Abstract
Ten palm leaf impressions are documented from the latest Maastrichtian (late Cretaceous) to early Danian (earliest Paleocene) sediments (K-Pg, c. 66–64 Ma) of the Mandla Lobe of the Deccan Inter-trappean Beds, Madhya Pradesh, central India. The palmate leaf shape along with a definite well-preserved costa support their placement in the subfamily Coryphoideae of the family Arecaceae. We place all recovered palm leaf specimens in the fossil genus Sabalites, report seven species of coryphoid palms and describe two new species namely, Sabalities umariaensis sp. nov. and Sabalites ghughuaensis sp. nov. The fossils indicate that coryphoid palms were highly diverse in central India by the latest Cretaceous. These and earlier reported coryphoid palm fossils from the same locality indicate that they experienced a warm and humid tropical environment during the time of deposition. These discoveries confirm the presence of a diversity of Coryphoideae in Gondwana prior to the India-Eurasia collision and provide information about coryphoid biogeographical history over geological time. Based on megafossil remains, we trace coryphoid palm migration pathways from India to mainland Southeast (SE) Asia and other parts of Asia after the docking of the Indian subcontinent with Eurasia early in the Paleogene.
Keywords: Coryphoideae, Leaf fossils, Late cretaceous-early paleocene, Paleobiogeography, Paleoclimate, Madhya Pradesh
Highlights
-
•
Indian Deccan fossils show K-Pg diversity of Coryphoideae in central India.
-
•
Two new species of coryphoid leaves are described.
-
•
Coryphoid palm megafossil occurrences in Asia are reviewed.
-
•
A warm humid climate prevailed in central India at the K-Pg boundary.
1. Introduction
The subfamily Coryphoideae constitutes the second largest subfamily of Arecaceae (palms) with respect to the number of genera and the third in terms of the number of species (Uhl and Dransfield, 1987; Dransfield et al., 2008). Coryphoideae consists of 47 genera and 518 species (Baker and Dransfield, 2016) and subdivides into eight tribes: Borasseae, Caryoteae, Chuniophoeniceae, Corypheae, Cryosophileae, Phoeniceae, Sabaleae and Trachycarpeae (Asmussen et al., 2006; Dransfield et al., 2008). This palm subfamily is sister to the Arecoideae and Ceroxyloideae clades (Asmussen et al., 2000) and includes all palms with a fused lamina and induplicate folded leaf segments (Matsunaga et al., 2019). Fan-shaped palmate leaves with radiating leaf segments are characteristic of the subfamily Coryphoideae (Dransfield et al., 2008), however some members of the tribes Caryoteae (Caryota L., and Arenga Labill. ex DC.) and Phoeniceae (Phoenix L.) of this subfamily possess pinnately compound leaves (Matsunaga and Smith, 2021). In Coryphoideae, truly palmate leaves (without costae) are rather unusual, while the costapalmate type is more widespread (Dransfield et al., 2008). Costapalmate leaves, exclusive for the subfamily Coryphoideae, are typically similar to a palmate leaf but have a definite costa (midrib) (Fig. 1). The costa or extension of the petiole into the lamina area is robust, armed or unarmed, very thick at the basal portion and gradually tapers towards the apex. The adaxial surface of the leaves may possess a hastula or ligule-like appendage at the junction of the petiole and leaf blade (Manchester et al., 2010). The above-mentioned diagnostic morphological features are known to occur among various coryphoid palm genera (Dransfield et al., 2008).
Fig. 1.
Different types of modern palm leaves.
Modern members of the Coryphoideae are distributed in a wide range of habitats from pantropical to some warm temperate areas of the southern part of North America, the northern part of South America, Africa, Madagascar, the Philippines, Indonesia, the southern part of Australia and Asia (Dransfield et al., 2008; Reichgelt et al., 2018). They can also tolerate mild frosts as adults and survive in gardens, but do not form naturally reproducing populations where frosts are frequent or severe (Reichgelt et al., 2018). In Asia, the Coryphoideae has a widespread distribution from the Solomon Islands through Vanuatu, New Caledonia, Fiji to French Polynesia, Malesia, and Hawaii (Prebble and Dowe, 2008). In India, the Coryphoideae are represented by a few common taxa with costapalmate type leaves such as Sabal, Borassus, Trachycarpus, Licuala, Livistona, Corypha, Hyphaene etc. (Govaerts and Dransfield, 2005).
Numerous coryphoid palm leaves have been reported from Mesozoic to Paleogene sediments outside of India (Berry, 1905, 1911, 1914, 1917, 1924; Endo, 1934; Lesquereux, 1878; Knowlton, 1917; Brown, 1962; Guo, 1965; Daghlian, 1978; van der Burgh, 1984; Tao, 1988; Mustoe and Gannaway, 1995; Newberry, 1898; Kvaček and Herman, 2004; Marmi et al., 2010; Zhou et al., 2013; Wang et al., 2015, 2016; Greenwood and West, 2017; Su et al., 2019; Greenwood and Conran, 2020; Song et al., 2021). The earliest coryphoid leaf fossils are from the late Cretaceous (upper Coniacian-lower Santonian) of South Carolina (Berry, 1914) and the Santonian Magothy Formation of New Jersey and Maryland (Berry, 1905, 1911). Based on fossils from the Late Cretaceous (Campanian) Aguja Formation of Texas it has been suggested that an ecological relationship between animals (dinosaur herbivores) and coryphoid palms may have played an important role in palm evolution (Manchester et al., 2010). However, only a few confirmed reports of fossil costapalmate leaf remains are known so far from the Late Cretaceous to Paleogene sediments of India (Table S1; Mahabale, 1966; Lakhanpal et al., 1983, 1984; Bonde, 1986; Srivastava et al., 2014; Roy et al., 2021). From this perspective, the occurrence of seven species of coryphoid palms, including two new species from the latest Maastrichtian (Late Cretaceous) to earliest Danian (early Paleocene) sediments (Chron 29R, 66–64 Ma) of the Deccan Inter-trappean Beds of India, several million years prior to the earliest likely time of India–Asia land contact, is noteworthy and seems to indicate a well-established Late Cretaceous Gondwanan presence of the subfamily Coryphoideae. This subfamily then probably dispersed to mainland Southeast (SE) Asia just before, or soon after, a land connection was established between the Indian subcontinent and Eurasia. The recovered palm fossils also indicate that, at the time of the Cretaceous-Paleogene (K-Pg) transition, the flora in Madhya Pradesh included a diversity of coryphoid palms, which also has some bearing on the reconstruction of the paleoclimate of that area.
The aims of the present study are to (1) report and describe, two new species of coryphoid palm leaf remains under the fossil genus Sabalites, (2) review the fossil history of Asian coryphoid palms in detail and compare our fossil leaf specimens with earlier reported reliable coryphoid fossil leaf species, and (3) review comprehensively the biogeographic patterns of this palm subfamily in Asia and discuss its possible migration patterns.
2. Materials and methods
2.1. Geological setting
The Deccan Inter-trappean Beds, one of the world's most important Large Igneous Provinces, covers an area of about 500,000 km2 in western, central, and southern parts of India, including Andhra Pradesh, Gujrat, Karnataka, Madhya Pradesh, and Maharashtra (Smith et al., 2015). The Deccan Volcanic Province records a massive accumulation of tholeiitic magmas in a relatively short time span (Chenet et al., 2007, 2009) from the latest Maastrichtian to the earliest Danian (c. 67–64 Ma, chrons 30N–29N) based on radiometric dating (40Ar/39Ar), planktonic foraminifera and magnetostratigraphy (Venkatesan et al., 1997; Khosla, 1999; Hofmann et al., 2000; Sheth et al., 2001; Keller et al., 2009; Chenet et al., 2007, 2009; Renne et al., 2015; Schoene et al., 2015; Smith et al., 2015; Srivastava et al., 2015). Bounding the K-Pg transition, lavas poured out through numerous fissures and in so doing inundated and created a range of terrestrial and lacustrine environments (Nair and Bhusari, 2001). The eruptions occurred in discrete episodes with intermittent quiescent periods marked by the accumulation of inter-trappean sediments comprising lacustrine shale, silt and carbonate deposits hosting floral and faunal fossils (Khosla and Verma, 2015; Verma and Khosla, 2019). The eruptive succession in western India began at ∼66.4 Ma and continued until 65.6 Ma (Schoene et al., 2015, 2019; Sprain et al., 2019). The palm fossils described here are from the Mandla Lobe, a 900-m thick package of 29 flows dated as primarily belonging to Chron 29R (Pathak et al., 2017), which lasted <1 Ma and straddles the K-Pg boundary, although Srivastava et al. (2015) argue for a mean age of 64.21 ± 0.33 Ma.
2.2. Materials and preparation
We recovered fossilized palm fronds bearing similarities with modern coryphoid palm leaves during fieldwork in 2021. All were collected from the latest Maastrichtian (Late Cretaceous) to earliest Danian (early Paleocene) sediments of the Mandla Lobe Deccan Inter-trappean beds of Umaria village (23°05′26.41″N, 80°37′35.25″E), Gughua Mal village (23°06′48.60″N, 80°37′22.89″E) in Dindori District, and Karondi village (N23° 12.274′, E80° 33.105′) in Jabalpur District of Madhya Pradesh, central India (Fig. 2). Umaria, a small village under Mehandwani Tehsil in Dindori District, is located 48 km westwards from the district headquarters of Dindori. Ghughua, a village under Shahdol Division in Dindori District, is situated between Niwas and Shahpura about 77 km east of Jabalpur, while Karondi is a small village (a total area of about 320.99 ha) under Karondi Panchayat in Kundam Tehsil in Jabalpur District, located 45 km eastward from the district headquarters in Jabalpur. We have recovered a large number of fossil woods, seeds, and leaves from the aforesaid localities; some of them are reported (Khan et al., 2019, 2020a, b; Roy et al., 2021). The inter-trappean sediments of Madhya Pradesh are very rich in permineralized woods (Mahabale, 1958; Lakhanpal et al., 1979; Ambwani, 1983; Ambwani and Mehrotra, 1989; Gayakwad and Patil, 1989; Bonde et al., 2008; Khan et al., 2019, 2020a), but leaf remains are very scarce (Srivastava et al., 2014; Roy et al., 2021).
Fig. 2.
Map showing Deccan Volcanic Province (DVP). The red star indicates fossil localities (modified after Smith et al., 2015).
