Abstract
Background
Previous research has supported the association between the triglyceride–glucose index (TyG index) and the incidence and prognosis of cardiovascular disease. However, the association between the TyG index and the prognosis of patients with acute coronary syndrome (ACS) without diabetes mellitus (DM) who underwent emergency percutaneous coronary intervention (PCI) with drug-eluting stents (DESs) has not been thoroughly investigated, and these patients may easily be neglected. Therefore, this study aimed to investigate the association between the TyG index and major adverse cardiovascular and cerebrovascular events (MACCEs) in Chinese ACS patients without DM who underwent emergency PCI with DES.
Methods
The total number of ACS patients without DM who underwent emergency PCI with DES for this study was 1650. Ln [fasting triglycerides (mg/dL) ×fasting plasma glucose (mg/dL)/2] is the formula used to calculate the TyG index. According to the TyG index, we classified the patients into two groups. The frequency of the following endpoint events was calculated and compared between the two groups: all-cause death, non-fatal myocardial infarction (MI), non-fatal ischemia stroke, ischemia-driven revascularization and cardiac rehospitalization.
Results
After a median of 47 months of follow-up [47 (40, 54)], 437 (26.5%) endpoint events were recorded in total. The TyG index was further demonstrated to be independent of MACCE by multivariable Cox regression analysis (hazard ratio [HR], 1.493; 95% confidence interval [CI], 1.230–1.812; p<0.001). The TyG index≥7.08 group had a considerably greater incidence of MACCE (30.3% vs. 22.7% in the TyG index<7.08 group, p<0.001), cardiac death (4.0% vs. 2.3% in the TyG index<7.08 group, p=0.047), and ischemia-driven revascularization (5.7% vs. 3.6% in the TyG index<7.08 group, p=0.046) than the TyG index<7.08 group. Between the two groups, there was no discernible difference in all-cause death (5.6% vs. 3.8% in the TyG index<7.08 group, p=0.080), non-fatal MI (1.0% vs. 0.2% in the TyG index<7.08 group, p=0.057), non-fatal ischemic stroke (1.6% vs. 1.0% in the TyG index<7.08 group, p=0.272), and cardiac rehospitalization (16.5% vs. 14.1% in the TyG index<7.08 group, p=0.171).
Conclusion
For ACS patients without DM who received emergency PCI with DES, the TyG index might be an independent predictor of MACCE.
Keywords: triglyceride-glucose index, insulin resistance, acute coronary syndrome, emergency percutaneous coronary intervention, major adverse cardiovascular and cerebrovascular event
1. Introduction
The most severe form of atherosclerotic cardiovascular disease (ASCVD), acute coronary syndrome (ACS), is responsible for the majority of cardiovascular disease (CVD)-related morbidity and mortality worldwide (1, 2). Since the significant development and implementation of superior evidence-based treatments, such as optimized medical therapy and revascularization, in recent years, the prognosis of ACS patients has noticeably improved. Nevertheless, the risk of recurrent poor cardiovascular events remains very high in patients with ACS (3, 4). As a result, clinical trials should identify the characteristics that put patients with ACS at a high risk of developing major adverse cardiovascular and cerebrovascular events (MACCEs), establish novel treatment targets, and develop tailored approaches that are line with risk levels.
Various variables, such as high glucose and lipid abnormalities, contribute to the development of CVD (5, 6). The primary pathophysiological underpinning for the beginning of ACS is thought to be the rupture and erosion of atherosclerotic plaque. Insulin resistance (IR) has been demonstrated to be a critical mediator in the development of metabolic disorders and atherosclerotic CVD (7, 8). Despite being widely acknowledged as the most accurate and trustworthy procedures to measure IR, the homeostasis model assessment of IR (HOMA-IR) and hyperinsulinemia-euglycemic clamp are complicated and costly to be applied in clinical practice (9). As a result, we require a straightforward, usable, and trustworthy marker to assess IR. The key elements of Mets are high levels of fasting blood glucose (FBG) and triglyceride (TG). The triglyceride–glucose (TyG) index, which is simply a combination of TG and FBG, has recently been revealed to have a substantial connection with the HOMA-IR and hyperinsulinemia-euglycemic clamp (10, 11). Furthermore, numerous studies have shown that the TyG index can indicate myocardial infarction (MI), stroke, or other adverse cardiovascular events (12–14). However, IR is easy to be ignored in non-diabetic patients. Studies have shown that IR is related to in-stent restenosis (ISR) in non-diabetic patients undergoing coronary stenting (15–17). The association between the TyG index and the prognosis of ACS patients without diabetes mellitus (DM) who underwent emergency percutaneous coronary intervention (PCI) with drug-eluting stents (DESs) has not been thoroughly investigated. Therefore, the purpose of the current study was to investigate the association between the TyG index and MACCEs in Chinese ACS patients without DM who underwent emergency PCI with DES.
