Abstract
Objective
Acute liver injury (ALF) is a potential factor of many serious hepatopathies. Carbon tetrachloride (CCl4) is a possible environmental toxicant that can induce ALF. Portulaca oleracea (PO) is one of the most popular edible herbs and has several biological activities such as antioxidant, antimicrobial, anti-inflammatory effects. We explored the significance of PO in regulating inflammatory function in animal models and cultured hepatocytes during liver damage caused by CCl4.
Methods
The effect of PO on ALF was evaluated by CCl4-induced mice models in vivo. Hepatic levels of transaminase activities and inflammatory factors were examined. The gene and protein expression of S100A8 and S100A9 were measured by RT-PCR and Western blot analysis. Meanwhile, the efficacy of PO was certified by HepG2 cells in vitro. The transaminase activities, inflammatory factors, and the protein expression of S100A8 and S100A9 were also detected.
Results
Animal tests showed that pretreatment with PO reduced the liver pathological tissue damage and the serum levels of ALT, AST, ALT and LDH, as well as reducing the pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) secretion in CCl4-induced liver injury mice. Simultaneously, HepG2 cells pretreated with PO exhibited a significant decrease in the activities of ALT and AST. Moreover, PO resulted in a significant downregulation of the pro-inflammatory markers S100A8, S100A9 gene and protein expression on CCl4 induced acute liver injury was demonstrated entirely in vivo and vitro experiments.
Conclusion
PO may down-regulate S100A8 and S100A9 and inhibit pro-inflammatory cytokines' release, indicating a potential clinical effect for controlling the disease.
Keywords: acute liver injury, CCl4, Portulaca oleracea L., S100A8, S100A9
1. Introduction
The liver is a vital organ and plays a crucial role in metabolism and detoxification, making the liver one of the most vulnerable organs. Acute liver injury is a potential factor of many serious hepatopathies, which ultimately progress to hepatic failure (Wu et al., 2010). Acute liver injury can be induced by viral hepatitis, medicine induction, alcoholism, toxins, and hepatic ischemia–reperfusion injury. Carbon tetrachloride (CCl4) is a potential environmental toxicant that can induce liver injury by generating reactive oxygen species (ROS) through the hepatic cytochrome P450 system (Chang et al., 2021, Munakarmi et al., 2020). The hepatotoxicity mechanism of CCl4 is multifactorial, involving inflammation, oxidative stress, immunological and apoptotic reactions (Shi et al., 2017, Jaeschke et al., 2012). CCl4-induced acute liver injury animal model has been widely used to assess the efficacy and mechanism of hepatoprotective medicine. Although hepatic diseases are responsible for a significant number of liver transplantations and deaths worldwide, the therapeutic options for liver diseases are still minimal, resulting in great demand for the development of safe and effective hepatoprotective drugs.
Portulaca oleracea L. (PO) is an annual herbaceous plant widely distributed in many parts of the world, which has been used as folk medicine and food nutrient supplements in different countries. In China, PO is one of the most popular edible herbs and is also used as a traditional medicine for alleviating a broad spectrum of diseases, including gastrointestinal diseases, kidneys, and bladder ulcers, fevers, insomnia, severe inflammations, headaches (Iranshahy et al., 2017, Qin et al., 2020). Modern pharmacological studies revealed that PO has several biological activities such as antioxidant, antimicrobial, anti-inflammatory, bronchodilator, renoprotective, neuroprotective, muscle relaxant, hepatoprotective effects (Zhou et al., 2015). As a natural anti-hepatotoxicity agent, the activity of PO extract is inseparable from the flavonoids present in their composition, such as kaempferol, apigenin, luteolin, myricetin, and quercetin. Although an excellent hepatoprotective effect of PO extract has been exhibited, the research on the mechanism of the hepatoprotective effect of PO is still limited (Zhou et al., 2015). In the present study, the anti-hepatotoxic effects of PO were evaluated on CCl4-induced acute liver injury in mice and HepG2 cells. In addition, the gene and protein expression level of S100A8 and S100A9 were assessed to reveal the mechanism of the hepatoprotective effect of PO.
2. Materials and methods
2.1. Herbal materials
PO was collected in the wild at Yumin County of Tacheng area in Xinjiang Province (46.1°N, 83.0°E) in July 2014. The herbal material was identified and authenticated as Portulaca oleracea L. (Portulacaceae) by Professor Chunlin Long, College of Life and Environmental Science, Minzu University of China (Beijing, China). A voucher specimen (No. 20140601) was deposited in the School of Pharmacy, Minzu University of China (Beijing, China).
