Abstract
Ischemia reperfusion injury can lead to further myocardiocyte damage in patients with ST-elevation myocardial infarction (STEMI). Pentoxifylline is a methylxanthine derivative with known anti-inflammatory, antioxidant, vasodilator, and rheological properties which can be a promising agent in preventing reperfusion injury. PENTOS-PCI is a single-center, randomized, double-blind, placebo-controlled trial which evaluated the efficacy and safety of preprocedural administration of intravenous pentoxifylline in patients undergoing primary percutaneous coronary intervention (PCI). Patients with acute STEMI who were eligible for PCI were randomized to receive either 100-mg intravenous infusion of pentoxifylline or placebo, prior to transferring to catheterization laboratory. Overall, 161 patients were included in our study of whom 80 patients were assigned to pentoxifylline and 81 to the control groups. Per-protocol analysis of primary endpoint indexing PCI’s success rate as measured by thrombolysis in myocardial infarction (TIMI) flow grade 3 was not significantly different between pentoxifylline and placebo (71.3% and 66.3% respectively, P = 0.40). In addition, pentoxifylline could not improve secondary angiographic endpoints including myocardial blush grade 3 (87.5% and 85.2%, P = 0.79) and corrected TIMI frame count (22.8 [± 9.0] and 24.0 [± 5.1], P = 0.33) in the intervention and placebo groups respectively. The rates of major adverse cardiac and treatment emergent adverse effects were not significantly different between the two groups. Administration of intravenous pentoxifylline before primary PCI did not improve the success rate of the procedure in patients with STEMI. Intravenous administration of pentoxifylline was well tolerated, and there were no significant differences regarding adverse drug reactions in the two groups.
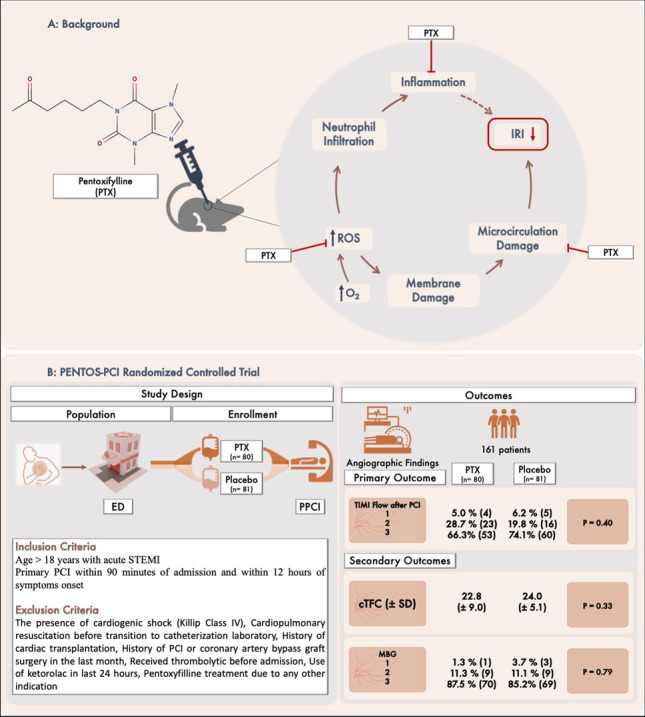
Graphical Abstract
Panel A, background: pentoxifylline is a methylxanthine derivative with known anti-inflammatory, antioxidant, vasodilator, and rheological properties which can be a promising agent in preventing reperfusion injury. Panel B: study design and main results of the PENTOS-PCI trial. cTFC corrected TIMI frame count, ED emergency department, IRI ischemia reperfusion injury, MBG myocardial blush grade, PCI percutaneous coronary intervention, PPCI primary PCI, PTX pentoxifylline, ROS reactive oxygen species, SD standard deviation, STEMI ST-elevation myocardial infarction, TIMI thrombolysis in myocardial infarction.
Keywords: Pentoxifylline, STEMI, Reperfusion injury, PCI
Introduction
Acute myocardial infarction (MI) caused by coronary occlusion can initiate time-dependent myocardiocyte death and may lead to irreversible myocardial scaring if not treated promptly (Sandoval and Jaffe 2019). In this regard, reestablishing efficient perfusion to salvage myocardium either pharmacologically or via percutaneous coronary intervention (PCI) is of top priority in patients with myocardial ischemia. However, the process of reperfusion can damage the myocardium and leads to further ischemia in a paradoxical manner called myocardial ischemia reperfusion injury (IRI) (Yellon and Hausenloy 2007). Despite numerous advances in the treatment of patients with acute coronary syndrome (ACS), the mortality and morbidity in this population are still high and clinical investigations into the prevention and treatment of IRI in order to improve outcomes in this setting are still ongoing.
