Abstract
The objective of this study was to develop a specific immunological diagnostic assay for yellow disease in hyacinths, using monoclonal antibodies (MAbs). Mice were immunized with a crude cell wall preparation (shear fraction) from Xanthomonas hyacinthi and with purified type IV fimbriae. Hybridomas were screened for a positive reaction with X. hyacinthi cells or fimbriae and for a negative reaction with X. translucens pv. graminis or Erwinia carotovora subsp. carotovora. Nine MAbs recognized fimbrial epitopes, as shown by immunoblotting, immunofluorescence, enzyme-linked immunosorbent assay (ELISA), and immunoelectron microscopy; however, three of these MAbs had weak cross-reactions with two X. translucens pathovars in immunoblotting experiments. Seven MAbs reacted with lipopolysaccharides and yielded a low-mobility ladder pattern on immunoblots. Subsequent analysis of MAb 2E5 showed that it specifically recognized an epitope on the O antigen, which was found to consist of rhamnose and fucose in a 2:1 molar ratio. The cross-reaction of MAb 2E5 with all X. hyacinthi strains tested showed that this O antigen is highly conserved within this species. MAb 1B10 also reacted with lipopolysaccharides. MAbs 2E5 and 1B10 were further tested in ELISA and immunoblotting experiments with cells and extracts from other pathogens. No cross-reaction was found with 27 other Xanthomonas pathovars tested or with 14 other bacterial species from other genera, such as Erwinia and Pseudomonas, indicating the high specificity of these antibodies. MAbs 2E5 and 1B10 were shown to be useful in ELISA for the detection of X. hyacinthi in infected hyacinths.
Xanthomonas hyacinthi (ex Wakker 1883) sp. nov., nom. rev. from Xanthomonas campestris pv. hyacinthi (36) infects hyacinths and some related ornamental bulbous crops (14). The spread of an X. hyacinthi infection (yellow disease) can be a fast process (35) and can cause considerable economic loss for the hyacinth growers; for example, 710,000 hyacinth bulbs were condemned because of yellow disease in 1997. Consequently, a rapid diagnosis for the presence of X. hyacinthi in plant material is a prerequisite for taking decisive actions to prevent further spread of disease. Xanthomonas pathovars are difficult to distinguish, as they are almost identical in bacteriological and biochemical traits (7). Pathovars of Xanthomonas can be differentiated by their ability to infect certain host plants, often economically important plant crops. A reclassification study of the genus Xanthomonas, using phenotypic, chemotaxonomic, and genotypic approaches, showed that X. hyacinthi strains constitute a homogenous group with a genotype distinct from that of X. campestris (36).
Various reports describe the identification and detection of Xanthomonas species and pathovars by serological (2, 4, 5) and DNA-based (17, 18, 30) methods. The detection of plant pathogens with antisera is still the method of choice for many plant inspection services because of the relatively low costs and the presence of technical infrastructure based on automated enzyme-linked immunosorbent assays (ELISA). Therefore, we initiated a study of the surface antigens of X. hyacinthi for the development of a serological detection assay. There are several reports describing the production of monoclonal antibodies (MAbs) specific for Xanthomonas pathovars (2, 4, 5). Most of the strategies used involved immunization of mice with whole-cell preparations; however, raising antibodies to well-characterized antigens would have obvious advantages.
Recently, the existence of type IV fimbriae among xanthomonads has been reported (24, 34). These filamentous protein polymers have been shown to have antigenic properties as good as those of type IV fimbriae of other bacterial species, such as Neisseria gonorrhoeae and Pseudomonas aeruginosa (22, 31). Immunoblot experiments indicated that Xanthomonas pathovars showed variation in molecular mass of the fimbrial subunit (34). These findings suggested that Xanthomonas type IV fimbriae may contain unique determinants, as found for other type IV fimbriae, and that this multimeric surface antigen from X. hyacinthi might be suitable for MAb production. Other components of Xanthomonas known to have antigenic properties include the outer membrane proteins and lipopolysaccharide (LPS) (1–5), and it has been shown that variation in outer membrane proteins and LPS is correlated with the pathovar grouping of X. campestris (21, 23).
In this report, we obtained MAbs by immunizing mice with purified fimbriae and shear fractions of X. hyacinthi and analyzed them for application to phytodiagnostic purposes. The antifimbria MAbs reacted with different fimbrial epitopes. We found that the anti-LPS MAbs recognized the O antigen of X. hyacinthi S148. This antibody was found to be X. hyacinthi specific. The O antigen of S148 was partially characterized and shown to consist of rhamnose (Rha) and fucose (Fuc). The cross-reaction of these MAbs with all strains of X. hyacinthi tested showed that this Rha-Fuc O antigen is conserved within the species.
MATERIALS AND METHODS
Cultures and media.
The bacterial strains used in this study are listed in Table 1. Bacterial cells were cultured on nutrient yeast agar (NYA; Difco Laboratories, Detroit, Mich.). Some Xanthomonas species and pathovars were grown on different media as prescribed by the LMG Culture Collection, Ghent, Belgium.
TABLE 1.
Bacterial strains used in this study
| Species | Strain designation | Sourcea |
|---|---|---|
| Xanthomonas albilineans | 887 | LMG |
| Xanthomonas arboricola pv. pruni | 852 | LMG |
| Xanthomonas axonopodis pv. begoniae | 241, 1925 | NCPPB |
| Xanthomonas axonopodis pv. citri | 682, 990 | LMG |
| Xanthomonas axonopodis pv. dieffenbachiae | 1104 | IPO-DLO |
| Xanthomonas axonopodis pv. malvacearum | 761, 996 | LMG |
| Xanthomonas axonopodis pv. manihotis | 784 | LMG |
| Xanthomonas axonopodis pv. phaseoli | 7488, 7455 | LMG |
| Xanthomonas axonopodis pv. vasculorum | 901 | LMG |
| Xanthomonas axonopodis pv. vesicatoria | 512 | IPO-DLO |
| 901, 905, 910, 929 | LMG | |
| Xanthomonas axonopodis pv. vignicola | 381 | IPO-DLO |
| Xanthomonas campestris pv. campestris | 43, 367, 372 | IPO-DLO |
| 10, 11 | NAKB | |
| 173 | LBO | |
| 568 | LMG | |
| Xanthomonas campestris pv. fici | 701 | LMG |
| Xanthomonas campestris pv. gummisudans | 732 | LMG |
| Xanthomonas fragariae | 708 | LMG |
| Xanthomonas hortorum pv. pelargonii | 7314 | LMG |
| Xanthomonas hyacinthi | S148, S133, S171, S172, NN1, NAD55, TV43, HK60 | LBO |
| Xanthomonas oryzae | 630 | LMG |
| Xanthomonas oryzicola | 797 | LMG |
| Xanthomonas populi | 889 | PD |
| 730 | LMG | |
| Xanthomonas translucens pv. cerealis | 3213, 890 | LMG |
| Xanthomonas translucens pv. graminis | 726 | LMG |
| 2700, 3041 | NCPPB | |
| Xanthomonas translucens pv. hordei | 737 | LMG |
| Xanthomonas translucens pv. phlei | 3231 | NCPPB |
| Xanthomonas translucens pv. poae | 3230 | NCPPB |
| 728 | LMG | |
| Xanthomonas translucens pv. translucens | 2904 | NCPPB |
| 876, 5263 | LMG | |
| Xanthomonas vasicola pv. holcicola | 736 | LMG |
| Xanthomonas vesicatoria | 911, 920 | LMG |
| 512 | IPO-DLO | |
| Agrobacterium tumefaciens | 85 | LMG |
| Bacillus subtilis | 7135 | LMG |
| Erwinia amylovora | 2024 | LMG |
| Erwinia carotovora subsp. carotovora | 550 | LBO |
| 2417 | LMG | |
| Erwinia chrysanthemi | 108 | LBO |
| 2488 | LMG | |
| Erwinia cytolytica | 75 | LBO |
| Erwinia rhapontici | 164 | LBO |
| Klebsiella oxytoca | 3055 | LMG |
| Pseudomonas aeruginosa | 1242 | LMG |
| Pseudomonas fluorescens | 2434 | PD |
| Pseudomonas marginata | 570 | LBO |
| Pseudomonas syringae pv. syringae | 1247 | LMG |
| Stenotrophomonas maltophilia | 958 | LMG |
| Xylophilus ampelinus | 5856, 523 | LMG |
Abbreviations: LMG, Bacteria Collection Laboratorium Microbiologie, Universiteit Gent, Ghent, Belgium; NCPPB, National Collection of Plant Pathogenic Bacteria, Harpenden Laboratory, Harpenden, Hertsfordshire, United Kingdom; IPO-DLO, Research Institute for Plant Protection, Wageningen, The Netherlands; NAKB, Inspection Service for Floriculture and Arboculture, Roelofarendsveen, The Netherlands; LBO, Bulb Research Centre, Lisse, The Netherlands; PD, Plant Protection Service, Wageningen, The Netherlands.
