Abstract
Fibroblast-activation protein (FAP) is a serine protease classified in the dipeptidyl peptidase 4 (DPP4) family. FAP is predominantly expressed in activated fibroblasts such as the cancer-associated fibroblasts (CAFs). FAP expression in CAFs is associated with tumor progression and poor prognosis in solid cancers. Recently, radiolabeled FAP inhibitors (FAPI) has been developed, which enables positron emission tomography (PET) imaging of FAP. FAPI PET/CT can provide a higher tumor-to-background ratio (TBR) than 18F-fludeoxyglucose PET/CT in various cancers, and thus has attracted substantial attention. As studies on FAPI PET grow in number and size, incidental findings related to non-oncologic conditions have been increasingly reported. FAPI PET uptake has been reported in various conditions such as benign tumors, fibrotic, granulomatosis, scarring/wound, degenerative diseases, and inflammatory diseases.
The knowledge of physiological and non-oncologic causes of FAPI uptake is indispensable for accurate FAPI PET/CT interpretation and can help appropriate management of incidental findings on FAPI PET/CT in patients referred for cancer staging indications. In this review article, we describe for each organ system (Brain, Oral mucosa, Salivary Glands, Thyroid, Lung, Myocardium, Breast, Esophagus, Stomach, Intestine, Liver, Gallbladder, Pancreas, Spleen, Kidney, , Uterus, Bone marrow, Joints, Muscle, Vessels, Lymph nodes), the patterns of physiological FAPI uptake and the main causes of non-oncological uptake reported from the literature with FAPI-02, FAPI-04 and FAPI-46. We also illustrate some examples from our institutional database at UCLA.
Introduction
Fibroblast-activation protein (FAP) is a Type II transmembrane serine protease belonging to the dipeptidyl peptidase 4 (DPP4) family. FAP is predominantly expressed in activated fibroblasts such as the cancer-associated fibroblasts (CAFs) of various types of cancers. FAP expression in CAFs is associated with tumor cell migration, invasion, and angiogenesis, 1,2 thus FAP overexpression is associated to poor prognosis in solid tumors. 3 FAP has become a molecular target of high interest for cancer diagnosis and treatment. The development of radiolabeled FAP inhibitors (FAPI) that binds to FAP with high affinity enabled the positron emission tomography (PET) imaging of FAP. FAPI- /PET CT can potentially identify tumor lesions with a higher tumor-to-background ratio (TBR) than 4 18F-fludeoxyglucose (FDG) PET/CT in a variety of tumor entities, 5–7 which has sparked considerable interest in the oncologic community. 8–12 For cancer staging, FAPI PET/CT can be a promising modality, however false-positive results (i.e. non-oncologic findings) have been reported. 13–18 FAP is expressed not only in the CAFs but also in most of any activated fibroblasts involved in various processes such as wound healing, scar, fibrosis or inflammation. Also, FAP is expressed to some extent in neovasculature cells, endothelial, malignant epithelial, embryologic, and immunologic tissues. 4,19 Thus, FAPI uptake can be seen in non-malignant diseases. 19 Radiologists and nuclear medicine physicians need to be familiar with non-oncologic incidental FAPI PET findings, to avoid erroneous diagnosis.
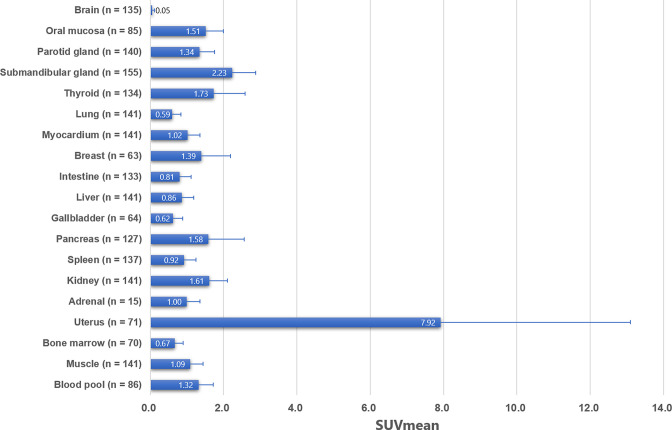
In this review article, we describe for each organ system (Brain, Oral mucosa, Salivary Glands, Thyroid, Lung, Myocardium, Breast, Esophagus, Stomach, Intestine, Liver, Gallbladder, Pancreas, Spleen, Kidney, Uterus, Bone marrow, Joints, Muscle, Vessels, Lymph nodes), the patterns of physiological FAPI uptake and the main causes of non-oncological uptake reported from the literature with FAPI-02, FAPI-04 and FAPI-46. Figure 1 summarize from the literature the pooled mean standardized uptake values (SUVmean) with standard deviation (SD) of FAPI-02, FAPI-04, and FAPI-46 at 60 min after injection in each normal organ. 4,6,16,20,21 Table 1 list the main non-oncological causes of increased FAPI uptake reported in the literature and Figure 2 depicts their SUVs. We also illustrate some examples from our institutional database at UCLA.
Figure 1.
Pooled SUVmean and SD of each organ system. Error bars show SD, SD, standard deviation; SUV, standardized uptake value.
Table 1.
Summary of non-oncologic FAPI uptake in each organ system
| Organ systems | Conditions |
|---|---|
| Brain | Progressive multifocal leukoencephalopathy, tuberculosis meningitis |
| Head and neck | [Thyroid] follicular adenoma, thyroiditis [Salivary gland] IgG4-related sialadenitis [Dental] periodontitis |
| Thorax | [Lung] pneumonia (infectious), organizing pneumonia, interstitial lung disease, tuberculosis, Cryptococcosis, necrotizing granuloma [Cardiac] myocardial ischemia, myocardial infarction, amyloidosis, sarcoidosis, dilated cardiomyopathy, pulmonary hypertension, cardiotoxicity induced by chemotherapy/ immunotherapy [Breast] mastopathy |
| Abdomen and pelvis | [Gastrointestinal] esophagitis, Crohn’s disease, hemorrhoids [Liver] chronic hepatitis, cirrhosis, focal nodular hyperplasia, hepatic adenoma [Pancreas] acute pancreatitis, IgG4-related pancreatitis, pseudocyst [Bile duct] IgG4-related sclerosing cholangitis, portal biliopathy [Spleen] splenic hemangioma [Kidney] renal fibrosis, angiomyolipoma [Uterus] leiomyoma |
| Musculoskeletal | [Bone] degenerative change, Schmorl’s node, bone fracture, fibrous dysplasia, avascular necrosis, bone tuberculosis, mastoiditis, myositis ossificans [Joint] osteoarthritis, rheumatoid arthritis, enthesopathy, arthritis induced by immune-checkpoint inhibitor [Soft tissue] scarring/wound, juvenile polymyositis, hematoma, elastofibroma dorsi |
| Others | [Vascular] unstable atherosclerotic plaque, large-vessel vasculitis [Lymph node] reactive lymph node |
FAPI, fibroblast-activation protein inhibitor.
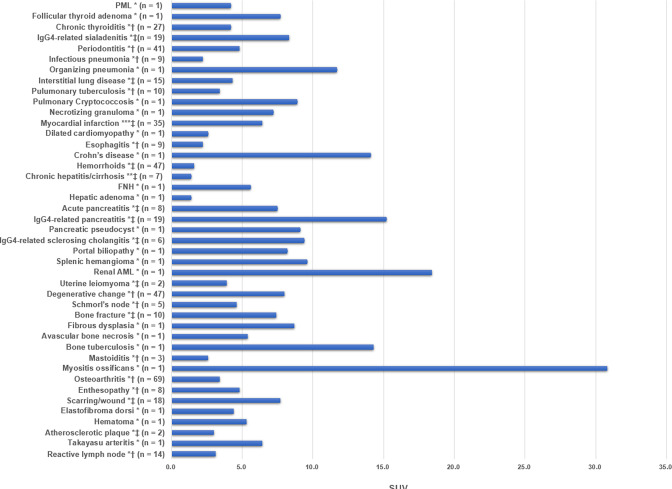
Figure 2.
Reported SUVs of non-oncologic diseases. (* SUVmax, ** SUVmean, *** SUVpeak, † median, ‡ average). SUV, standardized uptake value.
Brain
Brain exhibits very low FAPI uptake (pooled SUVmean: 0.05 ± 0.05), as FAPI does not cross-the blood–brain barrier. 22,23 In primary brain tumors, FAP expression by immunohistochemistry and FAPI uptake (SUVmax: 4.2 ± 2.4, n = 15) in high-grade glioma/glioblastoma has been reported. 23 Also, high FAPI uptake in brain metastasis has been reported in patients with lung cancer (average SUVmax: 9.0 [95%CI: 4.0–14.0], n = 23). 24 In non-oncologic conditions, progressive multifocal leukoencephalopathy (PML) can show multifocal FAPI uptake (SUVmax: 2.2–4.2) in the brain. 25 Also, tuberculosis meningitis accumulates FAPI, which may mimic brain metastasis. 18,26 Thus, they should be included in the differential diagnosis of focal FAPI uptake in the brain.