2. Methods
2.1. Study population
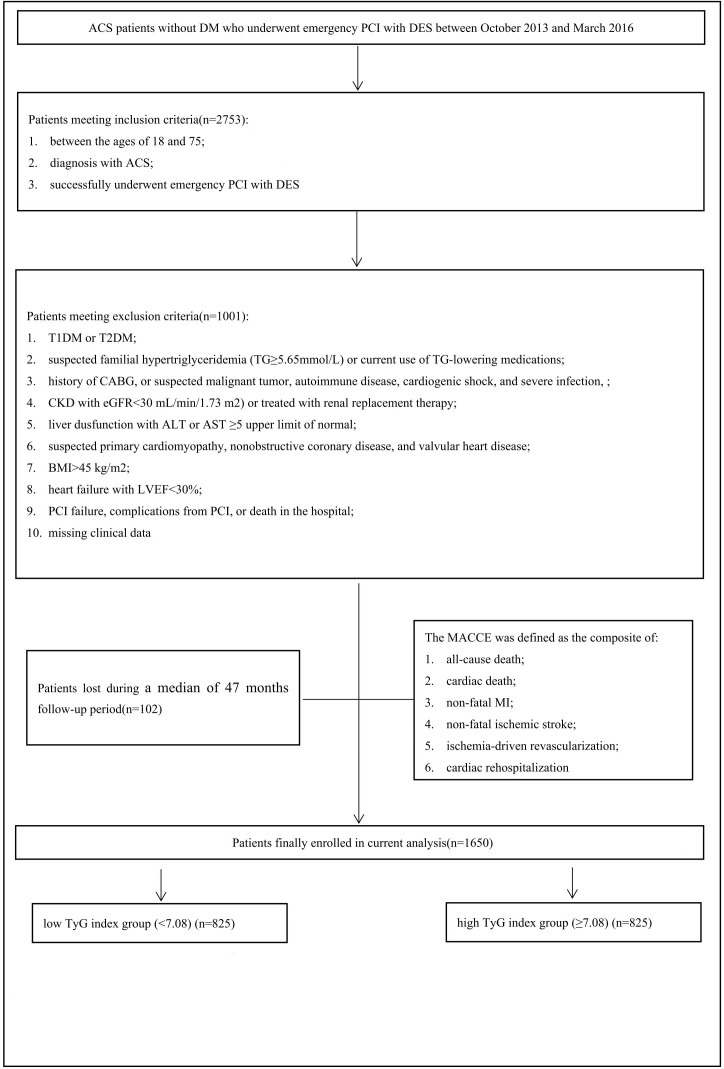
The current research was conducted between October 2013 and March 2016 on ACS patients without DM who received emergency PCI with DES at the First Affiliated Hospital of Xi’an Jiaotong University. It is an observational, retrospective cohort study with a single center. The following were the inclusion requirements: (1) between the ages of 18 and 75; (2) with an ACS diagnosis; and (3) successfully completed emergency PCI with DES. A total of 2753 consecutive ACS cases were examined. The following were the primary exclusion criteria for this study: (1) type 1 DM (T1DM) or type 2 DM (T2DM); (2) suspected familial hypertriglyceridemia (TG≥5.65 mmol/L) or current use of TG-lowering drugs; (3) history of coronary artery bypass grafting (CABG), or suspected malignant tumor, autoimmune disease, cardiogenic shock, and severe infection; (4) chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR) < 30mL/min/1.73 m2) or treated with renal replacement; (5) body mass index (BMI) > 45 kg/m2; (6) suspected primary cardiomyopathy, nonobstructive coronary disease, and valvular heart disease; (7) liver dysfunction with aspartate transaminase (AST) or alanine transaminase (ALT) ≥5 times upper limit of normal; (8) heart failure with left ventricular ejection fraction (LVEF) < 30%; (9) PCI failure, complications from PCI, or death in the hospital; and (10) absence of clinical data. The exclusion criteria resulted in the exclusion of 1101 patients. Over the duration of the follow-up period, 102 patients were lost. Finally, 1650 ACS patients were included in the study. According to the cohort’s median TyG index level (7.08), the patients were separated into two groups: low TyG index group (<7.08) and high TyG index group (≥7.08) ( Figure 1 ).
Figure 1.
The flowchart of study subject enrollment. ACS, acute coronary syndrome PCI, percutaneous coronary intervention. DES, drug-eluting stent; TG, triglyceride; TIDM, type I diabetes mellitus; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index, LVEF, left ventricular ejection fraction; MACCE, major adverse cardiovascular and cerebrovascular events, MI, myocardial infarction; TYG, triglyceride-glucose.
2.2. Data collection and follow-up
Clinicians with the appropriate training gathered clinical data from the electronic medical records. These records contained information on people’s anthropometric characteristics, laboratory test results, and their medical and procedural histories. After fasting for the previous night, the venous blood samples were obtained in the morning and examined in the central laboratory on the same day according to established laboratory procedures. All patients were routinely observed by professional clinicians for MACCEs at 3, 6, and 12 months following hospitalization, as well as every 6 months for up to 66 months. Follow-up data were collected from hospital records or through telephone or in-person interviews with patients and their families.
MACCE, the observational endpoint of the current trial, was the composite of cardiac rehospitalization (admission because of angina or heart failure), ischemia-driven revascularization, non-fatal ischemic stroke, non-fatal myocardial infarction (MI), cardiac death, and all-cause death. Only the most serious event (all-cause death > non-fatal MI > non-fatal ischemic stroke > ischemia-driven revascularization > cardiac rehospitalization) was chosen for our analysis for patients with several poor outcomes almost occurring at the same time throughout the follow-up. Only the first instance of the same incident for patients with numerous occurrences was chosen for our analysis.
2.3. Definitions
The diagnostic criteria for ACS, which comprised ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation acute coronary syndrome (NSTE-ACS: non-ST-segment elevation MI [NSTEMI] or unstable angina [UA]), were directed to appropriate guidelines (18, 19). Patients were considered to have hypertension if they had previously received a conclusive diagnosis or if their systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg on more than two separate days during their initial hospitalization. According to the practical guidelines, patients with T1DM or T2DM were those who had a prior, conclusive diagnosis or who have just had their T1DM or T2DM confirmed (20). Patients were considered to have hypercholesterolemia if their fasting total cholesterol (TC) >6.22 mmol/L, their low-density lipoprotein cholesterol (LDL-C)>4.14 mmol/L, or they were on lipid-lowering medication. Patients with cerebral infarction or cerebral hemorrhage were considered to have a stroke. Patients with peripheral artery disease (PAD) were those who had artery disease that affected arteries other than the aorta and coronary arteries with stenosis ≥ 50% and related ischemic symptoms and/or signs. Patients were considered to have renal dysfunction if their eGFR was between 30 and 60mL/min/1.73m2. Emergency PCI was defined as PCI performed within 24 h of hospital admission for NSTE-ACS patients or within 12 h of symptom onset for STEMI patients.
The following equation was used to determine the TyG index: ln[fasting TG (mg/mL)×FBG (mg/mL)/2] (21). BMI was calculated as follows: weight (kg)/[height (m)]2. The formula for eGFR was 186×serum creatinine (mg/dL)-1.154×age-0.203 (×0.742 if female) (22).
A number of main coronary vessels, including left anterior descending artery, left circumflex artery, and right coronary artery, must have a stenosis ≥50% to be considered multivessel lesions. According to the prior medical history or coronary angiography, a chronic total occlusion (CTO) lesion was a complete obstruction lesion that lasted more than 3 months. A single stenotic lesion that was longer than 20 mm was considered a diffuse lesion. Stenosis of more than 50% appearing in the segment inside the stent, 5 mm proximal or distal to the stent, was described as ISR (23).