Cultivation and inoculation of hyacinths.
The cultivars Anna Marie, Carnegie, Pink Pearl, and King of the Blues were maintained in a greenhouse with a day-night regimen of 12 h of light (25°C; relative humidity, 70%) and 12 h of darkness (10°C; relative humidity, 90%). Leaves were spray inoculated with bacterial suspensions (106 to 107 CFU/ml) or with phosphate-buffered saline (PBS) as a control. X. hyacinthi S148 and TV45 were used for these experiments. After 2 to 3 weeks, lesions were visible and leaf material was collected for experimental use.
Preparation of antigens and immunization of mice.
Cell extracts were prepared by ultrasonication (Branson, Danbury, Conn.) of bacterial suspensions (109/ml), as previously described (34). Protein-free samples were prepared by incubation with 50 μg of proteinase K (Sigma, St. Louis, Mo.) per ml for 120 min at 60°C. A crude outer bacterial surface preparation was obtained by shearing cells of strain S148 as previously described (34) and concentrating to 0.5 mg of protein/ml in PBS. Two 6-week-old New Zealand mice were injected intraperitoneally with 0.25 ml of this preparation, mixed 1:1 with Freund’s complete adjuvant (FCA), three times at 2-week intervals. Native fimbriae were isolated and purified from strain S148 (34) and concentrated to 0.5 and 0.1 mg/ml in PBS, respectively. The fimbrial preparations were mixed 1:1 with FCA, and from each preparation, 0.25 ml was injected intraperitoneally (three times with 2-week intervals) in two 6-week-old BALB/c mice. Two weeks after the third injection, the mice received a final booster injection with the same preparation but without FCA, and after 4 days, the spleens were collected.
Modification of protein antigens.
Differential epitope recognition of the antifimbrial MAbs was visualized by immunoblotting proteolytic fragments of the fimbrial 17-kDa protein subunit following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). These fragments were obtained by partial proteolysis of the 17-kDa protein with γ-chymotrypsin (Sigma) using 1 U/μg of protein for 10 min at 25°C.
Hybridoma production.
Hybridoma cells were produced by using equal amounts of the myeloma cell line Sp2/0-Ag-14 and spleen cells from the immunized mice which showed the highest titer in ELISA against fimbrial preparations or X. hyacinthi whole-cell preparations. Fusion and culturing of hybridoma cell lines were performed by the method of Boonekamp et al. (6). To prevent growth of yeast, 5 μg of amphotericin B (Fungizone; Imperial Laboratories Ltd., Andover, United Kingdom) was added per ml of medium. Selected hybridoma cultures were subcloned by the limiting dilution technique.
MAb production and isotyping.
Hybridoma fluid samples were centrifuged for 10 min at 1,000 × g (Sigma 2-15 centrifuge, 4×25 ml rotor, 4°C) to remove cellular debris. Culture supernatants were used directly for immunological experiments or stored at −20°C. Immunoglobulin isotyping was performed with the Sigma Mouse Monoclonal Antibody Isotyping kit.
ELISA.
The antigen-coated plate (ACP)-ELISA was used for the screening of positively reacting hybridoma supernatants. Polyvinyl chloride 96-well plates (Costar, Cambridge, Mass., and Greiner, Frickenhausen, Germany) were coated with 100-μl portions of bacterial suspension (approximately 108 bacterial cells/ml) in coating buffer (0.05 M carbonate-bicarbonate buffer [pH 9.6]) by drying in a 37°C ventilated incubator and stored at −20°C until used. In some experiments, microtiter plates were coated with purified fimbriae (3 μg/well). For direct antibody sandwich (DAS)-ELISA, plates were coated with gamma globulin, isolated from polyclonal rabbit antisera raised against whole cells of X. hyacinthi S148, with 0.1 μg of gamma globulin/well. Subsequently, bacterial dilutions or infected-hyacinth extracts were added and incubated at 20°C. ACP-ELISA and DAS-ELISA plates were blocked with 0.5% skim milk powder (Oxoid, Basingstoke, United Kingdom) in PBS with 0.5% Tween 20 (PBST), washed three times with PBST, and hybridoma supernatant (100 μl/well) was added. Following incubation for 2 h at 37°C, plates were washed, and goat anti-mouse alkaline phosphatase (American Qualex, La Miranda, Calif.) diluted 1:10,000 was added for 1 h at 37°C. Plates were washed with PBST and developed by adding substrate, consisting of p-nitrophenyl-phosphate in 10% (vol/vol) diethanolamine buffer, pH 9.8. Absorbance at 405 nm was measured with an Anthos Labtec ELISA reader after 1 and 2 h of incubation at 37°C. The positive-negative threshold of the ELISA was determined as three times the mean of the absorbance of PBS control samples or healthy hyacinth samples. In practice, this meant that absorbance values above 0.08 were considered positive.
LPS isolation.
For preparation of LPS, the cell pellet obtained from a 5-liter culture of strain S148 was extracted by a modified hot phenol-water method (10) and the aqueous-phase material was fractionated by size exclusion chromatography on a Sephadex G-150 superfine column (Pharmacia, Uppsala, Sweden), as previously described (26). The eluted fractions were assayed colorimetrically for 3-deoxy-d-manno-2-octulosonic acid (Kdo) by the thiobarbituric acid assay (38) and for hexose with phenol-sulfuric acid (40); LPS-containing fractions were identified by PAGE analysis.
PAGE and immunoblot analyses.