It has been documented that in a significant number of patients, microvascular damage can occur despite proper recanalization of culprit artery (Rezkalla and Kloner 2002). Pentoxifylline is a methylxanthine derivative used to be prescribed for patients with intermittent claudication in previous decades (Salhiyyah et al. 2012). Given the multifactorial nature of IRI, pentoxifylline could be a promising agent for prevention of microvascular damage and reperfusion injury by different mechanisms. These include rheological properties such as decreasing blood viscosity, reducing fibrinogen levels, diminishing platelet aggregation, and increasing red blood cells’ distensibility and blood filterability (Strano et al. 1984). Altogether, these properties can improve microvascular circulation and prevent coronary no-reflow or slow-flow phenomena which both play an important role in the pathophysiology of IRI. Pentoxifylline is known to inhibit phosphodiesterase, an enzyme that breaks down cyclic adenosine monophosphate (cAMP), which elevates the level of intracellular cAMP and, thus, lowers platelet aggregation and depresses the production of tumor necrosis factor-alpha (TNF-α) (Strieter et al. 1988). Oxidative stress and inflammation are also involved in the pathophysiology of IRI, and by pleiotropic antioxidant and anti-inflammatory properties, pentoxifylline may have favorable effects for the prevention of IRI (Horton & White 1993). In addition, pentoxifylline has been shown to augment the production of prostacyclins and eicosanoid which is an endogenous vasodilator (Matzky et al. 1982).
In light of these mechanisms, it was hypothesized that pentoxifylline can provide benefit on top of the optimal interventional and pharmacological treatments in patients with ACS and the present study designed to evaluate the possible role of preprocedural administration of pentoxifylline in patients with ST-elevation MI (STEMI) undergoing primary PCI.
Methods
Evaluating the role of intravenous pentoxifylline administration on increasing primary percutaneous coronary intervention success in patients with ST-elevation myocardial infarction (PENTOS-PCI) is a prospective single-center randomized double-blind placebo-controlled clinical trial which was conducted at Tehran Heart Center, Tehran, Iran, from September 2019 to December 2020. The trial was performed in accordance with the principles of the Declaration of Helsinki and the European Guidelines for Good Clinical Practice. The study protocol was approved by the institutional review board and ethics committee and was registered in the Clinical Trial Database on 2 Aug. 2019 (IRCT20120111008698N24 available at https://www.irct.ir/trial/34950).
Eligible patients were randomized and immediately received intravenous (IV) pentoxifylline (SANTA FARMA, Turkey) just after diagnosis, prior to transferring to catheterization laboratory for primary PCI, and written informed consent was obtained from all the participants prior to being taken to the study intervention.
Study group
Patients older than 18 years were considered eligible for enrollment if they had experienced chest discomfort lasting more than 20 min and had been diagnosed with acute STEMI based on clinical, laboratory, and electrocardiographic (ECG) findings and are eligible for undergoing primary PCI within 90 min of admission and within 12 h of symptom onset. The definition of acute STEMI according to the European Society of Cardiology Guidelines is a persistent chest discomfort with the elevation of at least one cardiac troponin value above the 99th percentile upper limit and ST-segment elevation in at least 2 contiguous leads (Ibanez et al. 2018). The exclusion criteria are listed in Table 1.
Table 1.
Exclusion criteria of the study; PCI percutaneous coronary intervention
| Patients unwilling to participate |
| Symptoms for more than 12 h |
| Presence of cardiogenic shock (Killip Class IV) |
| Cardiopulmonary resuscitation before transition to catheterization laboratory |
| History of cardiac transplantation |
| History of PCI or coronary artery bypass graft surgery in the last month |
| Patients who received thrombolytic before admission to our center |
| Current use of pentoxifylline for another indication |
| Use of ketorolac in the last 24 h |
Randomization
Patients were randomly assigned in blocks of four via a permuted randomization block to receive either pentoxifylline or placebo. The intervention group received a 100 mg of IV pentoxifylline in which half of the dose (50 mg) was administered via IV bolus and the remaining 50 mg was administered in a 30-min slow infusion (Beermann et al. 1985). The placebo group received only the solvent (sodium chloride 0.9%). The entire study population received routine medications for the treatment of STEMI, and clopidogrel was the P2Y12 inhibitor administered in all patients, which was the general P2Y12 inhibitor of choice based on institutional protocols at the time of randomization in our center. The choice of using thrombus aspiration, stent type, and administration of parenteral antiplatelet agents were left to the preference of the interventional cardiologist.
After enrollment, the following patient information was recorded: demographic information, laboratory findings, ischemic time (pain-to-balloon), number of culprit arteries, characteristics of the coronary angiography including infarct-related artery, thrombolysis in MI (TIMI) flow grade before PCI, use of glycoprotein (GP) IIb/IIIa inhibitors, use of medications during the procedure (i.e., adenosine, nitroglycerin, and verapamil) or thrombectomy devices, and the type of PCI procedure.
Study endpoints
The primary endpoint was the success rate of PCI based on angiographic findings which was defined by TIMI flow criteria after intervention. Secondary endpoints were corrected TIMI frame count (cTFC), myocardial blush grade (MBG), troponin I (TnI) levels 24 and 48 h after the procedure, global left ventricular ejection fraction (LVEF) measured by echocardiography before discharge, and adjudicated 30-day and 3-month major adverse cardiac events (MACE). Adverse effects related to investigational treatment, any major or clinically relevant non-major bleeding, hypotension, and allergy have been recorded.
