB: tisagenlecleucel and lisocabtagene maraleucel). Likewise, resistance mechanisms of other CD19-positive hematological malignancies, including leukemia, most likely will differ. Thus, preventing relapse post CD19-CAR T cell therapy will most likely require a personalized medicine approach.
Declaration of interests
S.G. is a co-inventor on patent applications in the fields of cell or gene therapy for cancer, a consultant of TESSA Therapeutics, a member of the Data and Safety Monitoring Board (DSMB) of Immatics, and has received honoraria from Tidal, Catamaran Bio, and Sanofi within the last 2 years.
References
- 1.Sworder B.J., Kurtz D.M., Alig S.K., Frank M.J., Shukla N., Garofalo A., Macaulay C.W., Shahrokh Esfahani M., Olsen M.N., Hamilton J., et al. Determinants of resistance to engineered T cell therapies targeting CD19 in large B cell lymphomas. Cancer Cell. 2023;41:210–225.e5. doi: 10.1016/j.ccell.2022.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., Lin Y., Braunschweig I., Hill B.T., Timmerman J.M., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain M.D., Ziccheddu B., Coughlin C.A., Faramand R., Griswold A.J., Reid K.M., Menges M., Zhang Y., Cen L., Wang X., et al. Whole-genome sequencing reveals complex genomic features underlying anti-CD19 CAR T-cell treatment failures in lymphoma. Blood. 2022;140:491–503. doi: 10.1182/blood.2021015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah N.N., Fry T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019;16:372–385. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson R.C., Maus M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer. 2021;21:145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank M.J., Hossain N.M., Bukhari A., Dean E., Spiegel J.Y., Claire G.K., Kirsch I., Jacob A.P., Mullins C.D., Lee L.W., et al. Monitoring of circulating tumor DNA Improves early relapse detection after axicabtagene ciloleucel infusion in large B-cell lymphoma: Results of a Prospective Multi-Institutional trial. J. Clin. Oncol. 2021;39:3034–3043. doi: 10.1200/JCO.21.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fares C.M., Van Allen E.M., Drake C.G., Allison J.P., Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: Why Does checkpoint Inhibitor Immunotherapy not work for All patients? American Society of clinical Oncology educational book/ASCO. American Society of Clinical Oncology. Meeting. 2019;39:147–164. doi: 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]