LPS samples were analyzed by deoxycholic acid PAGE, using 18% acrylamide gels. The gels were either silver stained for LPS (32) or Alcian blue-silver stained for possible K antigens (26). For immunological analysis of LPS samples, polyacrylamide gels were blotted to Nytran+ membranes (Schleicher and Schuell, Keene, N.H.) with a Trans-Blot SD apparatus (Bio-Rad). Fimbriae or other protein samples were analyzed by SDS-PAGE, using 12% Laemmli gels (15). Immunoblotting of separated protein samples was performed as previously described (34). In some experiments, a slot blot apparatus (Miniblotter 16; Immunetics, Cambridge, Mass.) was used for simultaneous testing of several antisera for recognition of blotted antigens.
Processing of leaf tissue samples of hyacinths.
Inoculated areas of hyacinth leaves were excised (1 cm2; average wet weight of 60 mg) and immersed in 1-ml portions of PBST in small plastic bags. Homogenization of the samples was performed with a hand homogenizer (Bioreba AG, Basel, Switzerland). Subsequently, the plant extracts were serially diluted in antibody-coated microtiter plates and processed as described above.
Immunofluorescence experiments.
Samples of hyacinths infected with X. hyacinthi and samples of bacteria were incubated on microscope slides and examined as previously described (34). For fluorescence labeling, fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse polyvalent immunoglobulins (immunoglobulin A [IgA], IgG, and IgM) and goat anti-rabbit IgG (Sigma) were used.
Glycosyl residue analysis.
Sugar composition analysis was performed by gas chromatography-mass spectrometry of the trimethylsilyl methyl glycoside derivatives (40), using a 30-m DB1 fused silica column (J&W Scientific, Folsum, Calif.) on a 5890A GC-MSD (Hewlett-Packard, Palo Alto, Calif.). Inositol was used as an internal standard, and retention times were compared to those of authentic monosaccharide standards.
1H NMR analyses.
Proton nuclear magnetic resonance (1H NMR) spectroscopy was performed with a Brüker AM 250 apparatus. The samples were dissolved in 2H2O, and the spectra were obtained at 308 K. Chemical shifts were established relative to acetone.
Immunoelectron microscopy.
Incubation of X. hyacinthi cells on Formvar-carbon-coated nickel grids with antisera was performed as previously described (34). MAbs were used in dilutions of 1:5 or 1:10 in PBS. Goat anti-mouse IgG and IgM, conjugated with 10-nm-diameter gold particles (Biocell Laboratories International, Cardiff, United Kingdom), was used as the secondary antibody. After counterstaining with phosphotungstic acid and air drying, grids were examined with a Philips transmission electron microscope type 201 or CM100, each operated at 60 kV.
RESULTS
Selection of MAbs specific for X. hyacinthi.
Immunizations with LPS and fimbrial fractions yielded numerous hybridoma cell lines. About 60 clones reacted positively with X. hyacinthi samples in ELISA or immunoblot analyses. Subsequent screening by ELISA and in immunoblotting experiments of the hybridoma clones for negative reactions with Erwinia carotovora subsp. carotovora 550 and X. campestris pv. graminis LMG 726 identified 15 clones which recognized exclusively X. hyacinthi antigens (Table 2).
TABLE 2.
MAbs obtained after immunization with purified fimbriae (15) and partly purified LPS (8) from X. hyacinthi S148
| MAb | Isotype | Reactivity of MAbs ina:
|
|||
|---|---|---|---|---|---|
| Immunoblot | ELISA | IF | Immuno-EM | ||
| Group 15 | |||||
| 12G9 | IgG1 | + | − | − | − |
| 8C11 | IgG2a | ++ | − | ND | − |
| 6A3 | IgG1 | + | − | ND | − |
| 5D12 | IgG3 | + | − | + | − |
| 3B7 | ?b | + | +/− | ND | − |
| 1A7c | IgG1 | + | − | + | − |
| 6C9c | IgM | + | − | + | + |
| 5G8c | IgG1 | + | − | ND | + |
| 9A2 | ? | +/− | − | + | + |
| Group 8 | |||||
| 2E5 | IgM | + | ++ | + | + |
| 1B10 | IgG1 | + | ++ | + | − |
| 3E10 | IgG3 | + | ++ | + | − |
| 6A1 | IgG1 | + | ++ | + | − |
| 6A12 | IgG2b | + | ++ | + | − |
| 9H3 | IgG1 | + | ++ | + | − |
| 12G7 | IgG3 | + | ++ | + | − |
| 2D10 | IgG1 | + | ++ | + | − |
IF, immunofluorescence labeling experiments; immuno-EM, immunoelectron microscopy. Intensity of the reaction: ++, strong reaction; +, medium reaction; +/−, weak reaction; −, no reaction; ND, not determined.
?, no distinct isotype.
Weak cross-section with X. translucens pv. hordei and pv. translucens.
Specificities of the MAbs.
Two distinct groups of MAbs were identified: group 8 MAbs, resulting from immunization with a shear fraction of X. hyacinthi S148, reacted strongly in ACP-ELISA with X. hyacinthi isolates. Group 15 MAbs, resulting from immunization with fimbriae, reacted strongly with a protein band in immunoblotting but only weakly with whole-cell antigens in ACP-ELISA. The MAbs belonged to the immunoglobulin classes IgG1, IgG3, IgG2a, IgG2b, and IgM (Table 2).
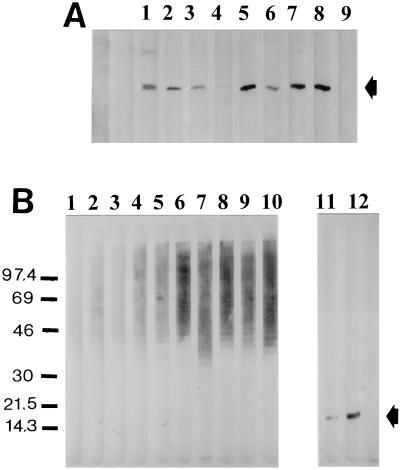
To assess whether the MAbs reacted with a polysaccharide component or a protein epitope, X. hyacinthi cells were sonicated and incubated with or without proteinase K. Next, these extracts were separated by SDS-PAGE and blotted onto nitrocellulose membranes. Analyses of the immunoblots showed that the group 15 MAbs reacted with the 17-kDa fimbrial subunit protein (Fig. 1A). The group 8 MAbs yielded a ladder-like pattern, suggesting that the reaction was with a polysaccharide component of X. hyacinthi (Fig. 1B).
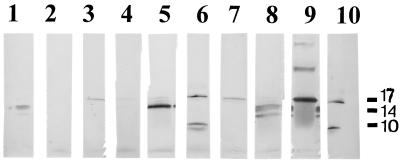
FIG. 1.
