Abstract
Objective
This study aimed to identify the main medicinal active components of Panax ginseng (P. ginseng) in the compatibility environment of clinical application. For this purpose, the anti-inflammatory ingredients of P. ginseng were investigated based on its therapeutic effect in Sijunzi Decoction (SJD) which is a widely used traditional Chinese formula.
Methods
The fingerprints of 10 batches of SJD consisting of different sources of P. ginseng were established by UPLC technique to investigate the chemical components. At the same time, the anti-inflammatory effects of these components were evaluated by dextran sulfate sodium-induced ulcerative colitis mouse model. Grey relational analysis was applied to explore the correlation degree between fingerprints and anti-inflammatory effects in SJD. Lipopolysaccharide-stimulated RAW264.7 murine macrophages were established to evaluate the anti-inflammatory action of the screened effective substances of P. ginseng.
Results
According to grey relational analysis, notoginsenoside R1, ginsenoside Rg2 and ginsenoside Rb3 of P. ginseng were the major anti-inflammatory contributions in SJD. They had been proven to be closely associated with the anti-inflammatory process of SJD and displayed a close effect compared with SJD by LPS-stimulated RAW264.7 murine macrophages.
Conclusion
Our work provides a general strategy for exploring the pharmacological ingredients of P. ginseng in traditional Chinese formulas which is beneficial for establishing the quality standards of traditional herbs in traditional Chinese medicine prescription based on their clinical therapeutic effect.
Keywords: anti-inflammation, ginsenosides, Panax ginseng C.A. Mey, Sijunzi Decoction, spectrum-effect relationship, ulcerative colitis
1. Introduction
Roots of Panax ginseng C. A. Mey are a well-known traditional Chinese medicine (TCM) in China and widely used clinically in traditional Chinese formulas. Ginsenosides are the main active constituents that show various pharmacological effects such as immunoregulation effect (Hao et al., 2019), antioxidant activity (Chen & Huang, 2019), anti-inflammatory, anti-nociceptive effects, etc (Cao, Li, Yuan, Zhang, & Liu, 2021, Lee et al., 2019). The growth of P. ginseng was affected by plant origin, species, ages, parts, soils or climates, which may lead to the differences of chemical compositions and bioactivities (Liu and Xiao, 1992, Shi et al., 2007, Kang et al., 2008, Li et al., 2012, Shan et al., 2014, Zhang et al., 2018). Consequently, it is urgent to investigate the main active components of P. ginseng to establish a quality standard ensuring clinical efficacy.
The characteristics of multiple-effects of TCM are conveyed through prescriptions for the specific disease in clinic. Therefore, the orientated effective components of TCM ought to be discovered under the condition of prescription compatibility, which provides an environment for exerting its efficacy. Sijunzi Decoction (SJD) is a classic traditional Chinese formula consisting of P. ginseng, Atractylodes macrocephala Koidz. Porica cocos (Schw.) Wolf and Glycyrrhiza uralensis Fisch. (Wang et al. 2013). It has been widely applied in gastrointestinal diseases for thousands of years in China. Previous studies demonstrated that SJD can significantly improve the inflammatory response to colitis (Gao, Peng, & Li, 2018, Lu et al., 2017, Song et al., 2016, Yu et al., 2016). According to the compatibility theory of TCM, P. ginseng is the monarch drug of SJD, which means that P. ginseng plays a major therapeutic role in the prescription. Therefore, SJD was regarded as a carrier to study the pharmacodynamic substances of P. ginseng in this research.
Chromatographic fingerprint analysis is considered to be an appropriate method to assess the consistency of botanical drugs approved by both the Food and Drug Administration and the European Medicines Agency (Li, Chen, Jia, & Xie, 2013). Therefore, chromatographic fingerprint analysis is a rational approach for quality assessment of traditional Chinese herbal medicine (Xie et al., 2006). However, chemical components are not identical to effective substances. For this reason, a spectrum‐effect relationship integrating the chemical fingerprints with pharmacological effects is established to hunt for the functional constituents. It already has extensive use in guaranteeing the quality and ascertaining bioactive ingredients in TCM (Liu et al., 2019, Zhang et al., 2019, Shen et al., 2018, He and Zhou, 2021).