2.2. In vivo assessment of anti-hepatotoxic activity
2.2.1. Preparation of aqueous herbal extract
The aqueous herbal extract was prepared to evaluate the anti-hepatotoxic activity of PO in mice. The plant (1.0 kg) was ground and soaked in 10 times of distilled water for 2 h to improve the extraction rates, then heated by 100 °C, reflux extracted for two times, and each time for 2 h. The collected extracts were evaporated under a vacuum, and finally, 4.0 g/mL concentration of the extracts was remained as stock solution and kept at − 20 °C refrigerator. Before being administered to animals, the stock solution was dissolved completely, mixed evenly, and then diluted to each application dose by water.
2.2.2. Animal and treatment
Male Kunming mice (21–25 g) were purchased from the Vital River Laboratory Animal Technology Co., ltd., (Beijing, China). All animal experiments complied with the National Research Council's Guide for the Care and Use of Laboratory Animals. The Institutional Animal Ethics Committee approved the protocol of the Minzu University of China, (Beijing, China) (approval number: ECNUC2019008AO). The animals were housed in standard temperature and humidity conditions with a 12 h light–dark cycle and free access to food and water. Animal care and experimental procedures were approved by the biological and medical ethics committee of the Minzu University of China. After adaptation for 3 d, the mice were randomly divided into six groups consisting of 10 animals per group. Control group (Group 1): mice not given CCl4 and PO; CCl4 model group (Group 2): mice treated with CCl4 alone; CCl4 + PO group (Groups 3–5): mice treated with CCl4 and administered with PO at a dose of 1.0 g/kg, 2.0 g/kg and 4.0 g/kg, representative of low, medium and high dosages, respectively; CCl4 + Silibinin group (Group 6): mice treated with CCl4 and administered with silibinin at a dose of 0.2 g/kg. The mice in Group 1 and Group 2 were pretreated with water alone; Groups 3–5 and Group 6 were intragastrically treated daily with PO (1.0 g/kg, 2.0 g/kg, and 4.0 g/kg) and silibinin (0.2 g/kg) for 5 d, respectively. On the 5th d after administration for two hands fasting for 12 h, mice in Groups 2–6 received an intraperitoneal injection of 0.2% CCl4 in peanut oil (volume percentage, 10 mL/kg). In contrast, mice in Group 1 received an intraperitoneal injection of the same volume of peanut oil used as a vehicle. Twenty-four hours after CCl4 injection, all the animals were anesthetized using pentobarbitone (35 mg/kg, i.p.). The abdominal artery was isolated, and blood was collected. The blood was allowed to clot for 1 h at room temperature, and the serum was separated by centrifugation at 3500 rpm for 15 min and used for biochemical estimations. After collection of the blood, livers were excised and rinsed in saline. Some part of the liver was taken for biochemical examination, and some part preserved was placed in 10% formalin in PBS for histopathological analysis. The remaining liver was frozen in liquid nitrogen and stored at –80 °C refrigerator.
2.2.3. Serum measurements
On the fifth day after administration for two hands, Serum Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Lactate dehydrogenase (LDH), and Alkaline phosphatase (ALP) were measured with an Automatic Biochemistry Analyzer (Hitachi 7020, Tokyo, Japan). Serum TNF-α, IL-6, and IL-1β were determined using commercially available ELISA kits (BOSTER Biological Technology Co., ltd., Wuhan, China).
2.2.4. Liver histology
The liver samples were fixed in 10% formalin, processed by routine histology procedures, embedded in paraffin, cut in 5 μm pieces, and mounted on the slide. The samples were stained with hematoxylin and eosin (H&E) for histopathological examination. The images were examined and evaluated for pathological changes.
2.2.5. RT-PCR analysis
S100A8 and S100A9 in 220 differential genes (Zhou et al., 2020) from transcriptome analysis were selected for quantitative confirmation by quantitative real-time PCR (qRT-PCR) analysis with Applied Biosystems 7500 Real-Time PCR Systems (Thermo, USA). According to the manufacturer's instructions, total RNA from liver specimens was isolated using TRIzol reagent (Invitrogen). After reverse transcription by PrimeScript RT reagent Kit (Takara, JP), we obtained the cDNA of the total mRNA. Then, SYBR Premix Ex Taq (Takara, JP) was used in the quantitative PCR reaction, and primers were shown in Table 1. The expression levels of β-actin were regarded as a reference for the gene. The fold changes were calculated by the 2-ΔΔCT method, and the qPCR reactions were performed in biological triplicates.
Table 1.