After the completion of primary PCI, the TIMI flow grade, MBG, and the cTFC were evaluated by two blinded senior interventional cardiologists. Regarding cases in which they disagreed, another blinded interventional cardiologist read the angiography footages for adjudication. The TIMI flow grade classifies the success/failure reperfusion after thrombolysis in grades, with grade 0 indicating no perfusion, grade 1 penetration without perfusion, grade 2 partial perfusion, and grade 3 complete perfusion. Corrected TFC was calculated for the infarct-related artery according to the technique described by Gibson and coworkers after the first balloon inflation and after stent placement (Gibson et al. 1996). MBG has been defined previously as follows: 0, no myocardial blush or contrast density; 1, minimal myocardial blush or contrast density; 2, moderate myocardial blush or contrast density but less than that obtained during angiography of a contralateral or ipsilateral non–infarct-related coronary artery; and 3, normal myocardial blush or contrast density, comparable with that obtained during angiography of a contralateral or ipsilateral non–infarct-related coronary artery. When myocardial blush persisted (“staining”), this phenomenon suggested leakage of contrast medium into the extravascular space and was graded 0 (Henriques et al. 2003).
MACEs were comprised of target vessel revascularization (TVR), target lesion revascularization (TLR), and new hospitalization due to heart failure, stroke, nonfatal MI, and cardiac death. MACEs were recorded 1 month and 3 months after primary PCI through clinical visits, and the investigators were blinded and adjudicated for the events.
Statistical analysis
All statistical analyses were conducted applying IBM SPSS Statistics for Windows, version 26.0 (Armonk, NY: IBM Corp.). Continuous variables are described as mean with standard deviation (SD) or median with 25th and 75th percentiles (as interquartile range (IQR) boundaries) for normally and skewed distributed variables, respectively. Normality of the variables was checked using histogram chart and descriptive measures. The continuous variables were compared between the two intervention groups applying the independent samples t-test or Mann–Whitney U-test for normally and skew distributed variables, respectively. Categorical variables were expressed as frequency with percentages and were compared between the groups using the chi-square test and Fisher’s exact test, as appropriate. The effect of intervention compared to the placebo was reported as odds ratio (OR) with its corresponding 95% confidence interval (CI). The time to the occurrence of MACE was assessed using Kaplan–Meier curve, and the effect of groups on it was assessed using the log-rank test. P values < 0.05 were considered statistically significant.
Results
Patient enrollment
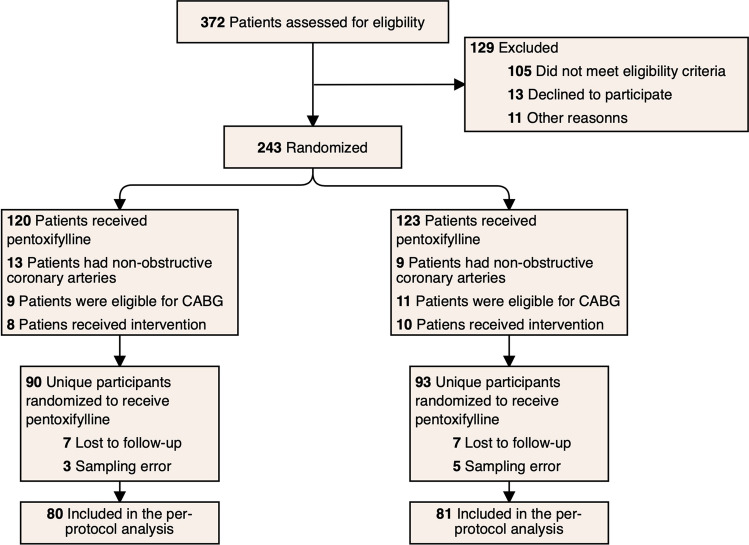
Randomization was carried out on 243 patients with acute STEMI: 120 patients were assigned to the pentoxifylline group while 123 patients were assigned to the control group. Thirty patients in the pentoxifylline group and 30 patients in the control group were excluded because they did not undergo intervention in the catheterization laboratory. Ten patients in the pentoxifylline group and 12 patients in the control group were also lost to follow-up. Ultimately, 161 patients were eligible for per-protocol analysis: 80 patients were assigned to the pentoxifylline group and 81 to the control group (Fig. 1). Patient enrollment was stopped prematurely due to drug shortage and COVID-19 pandemic–related complications.
Fig. 1.
Consort diagram of the patients through the study
Baseline characteristics
The baseline characteristics of the study population are depicted in Table 2, which shows that the mean age of the patients was 59.6 years and that 79.5% of the patients were male. There were no significant differences between the pentoxifylline and the control groups in terms of the baseline comorbidities such as the ischemic time, history of diabetes mellitus, dyslipidemia, hypertension, obesity, drug history, a positive family history of coronary artery disease, and active smoking. Infarct-related arteries, number vessel disease, and baseline levels of hemoglobin, serum creatinine, and hs-TnI were also not different significantly between groups (Table 2). Moreover, the differences between the pentoxifylline and control groups in terms of the initial TIMI flow grade, procedure types, thrombus burden, the use of parenteral antiplatelet (eptifibatide) or procedural medications, the use of thrombectomy devices, and pre- or postdilation ballooning were also statistically non-significant (Table 3).