The recovered leaf specimens (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7) required a little preparation before photography, as the morphological details are not exposed in the initial fracture but are revealed by careful removal of the overlying matrix using chisel and hammer. Photography was done with natural low-angled light using a digital camera (NIKON D3300). Line-drawings of holotype fossil specimens (Fig. 3, Fig. 4, Fig. 5b and d), additional specimens (Fig. 6b, d), and earlier reported coryphoid palm leaf specimens (Fig. 8, Fig. 9) were made using CorelDraw V. 20 software. The exact taxonomic determination required extensive literature and herbarium searches to compare the Deccan palm leaf specimens with both fossil and modern leaves of coryphoid palms sharing similar morphological features (nature of the costae, petioles, and leaf segments with their venation patterns). Identification of the fossil palm leaf specimens was based on the key and definitions provided by Read and Hickey (1972). We followed their classification and placed our specimens under the fossil genus Sabalites G. Saporta (Read and Hickey, 1972), which they proposed exclusively for costapalmate fossil palm leaves. Terms used to describe our Cretaceous palm leaf specimens are in conformity with the standard terminology for the morphological description of fossil palm leaves (Read and Hickey, 1972). Several modern coryphoid palm leaves were also examined critically using digital herbarium catalogues, specifically the Kew Herbarium catalogue (https://apps.kew.org/herbcat/navigator.do), and global biodiversity information facility (www.gbif.org). The holotype specimens (SKBU/PPL/Um/L/1a; SKBU/PPL/Gu/L/1) and additional specimens (SKBU/PPL/Um/L/1b; SKBU/PPL/Gu/L/2; SKBU/PPL/Um/L/2a, SKBU/PPL/Um/L/2b, SKBU/PPL/Um/L/3, SKBU/PPL/Um/L/5, SKBU/PPL/Um/L/6; SKBU/PPL/K/L/2) are housed in the Museum of the Department of Botany, Sidho-Kanho-Birsha University (SKBUH), Purulia, India.
Fig. 3.
a. Sabalites umariaensis Kumar, Spicer RA et Khan, sp. nov. (Holotype: SKBU/PPL/Um/L/1a)- a portion of leaf blade showing a short petiole with a distinct hastula and plicate leaf segments attached to a distinct long straight costa; b. drawing of the leaf blade of S. umariaensis (Scale bar = 2 cm).
Fig. 4.
Sabalites umariaensis Kumar, Spicer RA et Khan, sp. nov. a, b. Enlarged views of the basal portion of the leaf blade of S. umariaensis showing induplicate leaf segments with a distinct mid-vein (marked by the IL) attached to costa (marked by the C) and hastula (marked by the H); c. Basal portion of the leaf blade of modern coryphoid palm; d. a counterpart of leaf blade of S. umariaensis (specimen no.: SKBU/PPL/Um/L/1b) ; e, f. enlarged view of a part of the leaflet of fig. d showing mid-vein (marked by 1), secondary lateral veins (marked by 2) and cross veins (marked by 3); g. line drawing of a part of fossil leaflet showing mid-vein (marked by 1), secondary lateral veins (marked by 2), and cross veins (marked by 3) (Scale bar = 1 cm).
Fig. 5.
Sabalites ghughuaensis Kumar, Spicer RA et Khan, sp. nov. (Holotype: SKBU/PPL/Gu/L/1). a. A portion of leaf blade showing plicate leaf segments attached to a robust costa; b. line drawing of fig. a; c. enlarged view of a portion of the leaf blade of S. ghughuaensis showing plicate leaf segments with distinct mid-vein and secondary lateral veins; d. line drawing of fig. c showing plicate leaf segments with distinct mid-vein and secondary lateral veins (Scale bar = 2 cm).
Fig. 6.
Sabalites deccanensis Kumar, Spicer RA et Khan, sp. nov. (Holotype: SKBU/PPL/Gu/L/2). a. A portion of leaf blade showing a distinct petiole and induplicate leaf segments attached to a robust costa; b. line drawing of fig. a; c. enlarged view of a lower portion of the leaf blade of S. deccanensis with induplicate leaf segments emerging from the transition area of petiole and costa; d. line drawing of fig. c showing induplicate leaf segments attached to the costa (Scale bar = 1 cm).
Fig. 7.
a. Sabalites sp. Type 1 (specimen no. SKBU/PPL/Um/L/2a)- a portion of leaf blade showing induplicate leaf segments attached to a long straight costa (Scale bar = 1 cm); b. Sabalites sp. Type 1 (specimen no. SKBU/PPL/Um/L/2b)- counterpart showing induplicate leaf segments attached to a long straight costa (Scale bar = 1 cm); c. Sabalites sp. Type 2 (specimen no. SKBU/PPL/Um/L/6)- a portion of leaf blade showing induplicate leaf segments attached to a long straight costa (Scale bar = 1 cm); d. Sabalites sp. Type 3 (specimen no. SKBU/PPL/Um/L/3)- a portion of leaf blade showing leaf segments attached to a robust costa (Scale bar = 1 cm); e. Sabalites sp. Type 4 (specimen no. SKBU/PPL/Um/L/5)- a portion of leaf blade showing induplicate leaf segments attached to a long straight costa (Scale bar = 1 cm); f. enlarged view of the basal portion of the leaf blade of Sabalites sp. Type 4 (Scale bar = 1 cm); g. Sabalites sp. Type 5 (specimen no. SKBU/PPL/K/L/2)- a portion of leaf blade showing induplicate leaf segments attached to a stout costa (Scale bar = 2 cm).
Fig. 8.
Leaf architecture of fossil species of Coryphoid palms. a. Sabalites dindoriensis Srivastava et al. (Scale bar = 4 cm); b. S. guangxiensis Wang et al. (Scale bar = 2 cm); c. S. tibetensis Su et al. (Scale bar = 1 cm); d. Chuniophoenix slenderifolia Wang et al. (Scale bar = 1 cm); e. Sabalites cf. asymmetricus Wang et al. (Scale bar = 1 cm); f. S. longirhachis Marmi et al. (Scale bar = 5 cm); g. S. changchangensis Zhou et al. (Scale bar = 1 cm); h. S. geneseensis Greenwood and West (Scale bar = 1 cm); i. S. karondiensis Roy et al. (Scale bar = 1 cm); j. Livistona roundifolia Wang et al. (Scale bar = 2 cm).
Fig. 9.
Leaf architecture of fossil species of Coryphoid palms. a. Trachycarpus formosa Wang et al. (Scale bar = 2 cm); b. Sabalites tenufolius Zhou et al. (Scale bar = 1 cm); c. S. asymmetricus Zhou et al. (Scale bar = 1 cm); d. S. colaniae Song et al. (Scale bar = 1 cm); e. S. dowsonii Greenwood and Conran (Scale bar = 5 cm); f. S. robustus Zhou et al. (Scale bar = 1 cm); g. S. campbellii Mustoe and Gannaway (Scale bar = 5 cm); h. S. szei Zhou et al. (Scale bar = 1 cm); i. Sabal maior Vanderburgh (Scale bar = 2 cm).
A cluster analysis using the PAST 4.03.exe. software was employed to explore the relationships between the external morphological features (leaf form, petiole armature and striation, length and breadth of petiole, length and breadth of costa, base width of costa, number of leaf segments, width and length of leaf segment, leaf base, angle of divergence of leaflets and hastula) of twenty-one fossil species of coryphoid palm leaf remains including our two new fossil species (Tables S2 and S3). The characteristics included in this analysis were chosen because they are well-preserved in the fossil species. The dendrogram was constructed using the paired group (UPGMA) algorithm and the Bray–Curtis method, the most efficient clustering algorithm. Details of the characteristics and the scoring are given in Table S3.
3. Results
3.1. Taxonomic treatment of the recovered palm leaf specimens
As all the recovered Deccan leaf specimens are impressions lacking epidermal anatomy, we focus only on their leaf blade architecture. The characteristic features of the fossil leaf specimens, including palmate, plicate induplicate leaf segments with a well-defined costa (the extension of the petiole into the leaf blade-costapalmate), a triangular hastula, and an unarmed petiole, collectively demonstrate that they have affinities with modern leaves of the palm subfamily Coryphoideae. This is because a costapalmate leaf type is found today exclusively in the Coryphoideae (Harley, 2006; Dransfield et al., 2008). As Read and Hickey (1972, p. 129) stated, “Since it is very difficult to identify specimens of modern palms accurately from their leaves alone, no attempt should be made to place fossil palm fragments in genera of modern palms unless unquestionably identifiable with them.” Thus, it is difficult to assign the present fossil palm leaves to a taxonomic unit below the subfamily level based only on their external morphological characters. Under these circumstances, we place our fossil specimens under the morphogenus Sabalites (Saporta, 1865; Read and Hickey, 1972). Here, we report two new fossil species, namely, Sabalities umariaensis sp. nov., and Sabalites ghughuaensis sp. nov., and only their detailed systematic descriptions are provided. However, only diagnostic morphological features are provided for the other five species of Sabalites leaf specimens. Due to the lack of sufficient diagnostic characters, especially leaflet venation patterns, it is unreasonable to refer to them as new species at present. Therefore, we here tentatively describe them under morphotypes.
3.2. Cluster analysis
We compare the dissimilarities between the different coryphoid leaf fossil species through cluster analysis (Fig. 10). The first cluster contains only two species of Sabalites, namely, S. geneseensis and S. umariaensis. The second cluster containing the remaining fossil species consists of two sub-clusters. The first sub-cluster contains fifteen species such as,
Fig. 10.
Cluster analysis dendrogram using PAST software showing the characteristic relationship between the present two fossil species and the other reported fossil species of coryphoid palms (Linkage Algorithm-paired group (UPGMA); Similarity index- Bray–Curtis).
Sabaliteskarondiensis, S. dawsonii, Sabalites campbellii, Sabalites longirhachis, Trachycarpus formosa, S. cf. asymmetricus, S. ghughuaensis, Sabalites dindoriensis, Sabalites guangxiensis, Chuniophoenix slenderifolia, S. colaniae, S. szei, Sabalites tenuifolius, Sabalites asymmetricus and S. robustus. The second sub-cluster contains only four species: Sabal maior, Livistona roundifolia, S. tibetensis and S. changchangensis (Fig. 10). S. ghughuaensis is widely separated from the other species, T. formosa and S. cf. asymmetricus, within the same branch. These results, along with the detailed comparison of their morphological features with the earlier reported fossil species of coryphoid palm leaves in Table S2, are sufficient to further distinguish our new fossil species, namely, S. umariaensis and S. ghughuaensis. The thirteen features selected are based on the characteristics preserved and observed in the fossil specimens.
3.3. Systematics
Family: Arecaceae Bercht. & J. Presl
Sub-family: Coryphoideae Burnett
Genus: Sabalites G. Saporta emended Read et Hickey
Species: Sabalites umariaensis Kumar, Hazra T et Khan, sp. nov.
Holotype: SKBU/PPL/Um/L/1a (Fig. 3a)
Additional specimen: SKBU/PPL/Um/L/1b (Fig. 4d)
Repository: Repository: Department of Botany, Sidho-Kanho-Birsha University (SKBUH), West Bengal, India.