Primer sequences.
| Primers | Sequences (5′–3′) |
|---|---|
| S100A8 (Forward) | AAATCACCATGCCCTCTACA |
| S100A8 (Reverse) | TATCACCATCGCAAGGAACT |
| S100A9 (Forward) | CCAACAAAGCACCTTCTCAG |
| S100A9 (Reverse) | CCATCAGCATCATACACTCCTC |
2.2.6. Western blot analysis
Total protein was extracted from frozen liver samples by RIPA lysis buffer (Applygen, China) with protease inhibitor. Proteins were determined by the bicinchoninic acid (BCA) method. Samples were centrifuged for 15 min at 4 °C and 12 000 rpm and supernatants were collected. Protein concentrations were measured with a BCA Protein Assay Kit (Pierce). After denaturation, liver protein samples were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA, USA). Membranes were blocked for 2 h in 1 × TBST with 5% nonfat milk. Primary antibodies against S100A8 (1:1000; ab92331, Abcam), S100A9 (1:250; ab92507, Abcam), and β-actin (1:1000; ac026, Abclonal) were incubated with the membrane overnight at 4 °C. The membranes were washed three times with TBST. The membrane was then incubated with the corresponding secondary antibodies (HRP goat anti-rabbit IgG; AS014, Abclonal) at room temperature for 1 h and finally were scanned with the Tanon 4200SF fully automated chemiluminescence image analysis system (Shanghai Tianeng Technology Co., ltd., Shanghai, China). The lanes were analyzed by Image J software.
2.3. In vitro assessment of anti-hepatotoxic activity
2.3.1. Preparation of aqueous lyophilized powder
The plant (100 g) was ground and soaked in 10 times of distilled water for 2 h to improve the extraction rates, then heated by 100 °C, reflux extracted for two times, and each time for 2 h. The collected extracts were evaporated under a vacuum, then freeze-dried by lyophilizer, and stored at a cool and dry place.
2.3.2. Cell culture
Procell Life Science & Technology Co., ltd., supplied HepG2 (CL-0103) cell. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, high glucose) (SIGMA, USA) supplemented with 10% FBS (Gigco, USA) and 100 U/mL penicillin/streptomycin (Gibco, USA) and maintained at 37 °C and 5% CO2. Cells were passaged at 80% concentration. All experiments were conducted within five passages.
2.3.3. Cell viability experiment
HepG2 cells were seeded at a density of (1 × 104)/mL in 96-well plates. The cells were treated with PO lyophilized powder (0, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5 mg/mL) dissolved in DMEM and incubated for 24 h to select the highest dose concentration. The cells were treated with different ratio of saturated CCl4 (0%, 30%, 40%, 50%, 60%, 70%, 80%, 90%) dissolved in DMEM and incubated for 4 h, to select the modelling concentration. For efficacy study, HepG2 cells were pretreated with PO (0, 0.5, 0.75, 1, 2, 2.5. 3, 4 mg/mL) for 24 h, followed by CCl4 (55% saturated solution) intervened for 4 h. The cell viability was determined by Cell Counting Kit-8 (CCK-8) reagent. The absorbance values were read at 450 nm using an enzyme-labeled instrument (Flexstation3; Molecular Devices; America). Cell viability was calculated based on the absorbance value.
2.3.4. Transaminase viability detection
HepG2 cells were seeded at a density of (1 × 104)/mL in 24-well plates and incubated overnight. The cells were then pretreated with PO (0, 0.5, 0.75, 1, 1.5, 2, 3 mg/mL) for 24 h, followed by CCl4 (55% saturated solution) intervened for 4 h. After the above experiment, the ALT and AST activities in the supernatant were determined by the Reitman-Frankel method using an enzyme viability kit (Nanjing Jiancheng Bioengineering Institute, China).
2.3.5. Western blot analysis
HepG2 cells were seeded at a density of (4.8 × 105)/mL in 6-well plates and incubated overnight. Then the cells were pretreated with PO (0,1, 2, 2.5, 3, 4 mg/mL) for 24 h, followed by CCl4 (55% saturated solution) intervened for 4 h. HepG2 cells were collected and placed in RIPA lysis buffer (Applygen, China) with protease inhibitor and centrifuged (12 000 rpm for 5 min) to remove impurities at the end of the experiment. Proteins were determined by the bicinchoninic acid (BCA) method. Protein samples (total protein, 50 μg/lane) were separated by 12% polyacrylamide gel electrophoresis and then transferred to membranes (polyvinylidene fluoride, PVDF, Millipore, USA). Membranes were blocked for 1 h in 1 × TBS with 5% nonfat milk. Primary antibodies against S100A8 (1:1000; ab92331, Abcam), S100A9 (1:250; ab92507, Abcam), and β-actin (1:1000; ac026, Abclonal) were incubated with the membrane overnight at 4 °C. The membranes were washed three times with TBST. The membrane was then incubated with the corresponding secondary antibodies (HRP goat anti-rabbit IgG; AS014, Abclonal) at room temperature for 1 h and finally were scanned with the Tanon 4200SF fully automated chemiluminescence image analysis system. The lanes were analyzed by Image J software.