Head and neck
Thyroid
Mild physiological FAPI uptake is usually observed in the thyroid with a relatively wide range of normal variation (pooled SUVmean: 1.73 ± 0.86). Diffuse thyroid uptake elevation is commonly attributed to chronic thyroiditis. 27,28 Liu et al 28 found diffuse FAPI uptake in the thyroid in 4.8% (n = 39/815) of cancer patients, 28 patients (median SUVmax: 4.2, range 2.8–32) of them were subsequently evaluated with thyroid ultrasound and laboratory tests and 27/28 (96%) patients had a diagnosis of chronic thyroiditis (i.e. Hashimoto’s thyroiditis, Grave’s disease, and immune-related thyroiditis induced by immune-checkpoint inhibitors). In Grave’s disease, FAPI uptake can be seen in the extraocular muscles representing Grave’s ophthalmopathy (SUVmax: 4.2), 29 although physiologic activity can be seen in the extraocular muscles without ophthalmopathy. 16 Marked FAPI uptake has been reported in immune-related thyroiditis (SUVmax: 23.5, Figure 3), in which FDG uptake was moderate (SUVmax: 4.9) in contrast. 30 Cases of lymphoma showing diffuse uptake (SUVmax: 8.6) have been reported. 31
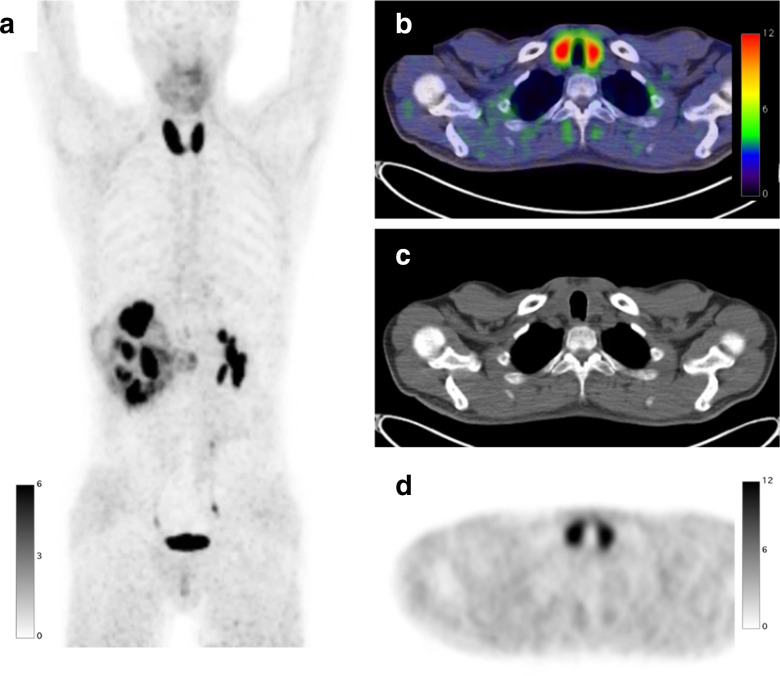
Figure 3.
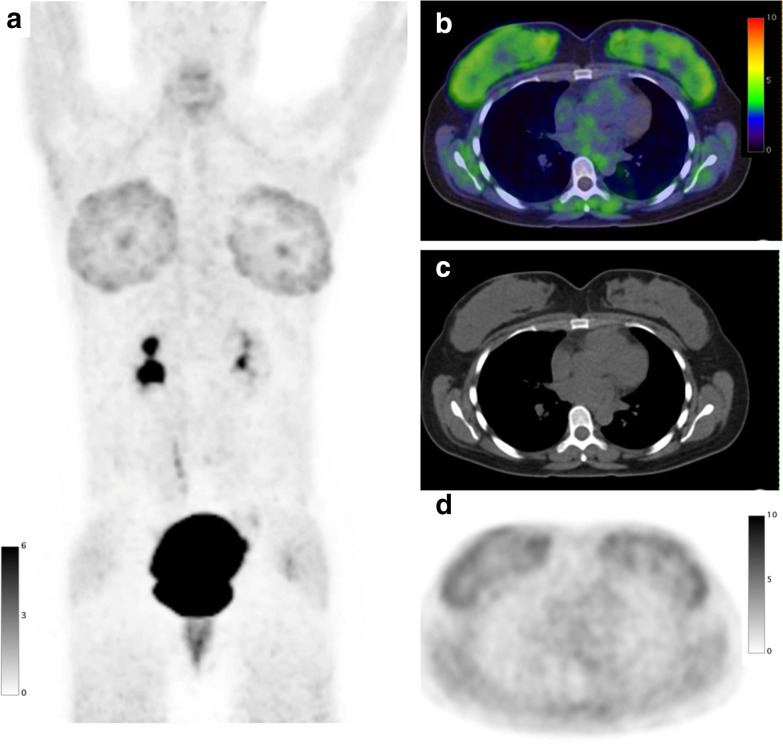
A 45-year-old male with right clear cell renal cell carcinoma treated with neoadjuvant immune checkpoint inhibitors underwent a FAPI PET/CT scan before surgery. FAPI PET MIP image (a) FAPI PET/CT (b), CT (c), and FAPI PET (d) demonstrate diffuse intense increased uptake (SUVmax: 23.5) in the enlarged thyroid indicating thyroiditis induced by immune checkpoint inhibitors. FAPI, fibroblast-activation protein inhibitor; PET, positron emission tomography; SUV, standardized uptake value.
Focal thyroid uptake can represent benign or malignant pathologies. Follicular adenoma, the most common form of benign thyroid neoplasm, can show FAPI uptake. Ou et al reported a case of follicular adenoma showing FAPI uptake (SUVmax: 7.7), potentially due to the fibrous tissue hyperplasia of the tumor. 32 Thyroid cancer is also associated with elevated FAPI uptake which is typically not very intense (SUVmax <6.0). 5,15,17,33–35 Thus, FAPI SUVmax cannot differentiate follicular adenoma from thyroid cancer. As with FDG PET/CT, 36 focal thyroid uptake should be examined by ultrasound sonography with or without fine-needle aspiration.
Salivary glands
Submandibular glands exhibit moderate to high physiological FAPI accumulation (pooled SUVmean: 2.23 ± 0.64). By contrast, parotid glands show low FAPI uptake (pooled SUVmean: 1.34 ± 0.42). In IgG4-related disease, FAPI uptake is commonly seen in the salivary glands (submandibular glands > parotid glands; average SUVmax 8.3 ± 3.9, n = 19) representing IgG4-related sialadenitis. 13,37
Dental
Oral mucosa exhibits mild physiological uptake (pooled SUVmean: 1.51 ± 0.49). Focal uptake in/around the teeth is one of the most common incidental FAPI PET/CT findings and can represent periodontitis (Figure 4). 15,16 Zheng et al reported periodontitis (median SUVmax 4.8 [range: 2.4–11.2]) in 11.3% of the benign uptake depicted on FAPI PET/CT in their cohort (n = 41/360). 15 Qin et al reported that focal dental uptake was more commonly seen in FAPI PET than in FDG PET and showed higher SUVmax (average SUVmax: FAPI 3.7 ± 0.9, FDG 2.8 ± 0.3, n = 33). 38
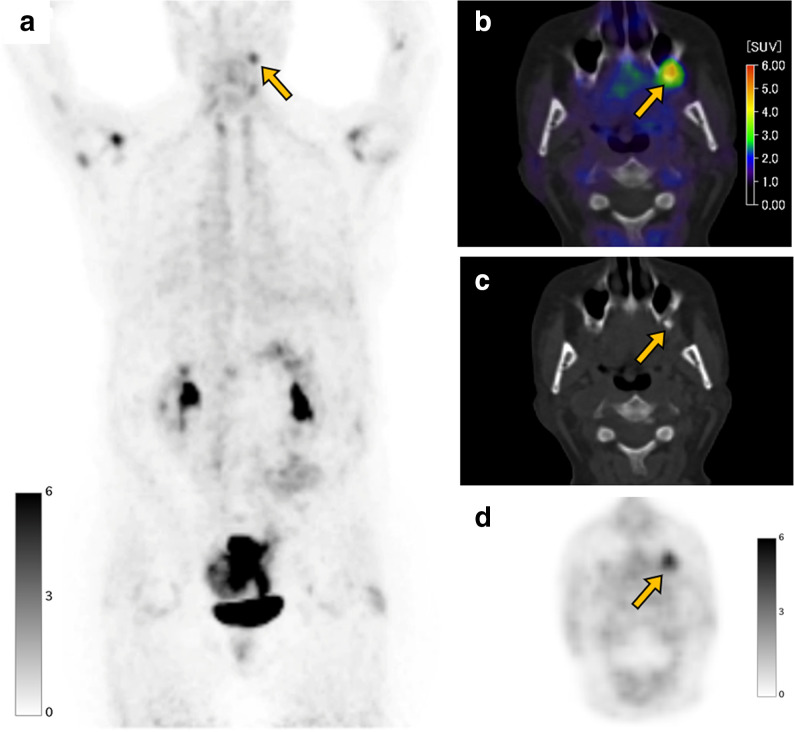
Figure 4.
A 56-year-old female with colon cancer underwent a FAPI PET/CT scan before surgery. FAPI PET MIP image (a) FAPI PET/CT (b), CT (c), and FAPI PET (d) demonstrate focal uptake (SUVmax: 5.0, arrow) in the tooth root in the maxillary bone representing periodontitis. Note that FAPI uptake is seen in the multiple joints such as shoulders (SUVmax: 6.3) and hips (SUVmax: 2.4), indicating osteoarthritis. FAPI, fibroblast-activation protein inhibitor; maximum intensity projection; PET, positron emission tomography; SUV, standardized uptake value.