Type locality: Umaria village (23°05′26.41″N, 80°37′35.25″E) in Dindori District, Madhya Pradesh, central India.
Type horizon and age: Deccan Inter-trappean beds; latest Maastrichtian (Late Cretaceous)-earliest Danian (early Paleocene)
Etymology: The specific epithet “umariaensis” refers to the fossils being found in Umaria village.
Specific diagnosis: Leaf costapalmate, large, fan-shaped with a long and strong costa; leaf blade symmetrically attached to the costa; petiole stout, unarmed, with pronounced longitudinal striations; triangular hastula present; costa slightly broad at the base and gradually tapers towards the apex; leaf segments narrow, plicate, fused at emerging point, emerging straight from the apex of the petiole and from the both sides of the costa at an acute angle, basal leaf segments strongly pendulous; plication induplicate; thick and prominent midvein in each leaf segment; mid-vein paralleled by prominent secondary lateral veins, oblique and transverse cross veins present on both sides of the secondary lateral vein.
Description: The holotype (Fig. 3a) preserves the basal and middle portion of a fan-shaped leaf blade and the top of petiole, apical portion broken; leaf frond large, costapalmate, maximum preserved length 24.3 cm and a maximum width of 6 cm; leaf blade symmetrically attached to the costa; the petiole clearly preserved at the base of the recovered leaf specimen (Fig. 3, Fig. 4a, b), short, length 1.5 cm and breadth 1.1 cm, texture fibrous, longitudinal striations clearly seen on the petiole (Fig. 4a, b), spineless with no prominent armouring found, the petiole tapering upward forming a strong long costa about 0.6 cm wide at the base; hastula (Fig. 3, Fig. 4a, b) prominent, triangular, 2 cm in length, 1.4 cm wide at base and 0.8 cm wide at the tip; costa (extension of the petiole into the leaf blade) well-preserved, long, straight, 22.8 cm in length, 0.6 cm in width, broad at the base and gradually tapering towards the apex (Fig. 3, Fig. 4a, b, d); some faint longitudinal fiber-like structures also seen on the surface of costa; leaf segments well-preserved along both sides of the costa (Fig. 3, Fig. 4a, d), narrow, folded, spineless, connate (attached to the adjoining leaf segments) at the base, plicate, emerging at an acute angle from the costa, basal segments strongly pendulous (Fig. 3, Fig. 4a, b), proximally the basal leaf segments of one side of the specimen first directed downward and then curve upward (Fig. 3a), the plication of the well-preserved leaf segments easily discernible and induplicate (V-shaped, i.e., midrib protruding downward) (Fig. 3, Fig. 4a, d); seemingly 25–31 number of leaf segments preserved on one side of the costa and 9 on another side, fused adjacently and attached to the costa by the entire base (Fig. 4a, d), angle of divergence approx. 25–35°, leaf segments maximum 17.3 cm in length, about 1.5 cm wide at the base and 1.3 cm at the distal region; each leaf segment with a characteristic thick, distinct mid-vein or primary vein (0.8 mm thick) (Fig. 4e, f, g), primary vein with numerous secondary lateral veins (Fig. 4e, f, g); secondary veins distinct, running parallel on either side of the mid-vein, distance between two secondaries 1.2–1.5 mm; some prominent oblique and transverse cross veins (Fig. 4e, f, g) present between secondary lateral veins.
3.3.1. Comparison with other coryphoid leaf fossil species
Numerous coryphoid costapalmate palm leaf fossils have been reported from the late Mesozoic and early Cenozoic sediments of India and elsewhere, and it is not possible to compare all of them with the present fossil specimen due to various differences in preservation state. We restricted our comparisons to those fossil species that are well-preserved (Table S2; Fig. 8, Fig. 9). In China, several reliable coryphoid costapalmate palm leaves have been reported from Eocene deposits, such as S. asymmetricus, S. robustus, S. tenuifolius, S. szei, and S. changchangensis (Zhou et al., 2013), and Oligocene sediments, namely, C. slenderifolia, Livistona rotundifolia, T. formosa (Wang et al., 2015), S. guangxiensis and S. cf. asymmetricus (Wang et al., 2016). S. asymmetricus, S. robustus, S. tenuifolius, S. szei and S. changchangensis reported by Zhou et al. (2013) from the Eocene Changchang Basin of Hainan Island of South China possess shorter costae with much narrower and shorter leaf segments than our specimen. Our specimen also differs from S. asymmetricus, S. robustus, S. szei and S. changchangensis in having a triangular hastula between the petiole and leaf blade. C. slenderifolia, L. rotundifolia, and T. formosa reported by Wang et al. (2015) from the Oligocene deposits of the Ningming Formation in Ningming County, Guangxi (China) possess a shorter costa than our specimen. In C. slenderifolia, leaf segments emerge asymmetrically from the sides of the costa. The petiole of L. rotundifolia has robust spines that differentiate it from the Deccan fossil specimen. T. formosa differs in having a cordate leaf base. Another two Oligocene (Ningming Formation) Chinese species, namely, S. guangxiensis and S. cf. asymmetricus reported by Wang et al. (2016) from the Chengzhong Town, Ningming County, Guangxi (China), possess a shorter costa, but have a longer petiole in comparison to the Deccan specimen. Our specimen also differs from S. guangxiensis and S. cf. asymmetricus in having a characteristic hastula between the leaf blade and petiole. Additionally, S. cf. asymmetricus differs in having an asymmetrical leaf base. S. tibetensis reported by Su et al. (2019) from the early late Eocene (Bartonian) sediments of Dayu Village, Lunpola Basin, central Tibetan Plateau differs in having spines at the base of the leaf blade. This material was originally described as being deposited at ∼25 Ma, but subsequent U/Pb dating has shown its age to be ∼38 Ma (Fang et al., 2020). The Tibetan fossil species also possesses a much longer petiole (65 cm) than the current specimen (1.5 cm).
A recently reported fossil species, S. colaniae (Song et al., 2021) from the Oligocene Dong Ho Formation of Hoanh Bo Basin, northern Vietnam, also possess a longer petiole (38 cm) than our specimen. In the Vietnam species, leaf segments emerge asymmetrically from the petiole apex and along both sides of the costa. However, in our specimen, leaf segments emerge symmetrically from the petiole apex and along both sides of the long costa. A Canadian early Paleocene species S. geneseensis (Greenwood and West, 2017) differs in having an asymmetrical leaf base and a prominent oblique ladder-like zigzag pattern of transverse veins. However, our specimen possesses a longer costa (22.8 cm) than S. geneseensis (3.1 cm) and a hastula, which is absent in S. geneseensis. Another Canadian fossil species, S. dawsonii reported by Greenwood and Conran (2020) from Eocene (Huntingdon Formation) sediments, differs in having an acutely narrow short costa that extends less than 10% of the leaf blade length from its broad triangular base. S. campbellii Newberry reported from the Paleocene-Eocene of USA (Newberry, 1898; Mustoe and Gannaway, 1995) differs from the palm material presented here in possessing a truncate leaf base. S. campbellii leaves from the Bellingham Bay Member of the Chuckanut Formation of Washington, USA (Mustoe and Gannaway, 1995) possess a shorter costa (1.5–2 cm) than our Deccan specimen. S. longirhachis Kvaček and Herman reported from the lower Campanian of the Grünbach Formation of Austria (Kvaček and Herman, 2004) and from the lower Maastrichtian of Fumanya (Tremp Formation), Pyrénées, Spain (Marmi et al., 2010) possess a much thicker, longer costa and petiole than our Deccan specimen. A fossil costapalmate leaf species, S. maior van der Burgh, reported from the Miocene of the lower Rhenish Plain, Germany, by Vanderburgh (1984), shows a clear difference in costa lengths and shapes and leaf segment dissection compared to our palm leaf specimen.
The present specimen was also compared to S. dindoriensis Srivastava et al. and S. karondiensis Roy et al. from the Cretaceous-Paleogene (K-Pg) boundary of Madhya Pradesh, central India (Srivastava et al., 2014; Roy et al., 2021). However, both these earlier reported Deccan species have no hastula between petiole and costa, which is clearly seen in our specimen. Our specimen also differs from them in having a greater number of plicate leaf segments. In addition, the arrangement and angle of divergence of the leaf segments is also different. Thus, by comparing the present fossil leaf specimen with the previously published reliable fossil species of coryphoid palms, we conclude that our fossil specimen differs from them (Table S2; Fig. 8, Fig. 9) and is, therefore, identified as a new species known as S. umariaensis Kumar, Hazra T et Khan, sp. nov. This conclusion is also supported by the cluster analysis (Fig. 10).
Species: S.ghughuaensis Kumar, Hazra T et Khan, sp. nov.
Holotype: SKBU/PPL/Gu/L/1
Additional specimen: SKBU/PPL/Gu/L/2
Repository: Department of Botany, Sidho-Kanho-Birsha University (SKBUH), West Bengal, India.
Type locality: Ghughua mal (location: 23°06′48.60″N, 80°37′22.89″E) in Dindori District, Madhya Pradesh, central India.
Type horizon and age: Deccan Inter-trappean beds; latest Maastrichtian (Late Cretaceous)-earliest Danian (early Paleocene)
Etymology: The specific epithet “ghughuaensis” recognizes Ghughua Mal village, the locality from where the fossil specimens were collected.
Specific diagnosis: Leaf costapalmate, fan-shaped with wide costa; leaf blade with an asymmetrical base; petiole well-developed, wide, unarmed, with pronounced longitudinal striations; hastula absent; leaf segments narrow, plicate, connate, emerging at an acute angle from the costa, basal leaf segments pendulous; plication induplicate; prominent mid-vein present in each leaf segment, parallel secondary lateral veins on either side of mid-vein.
Description: The impression specimens (Fig. 5, Fig. 6a) preserve the basal-middle portion of the fan-shaped leaf blade with costa and petiole, apical portion totally broken; leaf frond costapalmate, preserved part about 7.5–15 cm in length and 5.7–5.9 cm in width; leaf blade asymmetrically attached to the costa; petiole evidently preserved at the base (Fig. 5, Fig. 6a), length 1.3–4.5 cm and breadth 1.5–2.5 cm, spineless, longitudinal striations clearly seen on the petiole of one specimen (Fig. 6a); hastula absent; costa (Fig. 5, Fig. 6a) well-preserved, wide, almost straight, 6.2–10.5 cm in length, 1.8–2.8 cm in width; some faint longitudinal fiber like striations or ribs seen on the surface of costa (Fig. 5a); leaf segments narrow, well-preserved along both sides of the costa in holotype specimen (Fig. 5a) and along one side of the costa in another specimen (Fig. 6a), emerge asymmetrically from the apex of petiole and bilateral sides of the costa at an acute angle, spineless, connate at the base, plicate, basal leaf segments in one specimen strongly pendulous (Fig. 6a, c), the plication induplicate (V-shaped) (Fig. 5, Fig. 6c); 24 leaf segments preserved on both sides of the costa in the holotype specimen (Fig. 5a) and 18 leaf segments on one side of the costa in another specimen (Fig. 6a), segments fused adjacently and attached to the costa by the entire base (Fig. 5, Fig. 6d), angle of divergence approx. 35–45°, leaf segments 2.5–3.5 cm in length and 1.3–1.5 cm wide; due to the missing apex, the orientation of the leaf segments unknown; each leaf segment with a distinct primary vein (0.9 mm thick) (Fig. 5, Fig. 6c, d), secondary veins distinct, running parallel on either side of the primary vein (Fig. 5, Fig. 6c, d), the distance between two secondaries 1–1.3 mm; cross veins not clearly visible between secondary lateral veins.