Table 2.
Baseline characteristics of the study population; ACEI/ARB angiotensin converting enzyme inhibitors or angiotensin receptor blockers, BMI body mass index, IQR interquartile range, LAD left anterior descending, LCx left circumflex artery, RCA right coronary artery, SD standard deviation
| Variable | Placebo | Pentoxifylline | P value |
|---|---|---|---|
| Age, mean (± SD) | 59.3 (± 10.8) | 59.9 (± 9.1) | 0.67 |
| Male sex (%) (n) | 80.2% (65) | 78.8% (63) | 0.81 |
| Cardiovascular risk factors | |||
| Diabetes mellitus (%) (n) | 34.6% (28) | 33.8% (27) | 0.91 |
| Active smoking (%) (n) | 51.9% (42) | 52.5% (42) | 0.93 |
| Dyslipidemia (%) (n) | 27.2% (22) | 28.7% (23) | 0.83 |
| Family history (%) (n) | 22.2% (18) | 20.0% (20) | 0.73 |
| Hypertension (%) (n) | 38.3% (31) | 42.5% (34) | 0.58 |
| Obesity (BMI ≥ 30 kg/m2) (%) (n) | 37% (30) | 31.3% (25) | 0.44 |
| Drug history | |||
| Aspirin | 16% (13) | 18.8% (15) | 0.65 |
| Statins | 44.4% (36) | 50% (40) | 0.48 |
| Beta blockers | 17.3% (14) | 17.5% (14) | 0.97 |
| ACEI/ARB | 37% (30) | 35% (28) | 0.79 |
| Laboratory findings | |||
| Hemoglobin (g/dL), mean (± SD) | 15.16 (± 1.93) | 15.17 (± 1.85) | 0.98 |
| Serum creatinine (mg/dL), median (IQR) | 0.90 (0.8, 1.1) | 0.99 (0.86, 1.15) | 0.37 |
| Troponin I (ng/mL), median (IQR) | 74.6 (24.2, 402.5) | 52.5 (20.9, 160.8) | 0.14 |
| Ischemic time (min), median (IQR) | 210 (150, 345) | 270 (210, 320) | 0.10 |
| Culprit artery | |||
| LAD (%) (n) | 43.2% (35) | 47.5% (38) | 0.24 |
| RCA (dominant or balanced) (%) (n) | 28.4% (23) | 35.0% (28) | |
| LCx (dominant or balanced) (%) (n) | 28.4% (23) | 17.5% (14) | |
| Number vessel disease | |||
| 1 (%) (n) | 39.5% (32) | 37.5% (30) | 0.63 |
| 2 (%) (n) | 35.8% (29) | 31.3% (23) | |
| 3 (%) (n) | 24.7% (20) | 31.3% (23) | |
Table 3.
Baseline procedural characteristics of the study population; TIMI thrombolysis in myocardial infarction, POBA percutaneous old balloon angioplasty, PTCA percutaneous transluminal coronary angioplasty
| Variable | Placebo | Pentoxifylline | P value |
|---|---|---|---|
| TIMI flow of the culprit artery | |||
| 0 (%) (n) | 63.0% (51) | 62.5% (50) | 0.26 |
| 1 (%) (n) | 11.1% (9) | 6.3% (5) | |
| 2 (%) (n) | 23.5% (19) | 31.3% (25) | |
| 3 (%) (n) | 2.5% (2) | 0.0% (0) | |
| Procedure type | |||
| PTCA (%) (n) | 60.5% (49) | 60.0% (48) | 0.59 |
| Direct Stenting (%) (n) | 25.9% (21) | 21.3% (17) | |
| POBA (%) (n) | 13.6% (11) | 18.8% (15) | |
| Glycoprotein IIb/IIIa use | |||
| Bolus only (%) (n) | 42.5% (34) | 48.8% (39) | 0.70 |
| Bolus and infusion (%) (n) | 21.3% (17) | 17.5% (14) | |
| Predilation (%) (n) | 65.4% (53) | 62.5% (50) | 0.69 |
| Postdilation (%) (n) | 48.1% (39) | 52.5% (42) | 0.58 |
| During procedure medications (%) (n) | 8.6% (7) | 6.3% (5) | 0.56 |
| Thrombus aspiration (%) (n) | 3.7% (3) | 8.8% (7) | 0.18 |
| TIMI thrombus grade | |||
| 1 (%) (n) | 2.5% (2) | 1.3% (1) | 0.59 |
| 2 (%) (n) | 8.6% (7) | 5.0% (4) | |
| 3 (%) (n) | 12.3% (10) | 18.8% (15) | |
| 4 (%) (n) | 24.7% (20) | 30.0% (24) | |
| 5 (%) (n) | 51.9% (40) | 45.0% (36) | |
Primary outcome
In the pentoxifylline group, 66.3% (53/80) of patients achieved a TIMI flow grade 3, while this amount was 74.1% (63/81) in the placebo, although these different values were not statistically meaningful (P = 0.40).