Thorax
Lung
Lung parenchyma exhibits low physiological uptake (pooled SUVmean: 0.59 ± 0.24). FAPI accumulates in non-oncologic lung diseases such as interstitial lung diseases (average SUVmax: 4.3 ± 1.6, n = 15), infectious pneumonia (median SUVmax 2.2 [range: 1.5–3.7], n = 9), organizing pneumonia (SUVmax: 11.7), tuberculosis (median SUVmax: 3.4 [range: 2.5–7.5], n = 10), pulmonary Cryptococcus (SUVmax: 8.9), necrotizing granuloma (SUVmax: 7.2) (Figure 5). 14,15,17,39,40 Diffuse/multifocal FAPI uptake can be seen in pneumonia 41 and interstitial lung diseases. 14,39,40
Figure 5.
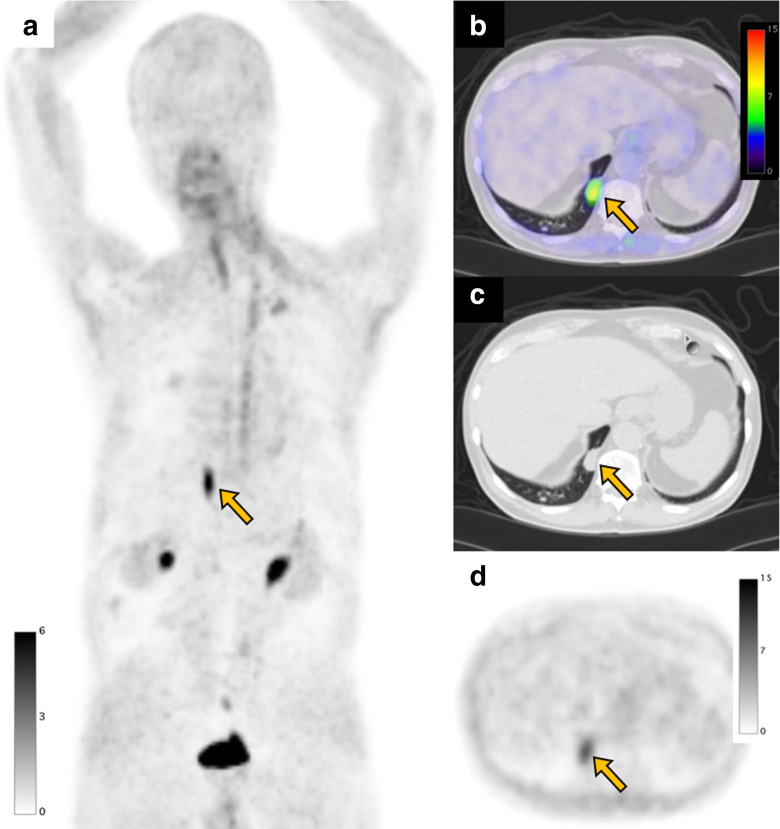
A 75-year-old male with a subcutaneous lipomatous tumor underwent a FAPI PET/CT scan before surgery. FAPI PET MIP image (a) FAPI PET/CT (b), CT (c), and FAPI PET (d) demonstrate focal uptake (SUVmax: 7.2, arrow) in a pulmonary nodule in the right lower lobe, which was diagnosed as necrotizing granuloma by biopsy. FAPI, fibroblast-activation protein inhibitor; maximum intensity projection; PET, positron emission tomography; SUV, standardized uptake value.
Focal FAPI uptake is seen in malignant lesions. Li et al analyzed 34 patients with lung adenocarcinoma and reported an average SUVmax of primary tumors of 12.5 ± 3.8. 42 Previously reported FAPI uptake in benign lung lesions (e.g. organizing pneumonia, old tuberculosis, pulmonary Cryptococcus, and necrotizing granuloma) is not very high (SUVmax<12). 15,17,40,43,44 Thus, marked FAPI uptake in lung nodules may suggest lung cancer rather than benign lesion. However, the range of SUVmax can overlap and the final diagnosis should not be made by FAPI PET/CT as it remains difficult to distinguish the benign or malignant nature of an incidentally found FAPI-avid solid pulmonary nodule. 24
Cardiac
Myocardial wall shows low physiological uptake (pooled SUVmean: 1.02 ± 0.35). Increased cardiac FAPI uptake has been reported in various non-oncologic conditions such as myocardial ischemia, acute myocardial infarction (mean SUVpeak: 6.4 ± 1.5, n = 35), cardiac amyloidosis, cardiac sarcoidosis, dilated cardiomyopathy (SUVmax: 2.6), pulmonary hypertension (SUVmax: 2.5), and cardiotoxicity induced by chemotherapy and immune-checkpoint inhibitor. 45–54 Siebermair et al detected focal myocardial uptake (SUVmax 2.2 ± 0.6) incidentally in 6/32 (18.8%) patients who underwent FAPI PET for cancer staging. Focal myocardial uptake was statistically significantly associated with patients’ conditions such as older age, coronary artery disease, myocardial infarction, and platinum-based chemotherapy. 55 Similarly, Heckmann et al also reported a significant correlation between cardiac FAPI uptake and cardiovascular risk factors including obesity, diabetes, hypertension, platinum-based chemotherapy, and radiation therapy. 56
Breast
Breast exhibits mild to moderate physiological uptake (pooled SUVmean: 1.39 ± 0.81) depending on the hormonal status (pre-menopausal (<35 years) vs post-menopausal (>65 years): [average SUVmax] 1.8 (n = 12) vs 1.0 (n = 68)). 57,58 Also, physiological uptake in the nipples can be seen (SUVmean: 1.00 ± 0.61, n = 49). 20 Elevated and diffuse uptake in the bilateral breasts can be seen in patients under hormonal stimulation (SUVmax4.0, Figure 6). 59 Increased diffuse uptake has been reported in middle-aged females and males with gynecomastia (SUVmax: 4.5 ± 1.5, n = 7). 16 FAPI uptake in the accessory breast has also been reported (SUVmax: 4.5). 60 This can mimic lymph node metastasis. High FAPI uptake has been reported in breast cancer (average SUVmax: 10.0 [range: 2.6–17.0]). 61,62 Thus, breast nodule showing marked FAPI uptake is highly suspected of breast cancer. We did not find any report on FAPI uptake in benign breast tumors.
Figure 6.
A 36-year-old female with invasive squamous cell carcinoma of the cervix after hormonal stimulation with gonadotropin injections underwent a FAPI PET/CT scan before surgery. FAPI PET MIP image (a) FAPI PET/CT (b), CT (c), and FAPI PET (d) demonstrate diffuse bilateral uptake (SUVmax: 4.0) in the breasts suggesting fibroglandular tissue composition after hormonal stimulation. FAPI, fibroblast-activation protein inhibitor; MIP, maximum intensity projection; PET, positron emission tomography; SUV, standardized uptake value.
Esophagus
Esophagus exhibits low to mild physiological uptake (pooled SUVmean: 1.39 ± 0.81) . Esophagitis can lead to increased FAPI uptake. Zheng et al reported that 4.9% (n = 9/182) of patients with various cancers had focal/diffuse mild uptake in the esophagus due to esophagitis (median SUVmax 2.2 [range: 1.5–3.8]). 15 In contrast, intense focal FAPI uptake has been reported in esophageal cancer (median SUVmax: 16.7, [range: 7.8–26.7], n = 21). 63 Thus, uptake distribution pattern and intensity can help to distinguish esophagitis from esophageal cancer.
Abdomen and pelvis
Gastrointestinal
Low physiological accumulation has been reported in the intestinal tract (pooled SUVmean: 0.81 ± 0.31). FAPI uptake in non-oncologic gastric conditions has not been reported. Pang et al have reported higher FAPI SUVmax and TBR in gastric cancer than with FDG (median SUVmax: 12.7 vs 3.7, n = 11). 64 Given the high detectability of FAPI PET, gastric cancer can be incidentally found as wall thickening with focal or diffuse FAPI uptake.
Luo et al reported a case with intense focal FAPI uptake (SUVmax: 14.1) in Crohn’s disease induced colonic stenosis. 65 Interestingly, the authors reported negative FAPI uptake in ulcerative colitis. Crohn’s disease should be included in the differential diagnosis of incidental, focal FAPI accumulation in the colon especially in the ileocecum, the most frequently affected region in Crohn’s disease.
Zheng et al 15 reported that 25.8% (n = 47/182) of cancer patients incidentally showed FAPI uptake in hemorrhoids, depicted as moderate focal uptake in the anal canal without remarkable CT changes. In their study, FAPI uptake was lower in hemorrhoids than in colorectal cancer (average SUVmax: 3.7 ± 0.7 vs 9.8 ± 3.5). Given this, SUVmax and CT features may help differential diagnosis of incidental uptake in the anal canal.
Liver
Normal liver exhibits low FAPI uptake (pooled SUVmean: 0.86 ± 0.33). Diffusely increased liver parenchymal FAPI uptake has been confirmed in patients with cirrhosis. 66,67 Parenchymal uptake is higher in patients with cirrhosis (average SUVmean: 1.4 [range: 0.44–2.4], n = 7). 67 FAPI PET/CT can provide higher detectability of hepatocellular carcinoma (HCC) than FDG PET/CT as it provides greater TBR. 66,68 However, increased FAPI uptake in the cirrhosis liver parenchyma reduces the TBR of HCC, which may lower the detectability. 66 As a potential pitfall, FAPI uptake can be seen in benign hepatic tumors such as focal nodular hyperplasia (FNH) (SUVmax 5.6) and hepatic adenoma (SUVmax 1.4). 18,69,70 Thus, final diagnosis of the incidental FAPI uptake should be made in combination with other modalities including contrast-enhanced CT and MRI.