2.4. Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median. Comparisons between the two groups were analyzed by independent-sample t-test or Mann–Whitney U-test. Categorical variables were described as counts (percentages) and compared by Pearson chi-square test (Pearson 2 test) or Fisher’s exact test. The predictive value of the variables for MACCE was evaluated by univariate and multivariable Cox proportional hazards analysis. Several risk factors including important clinical and significant variables (p<0.2) in the univariate model were included in the multivariate. The cumulative incidence of MACCE was estimated by Kaplan–Meier survival curves. Further stratified analysis was conducted to determine the consistency of the prognostic impact of the TyG index for MACCE.
IBM SPSS Statistics (version 24.0) was used for data analysis. A two-tailed p value <0.05 was regarded as statistically significant.
3. Results
Final enrollment in the research included 1650 patients (60.50 ± 10.19 years; 22.5% female). A total of 77 (4.7%) all-cause deaths including 52 (3.1%) cardiac deaths, 10 (0.6%) non-fatal MIs, 21 (1.3%) non-fatal ischemic strokes, 77 (4.7%) ischemia-driven revascularizations, and 252 (15.3%) cardiac rehospitalizations occurred throughout the duration of the 66-month follow-up. Finally, 437 (26.5%) MACCEs in total were included in the analysis.
3.1. Baseline characteristics of the low and high TyG index groups
According to the median TyG index level (7.08) of the cohort, the patients were separated into two groups: low TyG index group (<7.08) and high TyG index group (≥7.08). Table 1 shows the baseline characteristics of the two groups. Patients in the high TyG index group had a faster heart rate (74.53 ± 12.11 vs. 73.29 ± 11.96, p=0.036), higher percentage of males (80.1% vs. 74.9%, p=0.011), higher previous history of hyperlipidemia (15.3% vs. 6.5%, p<0.001), and lower percentage of smoking (52.6% vs. 59.9%, p=0.003) than those in the low TyG index group. In terms of laboratory findings, patients in the high TyG index group had raised levels of white blood cell (WBC; 7.46 ± 2.54 vs. 7.14 ± 2.58, p=0.013), hemoglobin (Hb; 139.51 ± 18.30 vs. 136.79 ± 18.41, p=0.003), blood urea nitrogen (BUN; 5.46 ± 1.70 vs. 5.13 ± 1.93, p<0.001), FBG (6.64 ± 2.07 vs. 5.19 ± 1.24, p<0.001), glycosylated hemoglobin A1c (HbA1c; 5.74 ± 0.70 vs. 5.49 ± 0.54, p<0.001), TC (4.01 ± 1.16 vs. 3.41 ± 1.09, p<0.001), TG (1.98 ± 1.05 vs. 1.07 ± 0.33, p<0.001), and LDL-C (1.96 ± 0.95 vs. 1.67 ± 0.70, p<0.001) but decreased level of high-density lipoprotein cholesterol (HDL-C; 0.95 ± 0.22 vs. 1.00 ± 0.23, p<0.001). No differences were found between the two groups in terms of coronary intervention information and medication at discharge.
Table 1.
Baseline clinical characteristics of patients in the low and high TyG index groups.
| Characteristics | Total population | Low TyG index group | High TyG index group | P value |
|---|---|---|---|---|
| (n=1650) | (n=825) | (n=825) | ||
| Age, years | 60.50 ± 10.19 | 61.14 ± 10.34 | 59.85 ± 10.01 | 0.010 |
| Gender, male, n (%) | 1279 (77.5%) | 618 (74.9%) | 661(80.1%) | 0.011 |
| BMI, kg/m2 | 23.26 ± 2.46 | 23.36 ± 2.48 | 23.17 ± 2.43 | 0.118 |
| SBP, mmHg | 126.93 ± 20.74 | 126.46 ± 20.96 | 127.39 ± 20.51 | 0.363 |
| DBP, mmHg | 77.67 ± 11.97 | 77.28 ± 11.87 | 78.06 ± 12.07 | 0.188 |
| Heart rate, bpm | 73.91 ± 12.05 | 73.29 ± 11.96 | 74.53 ± 12.11 | 0.036 |
| Smoking history, n (%) | 928(56.2%) | 494(59.9%) | 434(52.6%) | 0.003 |
| Drinking history, n (%) | 459(27.8%) | 239(29.0%) | 220(26.7%) | 0.297 |
| Family history of CAD, n (%) | 145(8.8%) | 70(8.5%) | 75(9.1%) | 0.664 |
| Initial diagnosis, n (%) | 0.134 | |||
| UA | 976(59.2%) | 492(59.6%) | 484(58.7%) | |
| NSTEMI | 118(7.2%) | 68(8.2%) | 50(6.1%) | |
| STEMI | 556(33.7%) | 265(32.1%) | 291(35.3%) | |
| Medical history, n (%) | ||||
| Hypertension | 886(53.7%) | 434(52.6%) | 452(54.8%) | 0.374 |
| Hyperlipidemia | 180(10.9%) | 54(6.5%) | 126(15.3%) | < 0.001 |
| Renal dysfunction | 14(0.8%) | 7(0.8%) | 7(0.8%) | > 0.999 |
| Previous MI | 148(9.0%) | 77(9.3%) | 71(8.6%) | 0.605 |
| Previous PCI | 158(9.6%) | 86(10.4%) | 72(8.7%) | 0.241 |
| Previous stroke | 321(19.5%) | 157(19.0%) | 164(19.9%) | 0.663 |
| Previous PAD | 260(15.8%) | 119(14.4%) | 141(17.1%) | 0.137 |
| Laboratory results | ||||
| WBC (×109/L) | 7.30 ± 2.57 | 7.14 ± 2.58 | 7.46 ± 2.54 | 0.013 |
| PLT (×109/L) | 158.49 ± 55.42 | 156.37 ± 53.36 | 160.61 ± 57.35 | 0.120 |