2.4. Statistical analysis
Statistical analysis was performed using R software. The results were presented as mean ± SD when obeying normal distribution. If one-way ANOVA was used to analyze homogeneity of variance, statistical differences between groups and multiple comparisons are made by LSD-t-test; The heterogeneity of variance, differences among multiple groups were compared using the Kruskal-Wallis test and the Wilcox test between the two groups. The results were presented as the median (interquartile spacing), differences between multiple groups were compared using the Kruskal-Wallis test. Differences with P-values of < 0.05 were considered statistically significant.
3. Results
3.1. PO manifests significant anti-hepatotoxic activity on CCl4-induced acute liver injury
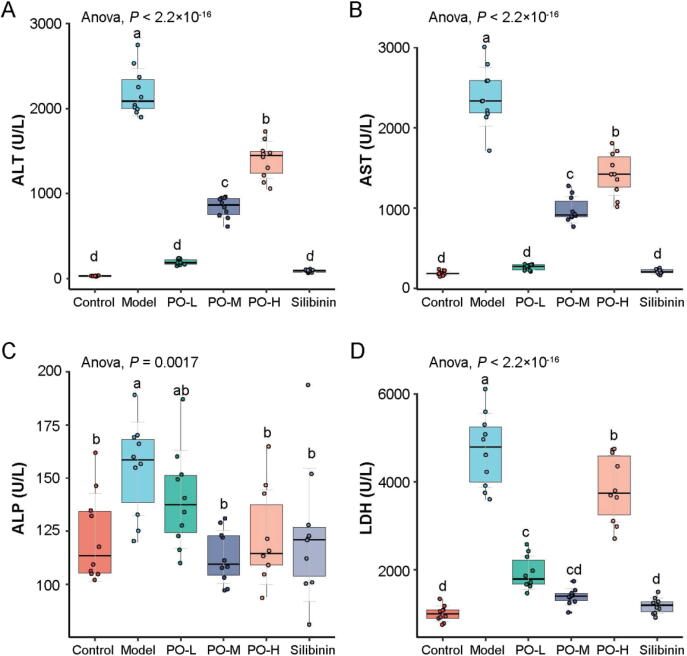
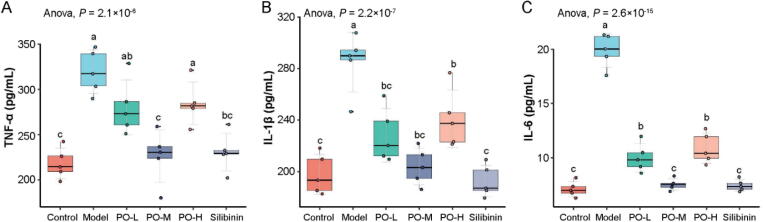
The effects of PO pretreatment on the CCl4-induced modifications in serum ALT, AST, ALP, and LDH levels were shown in Fig. 1. A single dose of CCl4 caused hepatotoxicity in mice, as indicated by an increase in serum ALT, AST, ALP, and LDH activities after CCl4 administration. In contrast, mice pretreated with PO exhibited a significant decrease in the activities of the hepatic enzymes (P < 0.01). Significantly, the effect of a low dose of PO was equivalent to silibinin, and the hepatic enzyme reduction effect was reduced with the dose increases. TNF-α, IL-1β, and IL-6 are critical inflammatory mediators involved in hepatocyte damage induced by CCl4. The levels of the cytokines were significantly increased in the CCl4-treated mice (P < 0.01). This increase was reduced in PO, especially in the middle dose group, equivalent to the silibinin-treated group (Fig. 2).
Fig. 1.
PO alleviates liver injury induced by carbon tetrachloride in mice. Effects of PO on ALT (A), AST (B) ALP (C) and LDH (D) of serum levels. Different letters represent the significant differences between each group.
Fig. 2.
PO reduces carbon tetrachloride-induced liver inflammation in mice. Effect of PO on serum TNF-α (A), IL-1β (B) and IL-6 (C) levels. Different letters represent the significant differences between each group.