Pancreas
Pancreas exhibits mild to moderate physiological uptake (pooled SUVmean: 1.58 ± 0.98). FAPI PET/CT may provide excellent detectability of pancreatic cancer (SUVmax: 21.4 [range: 11.6–34.9], n = 26). 71,72 However, diffuse pancreatic FAPI accumulation has also been confirmed in acute pancreatitis (average SUVmax: 7.5 ± 3.5, n = 8) 72 and IgG4-related pancreatitis (average SUVmax: 15.2 ± 9.0, n = 19). 37 Intense FAPI uptake in pancreatitis may mask the PET signal of pancreatic cancer, especially in case of tumor-induced pancreatitis (Figure 7). 71–73 Dual-time point scans (i.e. delayed scan at 3 h after injection) may help to distinguish pancreatitis and cancer because uptake in pancreatitis decreases over time whereas tumor uptake tends to remain. 71,72
Figure 7.
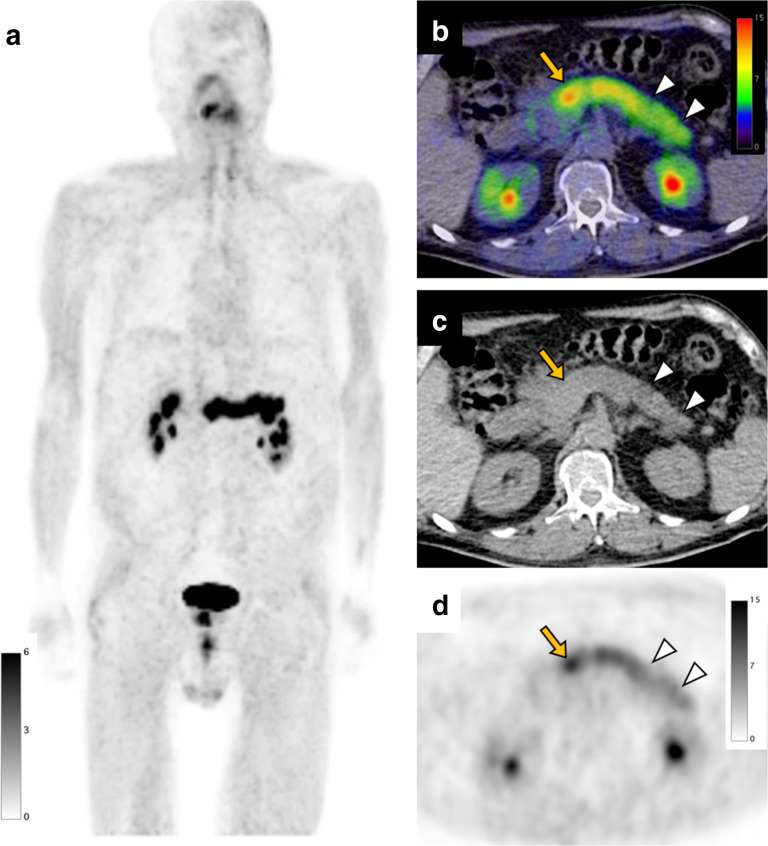
A 73-year-old male with pancreatic cancer underwent a FAPI PET/CT scan before surgery. FAPI PET MIP image (a) shows diffuse intense uptake in the pancreas. FAPI PET/CT (b), CT (c), and FAPI PET (d) demonstrate intense uptake in the enlarged pancreas head (arrow, SUVmax: 13.4) indicating pancreatic cancer. FAPI accumulation in the pancreas body to tail is slightly lower than primary tumor (arrowheads, SUVmax: 9.7) suggesting tumor-induced pancreatitis. FAPI, fibroblast-activation protein inhibitor; PET, positron emission tomography; SUV, standardized uptake value.
Non-oncological causes of focal FAPI uptake can mimic malignancy and false-positive findings have been reported. Zhang et al reviewed 103 various cancer patients who underwent FAPI PET/MR and found focal pancreatic uptake in 7 (6.8%) patients (median SUVmax: 4.4 [range: 3.1–9.1]). These lesions were followed-up by image(s) or biopsy and eventually diagnosed as non-specific focal uptake (median SUVmax: 4.3 [range: 4.2–8.7], n = 4) and benign conditions (e.g. prior pancreatitis (SUVmax: 3.1), pseudocyst (SUVmax: 9.1), and IgG4-related disease (SUVmax 5.1). 74 Clinical history and other modality images such as contrast-enhanced CT and MRI may help the diagnosis of focal pancreatic FAPI uptake.
Bile duct
Gallbladder exhibits low physiological uptake (pooled SUVmean: 0.62 ± 0.26). Intense uptake (average SUVmax >12, n = 12) has been reported in the cholangiocarcinoma. 5 Extrahepatic cholangiocarcinoma and pancreas cancer can cause tumor-induced obstructive cholangitis, in which high FAPI uptake (median SUVmax: 11.7, n = 4) has been reported. 71 Due to the high uptake, differentiating tumor-induced cholangitis from cancer on FAPI PET may be difficult. 71,75 In non-oncologic conditions, FAPI accumulation (average SUVmax: 9.4 ± 4.4, n = 6) in IgG4-related sclerosing cholangitis has been reported. 37,76 Also, Wang et al 77 reported FAPI uptake (SUVmax: 8.2) in portal biliopathy (also known as pseudosclerosing cholangitis) caused by cavernous transformation of the portal vein. They speculated that biliary fibrosis, portal phlebitis, perihepatic fibrosis, and thrombosis secondary to cavernous transformation may be the causes of FAPI uptake.
Spleen
Spleen exhibits low physiological uptake (pooled SUVmean: 0.92 ± 0.32). There has been one case study of a splenic incidental finding in the spleen in a cancer patient and the lesion was a splenic hemangioma (SUVmax: 9.6). 41
Kidney
Kidney shows mild to moderate physiological uptake (pooled SUVmean: 1.61 ± 0.49). Diffuse FAPI uptake in the kidney has been reported in patients with renal fibrosis. Zhou et al 78 compared kidney FAPI uptake with pathological grade of renal fibrosis, in a cohort of 13 patients with renal diseases. They found that positive correlation of SUVmax to fibrosis grade ([average SUVmax] Grade I: 3.9 ± 1.5,Grade II: 6.0 ± 1.7, Grade III: 7.7 ± 1.2). As a benign renal tumor, high FAPI uptake (SUVmax: 18.4) in AML has been reported. 79 FAPI accumulation in AML can be attributed to smooth muscle cells differentiated from fibroblast-like cells. There has been no FAPI PET research focused on renal cell carcinoma (RCC). Based on our experience, FAPI uptake in clear cell RCC, a most common RCC type, is generally mild. Given this, it might be difficult to differentiate RCC from benign renal tumor based on FAPI uptake.
Uterus
Uterus shows the highest physiological FAPI accumulation in the solid organs (pooled SUVmean: 7.92 ± 5.18). Uterine uptake is higher in pre-menopausal females than those in post-menopausal status (average SUVmax: 11.7 (n = 12) vs 3.0 (n = 68)). 58 Also, a negative correlation between uterine FAPI uptake and age has been reported. 16 The high physiological uptake may mask the uterine cancer ([average SUVmax] cervical cancer: 15.2, n = 4; endometrial cancer: 18.4, n = 2), 58 and detectability of small cancer lesion might be low particularly in females of childbearing age. In benign tumors, uterine leiomyoma typically shows FAPI accumulation (average SUVmax: 3.9 ± 3.7, n = 2), 17 which uptake degree is often similar to physiological uptake of the uterus (Figure 8).
Figure 8.
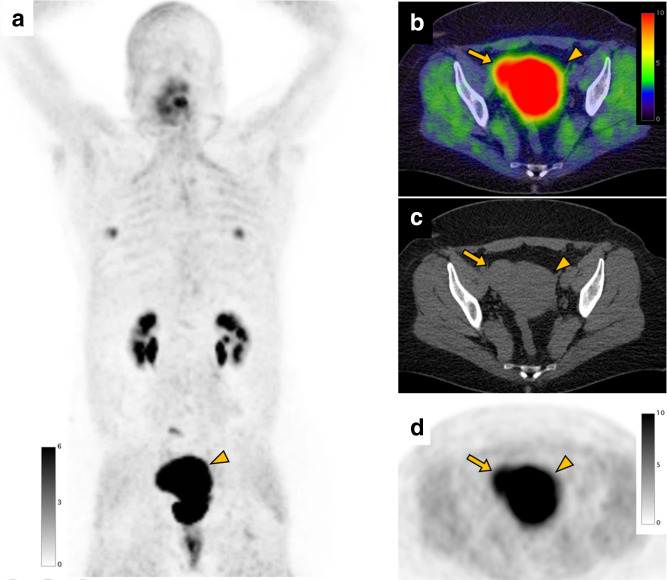
A 47-year-old female with gonadal vein tumor concerning for sarcoma underwent a FAPI PET/CT scan before surgery. FAPI PET MIP (a), FAPI PET/CT (b), CT (c), and FAPI PET (d) images show intense uptake in the uterus (arrowhead, SUVmax: 29.7). FAPI uptake is also shown in the uterine leiomyoma (arrow, SUVmax: 14.1) demonstrated as a slightly high-density mass on CT. FAPI uptake (SUVmax: 5.2) in the bilateral nipple (SUVmax: 5.2) is also seen. FAPI, fibroblast-activation protein inhibitor; PET, positron emission tomography; SUV, standardized uptake value.