Grey relational analysis (GRA) can estimate the degree of correlation between the two factors based on the comparison of similarities in the shapes of the various factors, which is often used to reveal quantitative comparisons of trends in dynamically changing systems (Kadier et al., 2015, Nelabhotla et al., 2016, Zhou et al., 2016). GRA has several advantages such as a small sample size, a small amount of calculation, and good intuition compared with other analysis methods (Shen et al., 2018).
In this study, GRA was applied to analyze the connection between chemical compositions in the fingerprints of SJD and pharmacodynamics evaluated by the ulcerative colitis (UC) mouse model. It aims to investigate the main activity substances with anti-inflammatory activities of P. ginseng in SJD during the process of treating UC. Compared with conventional fingerprints, this research restores P. ginseng to the “compatibility environment” of SJD which is coincident with the essential characteristics of the compatibility theory and clinical functions of TCM. Our results showed that notoginsenoside R1, ginsenoside Rg2 and ginsenoside Rb3 were the main activity substances of anti-inflammatory activities of P. ginseng in SJD. It can provide a strategy for establishing the quality standards of traditional herbs in traditional Chinese medicine prescription based on their clinical therapeutic effect.
2. Materials and methods
2.1. Chemicals and reagents
Notoginsenoside R1 (R1), ginsenoside Rb1 (Rb1), ginsenoside Rb2 (Rb2), ginsenoside Rb3 (Rb3), ginsenoside Rg1 (Rg1), ginsenoside Rg2 (Rg2), ginsenoside Rf (Rf), ginsenoside Ro (Ro), ginsenoside Rd (Rd), ginsenoside Re (Re), (20S)-ginsenoside Rg3 ((20S)-Rg3), (20R)-ginsenoside Rg3 ((20R)-Rg3), ginsenoside Rk2 (Rk2) (≥98% pure) were provided by the Shanghai Yuanye Biotechnology Co., ltd. (Shanghai, China). P. ginseng, A. macrocephala, P. cocos and G. uralensis applied in the study were purchased from Fusong Shenyuan Changbaishan Ginseng Technology Co., ltd. (Fusong, China).
Acetonitrile was purchased from Fisher Scientific (Waltham, MA, USA). Water was purified through a Milli-Q water purification system (Millipore, MA, USA). TNF-α, IL-1β, IL-6 and NO ELISA kits were provided by Jiangsu Mai Sha Industry Co., ltd (Yancheng, China). Dextran sulfate sodium (DSS) was obtained from MP Biomedicals (California, USA). Cell Counting Kit-8 (CCK-8) was obtained from Biosharp Life Science (Shanghai, China). Lipopolysaccaride (LPS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Griess reagents were purchased from Promega (Madison, USA).
2.2. Instruments and conditions
Chromatographic analysis was performed on a Waters ACQUITY UPLC system equipped with a photodiode array detector (Waters, USA).
2.2.1. UPLC conditions
Chromatographic separation was performed on a waters CSH-C18 column (2.1 mm × 150 mm, 1.7 μm) and maintained at 35 °C. The detection wavelength was monitored at 203 nm. The flow rate was 0.3 mL/min. The injection volume was 2 μL.
The mobile phase consisted of phosphoric acid aqueous solution (A) and acetonitrile (B) with the following optimized gradient elution: 0–7 min: 17%, 7–9 min: 17%−17.5%, 9–14 min: 17.5%−21%, 14–16 min: 21%−22%, 16–21 min: 22%−23%, 21–24 min: 23%−26%, 24–25 min: 26%−30%, 25–31 min: 30%−32%, 31–35 min: 32%−33%, 35–38 min: 33%−33%, 38–40 min: 33%−50%, 40–43 min: 50%−51%, 43–45 min: 51%−53%, 45–50 min: 53%−60%, 50–53 min: 60%−100%.
2.2.2. Method validation
Five sample solutions of SJD were analyzed to determine the precision. The repeatability was evaluated by five injections of one sample. Stability study of the sample was performed at different times in 0, 2, 4, 6, 12, and 24 h. Each sample solution was detected three times in parallel.