Secondary outcomes
There were no significant difference regarding angiographic findings including MBG (P = 0.79) and cTFC (P = 0.33) between the two groups (Table 4). Troponin levels 24 and 48 after procedure in the pentoxifylline were 1306 and 1197 and in the placebo group were 1610 and 1167 ng/mL respectively, not significantly different (P = 0.243 and P = 0.317, respectively). Predischarge LVEF was not different between groups (42.0% ± 7.3 vs. 40.4% ± 6.4; P value = 0.15). Only one patient experienced treatment emergent adverse effect which was a mild allergic reaction. MACEs after 1 month were 5 (6.3%) in the pentoxifylline group versus 7 (8.6%) in the placebo group, and MACEs after 3 months recorded 7 (8.8%) and 9 (11.1%), respectively (Table 5).
Table 4.
Angiographic endpoints of the study; cTFC corrected TIMI frame count, MBG myocardial blush grade, SD standard deviation, TIMI thrombolysis in myocardial infarction
| Primary endpoint | Placebo | Pentoxifylline | P value |
|---|---|---|---|
| TIMI flow after PCI | |||
| 0 (%) (n) | 0.0% (0) | 0.0% (0) | 0.40 |
| 1 (%) (n) | 6.2% (5) | 5.0% (4) | |
| 2 (%) (n) | 19.8% (16) | 28.7% (23) | |
| 3 (%) (n) | 74.1% (60) | 66.3% (53) | |
| Secondary endpoints | |||
| cTFC, median (± SD) | 23.9 (± 5.1) | 22.8 (± 9.0) | 0.33 |
| MBG | |||
| 0 (%) (n) | 0.0% (0) | 0.0% (0) | 0.79 |
| 1 (%) (n) | 3.7% (3) | 1.3% (1) | |
| 2 (%) (n) | 11.1% (9) | 11.3% (9) | |
| 3 (%) (n) | 85.2% (69) | 87.5% (70) | |
Table 5.
Clinical efficacy and safety outcomes; TLR target lesion revascularization, TVR target vessel revascularization
| Event | Placebo | Pentoxifylline | P value |
|---|---|---|---|
| Adjudicated major adverse cardiac events at 1 month | |||
| TLR (%) (n) | 3.7% (3) | 2.5% (2) | 0.56 |
| TVR (%) (n) | 1.2% (1) | 1.3% (1) | |
| New hospitalization (%) (n) | 3.7% (3) | 2.5% (2) | |
| Cardiovascular death (%) (n) | 1.2% (1) | 1.3% (1) | |
| Composite (%) (n) | 8.6% (7) | 6.3% (5) | |
| Adjudicated major adverse cardiac events at 3 months | |||
| TLR (%) (n) | 3.7% (3) | 2.5% (2) | 0.61 |
| TVR (%) (n) | 1.2% (1) | 1.3% (1) | |
| New hospitalization (%) (n) | 3.7% (3) | 2.5% (2) | |
| Cardiovascular death (%) (n) | 2.5% (2) | 2.5% (2) | |
| Composite (%) (n) | 11.1% (9) | 8.8% (7) | |
| Adjudicated treatment emergent adverse effects | |||
| All cause death (%) (n) | 1.2% (1) | 1.2% (1) | 0.43 |
| Bleeding (%) (n) | 0.0% (0) | 0.0% (0) | |
| Hypotension (%) (n) | 0.0% (0) | 0.0% (0) | |
| Allergy (%) (n) | 0.0% (0) | 1.2% (1) | |
| At least 1 event (%) (n) | 1.2% (1) | 2.5% (2) | |
Discussion
The PENTOS-PCI trial tested the effects of preprocedural administration of IV pentoxifylline on the success rate of PCI based on angiographic findings in patients with acute STEMI. To the best of our knowledge, this is the first RCT which evaluated the efficacy of this intervention in the acute STEMI setting. Although not powered to assess clinical outcomes, pentoxifylline failed to improve angiographic findings indexing PCI’s effectiveness and primary endpoint was not met. Analysis of secondary angiographic endpoints as measured by MBG and cTFC confirmed that there were no significant differences between pentoxifylline and placebo in reducing reperfusion injury.
IRI has been defined as myocardial injury caused by the restoration of blood flow after an ischemic episode which culminates in the death of cardiomyocytes that were viable right before myocardial reperfusion (Yellon and Hausenloy 2007). It has been proposed that IRI is responsible for up to half of the resulting infarct size in patients with ACS (Hausenloy et al. 2013). Key mechanisms in the pathophysiology of reperfusion injury include reactive oxygen species release, acute inflammatory response and cytokine surge, metabolic disturbances and intracellular acidosis, mitochondrial dysfunction, and intracellular calcium overload, all of which have been investigated as therapeutic modalities for the reduction of infarct size (Kakavand et al. 2021).