3.3.2. Comparison with other coryphoid leaf fossil species
Fossil costapalmate palm leaf species, namely, S. longirhachis, S. colaniae, S. tibetensis, S. cf. asymmetricus, S. guangxiensis, T. formosa, L. roundifolia, C. slenderifolia, S. szei, S. robustus and Sabal maiori, possess a longer petiole and leaf segments than our specimen. S. tenuifolius, S. colaniae, S. tibetensis and S. campbellii differ from the present specimen in having hastula between petiole and leaf blade.
The Chinese Oligocene species L. roundifolia has robust spines on the petiole, whereas the Ghughua specimen has an unarmed petiole. Four species of Sabalites, namely, S. dindoriensis, S. asymmetricus S. karondiensis and S. longirhachis, differ in having a longer costa with much longer leaf segments than S. ghughuaensis, and the current species differs from S. changchangensis and S. dawsonii in having a much longer costa. Additionally, longitudinal striations are clearly seen on the surface of the petiole and costa in S. ghughuaensis. Thus, in being different from earlier reported coryphoid fossil leaf remains (Table S2; Fig. 8, Fig. 9), a conclusion that is also supported by our cluster analysis (Fig. 10), the Ghughua specimen is here described under a new specific name, S. ghughuaensis Kumar, Hazra T et Khan, sp. nov.
Species: Sabalites sp. Type 1
Specimen numbers: SKBU/PPL/Um/L/2a (Fig. 7a); SKBU/PPL/Um/L/2b (Fig. 7b)
Locality: Umaria village (23°05′26.41″N, 80°37′35.25″E) in Dindori District, Madhya Pradesh, central India.
Description: Leaf strongly costapalmate, fan-shaped with a long and strong costa; leaf blade symmetrically attached to the costa; hastula absent; costa slightly broad at the base and gradually tapers towards the apex, costa 10.5 cm in length and 0.6–1.1 cm in breadth; leaf segments narrow, plicate, connate, emerging from the bilateral sides of the costa at an acute angle, 8–10 leaf segments preserved on one side and 4–5 on another side of the costa, about 0.9 cm wide, about 9.2 cm in length, angle of divergence of leaf segments approx. 30°–32°; prominent mid-vein in each leaf segment; mid-vein paralleled by faint secondary lateral veins, further venation details not clearly visible.
Species: Sabalites sp. Type 2
Specimen number: SKBU/PPL/Um/L/6 (Fig. 7c)
Locality: Umaria village (23°05′26.41″N, 80°37′35.25″E) in Dindori District, Madhya Pradesh, central India.
Description: Leaf strongly costapalmate, fan-shaped with a long and strong costa; costa slightly broad at the base and gradually tapers towards the apex, costa 10.4 cm in length and 0.5–1.6 cm in breadth, some faint longitudinal striations seen on costa; leaf segments narrow, plicate, connate, emerging symmetrically from the bilateral sides of the costa at an acute angle, 22 leaf segments preserved, about 0.7 cm wide, about 2.5–10 cm in length, angle of divergence of leaf segments approx. 35°–40°; prominent mid-vein seen in some leaf segment; mid-vein paralleled by faint secondary lateral veins, cross veins not clearly visible.
Species: Sabalites sp. Type 3
Specimen number: SKBU/PPL/Um/L/3 (Fig. 7d)
Locality: Umaria village (23°05′26.41″N, 80°37′35.25″E) in Dindori District, Madhya Pradesh, central India.
Description: Leaf costapalmate; costa strong and wide, 12 cm in length and 0.9–1.5 cm in breadth, longitudinal striations clearly seen on the surface of the costa; leaf segments connate, emerging from the costa at an acute angle, 7 leaflets preserved on one side of the costa, about 0.9 cm wide, about 5.2 cm in length, angle of divergence of leaf segments approx. 30°–32°; mid-vein preserved but other venation details of leaf segments not clearly preserved.
Species: Sabalites sp. Type 4
Specimen number: SKBU/PPL/Um/L/5 (Fig. 7e, f)
Locality: Umaria village (23°05′26.41″N, 80°37′35.25″E) in Dindori District, Madhya Pradesh, central India.
Description: Leaf strongly costapalmate, fan-shaped with a prominent costa; costa slightly broad at the base and gradually tapers towards the apex, costa 7.2 cm in length and 0.3–0.9 cm in breadth; leaf segments narrow, plicate, connate, emerging from the one side of the costa at an acute angle, 12 leaf segments preserved on one side and 7 on another side of the costa, about 0.7 cm wide, about 9.1 cm in length, angle of divergence of leaf segments approx. 18°–22°; prominent mid-vein in each leaf segment; mid-vein paralleled by faint secondary lateral veins, some faint cross veins also seen.
Species: Sabalites sp. Type 5
Specimen number: SKBUH/PPL/K/L/2 (Fig. 7g)
Locality: Karondi village (N23°12.274′, E80° 33.105′) in Jabalpur District, Madhya Pradesh, central India
Description: Leaf costapalmate; costa prominent, slightly broad at the base and gradually tapers towards the apex, costa 8.5 cm in length and 2.1 cm in breadth; leaf segments narrow, connate, emerging from the costa at an acute angle, 4 leaf segments clearly preserved on one side of the costa, about 1.9 cm wide, about 10.2 cm in length, angle of divergence of leaf segments approx. 30°–35°; prominent mid-vein in each leaf segment; mid-vein paralleled by faint secondary lateral veins.
4. Discussion
4.1. Biogeographical implications
4.1.1. The fossil history of coryphoid palms in Asia
The fossil record of Arecaceae has been extensively reviewed elsewhere by Harley (2006) and Dransfield et al. (2008), and among all the sub-families of Arecaceae, the sub-family Coryphoideae has by far the richest fossil record. However, the megafossil records of Coryphoideae in Asia have not yet been discussed specifically and so are presented here for the first time (Table S1). Megafossil records of coryphoid palms are geographically widespread in Asia, specifically in India and mainland SE Asia, and comprise a wide range of organs, namely, leaves, cuticles, leaf bases, leaf axes, stems, roots, fruits, peduncles, and inflorescences. For some of these organs records are rare, while for others, such as leaves and stems, records are abundant. Earlier fossil records show that coryphoid palms flourished at the K-Pg boundary (late Cretaceous-earliest Paleocene) in India and reached a peak of geographic distribution in the later Paleogene (Eocene and Oligocene) of China.
Leaves: Many coryphoid palm leaf fossils representing multiple species, such as Sabalites sp., S. cf. taishuensis, S. guangxiensis, S. colaniae, S, chinensis, S. asymmetricus, S. robustus, S. tenuifolius, S. szei, S. changchangensis, T. formosa, C. slenderifolia, L. roundifolia and Livistona sp., have been recovered from the Paleogene sediments of mainland SE Asia (Endo, 1934; Guo, 1965; Zhou et al., 2013; Wang et al., 2015, 2016; Song et al., 2021). In SE Asia, they are reported from the Oligocene of China (Wang et al., 2015, 2016) and Northern Vietnam (Song et al., 2021) and the Eocene of China (Endo, 1934; Guo, 1965; Zhou et al., 2013). Coryphoid palm leaf fossil specimens described as S. tibetensis are also recorded from early late Eocene sediments of the Lunpola Basin, central Tibetan Plateau (Su et al., 2019) and Livistona tibetica has been reported from the Eocene sediments of Xizang, China (Tao, 1988). It is interesting to note that most of them (S. guangxiensis, S. cf. asymmetricus, S. asymmetricus, S. robustus, S. tenuifolius, S. szei, S. changchangensis, T. formosa, C. slenderifolia, L. roundifolia and Livistona sp.) were described on the basis of both morphological and cuticular characteristics (nature of stomata, epidermal cells, trichome bases) (Zhou et al., 2013; Wang et al., 2015, 2016).
Chinese palm fossils are very rare in Neogene sediments, with only one coryphoid leaf species being reported from the Miocene sediments of Guangdong (Guo, 1965). Their limited distribution may be the result of taphonomic biases and/or fewer well-studied Neogene fossil localities in southern China compared to those of the Paleogene.
In India, fossil records of coryphoid leaf remains, namely, Sabalites sp., S. dindoriensis, S. karondiensis, S. microphylla, Livistona wadiai, Trachycarpus ladakhensis, Palmophyllum mohgaonense and Sabalophyllum livistonoides, are found from the Late Cretaceous through to the Miocene (Sahni, 1964; Mahabale, 1966; Lakhanpal et al., 1983, 1984; Bonde, 1986; Mathur et al., 1996; Srivastava et al., 2014; Roy et al., 2021). Besides leaf remains, other megafossil organs, namely sheathing leaf bases, petioles and leaf axes, have been reported from the Deccan Inter-trappean beds of India (Kulkarni and Patil, 1977; Shete and Kulkarni, 1980; Bonde et al., 2000). A permineralized sheathing leaf base described as Phoenicicaulon mahabalei, having affinity to the leaf base of extant members of the tribe Phoeniceae, was documented from the Deccan Intertrappean beds at Umaria, Madya Pradesh, India (Bonde et al., 2000). Shete and Kulkarni (1980) observed a fossil petiole described as Palmocaulon hyphaeneoides in the Deccan Inter-trappean Beds of Wardha District, Maharashtra, India. They observed that its anatomy strongly resembled a petiole of the modern coryphoid palm taxon Hyphaene. A petrified palm leaf axis described as Palmocaulon costapalmatum was also reported from the same locality (Kulkarni and Patil, 1977).