2.3. Preparation of 10 batches of SJD
All the herbs, P. ginseng, A. macrocephala, P. cocos, and G. uralensis, were crushed into powder and mixed in the proportion of 3:3:3:2 according to the composition recorded in “Taiping Huimin Heji Ju Fang”. Then, the four ingredient herbs with a total weight of 25 g were soaked in ten times the volume of deionized water (250 mL) and reflux extracted twice for 1.5 h, respectively. The merged aqueous solution was evaporated to a concentration of 0.5 g crude drug/mL under reduced pressure. Then, 10 mL aqueous solution was subjected to alcohol precipitation overnight, followed by evaporation of ethanol. The remaining aqueous solution was first extracted with 10 mL of ether and then extracted with 10 mL of water-saturated n-butanol three times. The n-butanol fraction was evaporated and diluted in 2.5 mL methanol. Then, after being filtered by a 0.22 µm syringe filter, the sample solution with a volume of 2 μL was analyzed by the UPLC system to obtain fingerprints. Individual standards were mixed and dissolved in methanol to obtain ginseng saponin combinations.
Considering the practical application, among the 10 batches of P. ginseng, farmland P. ginseng is the most widely applied in clinical practice due to its fertility and low cost. Therefore, in this study, SJD made of 5-year-old farmland P. ginseng (S10) was regarded as the model SJD generally representing SJD. The above sample solutions were further diluted 10 times to calculate the content of R1, Rg2 and Rb3 in model SJD.
2.4. Anti-inflammation evaluation of SJD
2.4.1. Animal handling and tissue collection
Male BABL/c mice (20–22 g) were provided by Changchun Institute of Biological Products Co., ltd. (Changchun, China). The animals were housed in a 12 h light/dark cycle at the animal center of Institute of Special Animal and Plant Sciences of Chinese Academy of Agricultural Sciences for 7 d before experiments.
All animals were handled in strict accordance with good animal practice according to the Animal Ethics Procedures and Guidelines of the People’s Republic of China, and the study was approved by The Animal Administration and Ethics Committee of the Institute of Special Animal and Plant Sciences, Chinese Academy of Agricultural Sciences (Permit No. ISAPSAEC-2018-001). The mice were randomly separated into 12 experimental groups: control group (n = 10), UC model group (n = 12), 10 different batches of SJD group (S1 − S10, n = 10). The control group was administrated with water. The UC model group and the SJD groups received 3% DSS in drinking water for 6 d. On the fourth day, SJD groups took SJD orally at a dose of 4.5 g crude drug/kg/d for the next 7 d.
Mice were euthanized and sacrificed at the end of experiment. After standing for 30 min, the blood sample was centrifuged at 4 °C (4 000 rpm for 20 min) and stored at − 80 °C for further study. The full-length colon was isolated and rinsed with normal saline. The images of colons in different treatments were collected.
2.4.2. Histological evaluation
The colon tissues of model SJD were fixed with 10% formaldehyde and paraffin embedded for histological evaluation. The colonic specimens (5 μm) were stained with hematoxylin & eosin (H&E) and the histological changes were observed by a light microscope.
2.4.3. Immunohistochemistry (IHC) analysis
Occludin and ZO-1 were detected by IHC staining according to the methods reported before (Guo et al., 2018). The samples of model SJD were incubated with the primary antibodies (occludin and ZO-1) overnight at 4 °C. Biotinylated immunoglobulin cocktail of goat anti-rabbit IgG was then applied at 37 °C for 20 min. DAB kit was used for the chromogenic reaction and the nucleus was then stained with hematoxylin for 1 min. The colon specimens were captured by a microscope at the ×400 magnification.
2.4.4. Cytokine analysis by ELISA
The serum levels of tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and nitric oxide (NO) were determined with ELISA kits following manufacturers' instructions.
2.4.5. NO production assay in vitro
RAW 264.7 macrophages were seeded in 24-well plates at (5.0 × 105)/mL and incubated for 12 h at 37 °C with 5% CO2. The macrophages were treated with LPS (1 μg/mL) for 30 h in the presence or absence of sample solutions. The concentration of samples in macrophages were determined according to the cell viability detected by a CCK-8 assay reported in the literature (Qiao et al., 2019). After incubation, each culture supernatant (100 μL) was mixed with an equal volume of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenedimine dihydrochloride in 2.5% phosphoric acid). The reaction was maintained for 10 min in the dark. Absorbance was measured at 520 nm in a microplate reader (Biotek Synergy Instrument, Vermont, USA). Nitrite concentrations were calculated using a NaNO2 standard calibration curve.