Musculoskeletal
Bone and joint
Bone marrow exhibits low physiological uptake (pooled SUVmean: 0.67 ± 0.22). Bone and joint FAPI uptake is one of the most common incidental FAPI PET/CT findings. 16 In the bone, FAPI uptake is often seen in the degenerative changes (median SUVmax: 8.0 [range: 3.1–17.3], n = 47) where osteophytes are typically shown on CT (Figure 9). Bone fracture also accumulates FAPI, 15,17 which has been reported to be significantly higher than FDG uptake (average SUVmax: 7.4 ± 4.1 vs 2.2 ± 1.7, n = 10). 17 Other bone conditions with reported increased FAPI uptake include Schmorl’s node (median SUVmax: 4.6 [range: 3.7–6.7], n = 5), 15 fibrous dysplasia (SUVmax: 8.7), 80 avascular necrosis (SUVmax: 5.4), 81 mastoiditis (median SUVmax: 2.6 [range: 2.5–3.3], n = 3), 15 bone tuberculosis (SUVmax: 14.3), 82 and myositis ossificans (SUVmax: 30.8). 83 Focal/multifocal bone uptake can mimic malignancy (e.g. Schmorl’s node and bone tuberculosis). Qin et al 38 compared FAPI uptake between bone metastases and benign bone conditions, and showed that the FAPI uptake is higher in metastases but with a large overlap (average SUVmax: 7.1 ± 4.3 [n = 94] vs 3.6 ± 1.6 [n = 201]). Thus, final diagnosis of incidental bone uptake should be made in combination with other imaging modalities, follow-up, and/or biopsy.
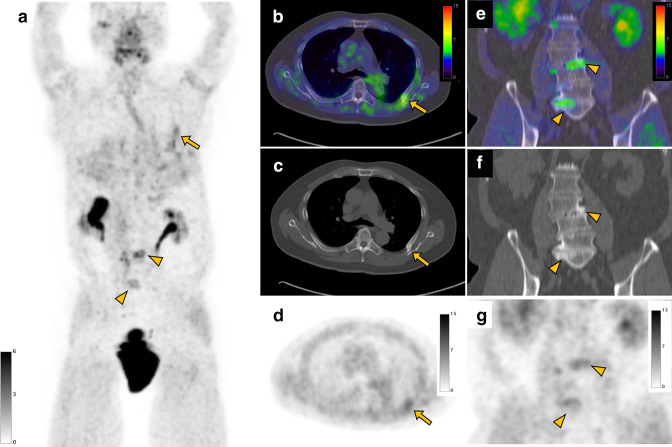
Figure 9.
A 78-year-old male with right clear cell renal cell carcinoma underwent a FAPI PET/CT scan before surgery. FAPI PET MIP (a), FAPI PET/CT (b), CT (c), and FAPI PET (d) images show uptake in a bone fracture of the left rib (arrow, SUVmax: 4.1). Also, coronal FAPI PET/CT (e), CT (f), and FAPI PET (g) images demonstrate FAPI uptake (arrowheads, SUVmax: 5.4) in the lumbar spines with osteophytes representing degenerative changes. FAPI, fibroblast-activation protein inhibitor; MIP, maximum intensity projection; PET, positron emission tomography; SUV, standardized uptake value.
In the joint, osteoarthritis is one of the most common conditions that shows FAPI uptake (median SUVmax: 3.4 [range: 2.0–5.9], n = 69), 15 which uptake is typically in the shoulders and hips (Figure 4). In addition, articular/entheseal FAPI uptake has been reported in the enthesopathy (median SUVmax: 4.8 [range: 2.2–8.7], n = 8), 15 rheumatoid arthritis, 84 and inflammatory arthritis induced by immune-checkpoint inhibitor (SUVmax: 12.0). 85
Soft tissue
Muscle exhibits mild physiological uptake (pooled SUVmean: 1.09 ± 0.35). FAPI uptake in the scarring/wound healing is commonly seen. As FAP is overexpressed by myofibroblasts in remodeling tissue, surgical procedures and implanted devices (e.g. surgical mesh, breast implant, and infusion port) cause FAPI uptake in the regarding region. Kessler et al reported 19.8% (n = 18/91) of cancer patients showed incidental FAPI uptake in scarring/wound healing with an average SUVmax of 7.7 (range: 2.4–13.3). 16 Focal or diffuse muscular FAPI uptake can be seen physiologically, but (poly)myositis should be considered when intense muscular uptake is observed as FAPI uptake in juvenile polymyositis has been reported. 86 An elastofibroma dorsi, a benign soft tissue tumor of the thoracic wall tumor in the infrascapular region without malignant potential, was incidentally detected as FAPI moderately-avid mass (SUVmax: 4.4). 87 As a potential pitfall, FAPI can accumulate focally in hematoma, which mimics malignancy. Yang et al reported a case that showed FAP uptake (SUVmax: 5.3) in intramuscular gluteal hematoma after bone marrow biopsy performed 2 days before the scan. 88
Other systems
Vascular
Blood pool exhibits mild physiological signal at 60 min (pooled SUVmean: 1.32 ± 0.41). FAPI signal may reflect the FAP expression in the vessel walls. The FAP expression is related to plaque vulnerability. 89,90 Case studies have reported FAPI uptake (SUVmax: 2.2–3.7) in unstable atherosclerotic plaques. 91,92 Interestingly, focal FAPI activity was observed only in the vulnerable plaque but not in stable plaques, 92 which may need cautious follow-up or intervention, depending on the patient’s background. Diffuse FAPI uptake in the arterial wall can represent large-vessel vasculitis such as giant cell arteritis and Takayasu arteritis (SUVmax: 6.4), 8,93 although further studies are warranted.
Lymph node
Reactive lymph nodes can show FAPI uptake. Zheng et al 15 reported that 7.7% (n = 14/182) of cancer patients showed FAPI uptake in reactive lymph nodes (median SUVmax: 3.1 [range: 1.4–11.7]). The uptake was most commonly seen in the mediastinum followed by the neck, axillary and inguinal region. Similar to other PET tracers, relatively low uptake and characteristic distribution may be a clue to distinguish reactive lymph nodes from metastasis. However, it can be difficult to differentiate them due to the overlap of FAPI signal between reactive lymph nodes (SUVmax: 3.6 ± 1.6, n = 69) and lymph node metastasis (SUVmax: 6.3 ± 3.4, n = 28). 15 For example, one case study reported FAPI uptake (SUVmax: 5.1) in intramammary lymphoid tissue that mimicked breast cancer, which was finally extracted by surgery. 22
Conclusion
Interpreting incidental FAPI uptake on PET/CT can be challenging in cancer patients, as FAPI uptake is not exclusively seen in malignant lesions but also in benign lesions, and there is a great overlap of SUV between them. 15 However, the knowledge of physiological and non-oncologic FAPI activity can help to perform accurate interpretation. Also, patients' characteristics including age, sex, pre-existing conditions and correlation with other available imaging modalities can provide further diagnostic information. As of now, the majority of the FAPI PET researches of benign lesions are based on case report series, and more systematic research is warranted to further optimize the management of incidental findings on FAPI PET/CT.
FAPI accumulation in non-oncologic conditions may limit the use of FAPI PET for cancer staging indications. Cancer imaging studies/trials should be carefully designed for specific indications and tumor types to show meaningful and reproducible diagnostic efficacy.
On the other hand, FAPI accumulation in non-oncologic conditions offers an immense opportunity to evaluate non-invasively fibroinflammatory processes. Ongoing clinical trials of FAPI PET in cardiovascular (e.g. myocardial infarction [NCT04723953, NCT04803864] and atherosclerosis [NCT05036759]) and fibro-inflammatory diseases (e.g., interstitial lung diseases [NCT05121779], rheumatoid arthritis (NCT4514614)), liver fibrosis [NCT05262647, NCT04533828, NCT04605939], and keloid [NCT05275699]) may show that the highest potential of FAPI PET imaging for diagnostic efficacy is outside of oncological applications.
Footnotes
CONFLICTS OF INTEREST: Jeremie Calais was the recipient of grants from the ERF-SNMMI (2019–2021 Molecular Imaging Research Grant for Junior Academic Faculty) and the Prostate Cancer Foundation (grant 20YOUN05 and 19CHAL02). Jeremie Calais reported receiving personal fees from Advanced Accelerator Applications, Astellas, Blue Earth Diagnostics, Curium Pharma, DS pharma, EXINI, GE Healthcare, IBA RadioPharma, Isoray, Janssen Pharmaceuticals, Lantheus, Lightpointmedical, Novartis, POINT Biopharma, RadioMedix, Progenics, Telix Pharmaceuticals, outside the submitted work.
Contributor Information
Masatoshi Hotta, Email: MHotta@mednet.ucla.edu.
Angela C Rieger, Email: acrodriguez@mednet.ucla.edu.
Mahbod G Jafarvand, Email: mjafarvand@mednet.ucla.edu.
Nandakumar Menon, Email: nandakumar.menon@va.gov.
Andrea Farolfi, Email: afarolfi@mednet.ucla.edu.
Matthias R Benz, Email: mbenz@mednet.ucla.edu.
Jeremie Calais, Email: jcalais@mednet.ucla.edu.