Stems: Fossil palm stems are usually included in the single genus Palmoxylon. Fossil coryphoid palm stem specimens assigned to different species of Palmoxylon have been found only in India (Mahabale, 1958; Sahni, 1964; Lakhanpal et al., 1979; Prakash and Ambwani, 1980; Ambwani and Mehrotra, 1989; Gayakwad and Patil, 1989; Bonde et al., 2008; Roy, 2013; Khan et al., 2020a). The significant anatomical attributes of the early reported Indian fossil species revealed their resemblance with Coryphoideae by having Cocos to Corphy-type general stem organization, the presence of well-preserved fibrovascular bundles with the reniform type of dorsal fibrous sclerenchymatous part, generally two metaxylem vessel elements in each fibrovascular bundle, compact ground parenchyma tissue, and the absence of centrifugal differentiation of the fibrous part of the fibrovascular bundle. Sahni (1964) and Roy (2013) documented silicified fossil wood Palmoxylon coronatum resembling Borassus from the late Tertiary (Miocene) of West Bengal. In the latest Maastrichtian to early Danian (earliest Paleocene) sediments (c. 66–64 Ma) of the Mandla Lobe of the Deccan Inter-trappean Beds of Madhya Pradesh, central India, fossil stem species, such as Palmoxylon sp., P. licualaense, P. mandlaensis, Palmoxylon shahpuraensis, P. taroides and P. dindoriensis, have been reported (Mahabale, 1958; Lakhanpal et al., 1979; Ambwani and Mehrotra, 1989; Gayakwad and Patil, 1989; Bonde et al., 2008; Khan et al., 2020a). A permineralized stem described as Palmoxylon livistonoides, having affinity with the modern stem of Livistona, was documented from the Deccan Inter-trappean beds of Nawargaon village in Wardha District, Maharashtra (Prakash and Ambwani, 1980).
Inflorescence axis: A fossil palm inflorescence axis or peduncle described as Palmostroboxylon indicum, and resembling that of modern Phoenix, has been reported from the Deccan Inter-trappean Beds of Dongargaon District Chandrapur, Maharashtra, India (Biradar and Bonde, 1979).
Fruits: Until recently there have been only three reports of fossil fruits of coryphoid palms from Asia, and all are from the Cretaceous-Paleocene sediments of India (Bande et al., 1982; Shete and Kulkarni, 1985; Patil et al., 2016). The fossil fruit Hyphaeneocarpon indicum from the latest Maastrichtian to early Danian (earliest Paleocene) sediments of the Deccan Inter-trappean Beds of Madhya Pradesh exhibits an affinity to the extant Hyphaene alliance (Bande et al., 1982). Patil et al. (2016) found a fruit described as Palmocarpon patanii from the Deccan Inter-trappean Beds of Maharashtra that has an affinity with the modern fruits of the coryphoid palm taxon Livistona. Shete and Kulkarni (1985) also found a coryphoid palm fossil fruit known as Palmocarpon coryphoidium at this locality.
Roots: So far only two borassoid fossil palm roots have been reported from the Deccan Inter-trappean Beds of Wardha District, Maharashtra, India (Ambwani, 1981, Awasthi et al., 1996a, b).
4.1.2. Possible migration routes of coryphoid palms from India to mainland SE Asia
Coryphoid palms originated in the Northern Hemisphere in the Late Cretaceous and diversified in boreotropical regions through the Cenozoic (Bjorholm et al., 2006; Baker and Couvreur, 2013a, b). They have a long fossil history in Asia, especially in India and mainland SE Asia (Table S1). However, scenarios for their biogeographical history, diversification and migratory routes in an Asian perspective through geological time remain poorly understood. Our fossil evidence of coryphoid palm leaf remains from the Deccan Inter-trappean beds of India provides important information regarding the paleobiogeography and palaeodiversity of Coryphoideae in Asia, and based on megafossil remains, we also trace Asian palm migration pathways.
The fossil findings presented and reviewed here demonstrate a Gondwanan presence of Coryphoideae. Previous fossil evidence of Coryphoid palms in Asia suggested that they had a broad distribution across India and mainland SE Asia during the late Mesozoic and Cenozoic (ranging from the Late Cretaceous to Miocene) (Table S1; Fig. 11). Until now, the oldest unequivocal fossils of Asian Coryphoideae are from the latest Maastrichtian (Late Cretaceous) to early Danian (earliest Paleocene) sediments (c. 66–64 Ma) of the Deccan Inter-trappean Beds of India (Table S1). Members of the Coryphoideae exhibit significant diversity with different fossil species bearing leaves, petioles, leaf axes, leaf bases, stems, roots, and peduncles; a diversity of form is seen in the Deccan Inter-trappean Beds of India since Late Cretaceous- earliest Paleocene time. This may imply that the tropical zone of Deccan Inter-trappean Beds was one of the centers for diversification of coryphoid palms. However, a large number of post K-Pg Coryphoideae palm leaf fossils have been reported mainly from China (Table S1), showing that in the late Paleogene (Eocene and Oligocene) Coryphoideae underwent a marked increase in geographic distribution, species diversity, and abundance, becoming an important group in the Palaeogene forests of China (Endo, 1934; Guo, 1965; Tao, 1988; Zhou et al., 2013; Wang et al., 2015, 2016). However, besides India, we do not have any convincing megafossil records of Coryphoideae from the Cretaceous or Paleocene sediments of other regions of Asia, especially mainland SE Asia. The only exception is that Ôyama and Matsuo (1964) noted a very small (4.2 cm wide and 8.6 cm long), incompletely preserved palm leaf fragment from the Upper Cretaceous (?) sediments of Japan, eastern Asia, and this identification may be insecure given the limited nature of the material; thus, the taxonomic position of this specimen is not clear. In addition, the Japanese specimen has only a long, triangular ligule and a wide rachis (?)/petiole (Please see Fig. 3, Fig. 4 pp. 244–245 in Ôyama and Matsuo, 1964) but lacks a definite costa, the most important morphological feature for the identification of costapalmate or coryphoid palm leaf. Sung et al. (1976) studied a fossil palynomorph Sabalpollenites areolatus recovered from Late Cretaceous-Early Palaeogene sedimentary deposits of Yunnan, China, and compared fossil pollen with the modern pollen of Sabal; but precise identification at a specific level was not definitive. Moreover, because pollen can be transported over long distances (Rousseau et al., 2008), and reworked from older to younger sediments prior to a final deposition without showing clear signs of abrasion or damage (Mander and Punyasena, 2014), megafossil evidence is more reliable than of pollen grains, especially in the case of palms.
Fig. 11.
Map showing the distribution of extinct Coryphoideae in Asia and its possible migratory routes.
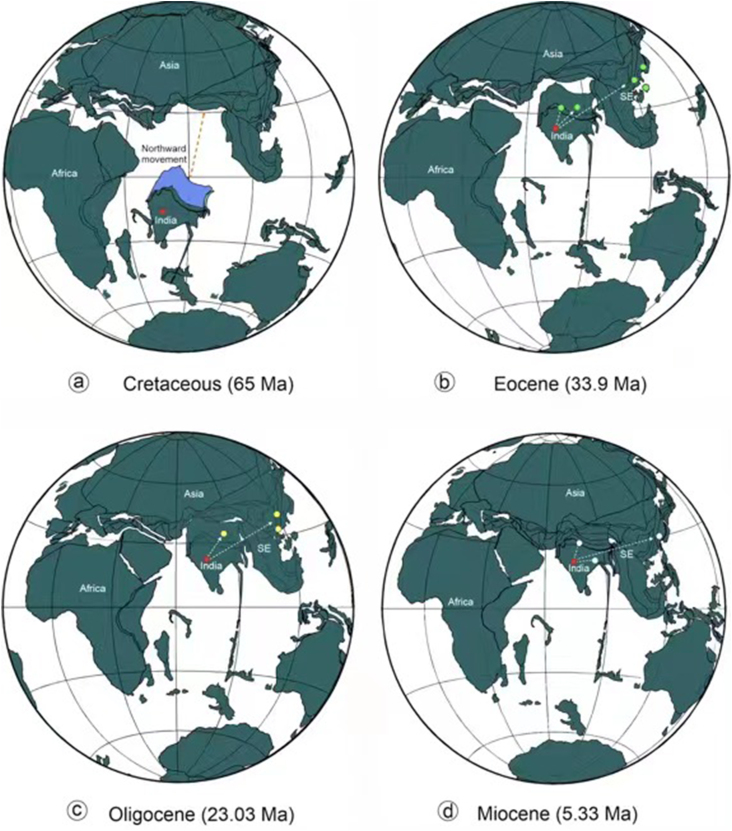
Our new findings as well as a previous megafossil-based investigation (Srivastava et al., 2014; Khan et al., 2020a; Roy et al., 2021) strongly suggest the presence of Coryphoideae on the Gondwana fragment that is now India, providing support for the “Out-of-India” hypothesis (Fig. 11, Fig. 12). This hypothesis holds that some Asian biotic elements had an ancient Gondwanan origin and reached Asia sometime close to 55 ± 10 Ma (Wang et al., 2014) by “rafting” on the Indian subcontinent after the breakup of Gondwana (Briggs, 2003). The geological history of India, including its split from other parts of Gondwana, its northward journey, and eventual collision with Eurasia, has been well documented (Briggs, 2003; Ali and Aitchison, 2005; Bouilhol et al., 2013).
Fig. 12.
Plate tectonic configuration from Cretaceous to Neogene and the possible dispersal routes of coryphoid palms. (a. Late Cretaceous (65 Ma); (b. Eocene (33.9 Ma); (c. Oligocene (23.03 Ma); and (d. Miocene (5.33 Ma). Blue area shows the possible extent of the subducted Indian plate.
The collision between India and Eurasia provided opportunities for exchanges of Cenozoic flora and fauna (Karanth, 2006). Many biotic elements from the Indian subcontinent therefore presumably spilled into Asia and differentiated in the Cenozoic mountains and valleys of southern China, Xizang, where for much of the Paleogene it was a lowland (<2 km) valley (Su et al., 2019), Indo-China, Thailand, and the Malayan peninsula after the collision. Some studies have also suggested that biotic exchange between India and Asia may not have required direct contact between the two landmasses (Chatterjee and Scotese, 2010). Instead, it may have occurred via African-Arabian connections between India and Eurasia (Greater Somalia or the Oman-Kohistan-Dras Island Arc) towards the end of the Cretaceous. This hypothesis is also consistent with the presence of Coryphoideae megafossils in Africa (Pan et al., 2006). Nevertheless, these considerations also support the “Out-of-India” hypothesis presented here that Coryphoid palms entered mainland masses of SE Asia (Vietnam) and other parts of Asia (Xizang, China) directly, or indirectly, from the Indian subcontinent (Fig. 11, Fig. 12). Previously, Srivastava et al. (2014) discussed the possible migratory path of the Coryphoideae from the Northern Hemisphere to the Indian subcontinent in a global perspective and suggested that this palm subfamily may have dispersed into India from Europe via Africa during the latest Cretaceous, before the Indian Plate collided with the Eurasian Plate. However, this route does not explain why SE Asian occurrences seem to post-date those in India and why Coryphoid palms did not move eastwards from Europe in the Late Cretaceous and early Paleogene, particularly as a high Tibetan plateau did not exist at that time (Spicer et al., 2020) and migration would have been possible both along the lower southern slopes of the Gangdese Mountains that pre-dated the rise of the Himalaya and through a wide east-west trending migratory corridor in central Tibet (Spicer et al., 2020). It may be that yet-to-be-discovered fossils will show palms were present in SE Asia prior to the K-Pg, but at present, it seems India provided the most likely coryphoid route to that region.