Recognition of fimbriae from X. hyacinthi S148 after separation of sonicated cells by SDS-PAGE and immunoslot blotting by group 15 MAbs. MAbs are 3B7 (lane 1), 1A7 (lane 2), 6A3 (lane 3), 5G8 (lane 4), 6C9 (lane 5), 5D12 (lane 6), 8C11 (lane 7), 12G9 (lane 8), and normal (preimmune) mouse serum (lane 9). The position of the 17-kDa fimbrial subunit is indicated by the arrow. (B) Reactions of group 8 and 15 MAbs on immunoslot blots with sonicated X. hyacinthi S148 cells after incubation with protease K (lanes 1 to 10) or without protease K treatment (lanes 11 and 12) and subsequent separation by SDS-PAGE. Antibodies are MAb 6C9 (lane 1), polyclonal rabbit IgG raised against the 17-kDa fimbrial subunit (lane 2), MAb 12G9 (lane 3), MAb 9H3 (lane 4), MAb 3E10 (lane 5), MAb 2D10 (lane 6), MAb 6A12 (lane 7), MAb 12G7 (lane 8), MAb 1B10 (lane 9), MAb 2E5 (lane 10), MAb 6C9 (lane 11), and MAb 12G9 (lane 12). The position of the fimbrial subunit is indicated by the arrow. The positions of molecular size standards (in kilodaltons) are shown to the left of the blot.
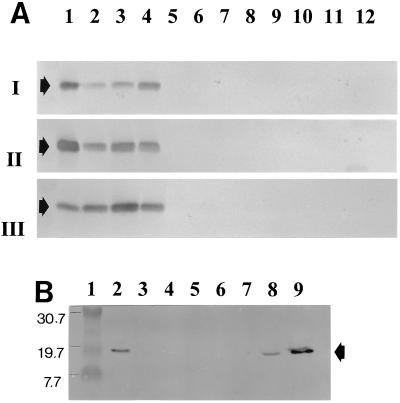
A large group of Xanthomonas pathovars and other bacterial species (Table 1) were analyzed with the MAbs in ACP-ELISA and in immunoblotting experiments to test the specificity of the two panels of MAbs. No cross-reaction of the group 8 MAbs was found with any other bacterial isolates; they reacted solely with the X. hyacinthi isolates in ELISA. Importantly, all strains of X. hyacinthi were recognized by the group 8 MAbs. The 17-kDa fimbrial subunit of the X. hyacinthi strains was recognized by the group 15 antibodies in immunoblotting experiments (Fig. 2 and Table 2). However, only X. translucens pv. hordei (LMG 737) and X. translucens pv. translucens (LMG 876) reacted in immunoblots with MAb 6C9 (Fig. 2B). These proteins also reacted weakly with MAbs 1A7 and 5G8 (not shown), but the other MAbs showed no visible reaction with the 17-kDa fimbrial subunit protein of these pathovars.
FIG. 2.
Immunoblotting analysis of sonicated bacterial cell fractions with group 15 antifimbrial MAbs. (A) Lanes 1 to 4, X. hyacinthi S148, TV43, NAD55, and S133, respectively; lane 5, X. fragariae; lane 6, X. vasicola pv. holcicola; lane 7, X. populi; lane 8, X. axonopodis pv. citri; lane 9, X. hortorum pv. pelargonii; lane 10, X. axonopodis pv. phaseoli; lane 11, X. oryzae; lane 12, X. vesicatoria. The immunoblots were developed after incubation with MAb 8C11 (I), 12G9 (II), or 5D12 (III). The position of the 17-kDa fimbrial subunit in each blot is indicated by an arrow. (B) Immunoblot of X. translucens pathovars, developed with MAb 6C9. Lane 1, marker proteins (positions [in kilodaltons] shown to the left of the blot]); lane 2, X. translucens pv. translucens LMG 876; lane 3, X. translucens pv. phlei LMG 730; lane 4, X. translucens pv. poae NCPPB 3230; lane 5, X. translucens pv. translucens LMG 5263; lane 6, X. translucens pv. cerealis LMG 679; lane 7, X. translucens pv. graminis LMG 726; lane 8, X. translucens pv. hordei LMG 737; lane 9, X. hyacinthi S148. The position of the fimbrial subunit is indicated by the arrow.
Recognition of antigens by group 8 and group 15 MAbs.
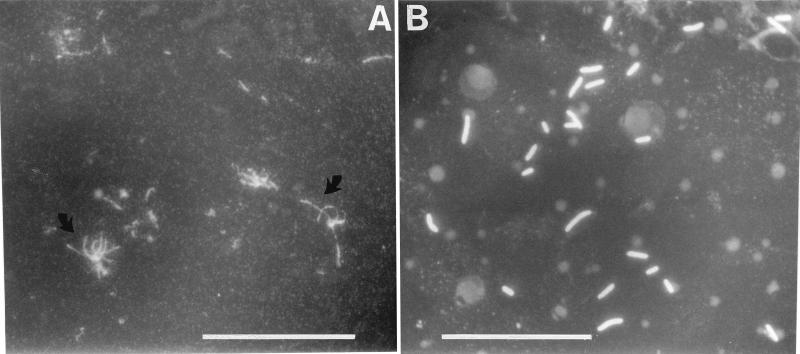
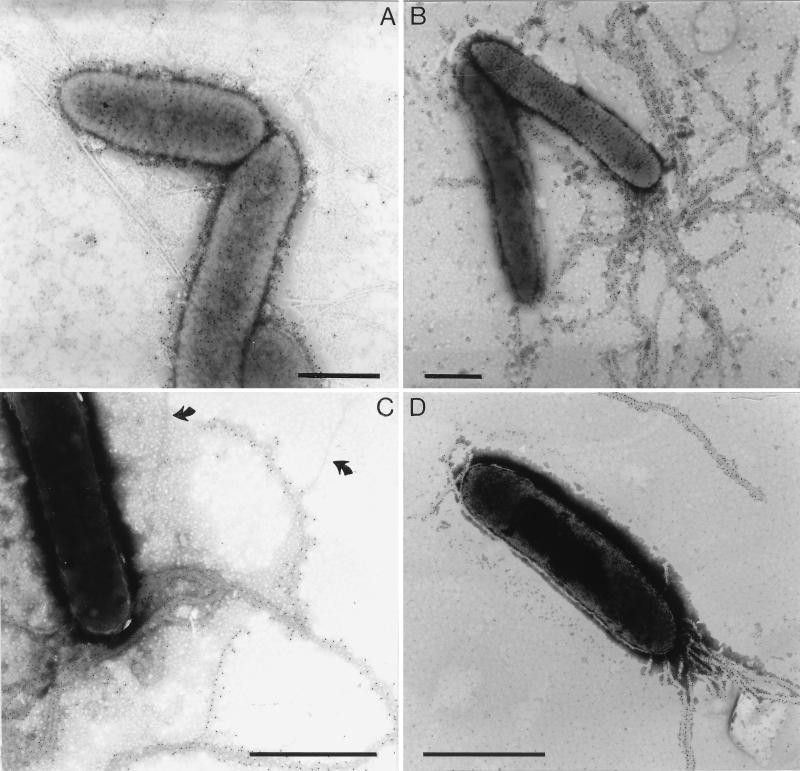
The group 15 MAbs were tested with different immunological techniques (Table 2). All nine MAbs reacted with the fimbrial subunit of X. hyacinthi isolates in immunoblotting experiments, but not with X. translucens pv. graminis. MAb 12G9 showed a relatively weak reaction, suggesting that it recognized a different subunit epitope than MAb 8C11. The group 15 MAbs reacted weakly in a DAS-ELISA with X. hyacinthi cells. When applied to immunofluorescence labeling, the group 15 MAbs 5D12, 1A7, 9A2, and especially MAb 6C9 showed polar labeling of individual cells; fluorescent fimbrial filaments could be visualized under the microscope (Fig. 3A). The group 15 MAbs were also used for immunogold localization studies using electron microscopy (Fig. 4B to D). A distinct tagging of the fimbriae from strain S148 with gold particles was found for MAbs 6C9, 5G8, and 9A2. Some unlabeled fimbriae were also visible in Fig. 4C. The fimbriae of the other strains of X. hyacinthi (Table 1) were labeled with gold as well (not shown). MAb 5D12 labeled only certain parts of the fimbriae but not the tips (not shown).