| Hb (g/L) | 138.15 ± 18.40 | 136.79 ± 18.41 | 139.51 ± 18.30 | 0.003 |
| BUN (mmol/L) | 5.29 ± 1.83 | 5.13 ± 1.93 | 5.46 ± 1.70 | < 0.001 |
| Cr (umol/L) | 67.93 ± 17.72 | 67.81 ± 18.22 | 68.05 ± 17.21 | 0.779 |
| eGFR (mL/min/1.73m2) | 97.48 ± 27.25 | 96.98 ± 26.14 | 97.98 ± 28.34 | 0.455 |
| FBG (mmol/L) | 5.92 ± 1.85 | 5.19 ± 1.24 | 6.64 ± 2.07 | < 0.001 |
| HbA1C (%) | 5.61 ± 0.64 | 5.49 ± 0.54 | 5.74 ± 0.70 | < 0.001 |
| HDL-C (mmol/L) | 0.98 ± 0.23 | 1.00 ± 0.23 | 0.95 ± 0.22 | < 0.001 |
| TC (mmol/L) | 3.71 ± 1.17 | 3.41 ± 1.09 | 4.01 ± 1.16 | < 0.001 |
| TG (mmol/L) | 1.52 ± 0.90 | 1.07 ± 0.33 | 1.98 ± 1.05 | < 0.001 |
| LDL-C (mmol/L) | 1.82 ± 0.85 | 1.67 ± 0.70 | 1.96 ± 0.95 | < 0.001 |
| NT-proBNP (pg/mL) | 690.55 ± 1233.19 | 729.52 ± 1239.91 | 651.57 ± 1225.95 | 0.199 |
| LVEF (%) | 60.07 ± 11.28 | 59.99 ± 11.21 | 60.16 ± 11.36 | 0.760 |
| Angiographic data | ||||
| LM disease, n (%) | 174(10.5%) | 88(10.7%) | 86(10.4%) | 0.873 |
| CTO, n (%) | 475(28.8%) | 243(29.5%) | 232(28.8%) | 0.550 |
| Number-vessel disease. n (%) | 0.574 | |||
| Single-vessel disease | 445(27.0%) | 231(28.0%) | 214(25.9%) | |
| Two-vessel disease | 497(30.1%) | 249(30.2%) | 248(30.1%) | |
| Three-vessel disease | 708(42.9%) | 345(41.8%) | 363(44.0%) | |
| Diffuse lesion, n (%) | 985(59.7%) | 506(61.3%) | 479(58.1%) | 0.175 |
| In-stent restenosis, n (%) | 48(2.9%) | 27(3.3%) | 21(2.5%) | 0.379 |
| Calcification lesion, n (%) | 38(2.3%) | 18(2.2%) | 20(2.4%) | 0.743 |
| Number of stents | 1.71 ± 1.15 | 1.68 ± 1.11 | 1.74 ± 1.20 | 0.276 |
| Medication at discharge, n (%) | ||||
| ACEI/ARB | 1345(81.5%) | 664(80.5%) | 681(82.5%) | 0.281 |
| β-blocker | 1338(81.1%) | 663(80.4%) | 675(81.8%) | 0.451 |
| Statins | 1643(99.6%) | 824(99.9%) | 819(99.3%) | 0.124 |
| P2Y12 inhibitor | 0.934 | |||
| Clopidogrel | 1491(90.4%) | 746(90.4%) | 745(90.3%) | |
| Ticagrelor | 159(9.6%) | 79(9.6%) | 80(9.7%) | |
| Aspirin | 1650(100.0%) | 825(100%) | 825(100%) | – |
| DAPT | 1650(100.0%) | 825(100%) | 825(100%) | – |
TyG, triglyceride-glucose; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CAD, coronary artery disease; UA, unstable angina; NSTEMI, non ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PAD, peripheral artery disease; WBC, white blood cell; PLT, platelet; Hb, hemoglobin; BUN, blood urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1C, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; LM, left main; CTO, chronic total occlusion; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; DAPT, dual antiplatelet therapy.
3.2. Predictive implications of the TyG index for MACCE
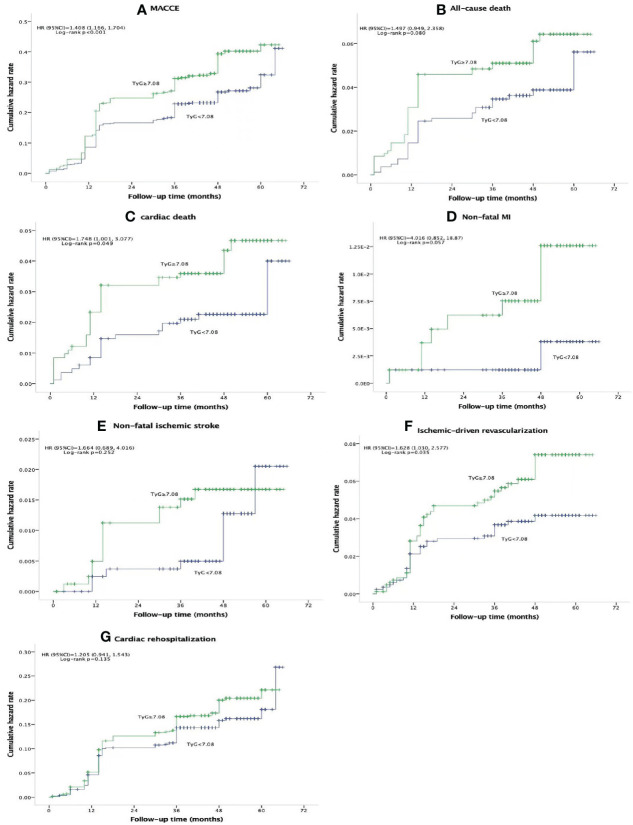
There were 437 (26.5%) MACCEs over the course of a median of 47 months follow-up [47 (40, 54)], including 77 (4.7%) all-cause deaths, 52 (3.2%) cardiac deaths, 10 (0.6%) non-fatal MIs, 21 (1.3%) non-fatal ischemic strokes, 77 (4.7%) ischemia-driven revascularizations, and 252 (15.3%) cardiac rehospitalizations. Patients in the high TyG index group had significantly greater incidences of MACCE (30.3% vs. 22.7%, p<0.001), cardiac death (4.0% vs. 2.3%, p=0.047), and ischemia-driven revascularization (5.7% vs. 3.6%, p=0.046) compared with those in the low TyG index group. However, there was no significant difference in the prevalence of all-cause death (5.6% vs. 3.8%, p=0.080), non-fatal MI (1.0% vs. 0.2%, p=0.057), non-fatal ischemic stroke (1.6% vs. 1.0%, p=0.272), and cardiac rehospitalization (16.5% vs. 14.1%, p=0.171) between the two groups ( Table 2 ). Figure 2 displays the Kaplan–Meier curves for the incidence of MACCE and each of its components for the low and high TyG groups. The difference between the low and high TyG index groups was clearly visible in the Kaplan–Meier curves for MACCE ( Figure 2A ; hazard ratio [HR] 1.408, 95% confidence interval [CI] 1.166–1.704, log-rank p<0.001). The difference was mostly due to a rise in cardiac death ( Figure 2C ; HR 1.748, 95% CI 1.001–3.077, log-rank p=0.049), and ischemia-driven revascularization ( Figure 2F ; HR 1.628, 95% CI 1.030–2.577, log-rank p=0.035). A statistically significant difference between the low and high TyG index groups could not be found in the Kaplan–Meier curves for all-cause death ( Figure 2B ; HR 1.497, 95% CI 0.949–2.358, log-rank p=0.080), non-fatal MI ( Figure 2D ; HR 4.016, 95% CI 0.852–18.87, log-rank p=0.057), non-fatal ischemic stroke ( Figure 2E ; HR 1.664, 95% CI 0.689–4.016, log-rank p=0.252), and cardiac rehospitalization ( Figure 2G ; HR 1.205, 95% CI 0.941–1.543, log-rank p=0.135).