Our materials, together with the previously reported coryphoid palms from the same locality (Table S1), indicate that costapalmate palms were one of the major components of the Madhya Pradesh K-Pg flora and exhibited a rich diversity. However, few coryphoid palms occur in the present-day tropical dry deciduous forests of Madhya Pradesh. In Madhya Pradesh, Coryphoideae is represented by only five genera (Borassus, Livistona, Caryota, Trachycarpus and Phoenix) with six species (B. flabellifer, Chuniophoenix urens, Livistona chinensis, P. sylvestris, P. dactylifera and Trachycarpus fortune) (Singh et al., 2001; Singh, 2014). Here, we assume that the narrow distribution of coryphoid palms today is likely due to climate, ecology, and vegetation change since the Late Cretaceous (Prasad et al., 2013; Bhatia et al., 2021).
4.2. Implications for past climate
Fossil palms, which can be very good indicators of past climatic conditions (Reichgelt et al., 2018), today have a near pantropical distribution (Harley, 2006). Palms are iconic plant fossils, providing evidence of warm climates in the geological past in different geographical areas (Wing and Greenwood, 1993; Greenwood and Wing, 1995; Archibald et al., 2014; Ding et al., 2017; Reichgelt et al., 2018; Su et al., 2019; Xiong et al., 2020). Early palm lineage fossils from the Cretaceous were most likely growing in tropical conditions (Manchester et al., 2010) and palms are interpreted to have diversified and spread out from tropical environments during the late Paleogene and Neogene (Svenning et al., 2008; Couvreur et al., 2011). The core distribution of modern palms is predominantly tropical (Walther et al., 2007; Reichgelt et al., 2018). Generally, high palm diversity is usually supposed to reflect both warm temperatures and a humid climate (Dransfield et al., 2008; Zhou et al., 2013). The highest diversity and abundance of palms are found in tropical regions where the mean annual temperature is 18–28 °C, the coldest month mean temperature ≥18 °C, and the mean annual range of temperature ≤10 °C (Greenwood and Wing, 1995; Reichgelt et al., 2018). Additionally, palm subfamilies including Coryphoideae, have their centres of distribution and diversification in the tropics (Svenning et al., 2008; Reichgelt et al., 2018). Thus, the discovery of present and previously reported coryphoid palm megafossils of different organs, namely, leaves (Srivastava et al., 2014; Roy et al., 2021), petioles (Trivedi and Verma, 1981), leaf bases (Bonde et al., 2000), stems (Mahabale, 1958; Lakhanpal et al., 1979; Ambwani, 1983; Ambwani and Mehrotra, 1989; Gayakwad and Patil, 1989; Bonde et al., 2008; Khan et al., 2020a) and fruits (Bande et al., 1982) is generally taken to indicate a warm and humid tropical climate prevailed across what is now Madhya Pradesh, central India, during Late Cretaceous-early Paleocene times. This suggested paleoclimatic condition is also supported by earlier published qualitative paleoclimatic reconstruction using nearest living relatives (NLR) analysis (Srivastava, 2010; Prasad et al., 2013; Srivastava and Srivastava, 2014; Manchester et al., 2016; Baas et al., 2017; Kapgate et al., 2017; Khan et al., 2019; Smith et al., 2021) and quantitative paleoclimatic data using Climate Leaf Analysis Multivariate Program (CLAMP) analysis (Bhatia et al., 2021). The CLAMP-derived results based on physiognomic features of woody dicot fossil leaf assemblages of the same fossil locality predict a mean annual temperature of 23.4 °C ± 2.3 °C; a cold month mean temperature of 17.2 °C ± 3.5 °C, a warm month mean temperature of 28.1 °C ± 2.9 °C, a relative humidity of 75.6% ± 10.1% and a growing season precipitation of 2320 mm ± 643 mm during Late Cretaceous-early Paleocene time (Bhatia et al., 2021).
5. Conclusions
The seven species of coryphoid palms, including two new species, recovered from the Deccan Inter-trappean Beds of Madhya Pradesh, central India provide important information for exploring the evolutionary history of coryphoid palms in the tropics and sub-tropics, and also provide solid evidence for the reconstruction of the paleoclimate of the Deccan region in latest Cretaceous to earliest Paleocene time. The fossil evidence of Coryphoideae presented and reviewed here suggests that members of the Coryphoideae were present in Gondwana in the Late Cretaceous and were “ferried” to Asia on the “raft” of India. Subsequently, they began a migration to mainland SE Asia around the time of the suturing of India and Eurasia 55 ± 10 Ma, possibly very soon after the K–Pg transition. A better understanding of the coryphoid palm biogeography in Asia requires further well-dated fossil discoveries.
Authors' contribution
All authors contributed to the study conception and design. Materials preparation, data collection, and analysis were performed by SK, TH, MH and MAK. The first draft of the manuscript was written by SK, MAK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
Financial support from the Department of Science and Technology (DST), New Delhi (Ref. no. DST/INSPIRE/03/2019/001456) is gratefully acknowledged. This work was supported by an INSPIRE fellowship awarded to S.K. by The Department of Science and Technology, New Delhi, INSPIRE Code (IF190496) S. K., T. H., M. H. and M. K. gratefully acknowledge the Department of Botany, Sidho-Kanho-Birsha University for providing infrastructural facilities to accomplish this work. RAS and TEVS were supported by NERC/NSFC BETR Project NE/P013805/1. SB acknowledges the Centre of Advanced Study (Phase-VII), Department of Botany, the University of Calcutta for providing necessary facilities.
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.01.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Earlier reported fossil coryphoid palms from Asia.
Comparison of the fossil species of coryphoid palms based on leaf morphological characters.
Morphological data matrix for character states of the present two fossil species and the other reported fossil species of coryphoid palms.
References
- Ali J.R., Aitchison J.C. Greater India. Earth-Sci. Rev. 2005;72:169–188. [Google Scholar]
- Ambwani K. Borassoid fossil palm root from the Deccan Intertrappean beds of Nawargaon in Wardha District, Maharashtra. Geophytology. 1981;11:13–15. [Google Scholar]
- Ambwani K. Palmoxylon shahpuraensis sp. nov., a fossil palm resembling Licuala from the Deccan Intertrappean beds of Mandla District, Madhya Pradesh. Palaeobotanist. 1983;31:52–59. [Google Scholar]
- Ambwani K., Mehrotra R.C. A new fossil palm wood from the Deccan Intertrappean bed of Shahpura, Mandla District, Madhya Pradesh. Geophytology. 1989;19:70–75. [Google Scholar]
- Archibald S.B., Morse G.E., Greenwood D.R., et al. Fossil palm beetles refine upland winter temperatures in the Early Eocene Climatic Optimum. Proc. Natl. Acad. Sci. U.S.A. 2014;111:8095–8100. doi: 10.1073/pnas.1323269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen C.B., Baker W.J., Dransfield J. In: Monocots: Systematics and Evolution. Melbourne (Australia) Wilson K.L., Morrison D.A., editors. CSIRO; 2000. Phylogeny of the palm family (Arecaceae) based on rps16 intron and trnL-trnF plastid DNA sequences; pp. 525–535. [Google Scholar]
- Asmussen C.B., Dransfield J., Deickmann, et al. A new subfamily classification of the palm family (Arecaceae): evidence from plastid DNA phylogeny. Bot. J. Linn. Soc. 2006;151:15–38. [Google Scholar]
- Awasthi N., Mehrotra R.C., Khare E.G. Maharashtra with Critical Remarks on Fossil Roots of Eichhornia. 1996. A borassoid palm root from the Deccan Intertrappean beds of Wardha district; pp. 57–61. [Google Scholar]
- Awasthi N., Mehrotra R.C., Srivastava R. Fossil woods from the Deccan Intertrappean beds of Madhya Pradesh. Geophytology. 1996;25:113–118. [Google Scholar]
- Baker W.J., Couvreur T.L.P. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. J. Biogeogr. 2013;40:274–285. [Google Scholar]
- Baker W.J., Couvreur T.L.P. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. II. Diversification history and origin of regional assemblages. J. Biogeogr. 2013;40:286–298. [Google Scholar]
- Baker W.J., Dransfield J. Beyond Genera Palmarum: progress and prospects in palm systematics. Bot. J. Linn. Soc. 2016;182:207–233. [Google Scholar]
- Bande M.B., Prakash U., Ambwani K. A fossil palm fruit Hyphaeneocarpon indicum gen. et sp. nov. from the Deccan Intertrappean Series, India. Palaeobotanist. 1982;30:303–309. [Google Scholar]
- Baas P., Manchester S.R., et al. Fossil wood with dimorphic fibers from the Deccan Intertrappean Beds of India-The oldest fossil Connaraceae? IAWA J. 2017;38:124–133. [Google Scholar]
- Berry E.W. A palm from the mid-Cretaceous. Torreya. 1905;5:30–33. [Google Scholar]
- Berry E.W. Contributions to the Mesozoic flora of the Atlantic coastal plain, VII. Bull. Torrey Bot. Club. 1911;38:399–424. [Google Scholar]
- Berry E.W. The upper cretaceous and Eocene floras of South Carolina and Georgia. US Geol. Sur. Prof. Paper. 1914;84:1–200. [Google Scholar]
- Berry E.W. The fossil plants from Vero, Florida. J. Geol. 1917;25:661–666. [Google Scholar]
- Berry E.W. Fossil plants and Unios in the Red Beds of Wyoming. J. Geol. 1924;32:488–497. [Google Scholar]
- Bhatia H., Khan M.A., Srivastava G., et al. Late Cretaceous–Paleogene Indian monsoon climate vis-à-vis movement of the Indian plate, and the birth of the South Asian Monsoon. Gondwana Res. 2021;93:89–100. [Google Scholar]