FIG. 3.
Fluorescent X. hyacinthi cells resulting from indirect FITC labeling after incubation with MAbs. (A) For cells incubated with MAb 6C9, fluorescent fimbrial strands are visible (arrows). (B) Cells incubated with MAb 2E5 are labeled all around. Bars, 15 μm.
FIG. 4.
Electron microscopy of immunogold-labeled cells of X. hyacinthi S148. The bacterial surface structures were labeled with 10-nm-diameter gold-conjugated anti-mouse IgG and IgM after incubation with MAbs 2E5 (A), 6C9 (B), 9A2 (C), and 5G8 (D). Panels B to D show fimbrial structures that are gold tagged; arrows indicate unlabeled fimbriae (C). In panel A, the cell surface LPS is labeled. Bars, 1 μm.
For further assessment of the differences in recognition sites of group 15 MAbs, attempts were made to localize the epitopes on the 17-kDa fimbrial subunit, by using partial proteolysis with γ-chymotrypsin (Fig. 5). MAbs 12G9 and 1A7 recognized only the intact 17-kDa fimbrial subunit, whereas MAbs 6C9, 8C11, and 5D12 recognized other, smaller peptide fragments as well. Polyclonal rabbit antiserum against the 17-kDa fimbrial subunit recognized multimers of the fimbrial subunit in immunoblotting experiments (Fig. 5, lane 9).
FIG. 5.
Immunoblotting analysis of protease-treated fimbrial subunits. Purified fimbriae of X. hyacinthi S148 were treated with γ-chymotrypsin. Subsequently, SDS-PAGE, immunoblotting, and incubation were performed. The following antifimbrial antibodies were used: MAb 9A3 (lane 1), MAb 2E5 (lane 2), MAb 3B7 (lane 3), MAb 1A7 (lane 4), MAb 6A3 (lane 5), MAb 5D12 (lane 6), MAb 12G9 (lane 7), MAb 6C9 (lane 8), polyclonal rabbit antiserum against the 17-kDa fimbrial subunit (lane 9), and MAb 8C11 (lane 10). The positions of markers are depicted in kilodaltons to the right of the blot.
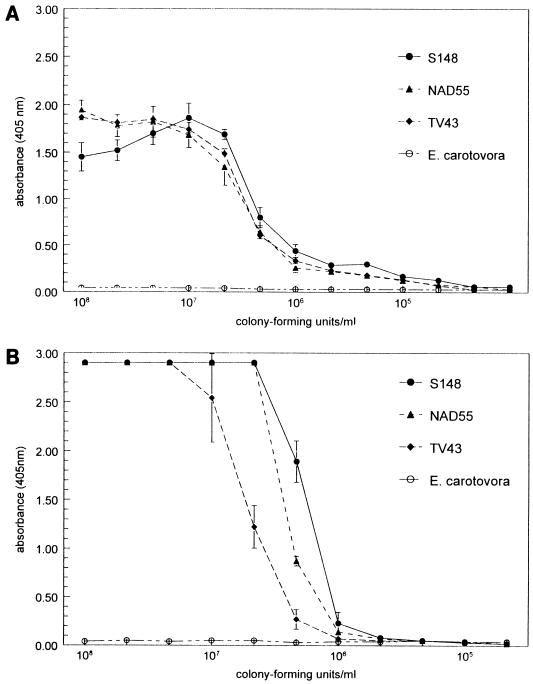
Compared to group 15 MAbs, the group 8 MAbs reacted differently in the immunological tests. Using ELISA, relatively high titers were found with most group 8 MAbs, especially with MAbs 2E5 and 1B10 (Fig. 6); however, only MAb 2E5 labeled the cell surface in immunoelectron microscopy of the X. hyacinthi cells (Fig. 4A), and the gold particles appeared to bind closely to the surface (the fimbriae were not recognized). When applied to immunofluorescence labeling experiments, the group 8 MAbs labeled X. hyacinthi cells, resulting in a halo of green light around the cell (Fig. 3B). Most members of the group 8 MAbs reacted on immunoblotting (Table 2 and Fig. 1B); the low-mobility ladder-like signal suggested the recognition of an LPS component of X. hyacinthi.
FIG. 6.
Titration of MAbs 2E5 (A) and 1B10 (B) in ELISA, using total cell preparations of X. hyacinthi S148, NAD55, and TV43 and Erwinia carotovora subsp. carotovora as the target. All MAb dilutions were tested in triplicate. The error bars represent the standard errors of the means.
Antigen characterization.
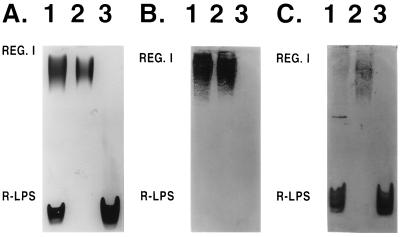
Polysaccharides were extracted from S148 cells and analyzed by SDS-PAGE and Alcian blue-silver staining to characterize the antigen recognized by group 8 MAbs (Fig. 7A, lane 1). Two major components were found in the extract. The high-mobility banding component is probably rough LPS (R-LPS); the low-mobility component showing a ladder pattern (region I) is probably the result of a sequential degree of polymerization of structurally constant repeating units, which could be from capsular antigens or smooth LPS (S-LPS) (9, 25). The fact that both components were visible without the Alcian blue prestain (not shown) suggested that the ladder pattern was the result of the presence of S-LPS (26). A portion of the preparation was subjected to polymyxin chromatography, which specifically binds LPS. The ladder pattern material was bound by the polymyxin, which also indicated that region I is due to S-LPS (data not shown).
FIG. 7.
Alcian blue-silver stained SDS-polyacrylamide gels (A) of crude (lanes 1) and purified (lanes 2 and 3) polysaccharide preparations of X. hyacinthi S148 and corresponding immunoblots, developed after incubation with MAb 2E5 (B) and polyclonal rabbit anti-Xanthomonas antiserum (C). REG. I, region I.
Immunoblotting analyses confirmed that MAb 2E5 recognized only the region I material, and not the R-LPS (Fig. 7B, lane 1). To demonstrate that there was no physical impediment to the binding of the low-molecular-weight R-LPS, a polyclonal antiserum that recognizes common epitopes in Xanthomonas spp. was employed (Fig. 7C). A distinct binding of the R-LPS was demonstrated. We concluded that MAb 2E5 was specific for the region I component.
Purification and structural analyses of the LPS component, recognized by MAb 2E5.