In 10–30% of patients, despite TIMI flow 3 of the culprit artery, microcirculation is impaired, which is manifested as a low myocardial perfusion grade in coronary angiography (Rezkalla and Kloner 2002). The embolization of the plaque debris, platelet and leukocyte aggregation, the physical damage to the capillary endothelium, microvascular vasoconstriction, and extravascular coronary microvascular compression due to intramyocardial edema are mechanisms involved in the microvascular integrity disruption (Kloner et al. 1974). Even patients with good contrast runoff (TIMI 3 flow) can have different outcomes due to microcirculation damage. Hence, the goal of treatment in acute MI is not only recanalization of the culprit epicardial artery but also adequate reperfusion of the coronary microcirculation and more importantly to the cardiomyocytes.
Although clinical outcomes have been considered as the gold standard for evaluating therapeutic efficacy of adjunctive therapies in reperfusion for AMI, surrogate endpoints have been encouraged for a variety of reasons. Large sample size needed to establish a significant survival is relatively the most important prohibitive factor for a phase II study like PENTOS-PCI. ST-segment resolution is a noninvasive predictor of mortality in a vast majority of patient populations, but it lacks sensitivity (Claeys et al. 1999). Cardiac magnetic resonance imaging can accurately determine infarct size but it is costly and not feasible in all centers (Wu et al. 2008). Doppler-derived coronary flow velocity reserve relates to recovery of myocardial contractile function, and the waveforms are predictive of functional recovery; however, this technique is invasive and not feasible in all centers (Akasaka et al. 2000). Myocardial contrast echocardiography (MCE) provides direct and immediate information on coronary microcirculation and is able to sense the changes after reperfusion and to expose perfusion abnormalities even in patients with excellent runoff; however, MCE is limited as far as feasibility is concerned (Agati et al. 1994). Angiographic parameters such as TIMI flow, cTFC, and MBG risk-stratify patients with acute MI. Although they are invasive and not easily repeatable, in our study, we operated angiography just once as the routine care and we did not use any additional follow-up imaging. For these reasons, we investigated the effects of pentoxifylline on myocardial perfusion with an integrated approach using TIMI, cTFC, and MBG. Global LVEF assessed by echocardiography has been used as a surrogate end point, but there are some problems for EF which has a limited technical feasibility in up to 30% of patients and it is so operator-dependent.
Despite numerous successful animal studies, clinical investigations into the prevention and treatment of IRI have still proven elusive. Approaches which have failed to reduce reperfusion injury and improve clinical outcome in clinical trials include drugs that can reduce oxidative stress (e.g., mitochondrial permeability transition pore (MPTP) inhibitors, Na+/H+ exchanger (NHE) inhibitors, or delta-protein kinase C (PKC) inhibitors) (Cung et al. 2015; Lincoff et al. 2014; Zeymer et al. 2001), anti-inflammatory agents (antibodies to CD18, CD11, or C5 complement) (Armstrong et al. 2007; Faxon et al. 2002), rheological agents (such as GP IIb/IIIa inhibitors or bivalirudin) (Thiele et al. 2008; Wöhrle et al. 2012), drugs that target cellular metabolism (glucose-insulin-potassium) (Selker et al. 2012), and agents with mixed mechanisms of action (statins or magnesium) (Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) Trial: a randomised controlled trial 2002; Kim et al. 2010). Based on the mechanism, pentoxifylline can be categorized into the latter group.
Although not powered, pentoxifylline has shown the potential to reduce pro-inflammatory markers, increase anti-inflammatory response, and also improve IRI in different settings previously. The addition of pentoxifylline into cardioplegic solution to avoid the myocardial inflammation and IRI injury during cardiopulmonary bypass graft surgery reduced the levels of interleukin (IL)-6, IL-8, and TNF-α (Ustunsoy et al. 2006). In patients with recent ACS, orally administration of pentoxifylline for 6 months significantly reduced the levels of C-reactive protein (CRP) and TNF-α (Fernandes et al. 2008). Pentoxifylline has also attenuated cardiac dysfunction in isolated ischemic-reperfused rat heart through reduction in TNF-α and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Zhang et al. 2005). Several hypotheses can explain this disparity between studies in different settings. The most important factor is the study group which is more homogenous in animal models as we know ischemia and IRI are personal concepts and patients with collateral circulations or shorter ischemic times may obtain more benefits by the preventive interventions and these two factors are better controlled in animal studies. In addition, prevention of IRI is more fitting in the setting of cardiac surgery and this can be elucidated by the important factor of time. Surgeons implicate the protective strategies before ischemia and induce cardioplegia before clamping; this may help the heart to maintain structural integrity without depleting adenosine triphosphate (ATP) and creatine phosphate (Kakavand et al. 2021). This is far too different from the present study with a mean ischemic time of about 4 h.