- Birader N.V., Bonde S.D. On a fossil palm peduncle from Dongargaon District Chandrapur, Maharashtra, India. Geophytology. 1979;9:132–138. [Google Scholar]
- Bonde S.D. Sabalophyllum livistonoides gen. et sp. nov.: a petrified palm leaf segment from Deccan Intertrappean bed at Nawargaon, District Wardha, Maharashtra, India. Biovigyanam. 1986;12:113–118. [Google Scholar]
- Bonde S.D., Chate S.V., Gamre P.G., et al. A dichotomously branched fossil palm stem from the Deccan Intertrappean beds of India. Curr. Sci. 2008;94:182–183. [Google Scholar]
- Bonde S.D., Kumbhojkar M.S., Aher R.T. Phoenicicaulon mahabalei gen. et sp. nov., a sheathing leaf base of Phoenix from the Deccan Intertrappean beds of India. Geophytology. 2000;29:11–16. [Google Scholar]
- Bjorholm S., Svenning J.C., Baker W.J., et al. Historical legacies in the geographical diversity patterns of New World palm (Arecaceae) subfamilies. Bot. J. Linn. Soc. 2006;151:113–125. [Google Scholar]
- Bouilhol P., Jagoutz O., Hanchar J.M., et al. Dating the India–Eurasia collision through arc magmatic records. Earth Planet. Sci. Lett. 2013;366:63–175. [Google Scholar]
- Briggs J.C. The biogeographic and tectonic history of India. J. Biogeogr. 2003;30:381–388. [Google Scholar]
- Brown R.W. Vol. 375. US Government Printing Office; Washington: 1962. (Paleocene flora of the Rocky Mountains and Great Plains). [Google Scholar]
- Chatterjee S., Scotese C. New aspects of Mesozoic biodiversity. Springer; Berlin, Heidelberg: 2010. The wandering Indian plate and its changing biogeography during the Late Cretaceous-Early Tertiary period; pp. 105–126. [Google Scholar]
- Chenet A.L., Quidelleur X., Fluteau, et al. 40K–40Ar dating of the Main Deccan large igneous province: Further evidence of KTB age and short duration. Earth Planet. Sci. Lett. 2007;263:1–15. [Google Scholar]
- Chenet A.L., Courtillot V., Fluteau F., et al. Determination of rapid Deccan eruptions across the Cretaceous-Tertiary boundary using paleomagnetic secular variation: 2. Constraints from analysis of eight new sections and synthesis for a 3500-m-thick composite section. J. Geophys. Res. 2009;114:B06103. [Google Scholar]
- Couvreur T.L.P., Forest F., Baker W.J. Origin and global diversification patterns of tropical rain forests: inferences from a complete genus-level of palms. BMC Biology. 2011;9:12. doi: 10.1186/1741-7007-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daghlian C.P. Coryphoid palms from the lower and middle Eocene of south-eastern North America. Palaeontogr. Abt. B. 1978;166:44–82. [Google Scholar]
- Ding L., Spicer R.A., Yang J., et al. Quantifying the rise of the Himalaya orogen and implications for the South Asian monsoon. Geology. 2017;45:215–218. [Google Scholar]
- Dransfield J., Uhl N.W., Asmussen C.B., et al. Royal Botanic Gardens; Kew, Kew Publishing: 2008. Genera Palmarum: The Evolution and Classification of Palms. [Google Scholar]
- Endo S. The geological age of the Fushun Group, South Manchuria. Proc. Imperial Acad. Sci. 1934;10:486–489. [Google Scholar]
- Fang X., Dupont-Nivet G., Wang C., et al. Revised chronology of central Tibet uplift (Lunpola Basin) Sci. Adv. 2020;6:eaba7298. doi: 10.1126/sciadv.aba7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayakwad B.B., Patil G. In: Proceedings of the Special Indian Geophytological Conference, Pune 1986. Biradar N.V., editor. University of Poona; Pune: 1989. On two palm woods from the Deccan Inter-trappean beds of Betul District, Madhya Pradesh; pp. 31–38. [Google Scholar]
- Govaerts R., Dransfield J. Kew; 2005. World Checklist of Palms: The Board of Trustees of the Royal Botanic Gardens; pp. 1–223. [Google Scholar]
- Greenwood D.R., Conran J.G. Fossil coryphoid palms from the Eocene of Vancouver, British Columbia. Int. J. Plant Sci. 2020;181:224–240. [Google Scholar]
- Greenwood D.R., West C.K. A fossil coryphoid palm from the Paleocene of western Canada. Rev. Palaeobot. Palynol. 2017;239:55–65. [Google Scholar]
- Greenwood D.R., Wing S.L. Eocene continental climates and latitudinal temperature gradients. Geology. 1995;23:1044–1048. [Google Scholar]
- Guo S.X. On the discovery of fossil palms from the Tertiary Formation of Kwangtung and Kuangsi. Acta. Palaeontol. Sin. 1965;13:598–605. (in Chinese) [Google Scholar]
- Harley M.M. A summary of fossil records for Arecaceae. Bot. J. Linn. Soc. 2006;151:39–67. [Google Scholar]
- Hofmann C., Feraud G., Courtillot V. 40Ar/39Ar dating of mineral separates and whole rocks from the Western Ghats lava pile: further constraints on duration and age of the Deccan traps. Earth Planet. Sci. Lett. 2000;180:13–27. [Google Scholar]
- Kapgate D.K., Manchester S.R., Stuppy W. Oldest fruit of Phyllanthaceae from the Deccan Inter-trappean beds of Singpur, Madhya Pradesh, India. Acta Palaeobot. 2017;57:33–38. [Google Scholar]
- Karanth P.K. Out-of-India Gondwanan origin of some tropical Asian biota. Curr. Sci. 2006;90:789–792. [Google Scholar]
- Keller G., Adatte T., Bajpai S., et al. K–T transition in Deccan Traps of central India marks major marine Seaway across India. Earth Planet. Sci. Lett. 2009;282:10–23. [Google Scholar]
- Khan M.A., Mandal K., Bera S. A new species of permineralized palm stem from the Maastrichtian–Danian sediments of Central India and its palaeoclimatic signal. Bot. Lett. 2019;166:189–206. [Google Scholar]
- Khan M.A., Roy K., Hazra T., et al. A new coryphoid palm from the Maastrichtian-Danian Sediments of Madhya Pradesh and its Palaeoenvironmental Implications. J. Geol. Soc. India. 2020;95:75–83. [Google Scholar]
- Khan M.A., Hazra M., Mahato S., et al. A Cretaceous Gondwana origin of the wax palm subfamily (Ceroxyloideae: Arecaceae) and its paleobiogeographic context. Rev. Palaeobot. Palynol. 2020;283:104318. [Google Scholar]
- Khosla S.C. Costabuntonia, a new genus of Ostracoda from the Inter-trappean beds (Paleocene) of east coast of India. Micropaleontology. 1999;45:319–324. [Google Scholar]
- Khosla A., Verma O. Paleobiota from the Deccan volcano-sedimentary sequences of India: paleoenvironments, age and paleobiogeographic implications. Hist. Biol. 2015;27:898–914. [Google Scholar]
- Knowlton F.H. Fossil floras of the Vermejo and Raton formations of Colorado and New Mexico. US Geol. Surv. Prof. Paper. 1917;101:323–455. [Google Scholar]
- Kulkarni A.R., Patil K.S. Palmocaulon costapalmatum, a petrified palm leaf axis from the Deccan Inter-trappean beds of Wardha District, Maharashtra. Geophytology. 1977;7:208–213. [Google Scholar]
- Kvaček J., Herman A.B. Monocotyledons from the early Campanian (Cretaceous) of Grünbach, lower Austria. Rev. Palaeobot. Palynol. 2004;128:323–353. [Google Scholar]
- Lakhanpal R.N., Prakash U., Ambwani K. Two petrified palm woods from the Deccan Intertrappean beds of Mandla District, Madhya Pradesh. Paleobotanist. 1979;26:119–129. [Google Scholar]
- Lakhanpal R.N., Sah S.C.D., Sharma K.K., et al. Geology of Indus suture zone of Ladakh, Wadia Institute of Himalayan Geology Dehradun. 1983. Occurrence of Livistona in the Hemis conglomerate horizon of Ladakh; pp. 179–185. [Google Scholar]
- Lakhanpal R.N., Thussu J., Guleria J.S. A fossil fan palm from the Liyan Formation of Ladakh (Jammu and Kashmir) Paleobotanist. 1984;31:201–207. [Google Scholar]
- Lesquereux L. Vol. 7. US Government Printing Office; 1878. (Contributions to the Fossil Flora of the Western Territories). [Google Scholar]
- Mahabale T.S. Resolution of the artificial palm genus, Palmoxylon: A new approach. Palaeobotanist. 1958;7:76–84. [Google Scholar]
- Mahabale T.S. Evolutionary trends in the palmae with special reference to fossil palms. Palaeobotanist. 1966;14:214–222. [Google Scholar]
- Manchester S.R., Bonde S.D., Nipunage D.S., et al. Trilocular palm fruits from the Deccan Inter-trappean Beds of India. Int. J. Plant Sci. 2016;177:633–641. [Google Scholar]
- Manchester S.R., Lehman T.M., Wheeler E.A. Fossil palms (Arecaceae, Coryphoideae) associated with juvenile herbivorous dinosaurs in the upper Cretaceous Aguja formation, Big Bend National Park, Texas. Int. J. Plant Sci. 2010;171:679–689. [Google Scholar]
- Mander L., Punyasena S.W. On the taxonomic resolution of pollen and spore records of Earth's vegetation. Int. J. Plant Sci. 2014;175:931–945. [Google Scholar]
- Marmi J., Gomez B., Martín-Closas C., et al. A reconstruction of the fossil palm Sabalites longirhachis (Unger) J. Kvaček et Herman from the Maastrichtian of Pyrenees. Rev. Palaeobot. Palynol. 2010;163:73–83. [Google Scholar]
- Mathur A.K., Mishra V.P., Mehra S. Significance of tephra at Dagshai kasauli and lower upper Dharamsala formational contacts, Himachal Pradesh. Visesa Prakasana-Bharatiya Bhuvaijñanika Sarveksana. 1996;21:29–32. [Google Scholar]
- Matsunaga K.K., Manchester S.R., Srivastava R., et al. Fossil palm fruits from India indicate a Cretaceous origin of Arecaceae tribe Borasseae. Bot. J. Linn. Soc. 2019;190:260–280. [Google Scholar]
- Matsunaga K.K., Smith S.Y. Fossil palm reading: using fruits to reveal the deep roots of palm diversity. Am. J. Bot. 2021;108:472–494. doi: 10.1002/ajb2.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe G.E., Gannaway W.L. Palm fossils from northwest Washington. Washington Geol. 1995;23:21–26. [Google Scholar]
- Nair K.K.K., Bhusari B. Stratigraphy of Deccan Traps: A Review. Geol. Surv. India Spec. Publ. 2001;64:477–492. [Google Scholar]
- Newberry J.S. Vol. 35. US Government Printing Office; 1898. (The later extinct floras of North America). [Google Scholar]