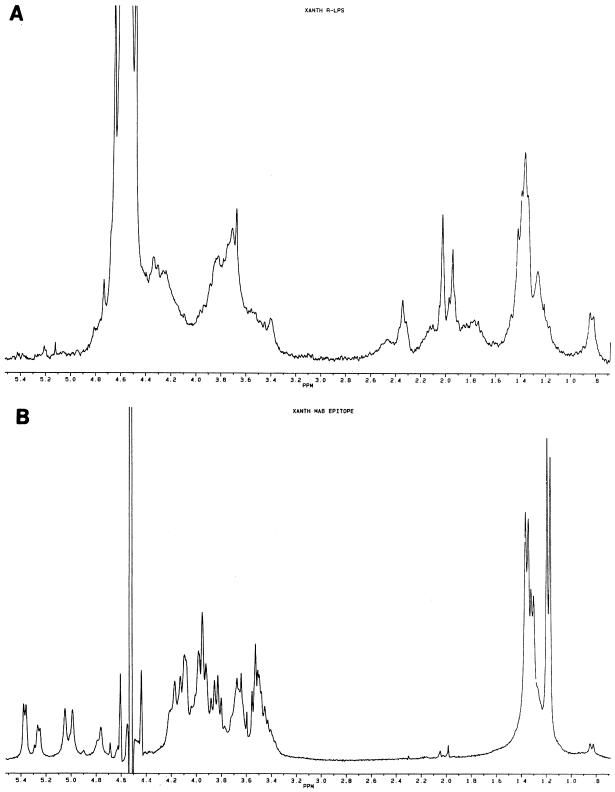
A crude polysaccharide preparation was fractionated by size exclusion chromatography which resulted in two major pools that were analyzed by SDS-PAGE (Fig. 7A, lanes 2 and 3). This showed that the chromatography completely separated the R-LPS from the region I material. Both were analyzed by immunoblotting (Fig. 7B, lanes 2 and 3). As expected, a ladder pattern in region I was found. In contrast, there was no recognition of the highly mobile R-LPS. Both components were then analyzed for glycosyl residue composition and by 1H NMR spectroscopy (Fig. 8).
FIG. 8.
1H NMR spectra of the R-LPS (A) and S-LPS (B) of X. hyacinthi S148.
The glycosyl residue composition of the R-LPS, as determined by gas chromatography-mass spectrometry analysis of the trimethylsilyl methyl glycosides, showed a predominance of mannose (Man), glucose (Glc), galacturonic acid (GalA), and N-acetylglucosamine (GlcNAc) in a 1:1:1:1 molar ratio. The latter sugar is probably a component of lipid A, so the core region would consist of the Man, Glc, and GalA. Minor amounts of Rha and Fuc were also detected. There was no Kdo or heptose (Hep) detected by this method. Neither Kdo nor Hep is present in the LPS of this strain, or they are modified in some way that does not allow detection by this method. The R-LPS also contained the β-OH fatty acids that are common components of LPS. Although a lack of standards precludes a molar comparison to the glycosyl components, the fatty acids were abundant and detected in a 1:1 ratio of 3-OH--C-12 and 3-OH--C-13, with minor amounts of 3-OH--C-14. 1H NMR analysis of the R-LPS showed the signals that arise from the fatty acids (Fig. 8A). The signals at 1.2 to 1.5 ppm are due to the various CH2 protons, and the signal at 0.8 ppm is from the terminal CH3. The resonances from the carbohydrate protons are found between 3.4 and 4.4 ppm, and the two sharp resonances at 1.95 and 2.05 ppm indicate the presence of acetyl groups on some of the glycosyl residues. Although poorly resolved (due to the poor solubility of the sample), the resonances at 1.8 and 2.4 ppm indicate that there is, in fact, some Kdo associated with the LPS core. In contrast to the R-LPS, the low-mobility ladder pattern material contained predominantly Rha and Fuc (>95% of total carbohydrate) in a 2:1 molar ratio. However, the fatty acids and GlcNAc of lipid A, as well as the core glycosyl components, were also present in the region I preparation. This demonstrated that MAb 2E5 is specific for the O antigen of the LPS. The NMR spectrum (Fig. 8B) showed that the signals associated with the C-6 methyl protons of the Rha and Fuc are found at 1.4 and 1.15 ppm, respectively, and those of the anomeric region are found at 4.6 to 5.4 ppm. A minor resonance at 0.8 ppm is due to the terminal methyl protons of the fatty acids, and the CH2 resonances are obscured by the Rha and Fuc signals. The 2:1 molar ratio of Rha to Fuc is supported by the relative areas of the C-6 methyl proton resonances; however, it is not clear from the present data if the O antigen is comprised of linear or branched trisaccharide repeats.
In conclusion, all data indicated that the ladder pattern is a result of the sequential degree of polymerization of O antigen in the S-LPS. Due to the fact that MAb 2E5 did not recognize the R-LPS, we can conclude that the epitope is the O antigen, which consists of Rha and Fuc.
Sensitivity of MAbs and application in routine detection of yellow disease.
To determine the lowest detection threshold for X. hyacinthi, microtiter plates were coated with serially diluted bacterial cells (ACP-ELISA). The range of healthy background was 0.00 to 0.02; which differed significantly (data not shown) from the lower limits of the ELISA. MAb 2E5 could detect as little as 10,000 CFU/100 μl; the detection limit of MAb 1B10 was about 85,000 CFU/100 μl (Fig. 6B). For routine detection of hyacinth plants suspected to be infected, leaf extracts were processed as described above and tested in DAS-ELISA with MAbs 2E5 and 1B10; the detection limits were about 20,000 CFU/100 μl (33 × 105 CFU per g of hyacinth leaf) and 100,000 CFU/100 μl (17 × 106 CFU per g of hyacinth leaf), respectively. When used in immunofluorescence labeling experiments, MAb 2E5 could detect approximately 1,000 cells/ml of hyacinth leaf extract.
DISCUSSION
In a previous study of X. hyacinthi fimbriae, polyclonal antisera raised against preparations of native or denatured fimbriae of X. hyacinthi recognized common epitopes of fimbriae and LPS from other xanthomonads (34). Although the signal in ELISA or immunoblots was considerably weaker than signal obtained with X. hyacinthi strains, these polyclonal sera cannot be used for routine detection purposes. The results of this study showed that two panels of anti-X. hyacinthi MAbs recognize two defined surface antigens: type IV fimbriae and LPS O antigen. The MAbs recognizing the O antigen of X. hyacinthi showed no cross-reaction with any other xanthomonads (Table 1). This was important, as we have occasionally isolated other pathovars from hyacinth plants, including X. translucens pv. graminis (33).