REFERENCES
- 1. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016; 16: 582–98. doi: 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 2. Koczorowska MM, Tholen S, Bucher F, Lutz L, Kizhakkedathu JN, De Wever O, et al. Fibroblast activation protein-α, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol Oncol 2016; 10: 40–58. doi: 10.1016/j.molonc.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu F, Qi L, Liu B, Liu J, Zhang H, Che D, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS ONE 2015; 10. doi: 10.1371/journal.pone.0116683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mona CE, Benz MR, Hikmat F, Grogan TR, Lueckerath K, Razmaria A, et al. Correlation of 68ga-fapi-46 PET biodistribution with FAP expression by immunohistochemistry in patients with solid cancers: interim analysis of a prospective translational exploratory study. J Nucl Med 2022; 63: 1021–26. doi: 10.2967/jnumed.121.262426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68)ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 2019; 60: 801–5. doi: 10.2967/jnumed.119.227967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, et al. 68 ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med 2019; 60: 386–92. doi: 10.2967/jnumed.118.215913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giesel FL, Kratochwil C, Schlittenhardt J, Dendl K, Eiber M, Staudinger F, et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of 68ga-FAPI and 18f-FDG PET/CT in cancer patients. Eur J Nucl Med Mol Imaging 2021; 48: 4377–85. doi: 10.1007/s00259-021-05307-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hicks RJ, Roselt PJ, Kallur KG, Tothill RW, Mileshkin L. FAPI PET/CT:will it end the hegemony of (18)F-FDG in oncology? J Nucl Med 2021; 62: 296–302. doi: 10.2967/jnumed.120.256271 [DOI] [PubMed] [Google Scholar]
- 9. Calais J, Mona CE. Will FAPI PET/CT replace FDG PET/CT in the next decade? point-an important diagnostic, phenotypic, and biomarker role. AJR Am J Roentgenol 2021; 216: 305–6. doi: 10.2214/AJR.20.24302 [DOI] [PubMed] [Google Scholar]
- 10. Jacobson FL, Van den Abbeele AD. (n.d.). Importance of (68)ga-FAPI PET/CT for detection of cancer. Radiology; 2022. doi: 10.1148/radiol.212884 [DOI] [PubMed] [Google Scholar]
- 11. Sharma P, Singh SS, Gayana S. Fibroblast activation protein inhibitor PET/CT: A promising molecular imaging tool. Clin Nucl Med 2021; 46: e141–50. doi: 10.1097/RLU.0000000000003489 [DOI] [PubMed] [Google Scholar]
- 12. Calais J. FAP: the next billion dollar nuclear theranostics target? J Nucl Med 2020; 61: 163–65. doi: 10.2967/jnumed.119.241232 [DOI] [PubMed] [Google Scholar]
- 13. Schmidkonz C, Rauber S, Atzinger A, Agarwal R, Götz TI, Soare A, et al. Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann Rheum Dis 2020; 79: 1485–91. doi: 10.1136/annrheumdis-2020-217408 [DOI] [PubMed] [Google Scholar]
- 14. Röhrich M, Leitz D, Glatting FM, Wefers AK, Weinheimer O, Flechsig P, et al. Fibroblast activation protein-specific PET/CT imaging in fibrotic interstitial lung diseases and lung cancer: A translational exploratory study. J Nucl Med 2022; 63: 127–33. doi: 10.2967/jnumed.121.261925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng S, Lin R, Chen S, Zheng J, Lin Z, Zhang Y, et al. Characterization of the benign lesions with increased 68ga-FAPI-04 uptake in PET/CT. Ann Nucl Med 2021; 35: 1312–20. doi: 10.1007/s12149-021-01673-w [DOI] [PubMed] [Google Scholar]
- 16. Kessler L, Ferdinandus J, Hirmas N, Zarrad F, Nader M, Kersting D, et al. Pitfalls and common findings in (68)ga-FAPI-PET - A pictorial analysis. J Nucl Med 2021. doi: 10.2967/jnumed.121.262808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lan L, Liu H, Wang Y, Deng J, Peng D, Feng Y, et al. The potential utility of [68 ga]ga-dota-fapi-04 as a novel broad-spectrum oncological and non-oncological imaging agent-comparison with [18f]fdg. Eur J Nucl Med Mol Imaging 2022; 49: 963–79. doi: 10.1007/s00259-021-05522-w [DOI] [PubMed] [Google Scholar]
- 18. Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y, et al. Usefulness of [68ga]ga-dota-fapi-04 pet/ct in patients presenting with inconclusive [18f]fdg pet/ct findings. Eur J Nucl Med Mol Imaging 2021; 48: 73–86. doi: 10.1007/s00259-020-04940-6 [DOI] [PubMed] [Google Scholar]
- 19. Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev 2020; 39: 783–803. doi: 10.1007/s10555-020-09909-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gündoğan C, Güzel Y, Can C, Kaplan İ, Kömek H. FAPI-04 uptake in healthy tissues of cancer patients in 68ga-FAPI-04 PET/CT imaging. Contrast Media Mol Imaging 2021; 2021: 9750080. doi: 10.1155/2021/9750080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang S, Zhou X, Xu X, Ding J, Liu T, Jiang J, et al. Dynamic PET/CT imaging of 68ga-FAPI-04 in chinese subjects. Front Oncol 2021; 11: 651005. doi: 10.3389/fonc.2021.651005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gündoğan C, Güzel Y, Can C, Alabalik U, Kömek H. False-positive 68ga–fibroblast activation protein-specific inhibitor uptake of benign lymphoid tissue in a patient with breast cancer. Clin Nucl Med 2021; 46: e433–35. doi: 10.1097/RLU.0000000000003594 [DOI] [PubMed] [Google Scholar]
- 23. Röhrich M, Loktev A, Wefers AK, Altmann A, Paech D, Adeberg S, et al. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein–specific PET/CT. Eur J Nucl Med Mol Imaging 2019; 46: 2569–80. doi: 10.1007/s00259-019-04444-y [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Tang G, Hu K, Liu X, Zhou W, Li H, et al. Comparison of (68)ga-FAPI and (18)F-FDG PET/CT in the evaluation of advanced lung cancer. Radiology 2022; 303: 191–99. doi: 10.1148/radiol.211424 [DOI] [PubMed] [Google Scholar]
- 25. Gong W, Fu M, Zhang Y, Yang X, Zhang C. Progressive multifocal leukoencephalopathy mimicking malignancy on 68ga-FAPI PET/CT. Clin Nucl Med 2022; 47: 430–32. doi: 10.1097/RLU.0000000000003976 [DOI] [PubMed] [Google Scholar]
- 26. Hao B, Wu X, Pang Y, Sun L, Wu H, Huang W, et al. 18F]FDG and [68ga]ga-DOTA-FAPI-04 PET/CT in the evaluation of tuberculous lesions[. Eur J Nucl Med Mol Imaging 2020; 48: 651–52. doi: 10.1007/s00259-020-04941-5 [DOI] [PubMed] [Google Scholar]
- 27. Can C, Gündoğan C, Güzel Y, Kaplan İ, Kömek H. 68Ga-FAPI uptake of thyroiditis in a patient with breast cancer. Clin Nucl Med 2021; 46: 683–85. doi: 10.1097/RLU.0000000000003637 [DOI] [PubMed] [Google Scholar]
- 28. Liu H, Yang X, Liu L, Lei L, Wang L, Chen Y. Clinical significance of diffusely increased uptake of 68ga-FAPI in thyroid gland. Front Med (Lausanne) 2021; 8: 782231. 10.3389/fmed.2021.782231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu H, Yang X, Wang Y, Wang P, Chen Y. 68Ga-FAPI PET/CT imaging of graves ophthalmopathy in a patient with esophageal cancer. Clin Nucl Med 2021; 46: 938–39. doi: 10.1097/RLU.0000000000003703 [DOI] [PubMed] [Google Scholar]
- 30. Hotta M, Sonni I, Benz MR, Gafita A, Bahri S, Shuch BM, et al. 68Ga-FAPI-46 and 18f-FDG PET/CT in a patient with immune-related thyroiditis induced by immune checkpoint inhibitors. Eur J Nucl Med Mol Imaging 2021; 48: 3736–37. doi: 10.1007/s00259-021-05373-5 [DOI] [PubMed] [Google Scholar]
- 31. Yang X, Gong W, Chen Y. 68ga-FAPI PET/CT imaging in a patient with primary thyroid lymphoma. Endocrine 2021; 73: 230–31. doi: 10.1007/s12020-021-02709-x [DOI] [PubMed] [Google Scholar]
- 32. Ou L, Wu J, Wu J, Mou C, Zhang C. Follicular thyroid adenoma showing avid uptake on 68ga-DOTA-FAPI-04 PET/CT. Clin Nucl Med 2021; 46: 840–41. doi: 10.1097/RLU.0000000000003762 [DOI] [PubMed] [Google Scholar]
- 33. Fu H, Fu J, Huang J, Pang Y, Chen H. 68Ga-FAPI PET/CT versus 18F-FDG PET/CT for detecting metastatic lesions in a case of radioiodine-refractory differentiated thyroid cancer. Clin Nucl Med 2021; 46: 940–42. doi: 10.1097/RLU.0000000000003730 [DOI] [PubMed] [Google Scholar]