- Ôyama T., Matsuo H. Notes on Palmaean leaf from the Ôarai flora (Upper Cretaceous), Oarai Machi, Ibaraki Prefecture, Japan. Trans. Proc. Palaeont. Soc. Japan N. S. 1964;55:241–246. [Google Scholar]
- Pan A.D., Jacobs B.F., Dransfield J., et al. The fossil history of palms (Arecaeae) in Africa and new records from the Late Oligocene (28–27 Mya) of north-western Ethiopia. Bot. J. Linn. Soc. 2006;151:69–81. [Google Scholar]
- Pathak V., Patil S.K., Shrivastava J.P. Tectonomagmatic setting of lava packages in the Mandla lobe of the eastern Deccan volcanic province, India: palaeomagnetism and magnetostratigraphic evidence. Geol. Soc. 2017;445:69–94. London, Special Publications. [Google Scholar]
- Patil S.P., Zilpe S.K., Kapgate D.K. Report of a palm fruit from the Deccan Inter-trappean series of Maraipatan, Chandrapur District (ms) Int. J. Res. Biosci. Agri. Technol. 2016:166–170. Special issue. [Google Scholar]
- Prakash U., Ambwani K. A petrified Livistona-like palm stem, Palmoxylon livistonoides sp. nov. from the Deccan Inter-trappean beds of India. Palaeobotanist. 1980;26:297–306. [Google Scholar]
- Prasad M., Khare E.G., Singh S.K. Plant fossils from the Deccan Inter-trappean sediments of Chhindwara district, Madhya Pradesh, India: their palaeoclimatic significance. J. Palaeontol. Soc. India. 2013;58:229–240. [Google Scholar]
- Prebble M., Dowe J.L. The late Quaternary decline and extinction of palms on oceanic Pacific islands. Quat. Sci. Rev. 2008;27:2546–2567. [Google Scholar]
- Read R.W., Hickey L.J. A revised classification of fossil palm and palm-like leaves. Taxon. 1972;21:129–137. [Google Scholar]
- Reichgelt T., West C.K., Greenwood D.R. The relation between global palm distribution and climate. Sci. Rep. 2018;8:4721. doi: 10.1038/s41598-018-23147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne P.R., Sprain C.J., Richards M.A., et al. State shift in Deccan volcanism at the Cretaceous-Paleogene boundary, possibly induced by impact. Science. 2015;350:76–78. doi: 10.1126/science.aac7549. [DOI] [PubMed] [Google Scholar]
- Rousseau D.D., Schevin P., Ferrier J., et al. Long-distance pollen transport from North America to Greenland in spring. J. Geophys. Res. 2008;113:G02013. [Google Scholar]
- Roy K., Hazra T., Hazra M., et al. A new coryphoid costapalmate palm leaf from the Maastrichtian-Danian of India. Bot. Lett. 2021;168:155–166. [Google Scholar]
- Roy S. On the occurrence of Palmoxylon coronatum Sahni resembling Borassus Linn. from the Tertiary of West Bengal, India. Ameghiniana. 2013;17:130–134. [Google Scholar]
- Sahni B. BSIP; Lucknow: 1964. Revision of Indian fossil plants III, Monocotyledons; pp. 1–89. Monograph No. I. [Google Scholar]
- Saporta G. Études sur la vegetation du sud-est de la France a lépoque tertiare. Ann. Sci. Nat. Bot. 1865;5:81–84. [Google Scholar]
- Schoene B., Samperton K.M., Eddy M.P., et al. U-Pb geochronology of the Deccan Traps and relation to the end-Cretaceous mass extinction. Science. 2015;347:182–184. doi: 10.1126/science.aaa0118. [DOI] [PubMed] [Google Scholar]
- Schoene B., Eddy M.P., Samperton K.M., et al. U-Pb constraints on pulsed eruption of the Deccan Traps across the end-Cretaceous mass extinction. Science. 2019;363:862–866. doi: 10.1126/science.aau2422. [DOI] [PubMed] [Google Scholar]
- Shete R.H., Kulkarni A.R. Palmocaulon hyphaeneoides sp. nov. from the Deccan Intertrappean beds of Wardha District, Maharashtra, India. Palaeontogr. Abt. B. 1980;172:117–124. [Google Scholar]
- Shete R.H., Kulkarni A.R. Palmocarpon coryphoidium sp. nov. a coryphoid palm fruit from Deccan Intertrappean beds of Wardha District, Maharashtra. J. Indian Bot. Soc. 1985;64:45–50. [Google Scholar]
- Sheth H.C., Pande K., Bhutani R. 40Ar-39Ar ages of Bombay trachytes: Evidence for a Palaeocene phase of Deccan volcanism. Geophys. Res. Lett. 2001;28:3513–3516. [Google Scholar]
- Singh N.P., Khanna K.K., Mudgal V., et al. III. Botanical Survey of India; Calcutta: 2001. (Flora of Madhya Pradesh). [Google Scholar]
- Singh V.P. Flora of Madhya Pradesh (western part) Sci. Publ. 2014;38:459–462. [Google Scholar]
- Smith S.Y., Manchester S.R., Samant B., et al. Integrating paleobotanical, paleosol, and stratigraphic data to study critical transitions: a case study from the Late Cretaceous–Paleocene of India. J. Palaeontol. Soc. India. 2015;21:137–166. [Google Scholar]
- Smith S.Y., Kapgate D.K., Robinson S., et al. Fossil fruits and seeds of Zingiberales from the Late Cretaceous–Early Cenozoic Deccan Intertrappean Beds of India. Int. J. Plant Sci. 2021;182:91–108. [Google Scholar]
- Song A., Liu J., Liang S.Q., et al. Leaf fossils of Sabalites (Arecaceae) from the Oligocene of northern Vietnam and their paleoclimatic implications. Plant Divers. 2022;44:406–416. doi: 10.1016/j.pld.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprain C.J., Renne P.R., Vanderkluysen L., et al. The eruptive tempo of Deccan volcanism in relation to the Cretaceous-Paleogene boundary. Science. 2019;363:866–870. doi: 10.1126/science.aav1446. [DOI] [PubMed] [Google Scholar]
- Spicer R.A., Su T., Valdes P.J., et al. The topographic evolution of the Tibetan region as revealed by palaeontology. Palaeobiodivers. Palaeoenviron. 2020 doi: 10.1007/s12549-020-00452-1. [DOI] [Google Scholar]
- Srivastava J.P., Duncan R.A., Kashyap M. Post-K/PB younger 40Ar–39Ar ages of the Mandla lavas: Implications for the duration of the Deccan volcanism. Lithos. 2015;224–225:214–224. [Google Scholar]
- Srivastava R., Srivastava G. Fossil fruit of Cocos L. (Arecaceae) from Maastrichtian-Danian sediments of central India and its phytogeographical significance. Acta Palaeobot. 2014;54:67–75. [Google Scholar]
- Srivastava R. Fossil dicotyledonous woods from Deccan Intertrappean sediments of Ghansor, Seoni District, Madhya Pradesh, India. Palaeobotanist. 2010;59:129–138. [Google Scholar]
- Srivastava R., Srivastava G., Dilcher D.L. Coryphoid palm leaf fossils from the Maastrichtian–Danian of Central India with remarks on phytogeography of the Coryphoideae (Arecaceae) PLoS One. 2014;9:e111738. doi: 10.1371/journal.pone.0111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T., Farnsworth A., Spicer R.A., et al. No high Tibetan plateau until the Neogene. Sci. Adv. 2019;5:eaav2189. doi: 10.1126/sciadv.aav2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung T.C., Lee M. Mesozoic and Early Paleogene sporo-pollen assemblages from Yunnan, China. Part II. Early Upper Cretaceous assemblages from Lufeng and Monding and late Upper Cretaceous-Early Paleogene assemblage from Mengla. Yunnan. Sci. Publ. Co. 1976;9-64:2–10. [Google Scholar]
- Svenning J.C., Borchsenius F., Bjorholm S., et al. High tropical net diversification drives the New World latitudinal gradient in palm (Arecaceae) species richness. J. Biogeogr. 2008;35:394–406. [Google Scholar]
- Tao J.R. Collected Papers of the Institute of Geology of the Chinese Academy of Science. 1988. Plant fossils from the Lêpuqu Formation in Lhazê County, Xizang and their palaeoclimatological significances; pp. 223–238. (in Chinese) [Google Scholar]
- Trivedi B.S., Verma C.L. Sabalocaulon intertrappeum gen. et sp. nov. from the Deccan Intertrappean beds of Madhya Pradesh, India. Palaeobotanist. 1981;28–29:329–337. [Google Scholar]
- Uhl N.W., Dransfield J. Allen Press; 1987. Genera Palmarum. A classification of palms based on the work of Harold E. Moore, Jr (No. L–0216) [Google Scholar]
- Van der Burgh J. Some palms in the Miocene of the Lower Rhenish Plain. Rev. Palaeobot. Palynol. 1984;40:359–374. [Google Scholar]
- Venkatesan T.R., Kumar A., Gopalan K., et al. 40Ar–39Ar age of Siberian basaltic volcanism. Chem. Geol. 1997;138:303–310. [Google Scholar]
- Verma O., Khosla A. Developments in the stratigraphy of the Deccan Volcanic Province, peninsular India. Geosciences. 2019;351:461–476. [Google Scholar]
- Walther G.R., Gritti E.S., Berger S., et al. Palms tracking climate change. Global Ecol. Biogeogr. 2007;16:801–809. [Google Scholar]
- Wang C.S., Dai J.G., Zhao X.X. Outward-growth of the Tibetan Plateau during the Cenozoic: a review. Tectonophysics. 2014;621:1–43. [Google Scholar]
- Wang Q.J., Ma F.J., Dong J.L., et al. New costapalmate palm leaves from the Oligocene Ningming Formation of Guangxi, China, and their biogeographic and palaeoclimatic implications. Hist. Biol. 2016;29:594–606. [Google Scholar]
- Wang Q.J., Ma F.J., Dong J.L., et al. Coryphoid palms from the Oligocene of China and their biogeographical implications. C. R. Palevol. 2015;14:263–279. [Google Scholar]
- Wing S.L., Greenwood D.R. Fossils and fossil climate: the case for equable continental interiors in the Eocene. Philos. Trans. R. Soc. London. Ser. B: Biol. Sci. 1993;341:243–252. [Google Scholar]
- Xiong Z., Ding L., Spicer R.A., et al. The early Eocene rise of the Gonjo Basin, SE Tibet: From low desert to high forest. Earth Planet. Sci. Lett. 2020;543:116312. [Google Scholar]
- Zhou W., Liu X., Xu Q., et al. New coryphoid fossil palm leaves (Arecaceae: Coryphoideae) from the Eocene Changchang Basin of Hainan Island. South China. Sci. China-Earth Sci. 2013;56:1493–1501. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Earlier reported fossil coryphoid palms from Asia.
Comparison of the fossil species of coryphoid palms based on leaf morphological characters.
Morphological data matrix for character states of the present two fossil species and the other reported fossil species of coryphoid palms.