Table 2.
Incidence of MACCE in low and high TyG index group.
| Endpoint event | Total population | Low TyG index group | High TyG index group | P value |
|---|---|---|---|---|
| (n=1650) | (n=825) | (n=825) | ||
| MACCE, n (%) | 437(26.5%) | 187(22.7%) | 250(30.3%) | < 0.001 |
| All-cause death, n (%) | 77(4.7%) | 31(3.8%) | 46(5.6%) | 0.080 |
| Cardiac death, n (%) | 52(3.2%) | 19(2.3%) | 33(4.0%) | 0.047 |
| Non-fatal MI, n (%) | 10(0.6%) | 2(0.2%) | 8(1.0%) | 0.057 |
| Non-fatal ischemic stroke, n (%) | 21(1.3%) | 8(1.0%) | 13(1.6%) | 0.272 |
| Ischemia-driven revacularization, n (%) | 77(4.7%) | 30(3.6%) | 47(5.7%) | 0.046 |
| Cardiac rehospitalization, n (%) | 252(15.3%) | 116(14.1%) | 136(16.5%) | 0.171 |
MACCE, major adverse cardiovascular and cerebrovascular events; TyG, triglyceride-glucose; MI, myocardial infarction.
Figure 2.
Kaplan-Meier curves for composite MACCE (A), all-cause death (B), cardiac death (C), non-fatal MI (D), non-fatal ischemic stroke (E), schemic-driven revascularization (F), and cardiac rehospitalization (G) of the TyG<7.08 group (blue line) versus the TyG<7.08 group (green line). TYG, triglyceride-glucose index; MACCE, major adverse cardiovascular and cerebrovascular event; MI, myocardial infarction; HR, hazard ratio; Cl, confidence interval.
3.3. Cox proportional hazard analysis to evaluate the prognostic implication of MACCEs
The relationship between the TyG index and MACCEs was analyzed using the Cox proportional hazards model. Univariate analysis revealed that the TyG index was substantially related to MACCEs (HR 1.408, 95% CI 1.166–1.704, p<0.001). Gender, STEMI, drinking history, number of stents implanted, left main (LM) disease, two-vessel and three-vessel disease, Hb, FBG, TG, and β-blocker medication at discharge were the additional significant risk variables. Several risk factors including important clinical and significant variables (p<0.05) in the univariate model were included in the multivariate.The TyG index continued to be an independent predictor of MACCEs (HR 1.493, 95% CI 1.230–1.812, p<0.001). STEMI, hyperlipidemia, Hb, and β-blocker medication at discharge were the additional independent predictors of MACCEs in the multivariate analysis ( Table 3 ).
Table 3.
Predictive value of TyG index for MACCE in univariate and multivariate analysis.
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR(95%CI) | P value | |
| Age | 1.006(0.997, 1.016) | 0.195 | ||
| Gender | 1.240(1.001, 1.537) | 0.049 | 1.068(0.824, 1.385) | 0.616 |
| Previous MI | 1.224(0.899, 1.666) | 0.199 | ||
| Previous PCI | 1.289(0.960, 1.731) | 0.091 | ||
| Initial diagnosis | 0.010 | 0.007 | ||
| UA | reference | reference | ||
| NSTEMI | 1.069(0.705, 1.621) | 0.753 | 1.094(0.720, 1.662) | 0.674 |
| STEMI | 1.372(1.111, 1.695) | 0.003 | 1.399(1.131, 1.731) | 0.002 |
| Hyperlipidemia | 1.377(0.984, 1.931) | 0.062 | 1.481(1.052, 2.083) | 0.025 |
| Smoking history | 1.202(0.996, 1.451) | 0.054 | ||
| Drinking history | 0.769(0.616, 0.960) | 0.020 | 0.843(0.667, 1.065) | 0.153 |
| HR | 1.006(0.998, 1.013) | 0.140 | ||
| Number-vessel disease | 0.045 | 0.141 | ||
| Single-vessel disease | reference | reference | ||
| Two-vessel disease | 1.266(1.012, 1.585) | 0.042 | 1.200(0.944, 1.527) | 0.135 |
| Three-vessel disease | 1.274(1.009, 1.608) | 0.039 | 1.230(0.978, 1.548) | 0.076 |
| LM disease | 1.361(1.033, 1.795) | 0.029 | 1.290(0.971, 1.713) | 0.079 |
| Number of stents | 1.096(1.014, 1.185) | 0.021 | 1.060(0.978, 1.150) | 0.158 |
| WBC | 1.026(0.998, 1.027) | 0.174 | ||
| Hb | 0.993(0.989, 0.998) | 0.010 | 0.994(0.988, 0.999) | 0.036 |
| HDL-C | 0.702(0.467, 1.056) | 0.089 | ||
| TyG index | 1.408(1.166, 1.704) | < 0.001 | 1.493(1.230, 1.812) | <0.001 |
| β-blocker medication | 0.761(0.588, 0.986) | 0.039 | 0.759(0.585, 0.984) | 0.037 |
TyG, triglyceride-glucose; MACCE, major adverse cardiovascular and cerebrovascular events; HR, hazard ratio; CI, confidence interval; MI, myocardial infarction; PCI, percutaneous coronary intervention; UA, unstable angina; NSTEMI, non ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; HR, heart rate; LM, left main; WBC, white blood cell; Hb, hemoglobin; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride.