- 34. Ballal S, Yadav MP, Moon ES, Roesch F, Kumari S, Agarwal S, et al. Novel fibroblast activation protein inhibitor-based targeted theranostics for radioiodine-refractory differentiated thyroid cancer patients: A pilot study. Thyroid 2022; 32: 65–77. doi: 10.1089/thy.2021.0412 [DOI] [PubMed] [Google Scholar]
- 35. Fu H, Fu J, Huang J, Su X, Chen H. 68Ga-FAPI PET/CT in thyroid cancer with thyroglobulin elevation and negative iodine scintigraphy. Clin Nucl Med 2021; 46: 427–30. doi: 10.1097/RLU.0000000000003569 [DOI] [PubMed] [Google Scholar]
- 36. Pencharz D, Nathan M, Wagner TL. Evidence based management of incidental focal uptake of fluorodeoxyglucose on PET-CT. BJR 2017; 91: 20170774. doi: 10.1259/bjr.20170774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo Y, Pan Q, Yang H, Peng L, Zhang W, Li F. Fibroblast activation protein-targeted PET/CT with (68)ga-FAPI for imaging igg4-related disease: comparison to (18)F-FDG PET/CT. J Nucl Med 2021; 62: 266–71. doi: 10.2967/jnumed.120.244723 [DOI] [PubMed] [Google Scholar]
- 38. Qin C, Song Y, Liu X, Gai Y, Liu Q, Ruan W, et al. Increased uptake of 68ga-DOTA-FAPI-04 in bones and joints: metastases and beyond. Eur J Nucl Med Mol Imaging 2021; 49: 709–20. doi: 10.1007/s00259-021-05472-3 [DOI] [PubMed] [Google Scholar]
- 39. Bergmann C, Distler JHW, Treutlein C, Tascilar K, Müller A-T, Atzinger A, et al. 68Ga-FAPI-04 PET-CT for molecular assessment of fibroblast activation and risk evaluation in systemic sclerosis-associated interstitial lung disease: a single-centre, pilot study. The Lancet Rheumatology 2021; 3: e185–94. doi: 10.1016/S2665-9913(20)30421-5 [DOI] [PubMed] [Google Scholar]
- 40. Tang W, Wu J, Yang S, Wang Q, Chen Y. Organizing pneumonia with intense 68ga-FAPI uptake mimicking lung cancer on 68ga-FAPI PET/CT. Clin Nucl Med 2022; 47: 223–25. doi: 10.1097/RLU.0000000000003855 [DOI] [PubMed] [Google Scholar]
- 41. Liu H, Wang Y, Zhang W, Cai L, Chen Y. Elevated 68ga-FAPI activity in splenic hemangioma and pneumonia. Clin Nucl Med 2021; 46: 694–96. doi: 10.1097/RLU.0000000000003638 [DOI] [PubMed] [Google Scholar]
- 42. Li Y, Lin X, Li Y, Lv J, Hou P, Liu S, et al. Clinical utility of F-18 labeled fibroblast activation protein inhibitor (FAPI) for primary staging in lung adenocarcinoma: a prospective study. Mol Imaging Biol 2022; 24: 309–20. 10.1007/s11307-021-01679-w [DOI] [PubMed] [Google Scholar]
- 43. Zhao L, Pang Y, Sun L, Lin Q, Chen H. Increased 68ga-FAPI uptake in the pulmonary cryptococcus and the postradiotherapy inflammation. Clin Nucl Med 2022; 47: 243–45. doi: 10.1097/RLU.0000000000003873 [DOI] [PubMed] [Google Scholar]
- 44. Hotta M, Benz MR, Allen-Auerbach MS, Crompton JG, Roth MD, Eilber FC, et al. High 68 ga-FAPI-46 uptake in a pulmonary necrotizing granuloma in a patient with subcutaneous lipoma. Eur J Nucl Med Mol Imaging 2022; 49: 1088–89. doi: 10.1007/s00259-021-05510-0 [DOI] [PubMed] [Google Scholar]
- 45. Chandra P, Nath S, Krishnamoorthy J, Sogunuru GP. Incidental detection of ischemic myocardium on 68 ga-FAPI PET/CT. Nucl Med Mol Imaging 2021; 55: 194–98. doi: 10.1007/s13139-021-00704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diekmann J, Koenig T, Thackeray JT, Derlin T, Czerner C, Neuser J, et al. Cardiac fibroblast activation in patients early after acute myocardial infarction: integration with magnetic resonance tissue characterization and subsequent functional outcome. J Nucl Med 2022: jnumed.121.263555. 10.2967/jnumed.121.263555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xie B, Wang J, Xi X-Y, Guo X, Chen B-X, Li L, et al. Fibroblast activation protein imaging in reperfused ST-elevation myocardial infarction: comparison with cardiac magnetic resonance imaging. Eur J Nucl Med Mol Imaging 2022; 49: 2786–97. 10.1007/s00259-021-05674-9 [DOI] [PubMed] [Google Scholar]
- 48. Zhu W, Guo F, Wang Y, Ding H, Huo L. 68Ga-FAPI-04 accumulation in myocardial infarction in a patient with neuroendocrine carcinoma. Clin Nucl Med 2020; 45: 1020–22. doi: 10.1097/RLU.0000000000003334 [DOI] [PubMed] [Google Scholar]
- 49. Totzeck M, Siebermair J, Rassaf T, Rischpler C. Cardiac fibroblast activation detected by positron emission tomography/computed tomography as a possible sign of cardiotoxicity. Eur Heart J 2020; 41(9): 1060. doi: 10.1093/eurheartj/ehz736 [DOI] [PubMed] [Google Scholar]
- 50. Niu N, Huo L, Zhang S, Liu Y, Li X. Immune checkpoint inhibitor-associated cardiotoxicity detected by 68ga-DOTATATE PET/CT and 68ga-FAPI PET/CT. Eur Heart J Cardiovasc Imaging 2022; 23(3): e123. doi: 10.1093/ehjci/jeab189 [DOI] [PubMed] [Google Scholar]
- 51. Shi X, Lin X, Huo L, Li X. Cardiac fibroblast activation in dilated cardiomyopathy detected by positron emission tomography. J Nucl Cardiol 2022; 29: 881–84. 10.1007/s12350-020-02315-w [DOI] [PubMed] [Google Scholar]
- 52. Wang L, Zhang Z, Zhao Z, Yan C, Fang W. 68ga-FAPI right heart uptake in a patient with idiopathic pulmonary arterial hypertension. J Nucl Cardiol 2022; 29: 1475–77. 10.1007/s12350-020-02407-7 [DOI] [PubMed] [Google Scholar]
- 53. Guo W, Chen H. 68)ga FAPI PET/MRI in cardiac amyloidosis. Radiology 2022; 303(1. doi: 10.1148/radiol.211951 [DOI] [PubMed] [Google Scholar]
- 54. Siebermair J, Kessler L, Kupusovic J, Rassaf T, Rischpler C. Cardiac fibroblast activation detected by 68gallium-FAPI-46 positron emission tomography-magnetic resonance imaging as a sign of chronic activity in cardiac sarcoidosis. Eur Heart J Case Rep 2022; 6: ytac005. doi: 10.1093/ehjcr/ytac005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siebermair J, Köhler MI, Kupusovic J, Nekolla SG, Kessler L, Ferdinandus J, et al. Cardiac fibroblast activation detected by ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J Nucl Cardiol 2021; 28: 812–21. doi: 10.1007/s12350-020-02307-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heckmann MB, Reinhardt F, Finke D, Katus HA, Haberkorn U, Leuschner F, et al. Relationship between cardiac fibroblast activation protein activity by positron emission tomography and cardiovascular disease. Circ Cardiovasc Imaging 2020; 13(9): e010628. doi: 10.1161/CIRCIMAGING.120.010628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dendl K, Koerber SA, Adeberg S, Röhrich M, Kratochwil C, Haberkorn U, et al. Physiological FAP-activation in a postpartum woman observed in oncological FAPI-PET/CT. Eur J Nucl Med Mol Imaging 2021; 48: 2059–61. doi: 10.1007/s00259-021-05203-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dendl K, Koerber SA, Finck R, Mokoala KMG, Staudinger F, Schillings L, et al. 68ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur J Nucl Med Mol Imaging 2021; 48: 4089–4100. doi: 10.1007/s00259-021-05378-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sonni I, Lee-Felker S, Memarzadeh S, Quinn MM, Mona CE, Lückerath K, et al. 68ga-fapi-46 diffuse bilateral breast uptake in a patient with cervical cancer after hormonal stimulation. Eur J Nucl Med Mol Imaging 2021; 48: 924–26. doi: 10.1007/s00259-020-04947-z [DOI] [PubMed] [Google Scholar]
- 60. Xu T, Wang W, Yang C, Cai L, Chen Y. 68Ga-DOTA-FAPI-04 uptake of accessory breast in a patient with breast cancer. Clin Nucl Med 2022; 47: 564–65. 10.1097/RLU.0000000000004069 [DOI] [PubMed] [Google Scholar]
- 61. Elboga U, Sahin E, Kus T, Cayirli YB, Aktas G, Uzun E, et al. Superiority of 68ga-FAPI PET/CT scan in detecting additional lesions compared to 18fdg PET/CT scan in breast cancer. Ann Nucl Med 2021; 35: 1321–31. doi: 10.1007/s12149-021-01672-x [DOI] [PubMed] [Google Scholar]
- 62. Backhaus P, Burg MC, Roll W, Büther F, Breyholz H-J, Weigel S, et al. Simultaneous FAPI PET/MRI targeting the fibroblast-activation protein for breast cancer. Radiology 2022; 302: 39–47. doi: 10.1148/radiol.2021204677 [DOI] [PubMed] [Google Scholar]