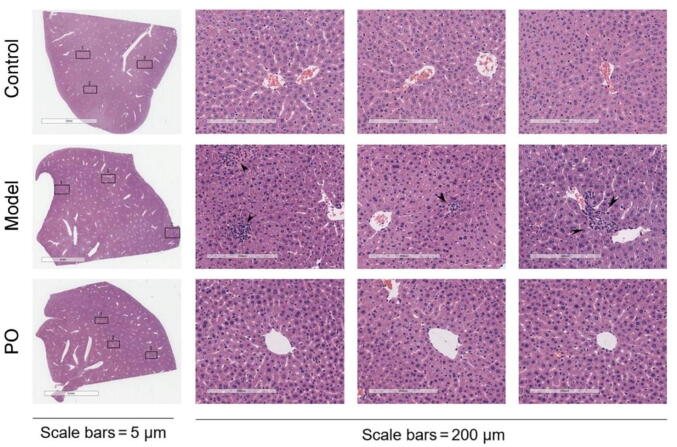
Histological evaluations provided direct evidence of the protective effects of treatment with PO (medium dose) on CCl4-induced liver injury. The control group showed lobular architecture with central veins and radiating hepatic cords. Hepatotoxicity induced by CCl4 was confirmed by abnormal histological findings manifested by morphological changes, such as inflammation around the portal triad, disorganization of hepatocytes, cell swelling, and congestion of sinusoids. In PO- and silibinin-treated groups, the histopathological abnormalities induced by CCl4 were restored (Fig. 3).
Fig. 3.
Histopathological changes of liver tissues (H&E staining; original magnification × 40, scale bar = 200 μm).
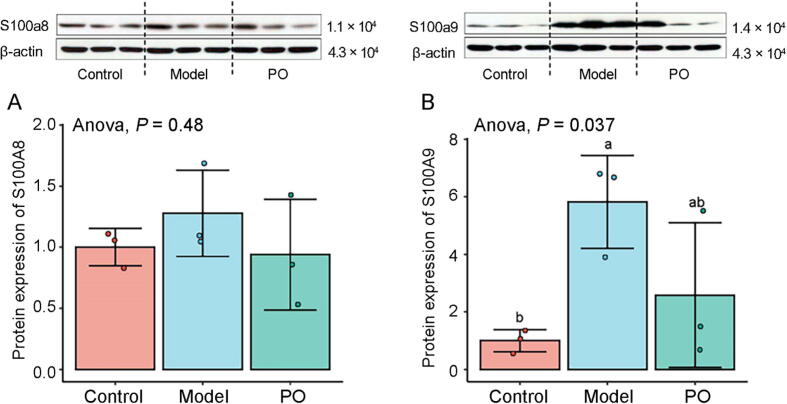
3.2. PO down-regulates S100A8 and S100A9 expression
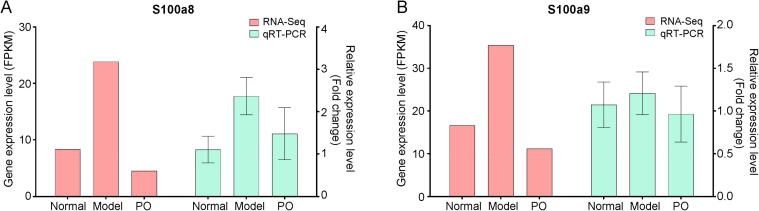
It was confirmed that S100A8/A9 was a biomarker in liver injury (Moles et al., 2014). Previous study has demonstrated that S100A8 and S100A9 were the differential genes in CCl4-induced ALF model according to the KEGG enrichment analysis (Zhou et al., 2020). The differential gene expression (DEGs) of KEGG enrichment analysis and pathways in the top 15 positions of significance were separately shown in Table S1. Therefore, the gene and protein expression levels of S100A9 and S100A8 were analyzed by RT-PCR and Western blot assay. The results showed that the gene expression of S100A8 and S100A9 were up-regulated in the model group and slightly down-regulated by PO, which was consistent with RNA-Seq. Meanwhile, the protein expression levels of S100A9 and S100A8 were analyzed by Western blot assay, and the results showed that PO down-regulated protein expression of S100A8 and S100A9, which significantly in S100A9 (P < 0.05). The results were certified in subsequent cell experiments (Fig. 4, Fig. 5).
Fig. 4.
Gene expression levels of S100A8 (A) and S100A9 (B).
Fig. 5.
Protein expression levels of S100A8 (A) and S100A9 (B).