3.4. Sensitivity analysis
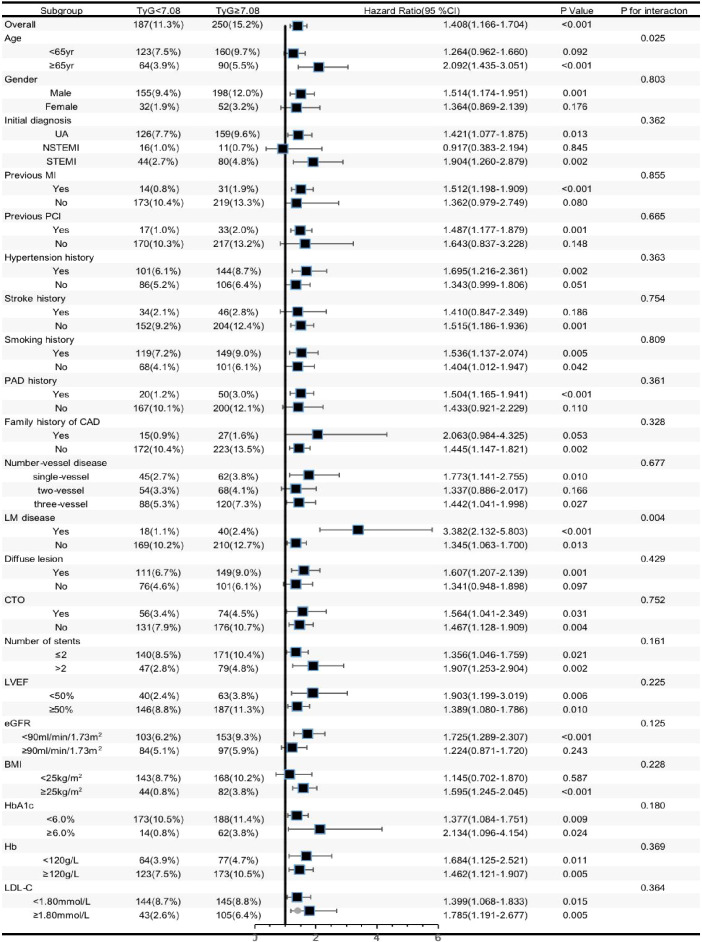
We conducted additional analysis among several subgroups to assess the independent relationship of the TyG index with MACCE. As shown in Figure 3 , the independent predictive effect of the TyG index on MACCE was primarily represented in the subgroups of male, age≥65 years, UAP and STEMI, with previous MI history, with previous PCI history, with hypertension history, without stroke history, with and without smoking history, with previous PAD history, without family history of CAD, single-vessel disease and three-vessel disease, with and without LM disease, with diffuse lesion group, with and without CTO, ≤2 and >2 stents implanted, LVEF≥50% and <50%, eGFR < 90mL/min/1.73 m2, BMI ≥25 kg/m2, HbA1c <6.0% and ≥6.0%, Hb≥120 g/L, and LDL-C <1.80 mmol/L and≥1.80 mmol/L. Interestingly, patients≥65 years of age (HR [95%CI] age≥65 years 2.092 (1.435–3.051) vs. age<65 years 1.264 (0.962–1.660), p for interaction =0.025) and those with LM disease (HR [95%CI] with LM disease 3.382 (2.132–5.803) vs. without LM disease 1.345 (1.063–1.700), p for interaction =0.004] appeared to have higher predictive values for the TyG index.
Figure 3.
Forest plot investigating the association between the TyG index and MACCE in different subgroups. TYG, triglyceride-glucose index; UA, unstable angina, NSTEMI, non ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention; DM, diabetes mellitus; CAD, coronary artery disease; LM, left main; CTO, chronic total occlusion; Hb, hemoglobin;LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; BMI, body mass index; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol, LDL-C, low-density lipoprotein cholesterol; C, confidence interval.
4. Discussion
The high TyG index group had a considerably higher incidence of MACCE than the low TyG index group in this cohort of Chinese ACS patients without DM who underwent emergency PCI with DES. The TyG index is an independent predictor of MACCEs. This work is the first study that investigated the association of between the TyG index and the prognosis of the Chinese ACS patients without DM who underwent emergency PCI with DES.
The knowledge of atherogenesis has advanced significantly. The advancement of atherosclerosis and cardiovascular events is related to LDL-C levels. Using statins (24), ezetimibe (25), and proprotein convertase subtilisin/kexin type9 (PCSK9) antibodies (26) to lower LDL-C levels can reduce risk by about 30%–50%; however, a significant amount of residual risk remains (24). Cardiologists that specialize in coronary heart disease have recently focused on inflammation inhibition. CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study) demonstrated for the first time that inhibiting the interleukin (IL)-1b pathway can lower the risk of cardiovascular events in patients with coronary artery disease (CAD) by about 17% (27). As one of the major risk factors for CVD, DM may be treated with hypoglycemic medications such as sodium-dependent glucose transporter 2 inhibitor (SGLT2i) and glucagon-like peptide-1 (GLP-1) receptor agonist to lower cardiovascular events (28). Therefore, the additional risk factors for MACCE in patients with ACS must be recognized. In our study, patients who belonged to the MACCE group had a perfect correlation with older age; initial diagnosis of STEMI; lower Hb; higher FBG, TG, and TyG index; more LM disease; more stents implanted, and low proportion of β-blocker medication at discharge ( Supplemental Table 1 ). Cox multivariate analysis revealed that the TyG index, STEMI, hyperlipidemia, Hb, and β-blocker medication at discharge were all substantially correlated with MACCE.
IR has a significant connection to dyslipidemia and inflammation (29), but it has not been given much attention. A significant portion of the residual risk following lipid-lowering treatment may be due to IR (30–32). A composite indicator composed of TG and FBG is named the TyG index. In clinical practice, the TyG index is frequently used as a straightforward index to assess the degree of IR. Furthermore, the TyG index has a high level of sensitivity and specificity as a marker for IR (21, 33). According to our study, patients in the high TyG index group had higher levels of LDL-C, TC, TG and WBC; lower levels of HDL-C; and a higher percentage of prior history of hyperlipidemia than those in the low TyG index group.
In terms of the underlying mechanisms, previous research has suggested that IR may have a role in the emergence of adverse cardiovascular events (34, 35). First, catecholamine secretion, sympathetic activity, and myocardial oxygen consumption may all rise as a result of IR. Second, the renin-angiotensin-aldosterone system (RAAS) to may be activated by IR, which results in cardiac insufficiency. Third, IR may enhance the synthesis of coagulation and inflammatory factors, causing an imbalance in coagulation and abnormalities of the fibrinolytic system, which can result in thrombosis. Fourth, cardiomyopathy and heart failure may result from changes in heart metabolism and impaired systolic function caused by IR. Fifth, IR may increase the risk of major adverse cardiovascular events (MACEs) by promoting atherosclerosis and causing plaque instability (34, 36).