Although serum levels of pentoxifylline were not measured in PENTOS-PCI, we know that a single 100 mg of IV pentoxifylline can achieve an area under the curve of ~ 500 ng·h/mL which is sufficient for other clinical indications (Beermann et al. 1985). It is probable that the administration of 100 mg pentoxifylline in this study was insufficient by intravenous administration; as long as the epicardial artery is partially or fully occluded during ACS, the drug delivery into cardiomyocytes is not reliable. In addition, since there were no previous studies regarding the safety of IV administration of pentoxifylline in this setting, we chose the aforementioned dose, but the lack of observed toxicity leaves unanswered the question of whether higher doses, longer infusions, or intracoronary administration of pentoxifylline may have been feasible or even effective. In addition to outcomes and dosing concerns, small sample size and being single center are other limitations of the present study.
Conclusion
In this double-blind, placebo-controlled trial, the administration of IV pentoxifylline before primary PCI did not improve the successfulness of procedure in patients with STEMI. The administration of pentoxifylline was well tolerated without any significant adverse drug reactions.
Acknowledgements
We are thankful for the support of the nurses and the staff at Tehran Heart Center and also express our gratitude to our patients for participating in this study.
Author contribution
Azita Talasaz, Hessam Kakavand, and Seyedmohammad Saadatagah contributed to the study conception and design. Material preparation and data collection were performed by Hessam Kakavand, Seyedmohammad Saadatagah, Mohammadreza Naderian, Ali Izadi Amoli, Farshad Sadri, Yaser Jenab, and Seyed Hossein Hosseini. Data analysis was performed by Hessam Kakavand, Maryam Aghakouchakzadeh, and Arash Jalali. The first draft of the manuscript was written by Hessam Kakavand, and all authors commented on previous versions of the manuscript. Seyedmohammad Saadatagah, Azita H. Talasaz, Hamidreza Pourhosseini, and Mojtaba Salarifar had a critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tehran University of Medical Sciences (2018–03-10, IR.TUMS.MEDICINE.REC.1396.4760).
Consent to participate
Informed consent was obtained from all individual participants.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agati L, Voci P, Bilotta F, Luongo R, Autore C, Penco M, Iacoboni C, Fedele F, Dagianti A. Influence of residual perfusion within the infarct zone on the natural history of left ventricular dysfunction after acute myocardial infarction: a myocardial contrast echocardiographic study. J Am Coll Cardiol. 1994;24(2):336–342. doi: 10.1016/0735-1097(94)90285-2. [DOI] [PubMed] [Google Scholar]
- Akasaka T, Yoshida K, Kawamoto T, Kaji S, Ueda Y, Yamamuro A, Takagi T, Hozumi T. Relation of phasic coronary flow velocity characteristics with TIMI perfusion grade and myocardial recovery after primary percutaneous transluminal coronary angioplasty and rescue stenting. Circulation. 2000;101(20):2361–2367. doi: 10.1161/01.cir.101.20.2361. [DOI] [PubMed] [Google Scholar]
- Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, Jr, O'Neill WW, Todaro TG, Vahanian A, Van de Werf F. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297(1):43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- Beermann B, Ings R, Månsby J, Chamberlain J, McDonald A. Kinetics of intravenous and oral pentoxifylline in healthy subjects. Clin Pharmacol Ther. 1985;37(1):25–28. doi: 10.1038/clpt.1985.6. [DOI] [PubMed] [Google Scholar]
- Claeys MJ, Bosmans J, Veenstra L, Jorens P, De Raedt H, Vrints CJ. Determinants and prognostic implications of persistent ST-segment elevation after primary angioplasty for acute myocardial infarction: importance of microvascular reperfusion injury on clinical outcome. Circulation. 1999;99(15):1972–1977. doi: 10.1161/01.cir.99.15.1972. [DOI] [PubMed] [Google Scholar]
- Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guérin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, ... Ovize M (2015) Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 373(11):1021-1031. 10.1056/NEJMoa1505489 [DOI] [PubMed]
- Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) trial: a randomised controlled trial (2002) Lancet 360(9341):1189–1196. 10.1016/s0140-6736(02)11278-5 [DOI] [PubMed]
- Faxon DP, Gibbons RJ, Chronos NA, Gurbel PA, Sheehan F. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J Am Coll Cardiol. 2002;40(7):1199–1204. doi: 10.1016/s0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- Fernandes JL, de Oliveira RTD, Mamoni RL, Coelho OR, Nicolau JC, Blotta M, Serrano CV., Jr Pentoxifylline reduces pro-inflammatory and increases anti-inflammatory activity in patients with coronary artery disease–a randomized placebo-controlled study. Atherosclerosis. 2008;196(1):434–442. doi: 10.1016/j.atherosclerosis.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Erik Bøtker H, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013;98(1):7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- Henriques JP, Zijlstra F, van't Hof AW, de Boer MJ, Dambrink JH, Gosselink M, Hoorntje JC, Suryapranata H (2003) Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 107(16):2115-2119. 10.1161/01.Cir.0000065221.06430.Ed [DOI] [PubMed]