3.3. PO reduce ALT, AST enzyme viabilities and down-regulate S100A8, S100A9 protein expression in HepG2 cells
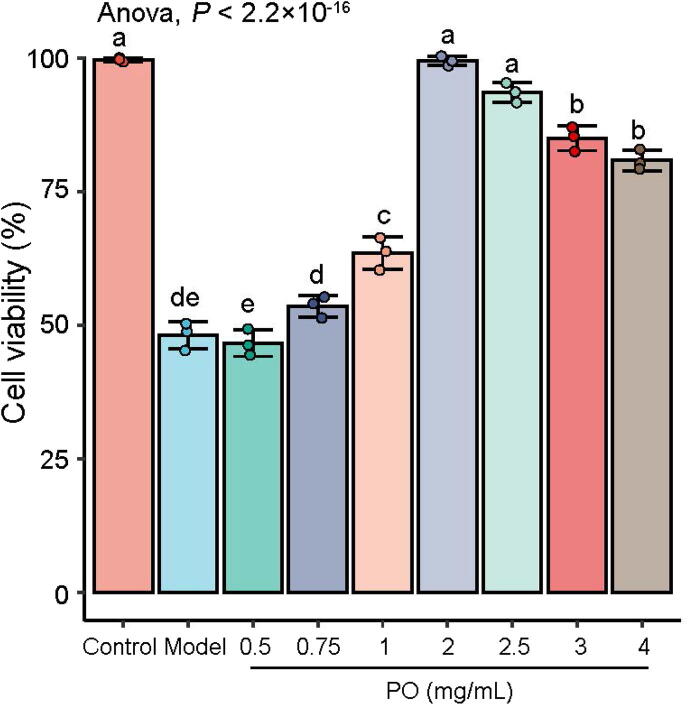
PO (100 g) were freeze-dried to 10.1 g lyophilized powder, and the extraction rate was 10.16%. Results obtained by the cell viability, the highest dose concentration of PO was 3 mg/mL, and the modeling concentration was 55% ratio of saturated CCl4 dissolved in DMEM. The effects of PO pretreatment on the CCl4-induced HepG2 cell viabilities were shown in Fig. 6. Cell viabilities were reduced by CCl4, while PO increased the cell viabilities, especially at 2 mg/mL.
Fig. 6.
Effect of PO on cell viability of HepG2 cells. Different letters represent the significant differences between each group.
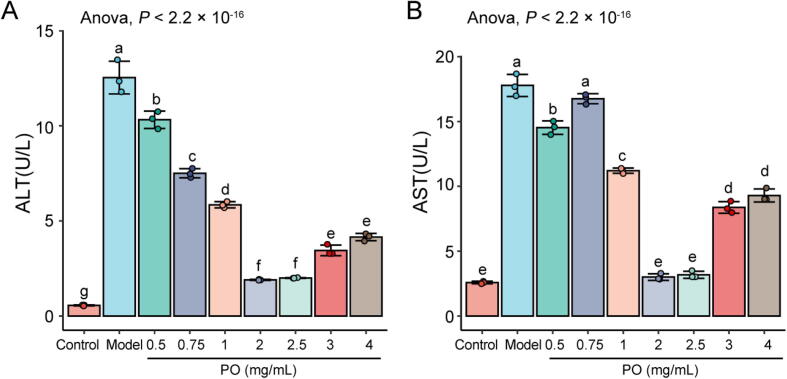
The modifications in cell supernatant ALT, AST levels were shown in Fig. 7. The CCl4 increased the ALT and AST activities in cell supernatants, whereas the HepG2 cells pretreated with PO exhibited a significant decrease in the activities of the transaminases. It is noteworthy that PO reduced transaminase activities were not dose-dependent; Doses at 2 mg/mL and 2.5 mg/mL were most significant (P < 0.01).
Fig. 7.
PO decrease in activities of ALT (A) and AST (B). Different letters represent the significant differences between each group.
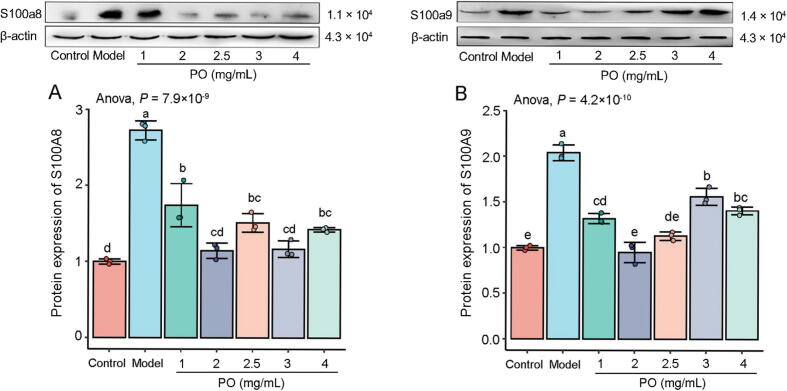
PO significantly downregulated the protein expression levels of S100A9 and S100A8 in HepG2 cells, but it was not dose-dependent (P < 0.01) (Fig. 8).
Fig. 8.
Expression levels of S100A8 (A) and S100A9 (B). Different letters represent the significant differences between each group.