- 63. Zhao L, Chen S, Chen S, Pang Y, Dai Y, Hu S, et al. 68Ga-fibroblast activation protein inhibitor PET/CT on gross tumour volume delineation for radiotherapy planning of oesophageal cancer. Radiotherapy and Oncology 2021; 158: 55–61. doi: 10.1016/j.radonc.2021.02.015 [DOI] [PubMed] [Google Scholar]
- 64. Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, et al. Comparison of (68)ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology 2021; 298: 393–402. doi: 10.1148/radiol.2020203275 [DOI] [PubMed] [Google Scholar]
- 65. Luo Y, Pan Q, Xu H, Zhang R, Li J, Li F. Active uptake of 68ga-FAPI in crohn’s disease but not in ulcerative colitis. Eur J Nucl Med Mol Imaging 2020; 48: 1682–83. doi: 10.1007/s00259-020-05129-7 [DOI] [PubMed] [Google Scholar]
- 66. Guo W, Pang Y, Yao L, Zhao L, Fan C, Ke J, et al. Imaging fibroblast activation protein in liver cancer: a single-center post hoc retrospective analysis to compare [68ga]ga-fapi-04 pet/ct versus mri and [18f]-fdg pet/ct. Eur J Nucl Med Mol Imaging 2020; 48: 1604–17. doi: 10.1007/s00259-020-05095-0 [DOI] [PubMed] [Google Scholar]
- 67. Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, et al. Fibroblast imaging of hepatic carcinoma with 68ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging 2020; 48: 196–203. doi: 10.1007/s00259-020-04882-z [DOI] [PubMed] [Google Scholar]
- 68. Shi X, Xing H, Yang X, Li F, Yao S, Congwei J, et al. Comparison of PET imaging of activated fibroblasts and 18f-FDG for diagnosis of primary hepatic tumours: a prospective pilot study. Eur J Nucl Med Mol Imaging 2020; 48: 1593–1603. doi: 10.1007/s00259-020-05070-9 [DOI] [PubMed] [Google Scholar]
- 69. Shang Q, Fu L, Pang Y, Meng T, Wu X, Sun L, et al. Increased [68 ga]ga-fapi uptake in focal nodular hyperplasia in a patient with sigmoid colon cancer. Eur J Nucl Med Mol Imaging 2021; 49: 415–16. doi: 10.1007/s00259-021-05519-5 [DOI] [PubMed] [Google Scholar]
- 70. Zhao L, Gu J, Fu K, Lin Q, Chen H. 68Ga-fapi pet/ct in assessment of liver nodules in a cirrhotic patient. Clin Nucl Med 2020; 45: e430–32. doi: 10.1097/RLU.0000000000003015 [DOI] [PubMed] [Google Scholar]
- 71. Pang Y, Zhao L, Shang Q, Meng T, Zhao L, Feng L, et al. Positron emission tomography and computed tomography with [68ga]ga-fibroblast activation protein inhibitors improves tumor detection and staging in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging 2022; 49: 1322–37. doi: 10.1007/s00259-021-05576-w [DOI] [PubMed] [Google Scholar]
- 72. Röhrich M, Naumann P, Giesel FL, Choyke PL, Staudinger F, Wefers A, et al. Impact of 68ga-FAPI PET/CT imaging on the therapeutic management of primary and recurrent pancreatic ductal adenocarcinomas. J Nucl Med 2021; 62: 779–86. doi: 10.2967/jnumed.120.253062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Luo Y, Pan Q, Zhang W, Li F. Intense FAPI uptake in inflammation may mask the tumor activity of pancreatic cancer in 68ga-FAPI PET/CT. Clin Nucl Med 2020; 45: 310–11. doi: 10.1097/RLU.0000000000002914 [DOI] [PubMed] [Google Scholar]
- 74. Zhang X, Song W, Qin C, Liu F, Lan X. Non-malignant findings of focal 68ga-FAPI-04 uptake in pancreas. Eur J Nucl Med Mol Imaging 2021; 48: 2635–41. doi: 10.1007/s00259-021-05194-6 [DOI] [PubMed] [Google Scholar]
- 75. Zheng J, Yao S. 68ga]ga-dota-fapi-04 and [18f] fdg pet/ct for the diagnosis of primary and metastatic lesions in patients with hepatic cancer[. Eur J Nucl Med Mol Imaging 2020; 47: 2078–79. doi: 10.1007/s00259-020-04847-2 [DOI] [PubMed] [Google Scholar]
- 76. Qin C, Yang L, Ruan W, Shao F, Lan X. Immunoglobulin G4-related sclerosing cholangitis revealed by 68ga-FAPI PET/MR. Clin Nucl Med 2021; 46: 419–21. doi: 10.1097/RLU.0000000000003552 [DOI] [PubMed] [Google Scholar]
- 77. Wang R, Gao X, Han X, Zhu Z, He X. Portal biliopathy and cavernous transformation of the portal vein revealed by 68ga-FAPI PET/CT. Clin Nucl Med 2022; 47: 161–63. doi: 10.1097/RLU.0000000000003815 [DOI] [PubMed] [Google Scholar]
- 78. Zhou Y, Yang X, Liu H, Luo W, Liu H, Lv T, et al. Value of [68ga]ga-fapi-04 imaging in the diagnosis of renal fibrosis. Eur J Nucl Med Mol Imaging 2021; 48: 3493–3501. doi: 10.1007/s00259-021-05343-x [DOI] [PubMed] [Google Scholar]
- 79. Guo YH, Yang MF. Increased 18F-ALF-NOTA-FAPI and 18F-FDG uptake in renal angiomyolipoma. Clin Nucl Med 2022; 47: e306–10. doi: 10.1097/RLU.0000000000004022 [DOI] [PubMed] [Google Scholar]
- 80. Wang Y, Wu J, Liu L, Peng D, Chen Y. 68Ga-FAPI-04 PET/CT imaging for fibrous dysplasia of the bone. Clin Nucl Med 2022; 47: e9–10. doi: 10.1097/RLU.0000000000003896 [DOI] [PubMed] [Google Scholar]
- 81. Liu H, Fu W, Yang X, Chen Y. Increased 68ga-FAPI uptake in avascular necrosis of femoral head in a patient with nasopharyngeal carcinoma. Clin Nucl Med 2022; 47: 449–50. 10.1097/RLU.0000000000003996 [DOI] [PubMed] [Google Scholar]
- 82. Gong W, Yang X, Mou C, Liu H, Zhang C. Bone tuberculous granulomatous inflammation mimicking malignancy on 68ga-FAPI PET/CT. Clin Nucl Med 2022; 47: 348–49. doi: 10.1097/RLU.0000000000003990 [DOI] [PubMed] [Google Scholar]
- 83. Gong W, Chen S, He L, Liu W, Zhang C. Intense 68ga-FAPI uptake in a patient with myositis ossificans: mimicking bone malignancy. Clin Nucl Med 2022; 47: 638–39. 10.1097/RLU.0000000000004213 [DOI] [PubMed] [Google Scholar]
- 84. Dorst DN, Rijpkema M, Buitinga M, Walgreen B, Helsen MMA, Brennan E, et al. targeting of fibroblast activation protein in rheumatoid arthritis patients: imaging and ex vivo photodynamic therapy . Rheumatology 2021. 10.1093/rheumatology/keab664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Luo Y, Pan Q, Yang H, Li F, Zhang F. Inflammatory arthritis induced by anti-programmed death-1 shown in 68ga-FAPI PET/CT in a patient with esophageal carcinoma. Clin Nucl Med 2021; 46: 431–32. doi: 10.1097/RLU.0000000000003608 [DOI] [PubMed] [Google Scholar]
- 86. Zheng J, Chen H, Lin K, Yao S, Miao W. 68ga]ga-fapi and [18f]fdg pet/ct images in a patient with juvenile polymyositis[. Eur J Nucl Med Mol Imaging 2021; 48: 2051–52. doi: 10.1007/s00259-020-05185-z [DOI] [PubMed] [Google Scholar]
- 87. Hayrapetian A, Girgis MD, Yanagawa J, French SW, Schelbert HR, Auerbach MS, et al. Incidental detection of elastofibroma dorsi with 68ga-FAPI-46 and 18F-FDG PET/CT in a patient with esophageal cancer. Clin Nucl Med 2021; 46: e86–87. doi: 10.1097/RLU.0000000000003218 [DOI] [PubMed] [Google Scholar]
- 88. Yang X, Liu H, You Z, Gong W, Chen Y. Increased 68ga-FAPI uptake in intramuscular gluteal hematoma in a patient with hemophagocytic syndrome. Clin Nucl Med 2021; 46: 1022–23. doi: 10.1097/RLU.0000000000003771 [DOI] [PubMed] [Google Scholar]
- 89. Brokopp CE, Schoenauer R, Richards P, Bauer S, Lohmann C, Emmert MY, et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur Heart J 2011; 32: 2713–22. doi: 10.1093/eurheartj/ehq519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meletta R, Müller Herde A, Chiotellis A, Isa M, Rancic Z, Borel N, et al. Evaluation of the radiolabeled boronic acid-based FAP inhibitor MIP-1232 for atherosclerotic plaque imaging. Molecules 2015; 20: 2081–99. doi: 10.3390/molecules20022081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hu Z, Yang X, You Z, Chen Y, Liu H. Increased 68ga-FAPI uptake in active atherosclerotic plaque. Clin Nucl Med 2022; 47: 545–46. 10.1097/RLU.0000000000004103 [DOI] [PubMed] [Google Scholar]
- 92. Yang Q, Zhang Z, Li M, Xu WH, Huo L. Increased 68ga-FAPI uptake of symptomatic intracranial atherosclerotic plaque revealed by PET/MR. Clin Nucl Med 2022; 47: 469–70. 10.1097/RLU.0000000000004050 [DOI] [PubMed] [Google Scholar]
- 93. Wu S, Pang Y, Zhao L, Zhao L, Chen H. 68Ga-FAPI PET/CT versus 18F-FDG PET/CT for the evaluation of disease activity in takayasu arteritis. Clin Nucl Med 2021; 46: 847–49. doi: 10.1097/RLU.0000000000003692 [DOI] [PubMed] [Google Scholar]