Pathovar- or species-specific MAbs have been made against several xanthomonads, including X. campestris pv. pelargonii, pv. begoniae, and pv. oryzicola. The antibodies recognizing these pathovars have been shown or suggested to react with LPS or other cell surface polysaccharides (4, 5). We found that the epitope recognized by anti-X. hyacinthi MAb 2E5 is the LPS O antigen. Although there was no reaction of MAb 2E5 with the R-LPS, composition analysis showed that the region I material contained typical lipid A fatty acid and the same sugars found in the R-LPS. We also found that the O antigen from X. hyacinthi S148 consists of rhamnose and fucose in a 2:1 molar ratio, which is different from the structurally characterized O antigen of X. campestris pv. campestris NCPPB 45. The latter has a complex hexasaccharide repeating structure consisting of rhamnose, galactose, and galacturonic acid in a molar ratio of 4:1:1 (8). There are other Xanthomonas pathovars (pv. pelargonii, malvacearum, and vasculorum) that produce fucose-containing LPSs (23, 37). However, these bacteria must have epitopes that are immunologically from those of X. hyacinthi, since no cross-reaction was found with O-antigen-specific MAbs or antifimbrial MAbs. All strains of X. hyacinthi were recognized by MAb 2E5, indicating that the O antigen is not strain specific. This is in contrast to what has been found for enteropathogens, such as Escherichia coli or Salmonella spp., which produce highly variable O antigens (39). Reports on the occurrence of X. hyacinthi in the field are (with a few exceptions) restricted to The Netherlands, where by far the greater part of hyacinth culture is situated (35). Recently, X. hyacnthi was reclassified as a new Xanthomonas species, based on biochemical characteristics and its high G+C content (69%) of DNA (36). The collection of X. hyacinthi isolates used in this study were isolated from sites separated in time, place, and host plant. The isolates showed differences in growth rate, production of extracellular polysaccharides, and rate of pathogenicity in hyacinths (33).
Analysis of X. hyacinthi S148 R-LPS core indicated the presence of Kdo and equimolar amounts of mannose, glucose, and galacturonic acid. The presence of low levels of Kdo is in agreement with the finding that the LPS of Xanthomonas sinensis contains one Kdo per LPS molecule (38). In addition, the core composition of X. hyacinthi S148 is generally similar to that of Xanthomonas sinensis (19) and, interestingly, the R-LPSs of Sinorhizobium spp. (27, 28). Future studies may determine whether the LPS core regions of these bacterial species are structurally related.
Fimbriae were only recently described for Xanthomonas (24, 34), and this is the first report of MAbs that recognize these filamentous protein structures. Most antifimbria (group 15) MAbs showed good reaction when applied in immunoblotting experiments, in contrast to their low titer in DAS-ELISA. Immunization of mice with purified type IV fimbriae from X. hyacinthi S148 resulted in nine MAbs. Three of these MAbs showed only weak cross-reactivity with X. translucens pv. hordei and pv. translucens, both of which were isolated from Hordeum vulgare (barley). The type IV fimbriae from X. hyacinthi and X. translucens might have common features and bind to compatible leaf surface receptors of these monocots. The type IV fimbriae are expressed by a number of bacterial genera, and many aspects of their structure and immunological properties have been described (29). The fimbrial subunit of type IV fimbriae has a conserved N-terminal amino acid sequence and an immunodominant variable central and C-terminal domain. The specificities of the antifimbrial MAbs described here suggest that these parts are recognized by the MAbs. Type IV fimbriae are found and expressed by a number of Xanthomonas species and their pathovars (24, 34). A strategy of raising MAbs against type IV fimbriae of other Xanthomonas species and their pathovars might be feasible. Another approach is the production of polyclonal sera against synthetic peptides, homologous to the variable and immunodominant domains of the fimbriae (12, 22), resulting in diagnostically applicable antibodies.
A drawback for using type IV fimbriae as the target antigen might be the existence of phase variation or antigenic variation as described for N. gonorrhoeae and Moraxella bovis (13, 20). However, these phenomena were not found for any of the isolates of X. hyacinthi under study. The presence of fimbria-like structures that are not recognized by the antifimbrial MAbs (Fig. 4C) indicates the existence of at least one other type of fimbriae. The expression of different fimbriae in the genus Xanthomonas has been detected with X. campestris pv. vesicatoria mutants (24). The attachment of X. hyacinthi and its type IV fimbriae to stomata of hyacinth leaves (34) suggests a role for these surface antigens in the first stages of yellow disease. Attachment could be inhibited by incubation with antifimbria Fab fragments (34). The adherence of Pseudomonas aeruginosa to receptors on human epithelial cells can be blocked by MAbs binding to the C-terminal region of the fimbrial subunit located at the tip (11, 16). As the X. hyacinthi antifimbria MAbs showed variation in epitope recognition (Fig. 5), we are currently investigating the application of these MAbs in inhibition experiments of fimbrial attachment.
Importantly, six of the antifimbrial MAbs and in particular the anti-LPS MAbs may be used for the specific detection of X. hyacinthi. MAbs 2E5 and 1B10 are currently being tested by the Bulb Inspection Service of The Netherlands. The sensitivity under laboratory conditions of MAb 2E5 is limited to 20,000 CFU/100 μl; under routine conditions, the detection limit in hyacinth leaf extract is close to 50,000 CFU/100 μl. In most cases, this is sufficient to detect the presence of X. hyacinthi in the infection sites; the application of these MAbs in immunofluorescence labeling experiments results in higher sensitivity of the detection of this bacterium. However, the use of the MAbs for routine detection with DAS-ELISA is preferred by most inspection services, due to the fast and automated logistics developed over the last decade.
ACKNOWLEDGMENTS
T.O.-R. was supported by the Academy of Finland. B.L.R. was funded by grant MCB-9728564 from the National Science Foundation and by the U.S. Department of Energy-funded Center for Plant and Microbial Complex Carbohydrate Research under grant DE-FG02-93ER-20097.