4. Discussion
Modern pharmacological studies have indicated the anti-hepatotoxic effects of P. oleracea (PO), such as tumors and toxicant damage. The protective effect of PO extract against CCl4-induced hepatic toxicity has been reported (Shi et al., 2014). Furthermore, PO extract treatment inhibited the increased serum activities of hepatic marker enzymes and ameliorated the histological alterations in the liver tissue induced by CCl4. Consistently, the present study demonstrated the hepatoprotective effects of PO aqueous extract against CCl4-induced acute liver injury in mice. Meanwhile, PO aqueous extract inhibited the increased serum activities of hepatic marker enzymes (ALT, AST, ALP, GSH) and ameliorated the histological alterations in the liver tissue induced by CCl4. In addition, PO administration decreased serum levels of IL-6, IL-1β, and TNF-α in mice, suggesting that PO may protect against CCl4-induced hepatotoxicity by ameliorating inflammation responses. From the overall experimental results, the effect of PO on each index was not dose-dependent. Briefly, it does not mean that the higher the dose is, the better the effect is. The hepatic marker enzymes were relatively reduced in high doses of PO.
Meanwhile, it had the same appearances in cell viability experiments. It may be considered the high dose of medication burden or damage to the liver metabolism. Therefore, the middle dose (2.0 g/kg) can be defined as a beneficial administration dose from this experiment; This claim can also be validated in cell experiments. It has been widely accepted that oxidative stress plays a prominent role in the progression of hepatic damage induced by CCl4, and ROS are the leading causes of CCl4-induced acute liver injury. Therefore, anti-oxidative therapy is the primary strategy for preventing and attenuating oxidative stress-related liver diseases (Liu et al., 2018). However, RNA-sequencing analysis in the previous study revealed that inflammation and immunity-related signaling pathways were involved in the mechanisms of hepatoprotective effects of PO, including cytokine-cytokine receptor interaction, inflammatory mediator regulation of TRP channels, Toll-like receptor signaling pathway, chemokine signaling pathway, and IL-17 signaling pathway. Inflammation is an essential defense mechanism in the human body (Zhou et al., 2020).
Various immunocytes and molecules form a massive regulatory network during inflammation, eliminating endogenous and exogenous pathogenic substances to protect the body. However, imbalance of the network, such as excessive inflammatory reactions and prolonged inflammatory status, may lead to further tissue damage. S100A8 and S100A9 have already been confirmed to play a decisive role in developing inflammation (Wang et al., 2018, Ishihara et al., 2009). S100A8 and S100A9 (also known as MRP8 and MRP14, respectively) are Ca2+ binding proteins belonging to the S100 family, which often exist in the heterodimer form (Wang et al., 2018). Under physiological conditions, constitutive expression of S100A8/A9 is mainly restricted to myeloid cells, including neutrophils and monocytes. However, prominent expression is also observed in epithelial and endothelial cells during inflammatory processes and cell damage. Increased levels of S100A8 and S100A9 are documented in several chronic inflammatory disorders, such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, cystic fibrosis, and psoriasis. Both proteins amplify proinflammatory responses in these settings by promoting leukocyte migration and inducing the release of cytokines and chemokines (De Ponti et al., 2015).
Regarding the liver, there have been some publications exploring the relationship between liver diseases and S100A9 recently (De Ponti et al., 2015, Wu et al., 2018, Rodrigues et al., 2021). One of the studies demonstrated that S100A9 showed a significant positive correlation with hepatic histologic features and biochemical indexes indicative of liver injury. It might be helpful as a reliable biomarker for developing the non-alcoholic fatty liver disease (NAFLD) (Eidi et al., 2015). Another study has used a mouse gene knockout approach to investigate the role of TLR2 in response to CCl4-induced liver injury and found that mice lacking TLR2 or S100A9 failed to recruit neutrophils to the injured liver and had a defective hepatic induction of the neutrophil chemokine CXCL-2. It is indicated that TLR2 and S100A8/S100A9 are the critical regulators of hepatic CXCL-2 expression and neutrophil recruitment (Moles et al., 2014). These findings suggested that S100A9 might have enormous potential as a biomarker for liver diseases related to inflammation (Wu et al., 2018).
5. Conclusion
The present study results demonstrated that PO may down-regulate S100A8 and S100A9 and inhibit the release of pro-inflammatory cytokines. The results of this study provide an experimental basis for using S100A9-based therapy in the treatment of ALP. It should be noted that the mechanisms between S100A9 related signaling pathways require further analysis, and further clinical trials are needed in order to assess whether the key findings of this study can be applied to human beings.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by Independent Research Projects for young teachers of Minzu University of China [No. 2021NQPY90].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chmed.2022.05.004.
Contributor Information
Zongran Pang, Email: pangzongran@muc.edu.cn.