2.5. Statistical analysis
Statistical analysis was analyzed by GraphPad Prism V7.0. The levels of proinflammatory cytokines TNF-α, IL-1β, IL-6 and NO were presented as mean ± standard deviation (SD). IHC assays were analyzed with one-way analysis of variance (ANOVA) followed by Tukey’s test. P < 0.05 was considered statistically significant.
2.6. Gray relational analysis
Grey theory is an effective model established by Professor Deng for acquiring uncertain knowledge. It mainly focuses on quantitatively describing and comparing the development tendency of a system. Grey relation degree is the key of GRA, which is obtained by comparing the geometrical similarity between reference data sequence and several comparative data sequences. The higher the grey relation degree, the closer development direction and rate of these sequences, and their relationship will be closer to each other (Dai, Liu, & Hu, 2014).
Steps in GRA include (1) data processing and normalizing and (2) calculating grey relational coefficient. The normalization process is calculated by Eqs. (1) and (2). The grey relational coefficient can be obtained by Eq. (3) from the normalized values. In the middle of the calculation, the coefficient constant is assumed to be ρ = 0.5.
| (1) |
| (2) |
where Eq. (1) is for the higher the better attributes or criterion; Eq. (2) is used for the smaller the better attributes or criterion. i = 0, 1……, n; k = 1, 2, ……, m.
| (3) |
where,
-
(1)
is the smallest value of Xi(k);
-
(2)
is the largest value of Xi(k);
-
(3)
is the absolute value of the difference between and ;
-
(4)
ρ is the coefficient constant, which is defined in the range 0 ≤ ρ ≤ 1. In the middle of the calculation, the coefficient constant is assumed to be ρ = 0.5.
3. Results
3.1. Results of UPLC fingerprints
In order to obtain a good resolution among the numerous chemical compounds in Chinese herbal formula, we compared Acquity UPLC CSH C18 RP column (150 mm × 2.1 mm, particle size of 1.7 µm) and Acquity UPLC BEH C18 RP column (50 mm × 2.1 mm, particle size of 1.7 µm). The CSH column showing better peak-to-peak resolution of the constituents was chosen to separate the compounds in SJD. Moreover, the UV absorption wavelength was set at 203 nm to reduce interference of chemical constituents from other herbs in SJD. Method validation data listed in Table 1 showed the relative standard deviation (RSD) of the precision, repeatability and sample stability which were calculated by the peak area of compounds. The precision and repeatability of the results were both <3% which certified that the chromatographic conditions were suitable. In the sample stability test, RSD of the samples was also <3% which demonstrated the samples were stable in 24 h.
Table 1.
Precision, repeatability and stability of SJD samples.