REFERENCES
- 1.Alvarez A M, Benedict A A, Mizumoto C Y, Hunter J E, Gabriel D W. Serological, pathological, and genetic diversity among strains of Xanthomonas campestris infecting crucifers. Phytopathology. 1994;84:1449–1457. [Google Scholar]
- 2.Alvarez A M, Benedict A A, Mizumoto C Y, Pollard L W, Civerolo E L. Analysis of Xanthomonas campestris pv. citri and X. c. citrumelo with monoclonal antibodies. Phytopathology. 1991;81:857–865. [Google Scholar]
- 3.Azad H, Schaad N W. The relationship of Xanthomonas campestris pv. translucens to frost and the effect of frost on black chaff development in wheat. Phytopathology. 1988;78:95–100. [Google Scholar]
- 4.Benedict A A, Alvarez A M, Berestecky J, Imanaka W, Mizumoto C Y, Pollard L W, Mew T W, Gonzalez C F. Pathovar-specific monoclonal antibodies for Xanthomonas campestris pv. oryzae and for Xanthomonas campestris pv. oryzicola. Phytopathology. 1989;79:322–328. [Google Scholar]
- 5.Benedict A A, Alvarez A M, Pollard L W. Pathovar-specific antigens of Xanthomonas campestris pv. begoniae and X. campestris pv. pelargonii detected with monoclonal antibodies. Appl Environ Microbiol. 1990;56:572–574. doi: 10.1128/aem.56.2.572-574.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boonekamp P M, Pomp H, Gussenhoven G C. Production and characterization of monoclonal antibodies to potato virus A. J Phytopathol. 1990;128:112–124. [Google Scholar]
- 7.Bradbury J F. Genus II. Xanthomonas Dowson 1939, 187AL. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 199–210. [Google Scholar]
- 8.Bukharov A V, Skvortsov I M, Ignatov V V, Shashkov A S, Knirel Y A, Dabrowski J. Structure of the O-specific polysaccharide of Xanthomonas campestris NCPPB 45 lipopolysaccharide. Carbohydr Res. 1993;241:309–316. doi: 10.1016/0008-6215(93)80121-t. [DOI] [PubMed] [Google Scholar]
- 9.Carlson R W, Bhat U R, Reuhs B. Rhizobium lipopolysaccharides: their structures and evidence for their importance in the nitrogen-fixing symbiotic infection of their host legumes. In: Gresshoff P M, editor. Plant biotechnology and development. Boca Raton, Fla: CRC Press; 1992. pp. 33–44. [Google Scholar]
- 10.Carlson R W, Sanders R E, Napoli C, Albersheim P. Host-symbiont interactions. III. Purification and characterization of Rhizobium lipopolysaccharides. Plant Physiol. 1978;62:912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doig P, Sastry G R K, Hodges R S, Lee K K, Paranchych W, Irvin R T. Inhibition of pilus-mediated adhesion of Pseudomonas aeruginosa to human buccal epithelial cells by monoclonal antibodies directed against pili. Infect Immun. 1990;58:124–130. doi: 10.1128/iai.58.1.124-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forest K T, Bernstein S L, Getzoff E D, So M, Tribbick G, Geysen H M, Deal C D, Tainer J A. Assembly and antigenicity of the Neisseria gonorrhoeae pilus mapped with antibodies. Infect Immun. 1996;64:644–652. doi: 10.1128/iai.64.2.644-652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas R, Meyer T F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 14.Janse J D, Miller H J. Yellow disease in Scilla tubergeniana and related bulbs caused by Xanthomonas campestris pv. hyacinthi. Neth J Plant Pathol. 1983;89:203–206. [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lee K K, Sheth H B, Wong W Y, Sherburne R, Paranchych W, Hodges R S, Lingwood C A, Krivan H, Irvin R T. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol Microbiol. 1994;11:705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 17.Leite R P, Minsavage G V, Bonas U, Stall R E. Detection and identification of phytopathogenic Xanthomonas strains by amplification of DNA sequences related to the hrp genes of Xanthomonas campestris pv. vesicatoria. Appl Environ Microbiol. 1994;60:1068–1077. doi: 10.1128/aem.60.4.1068-1077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louws F J, Fulbright D W, Stephens C T, de Bruijn F J. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl Environ Microbiol. 1994;60:2286–2295. doi: 10.1128/aem.60.7.2286-2295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lüderitz O, Freudenberg M A, Galanos C, Lehmann V, Rietschel E T, Shaw D H. Lipopolysaccharides of gram-negative bacteria. Curr Top Membr Transp. 1982;17:79. [Google Scholar]
- 20.Marrs C H, Ruehl W W, Schoolnik G K, Falkow S. Pilin gene phase variation of Moraxella bovis is due to an inversion of the pilin genes. J Bacteriol. 1988;170:3032–3039. doi: 10.1128/jb.170.7.3032-3039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minsavage G, Schaad N W. Characterization of membrane proteins of Xanthomonas campestris pv. campestris. Phytopathology. 1983;73:747–755. [Google Scholar]
- 22.Nicolson I J, Perry A C F, Virji M, Heckels J E, Saunders J R. Localization of antibody-binding sites by sequence analysis of cloned pilin genes from Neisseria gonorrhoeae. J Gen Microbiol. 1987;133:825–833. doi: 10.1099/00221287-133-4-825. [DOI] [PubMed] [Google Scholar]
- 23.Ojanen T, Helander I M, Haahtela K, Korhonen T K, Laakso T. Outer membrane proteins and lipopolysaccharides in pathovars of Xanthomonas campestris. Appl Environ Microbiol. 1993;59:4143–4151. doi: 10.1128/aem.59.12.4143-4151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojanen-Reuhs T, Kalkkinen N, Westerlund-Wikström B, van Doorn J, Haahtela K, Nurmiaho-Lassila E-L, Wengelnik K, Bonas U, Korhonen T K. Characterization of the fimA gene encoding bundle-forming fimbriae of the plant pathogen Xanthomonas campestris pv. vesicatoria. J Bacteriol. 1997;179:1280–1290. doi: 10.1128/jb.179.4.1280-1290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reuhs B L. Acidic capsular polysaccharides (K antigens) of Rhizobium. In: Stacey G, Mullin B, Gresshoff P M, editors. Biology of plant-microbe interactions. St. Paul, Minn: IS-MPMI; 1996. pp. 331–336. [Google Scholar]
- 26.Reuhs B L, Carlson R W, Kim J S. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J Bacteriol. 1993;175:3570–3580. doi: 10.1128/jb.175.11.3570-3580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuhs B L, Geller D P, Kim J S, Fox J E, Kumar Kolli V S, Pueppke S G. Sinorhizobium fredii and Sinorhizobium meliloti produce structurally conserved lipopolysaccharides and strain-specific K antigens. Appl Environ Microbiol. 1998;64:4930–4938. doi: 10.1128/aem.64.12.4930-4938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuhs B L, Kim J S, Badgett A, Carlson R W. Production of the cell-associated polysaccharides of Rhizobium fredii USDA205 is modulated by apigenin and host root extract. Mol Plant-Microbe Interact. 1994;7:240–247. doi: 10.1094/mpmi-7-0240. [DOI] [PubMed] [Google Scholar]
- 29.Strom M S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 30.Sulzinski M A, Schlagnhaufer B, Moorman G W, Romaine C P. PCR-based detection of artificial latent infections of geranium by Xanthomonas campestris pv. pelargonii. J Phytopathol. 1998;146:111–114. [Google Scholar]
- 31.Tennent J M, Mattick J S. Type 4 fimbriae. In: Klemm P, editor. Fimbriae, adhesins, genetics, biogenesis and vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 127–146. [Google Scholar]
- 32.Tsai C, Frisch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 33.van Doorn, J. 1998. Unpublished data.
- 34.van Doorn J, Boonekamp P M, Oudega B. Partial characterization of fimbriae of Xanthomonas campestris pv. hyacinthi. Mol Plant-Microbe Interact. 1994;7:334–344. doi: 10.1094/mpmi-7-0334. [DOI] [PubMed] [Google Scholar]
- 35.van Doorn J, Roebroeck E J A. Xanthomonas campestris pv. hyacinthi: cause of yellow disease in Hyacinthus. In: Swings J G, Civerolo E L, editors. Xanthomonas. London, United Kingdom: Chapman & Hall; 1993. pp. 83–91. [Google Scholar]
- 36.Vauterin L, Hoste B, Kersters K, Swings J. Reclassification of Xanthomonas. Int J Syst Bacteriol. 1995;45:472–489. [Google Scholar]
- 37.Volk W A. Cell wall lipopolysaccharides from Xanthomonas species. J Bacteriol. 1966;91:39–42. doi: 10.1128/jb.91.1.39-42.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissbach A, Hurwitz J. The formation of 2-keto-3-deoxyheptanoic acid in extracts of Escherichia coli B. J Biol Chem. 1958;234:705–709. [PubMed] [Google Scholar]
- 39.Whitfield C, Valvano M A. Biosynthesis and expression of cell-surface polysaccharides in Gram-negative bacteria. Adv Microb Physiol. 1993;35:135–246. doi: 10.1016/s0065-2911(08)60099-5. [DOI] [PubMed] [Google Scholar]
- 40.York W S, Darvill A G, McNeil M, Stevenson T T, Albersheim P. Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 1985;118:3–40. [Google Scholar]