Binan Lu, Email: binanlu@muc.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Chang S.N., Kim S.H., Dey D.K., Park S.M., Nasif O., Bajpai V.K.…Park J.G. 5-O-Demethylnobiletin alleviates CCl4-induced acute liver injury by equilibrating ROS-mediated apoptosis and autophagy induction. International Journal of Molecular Sciences. 2021;22(3):1083. doi: 10.3390/ijms22031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ponti A., Wiechert L., Stojanovic A., Longerich T., Marhenke S., Hogg N.…Angel P. Chronic liver inflammation and hepatocellular carcinogenesis are independent of S100A9. International Journal of Cancer. 2015;136(10):2458–2463. doi: 10.1002/ijc.29282. [DOI] [PubMed] [Google Scholar]
- Eidi A., Mortazavi P., Moghadam J.Z., Mardani P.M. Hepatoprotective effects of Portulaca oleracea extract against CCl4-induced damage in rats. Pharmaceutical Biology. 2015;53(7):1042–1051. doi: 10.3109/13880209.2014.957783. [DOI] [PubMed] [Google Scholar]
- Iranshahy M., Javadi B., Iranshahi M., Jahanbakhsh S.P., Mahyari S., Hassani F.V., Karimi G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. Journal of Ethnopharmacology. 2017;205:158–172. doi: 10.1016/j.jep.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Namura T., Murayama H., Arai S., Totani M., Ikemoto M. Possibility of formation of the S100A8/A9-proinflammatory cytokine complexes in vivo in acute inflammation and their functional roles. The Japanese Journal of Clinical Pathology. 2009;57(4):324–331. [PubMed] [Google Scholar]
- Jaeschke H., McGill M.R., Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metabolism Reviews. 2012;44(1):88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wen P.H., Zhang X.X., Dai Y., He Q. Breviscapine ameliorates CCl4 induced liver injury in mice through inhibiting inflammatory apoptotic response and ROS generation. International Journal of Molecular Medicine. 2018;42(2):755–768. doi: 10.3892/ijmm.2018.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A., Murphy L., Wilson C.L., Chakraborty J.B., Fox C., Park E.J.…Mann D.A. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. Journal of Hepatology. 2014;60(4):782–791. doi: 10.1016/j.jhep.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakarmi S., Chand L., Shin H.B., Jang K.Y., Jeong Y.J. Indole-3-carbinol derivative DIM mitigates carbon tetrachloride-induced acute liver injury in mice by inhibiting inflammatory response, apoptosis and regulating oxidative stress. International Journal of Molecular Sciences. 2020;21(6) doi: 10.3390/ijms21062048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Y. W., Hou, J. L., Wang, P., Wang, L., Yin, X. J., Zhang, Y., Yu, L. P., Xu, H. Y. (2020). Research progress and correlation analysis on “phytochemistry-pharmacological effects-CMM efficacy-diseases” of Portulaca oleracea. Chinese Traditional and Herbal Drugs, 51(7), 1924–1938.
- Rodrigues R.M., He Y., Hwang S., Bertola A., Mackowiak B., Ait-Ahmed Y.…Gao B. E-selectin-dependent inflammation and lipolysis in adipose tissue exacerbate steatosis-to-NASH progression via S100A8/9. Cellular and Molecular Gastroenterology and Hepatology. 2021:1–21. doi: 10.1016/j.jcmgh.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Han W., Shi H., Ren F., Chen D., Chen Y., Duan Z. Augmenter of liver regeneration protects against carbon tetrachloride-induced liver injury by promoting autophagy in mice. Oncotarget. 2017;8(8):12637–12648. doi: 10.18632/oncotarget.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Liu X., Tang G., Liu H., Zhang Y., Zhang B.…Wang W. Ethanol extract of Portulaca oleracea L. reduced the carbon tetrachloride induced liver injury in mice involving enhancement of NF-kappaB activity. American Journal of Translational Research. 2014;6(6):746–755. [PMC free article] [PubMed] [Google Scholar]
- Wang S., Song R., Wang Z., Jing Z., Wang S., Ma J. S100A8/A9 in inflammation. Frontiers in Immunology. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Zhang Y., Xiang Y., Tang Y., Cui F., Cao J.…Duan L. Association between serum S100A9 levels and liver necroinflammation in chronic hepatitis B. Journal of Translational Medicine. 2018;16(1):83. doi: 10.1186/s12967-018-1462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Han M., Chen T., Yan W., Ning Q. Acute liver failure: Mechanisms of immune-mediated liver injury. Liver Internationa. 2010;30(6):782–794. doi: 10.1111/j.1478-3231.2010.02262.x. [DOI] [PubMed] [Google Scholar]
- Zhou, Y. X., Xin, H. L., Rahman, K., Wang, S. J., Peng, C., & Zhang, H. (2015). Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. Biomed Research International, 2015, 925631. [DOI] [PMC free article] [PubMed]
- Zhou L., Song X.L., Lyv J.P., He Y.F., Pang Z.R., Lu B.N. Hepatoprotective effect of Portulacae Herba on carbon tetrachloride induced acute liver injury in mice. Chinese Journal of Experimental Traditional Medical Formulae. 2020;26:35–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.