| Retention time (min) | Compound names | Precision (n = 5)RSD (%) | Repeatability (n = 5)RSD (%) | Stability (n = 5)RSD (%) |
|---|---|---|---|---|
| 16.97 | R1 | 0.56 | 0.64 | 2.64 |
| 19.16 | Rg1 | 0.87 | 2.38 | 1.54 |
| 19.60 | Re | 0.64 | 1.12 | 0.92 |
| 28.73 | Rf | 0.57 | 1.07 | 0.71 |
| 31.49 | Rg2 | 0.24 | 1.63 | 2.23 |
| 32.30 | Rb1 | 0.46 | 0.75 | 1.33 |
| 33.98 | Ro | 0.96 | 0.46 | 0.63 |
| 35.98 | Rb2 | 0.75 | 0.84 | 1.95 |
| 36.61 | Rb3 | 1.26 | 1.37 | 1.84 |
| 40.13 | Rd | 0.74 | 1.73 | 0.73 |
| 43.64 | 20(S)-Rg3 | 0.64 | 0.63 | 0.63 |
| 44.01 | 20(R)-Rg3 | 0.63 | 0.42 | 1.74 |
| 52.60 | Rk2 | 0.06 | 0.21 | 0.93 |
UPLC fingerprints of 10 batches of SJD (S1–S10) made of different resources of P. ginseng shown in Fig. 1A were obtained and matched automatically by the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine, which was devised by the Chinese Pharmacopoeia Committee (Version 2012A) (Beijing, China). A representative reference fingerprint was formed automatically by the system. Thirteen compounds including R1, Rg1, Re, Rf, Rg2, Rb1, Ro, Rb2, Rb3, Rd, (20S)-Rg3, (20R)-Rg3, Rk2 in P. ginseng were identified by comparing their retention time with the reference standards displayed in Fig. 1B. The similarities of the entire chromatographic profiles of SJD and the collected information of different batches of P. ginseng were shown in Table 2. The similarity degrees of 10 batches of SJD samples were above 0.97 which indicated that all the SJD samples were of good consistency. The relative peak areas of the above 13 common peaks were listed in Table S1 (Supplemental Table S1). The RSD values of 10 batches of SJD were all over 20%, which indicated that there were significant differences in ginsenoside content among different batches.
Fig. 1.
UPLC chromatograms of SJD samples (A) and reference standards of P. ginseng (B). R: reference fingerprint.
Table 2.
Collected information and similarity of samples.
| Sample No. | Sources | Ages | Cultivation mode | Acquisition time | Similarity |
|---|---|---|---|---|---|
| S1 | Heilongjiang | 4 | Farmland cultivation | May 2017 | 0.991 |
| S2 | Heilongjiang | 4 | Farmland cultivation | April 2017 | 0.978 |
| S3 | Jilin | 4 | Farmland cultivation | April 2017 | 0.988 |
| S4 | Jilin | 4 | Farmland cultivation | May 2017 | 0.984 |
| S5 | Jilin | 4 | Under forest | October 2018 | 0.994 |
| S6 | Jilin | 5 | Under forest | October 2018 | 0.997 |
| S7 | Jilin | 6 | Under forest | October 2018 | 0.993 |
| S8 | Jilin | 15 | Under forest | October 2018 | 0.976 |
| S9 | Jilin | 4 | Farmland cultivation | October 2018 | 0.997 |
| S10 | Jilin | 5 | Farmland cultivation | October 2018 | 0.999 |
3.2. Results of anti-inflammatory experiments
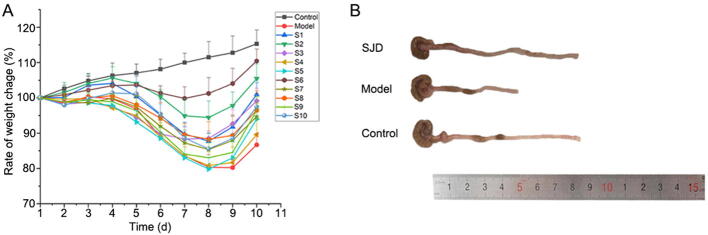
Previous studies showed that weight loss, colon length, and diarrhea rectal bleeding were the indicators to evaluate the ulcerative colitis induced by DSS (Kim et al., 2012, Nunes et al., 2019). As shown in Fig. 2A, the weight of mice in the control group gradually increased, while that of UC mice decreased obviously from the fourth day. After oral administration of SJD, the weight of UC mice in SJD groups (S1−S10) was gradually restored. The colon length of model group shown in Fig. 2B was significantly shorter than those of normal mice, which was one of the evidence of ulcerative colitis (Lee, Kwon, & Cho, 2018). In contrast with the UC model group, the length of colon in SJD group (S10) was ameliorated remarkably. From above, the increased body weight and colon length indicated good therapeutic effects of SJD on UC.
Fig. 2.
SJD improved DSS-induced colitis in mice. (A) The rate of body weight changes of mice; (B) The colon length of mice in different groups.