- Horton JW, White DJ. Free radical scavengers prevent intestinal ischemia-reperfusion-mediated cardiac dysfunction. J Surg Res. 1993;55(3):282–289. doi: 10.1006/jsre.1993.1141. [DOI] [PubMed] [Google Scholar]
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- Kakavand H, Aghakouchakzadeh M, Coons JC, Talasaz AH. Pharmacologic prevention of myocardial ischemia-reperfusion injury in patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Cardiovasc Pharmacol. 2021;77(4):430–449. doi: 10.1097/fjc.0000000000000980. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim J, Choi D, Lee CJ, Lee SH, Ko YG, Hong MK, Kim BK, Oh SJ, Jeon DW, Yang JY, Cho JR, Lee NH, Cho YH, Cho DK, Jang Y. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv. 2010;3(3):332–339. doi: 10.1016/j.jcin.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54(6):1496–1508. doi: 10.1172/jci107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoff AM, Roe M, Aylward P, Galla J, Rynkiewicz A, Guetta V, Zelizko M, Kleiman N, White H, McErlean E, Erlinge D, Laine M, Dos Santos Ferreira JM, Goodman S, Mehta S, Atar D, Suryapranata H, Jensen SE, Forster T, ... Krucoff M (2014) Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI Randomized Controlled Trial. Eur Heart J 35(37):2516-2523. 10.1093/eurheartj/ehu177 [DOI] [PubMed]
- Matzky R, Darius H, Schrör K. The release of prostacyclin (PGI2) by pentoxifylline from human vascular tissue. Arzneimittelforschung. 1982;32(10):1315–1318. [PubMed] [Google Scholar]
- Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002;105(5):656–662. doi: 10.1161/hc0502.102867. [DOI] [PubMed] [Google Scholar]
- Salhiyyah K, Senanayake E, Abdel-Hadi M, Booth A, Michaels JA (2012) Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev 1:Cd005262. 10.1002/14651858.CD005262.pub2 [DOI] [PubMed]
- Sandoval Y, Jaffe AS. Type 2 myocardial infarction: JACC review topic of the week. J Am Coll Cardiol. 2019;73(14):1846–1860. doi: 10.1016/j.jacc.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D'Agostino RB, Ruthazer R, Atkins JM, Sayah AJ, Levy MK, Richards ME, Aufderheide TP, Braude DA, Pirrallo RG, Doyle DD, Frascone RJ, Kosiak DJ, Leaming JM, Van Gelder CM, ... Udelson JE (2012) Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA 307(18):1925-1933. 10.1001/jama.2012.426 [DOI] [PMC free article] [PubMed]
- Strano A, Davi G, Avellone G, Novo S, Pinto A. Double-blind, crossover study of the clinical efficacy and the hemorheological effects of pentoxifylline in patients with occlusive arterial disease of the lower limbs. Angiology. 1984;35(7):459–466. doi: 10.1177/000331978403500709. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP, 3rd, Larrick J, Kunkel SL. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155(3):1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- Thiele H, Schindler K, Friedenberger J, Eitel I, Fürnau G, Grebe E, Erbs S, Linke A, Möbius-Winkler S, Kivelitz D, Schuler G. Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-elevation myocardial infarction trial. Circulation. 2008;118(1):49–57. doi: 10.1161/circulationaha.107.747642. [DOI] [PubMed] [Google Scholar]
- Ustunsoy H, Sivrikoz MC, Tarakcioglu M, Bakir K, Guldur E, Celkan MA. The effects of pentoxifylline on the myocardial inflammation and ischemia-reperfusion injury during cardiopulmonary bypass. J Card Surg. 2006;21(1):57–61. doi: 10.1111/j.1540-8191.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Wöhrle J, Merkle N, Kunze M, Cristea E, Mehran R, Rottbauer W, Stone GW. Effect of bivalirudin compared with unfractionated heparin plus abciximab on infarct size and myocardial recovery after primary percutaneous coronary intervention: the horizons-AMI CMRI substudy. Catheter Cardiovasc Interv. 2012;79(7):1083–1089. doi: 10.1002/ccd.23179. [DOI] [PubMed] [Google Scholar]
- Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, Carr JC, Holly TA, Lloyd-Jones D, Klocke FJ, Bonow RO. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94(6):730–736. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Zeymer U, Suryapranata H, Monassier JP, Opolski G, Davies J, Rasmanis G, Linssen G, Tebbe U, Schröder R, Tiemann R, Machnig T, Neuhaus KL (2001) The Na(+)/H(+) exchange inhibitor eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction. Results of the evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction (ESCAMI) trial. J Am Coll Cardiol 38(6):1644–1650. 10.1016/s0735-1097(01)01608-4 [DOI] [PubMed]
- Zhang M, Xu YJ, Saini HK, Turan B, Liu PP, Dhalla NS. Pentoxifylline attenuates cardiac dysfunction and reduces TNF-alpha level in ischemic-reperfused heart. Am J Physiol Heart Circ Physiol. 2005;289(2):H832–839. doi: 10.1152/ajpheart.00178.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.