Extensive research has been made on the TyG index. A strong correlation between high TyG index and cardiovascular events has also been demonstrated by a number of sizable prospective trials. High TyG index was found to be significantly related to an elevated risk of CVD in a study that included 5,014 people who appeared to be in good health (37). The TyG index and carotid intima media thickness were found to be related in a study of 473 postmenopausal non-diabetic women (38). Additionally, the TyG index can predict the ischemic stroke risk in the general population (39). Thomas B et al. reported that the TyG index may be beneficial in determining people who are at high risk for CVD (40). The findings of this research, which investigated thousands of people for sufficient sample size and average duration of 10 years, revealed that higher TyG index levels were linked to an increased likelihood of CVD events and were independent prognostic factors, regardless of fundamental information including weight, gender, smoking, accompanying diseases (such as hypertension and T2DM), and general cardiovascular risk factors such as HDL-C and LDL-C. The findings demonstrated the value of the TyG index as a CAD predictor. Additional research has revealed that individuals with metabolic syndrome and CAD have a poor prognosis, with an increased risk of MACE and much more severe CAD (41). The TyG index has also been linked to short-term MACE and mortality risk in individuals who have undergone PCI (42). According to Laxy M.’s research, STEMI individuals with a high TyG index are more likely to experience a higher incidence of cardiac death, malignant arrhythmias, unstable angina, and heart failure rehospitalization during 2 years following PCI (43). The TyG index is also associated with cardiovascular events in non-diabetic patients. According to a previous study by Quiroga B, et al., the TyG index is associated with the incidence of cardiovascular events in non-diabetic patients with non-dialysis-dependent CKD (44). A study of elder non-diabetic patients with STEMI showed that the TyG index can predict in-hospital and long-term mortality (45). Therefore, the TyG index is a predictor of adverse events in healthy people, patients with CVD or undergoing PCI, and patients with or without DM. To assess the effectiveness of the TyG index for predicting adverse cardiovascular and cerebrovascular events in Chinese ACS patients without DM who underwent emergency PCI with DES, we performed an analysis based on a cohort of 1650 ACS patients without DM. We discovered that the prevalence of MACCE in the high TyG index group was considerably greater compared with that in the low TyG index group. Thus, the TyG index is an independent prognostic factor of MACCEs, and it plays a key role in subgroup analysis.
This study has a number of limitations that must be acknowledged. First, this work is retrospective, observational, and single center in design. As a result, this study was unable to establish a causal relationship between the TyG index and MACCE. Second, both the sample size and follow-up period were insufficient. Third, the fact that only patients with ACS who received emergency PCI with DES were included in the current study may have led to selection bias and restricted the generalizability of our findings to patients with ACS who underwent elective PCI or were treated with drug-coated balloon and patients with chronic coronary syndrome. Fourth, despite adjusting for the usage of lipid-lowering treatment, the results may be skewed as we did not consider the type, intensity, and variations in those medications. Fifth, minimal information was available regarding the change in the TyG index level during the follow-up period. Moreover, the laboratory data and TyG index were only examined once, both of which could have resulted in bias because of measurement error.
5. Conclusion
In Chinese ACS patients without DM who underwent emergency PCI with DES, increasing IR extent as shown by the TyG index is a prominent risk factor of MACCE. The identification of the TyG index may help with early classification and intervention to prevent MACCE. To support our results, additional multi-center, large-scale, and prospective cohort trials with a sizable sample and prolonged follow-up period are required.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Academic Committee of the First Affiliated Hospital of Xi’an Jiaotong University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ and NG were responsible for designing the study and writing of the manuscript. YZ, ZZ, and Y-BL performed the follow-up of this cohort and collected the data. CC and F-FN analyzed the data and revised the manuscript. YZ, ZZ, and F-FN were responsible for research organization and coordination. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are indebted to all the members of the working group for their contribution and to the participants for their involvement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LF declared a shared affiliation with the authors YZ, CC, Y-BL, F-FN, and NG to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1101952/full#supplementary-material
References
- 1. Eisen A, Giugliano RP, Braunwald E. Updates on acute coronary syndrome: A review. JAMA Cardiol (2016) 1:718–30. doi: 10.1001/jamacardio.2016.2049 [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: A report from the American heart association. Circulation. (2018) 137:e67–e492. doi: 10.1161/cir.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 3. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide realworld data demonstrate the importance of a long-term perspective. Eur Heart J (2015) 36:1163–70. doi: 10.1093/eurheartj/ehu505 [DOI] [PubMed] [Google Scholar]
- 4. Fox KA, Carruthers KF, Dunbar DR, Graham C, Manning JR, De Raedt H, et al. Underestimated and under-recognized: The late consequences of acute coronary syndrome (GRACE UK-Belgian study). Eur Heart J (2010) 31:2755–64. doi: 10.1093/eurheartj/ehq326 [DOI] [PubMed] [Google Scholar]
- 5. Bays HE. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: Treating "sick fat" through improving fat function with antidiabetes therapies. Am J Cardiol (2012) 110:4b–12b. doi: 10.1016/j.amjcard.2012.08.029 [DOI] [PubMed] [Google Scholar]
- 6. Li XL, Guo YL, Zhu CG, Xu RX, Qing P, Wu NQ, et al. Relationship of high-density lipoprotein cholesterol with periprocedural myocardial injury following elective percutaneous coronary intervention in patients with low-density lipoprotein cholesterol below 70 mg/dL. J Am Heart Assoc (2015) 4:e001412. doi: 10.1161/jaha.114.001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol (2018) 17:122. doi: 10.1186/s12933-018-0762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: Implications for insulin-sensitizing agents. Endocr Rev (2019) 40:1447–67. doi: 10.1210/er.2018-00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab (2008) 294:E15–26. doi: 10.1152/ajpendo.00645.2007 [DOI] [PubMed] [Google Scholar]
- 10. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol (2014) 13:146. doi: 10.1186/s12933-014-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The vascular-metabolic CUN cohort. Prev Med (2016) 86:99–105. doi: 10.1016/j.ypmed.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 12. Wang A, Wang G, Liu Q, Zuo Y, Chen S, Tao B, et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: An 11-year follow-up. Cardiovasc Diabetol (2021) 20:46. doi: 10.1186/s12933-021-01238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: An 11-year prospective study in the kailuan cohort. Cardiovasc Diabetol (2021) 20:19. doi: 10.1186/s12933-020-01210-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo JW, Duan WH, Yu YQ, Song L, Shi DZ. Prognostic significance of triglyceride-glucose index for adverse cardiovascular events in patients with coronary artery disease: A systematic review and meta-analysis. Front Cardiovasc Med (2021) 8:774781. doi: 10.3389/fcvm.2021.774781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Radke PW, Voswinkel M, Reith M, Kaiser A, Haager PK, Hanrath P, et al. Relation of fasting insulin plasma levels to restenosis after elective coronary stent implantation in patients without diabetes mellitus. Am J Cardiol (2004) 93:639–41. doi: 10.1016/j.amjcard.2003.11.039 [DOI] [PubMed] [Google Scholar]
- 16. Piatti P, Di Mario C, Monti LD, Fragasso G, Sgura F, Caumo A, et al. Association of insulin resistance, hyperleptinemia, and impaired nitric oxide release with in-stent restenosis in patients undergoing coronary stenting. Circulation. (2003) 108:2074–81. doi: 10.1161/01.Cir.0000095272.67948.17 [DOI] [PubMed] [Google Scholar]
- 17. Hwang IK, Kim YK, Rha SW, Ra JE, Seo BS, Lee JK, et al. Impact of insulin resistance on 1-year clinical outcomes in non-diabetic patients undergoing percutaneous coronary intervention with drug-eluting stents. J Cardiol (2013) 61:113–6. doi: 10.1016/j.jjcc.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 18. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 19. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European society of cardiology (ESC). Eur Heart J (2016) 37:267–315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 20. Association Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care (2011) 34 Suppl 1:S62–9. doi: 10.2337/dc11-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab (2010) 95:3347–51. doi: 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med (2006) 145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 23. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol (2014) 63:2659–73. doi: 10.1016/j.jacc.2014.02.545 [DOI] [PubMed] [Google Scholar]
- 24. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. (2010) 376:1670–81. doi: 10.1016/s0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med (2015) 372:2387–97. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 26. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med (2017) 376:1713–22. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 27. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 28. Ray KK, Colhoun HM, Szarek M, Baccara-Dinet M, Bhatt DL, Bittner VA, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: A prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol (2019) 7:618–28. doi: 10.1016/s2213-8587(19)30158-5 [DOI] [PubMed] [Google Scholar]
- 29. Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-Art review. J Am Coll Cardiol (2020) 75:525–38. doi: 10.1016/j.jacc.2019.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PloS One (2012) 7:e52036. doi: 10.1371/journal.pone.0052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rewers M, Zaccaro D, D'Agostino R, Haffner S, Saad MF, Selby JV, et al. Insulin sensitivity, insulinemia, and coronary artery disease: The insulin resistance atherosclerosis study. Diabetes Care (2004) 27:781–7. doi: 10.2337/diacare.27.3.781 [DOI] [PubMed] [Google Scholar]
- 32. Tenenbaum A, Motro M, Fisman EZ, Adler Y, Shemesh J, Tanne D, et al. Effect of bezafibrate on incidence of type 2 diabetes mellitus in obese patients. Eur Heart J (2005) 26:2032–8. doi: 10.1093/eurheartj/ehi310 [DOI] [PubMed] [Google Scholar]
- 33. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord (2008) 6:299–304. doi: 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 34. Wu S, Liu W, Ma Q, Yu W, Guo Y, Zhao Y, et al. Association between insulin resistance and coronary plaque vulnerability in patients with acute coronary syndromes: Insights from optical coherence tomography. Angiology. (2019) 70:539–46. doi: 10.1177/0003319718809931 [DOI] [PubMed] [Google Scholar]
- 35. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: The bruneck study. Diabetes Care (2007) 30:318–24. doi: 10.2337/dc06-0919 [DOI] [PubMed] [Google Scholar]
- 36. Kedhi E, Kennedy MW, Maehara A, Lansky AJ, McAndrew TC, Marso SP, et al. Impact of TCFA on unanticipated ischemic events in medically treated diabetes mellitus: Insights from the PROSPECT study. JACC Cardiovasc Imaging (2017) 10:451–8. doi: 10.1016/j.jcmg.2015.12.023 [DOI] [PubMed] [Google Scholar]
- 37. Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest (2016) 46:189–97. doi: 10.1111/eci.12583 [DOI] [PubMed] [Google Scholar]
- 38. Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ (2018) 27:716–24. doi: 10.1016/j.hlc.2017.05.142 [DOI] [PubMed] [Google Scholar]
- 39. Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: Insights from a general population. Nutr Metab Cardiovasc Dis (2020) 30:245–53. doi: 10.1016/j.numecd.2019.09.015 [DOI] [PubMed] [Google Scholar]
- 40. Thomas B. The global burden of diabetic kidney disease: Time trends and gender gaps. Curr Diabetes Rep (2019) 19:18. doi: 10.1007/s11892-019-1133-6 [DOI] [PubMed] [Google Scholar]
- 41. Sung KC, Park HY, Kim MJ, Reaven G. Metabolic markers associated with insulin resistance predict type 2 diabetes in koreans with normal blood pressure or prehypertension. Cardiovasc Diabetol (2016) 15:47. doi: 10.1186/s12933-016-0368-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ho CK, Rahib L, Liao JC, Sriram G, Dipple KM. Mathematical modeling of the insulin signal transduction pathway for prediction of insulin sensitivity from expression data. Mol Genet Metab (2015) 114:66–72. doi: 10.1016/j.ymgme.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laxy M, Hunger M, Stark R, Meisinger C, Kirchberger I, Heier M, et al. The burden of diabetes mellitus in patients with coronary heart disease: A methodological approach to assess quality-adjusted life-years based on individual-level longitudinal survey data. Value Health (2015) 18:969–76. doi: 10.1016/j.jval.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 44. Quiroga B, Muñoz Ramos P, Sánchez Horrillo A, Ortiz A, Valdivieso JM, Carrero JJ. Triglycerides-glucose index and the risk of cardiovascular events in persons with non-diabetic chronic kidney disease. Clin Kidney J (2022) 15:1705–12. doi: 10.1093/ckj/sfac073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Şaylık F, Çınar T, Selçuk M, Tanboğa İH. The predictive value of triglyceride-glucose index for in-hospital and one-year mortality in elderly non-diabetic patients with ST-segment elevation myocardial infarction J Geriatr Cardiol (2022) 19:610–7 doi: 10.11909/j.issn.1671-5411.2022.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.