In order to observe the changes of colon tissue in control group, UC model group and SJD groups, sections of colon tissues were stained with H&E (Fig. 3). In the control group, the structure of each layer of colon was clear, the mucosa epithelium was intact, and the glands were arranged regularly. While in the model group, the colonic submucosa edema, infiltration of inflammatory cells (neutrophils or macrophages) in the mucosa and submucosa, loss and destruction of crypts and atrophy of glands were observed. For the SJD group, the structure of each layer of colon was clear, the mucosa epithelium was complete and the arrangement of glands was regular. The results demonstrate that SJD could recover the loss of barrier function and tissue damage caused by UC.
Fig. 3.
Sections of colon tissues stained with H&E. (Magnification: ×400).
Increasing evidence suggests that intestinal tight junction (TJ) proteins consisting of ZO-1 and occludin are key components of the intestinal mucosal barrier and participates in the development of UC (Perrier and Rutgeerts, 2011, Dokladny et al., 2016, Tan and Zheng, 2018). The effects of SJD on the expressions of occludin and ZO-1 in colons of mice of control and experimental groups were shown in Fig. 4. It is clear from the results that the amount of expression of occludin and ZO-1 in the model group both decreased markedly, while the expression in the UC mice was up-regulated under the treatment of SJD. This implies that SJD acted a protective effect on intestinal mucosal barrier by means of increasing the expression of TJ proteins.
Fig. 4.
Immunohistochemical expression of occludin (A) and ZO-1 (C) in colons. The expression of occludin and ZO-1 shown in (B) and (D) were calculated by the number of positive cells × intensity of positive cells. Data was analyzed by one-way ANOVA followed by post hoc Dunnett’s test. **P < 0.01 vs control group; ##P < 0.01 vs model group.
Four proinflammatory cytokines including TNF-α, IL-1β, IL-6 and NO in the serum of each group were presented in Fig. 5. The levels of TNF-α, IL-1β, IL-6 and NO in the serum of UC model group were remarkably increased compared to the control group. Whereas, the SJD groups (S1 − S10) decreased the expression of inflammatory factors in varying degrees. Overall, these results indicated that SJD alleviated inflammatory response in DSS-induced UC mice.
Fig. 5.
Effects of SJD on levels of proinflammatory cytokines of TNF-α (A), IL-1β (B), IL-6 (C) and NO (D) in serum of UC mice. Data was analyzed by one-way ANOVA followed by post hoc Dunnett’s test. **P < 0.01 vs control group; ##P < 0.01 vs model group.
3.3. Results of GRA with four pharmacodynamics indexes
TNF-α, IL-1β, NO and IL-6 related to inflammation were chosen to be four reference series and the 13 values of chromatogram peaks were regarded as compared series. The Grey relational coefficient between the compared and reference series was calculated with a resolution rate of 0.5. The Grey relational coefficient reflects the level of anti-inflammation. The higher the Grey relational coefficient, the greater the correlation between the compounds and the activity. According to the results exhibited in Fig. 6, the order of grey relational grade of the TNF-α was as follows: P1 > P9 > P5 > P7 > P4 > P13 > P8 > P2 > P6 > P3 > P12 > P11 > P10, the order of grey relational grade of the IL-1β was as follows: P1 > P9 > P5 > P4 > P13 > P8 > P7 > P3 > P12 > P6 > P11 > P2 > P10, the order of grey relational grade of the NO was as follows: P1 > P9 > P5 > P4 > P13 > P8 > P7 > P3 > P6 > P2 > P10 > P12 > P11, the order of grey relational grade of the IL-6 was as follows: P1 > P9 > P5 > P4 > P13 > P7 > P8 > P3 > P6 > P2 > P12 > P11 > P10. Following these results, we can conclude that the sequence above is coincident. P1 (R1) was the highest influence to resist inflammation. P5 (Rg2) and P9 (Rb3) also showed a prominent influence to resist inflammation with the correlation degree in all reference series greater than 0.7. P2, P3, P4, P6, P7, P8, P13 possessed smaller power to resist inflammation, and P10, P11, P12 had a relatively fainter effect. Therefore, R1 (P1), Rg2 (P5) and Rb3 (P9) were considered as key components to resisting inflammation due to their higher grey relational grade.
Fig. 6.

Calculated grey relational coefficient of 13 chromatographic peaks correlated with anti-inflammatory activity deduced by GRA.
3.4. Verification experiment of anti-inflammatory activity
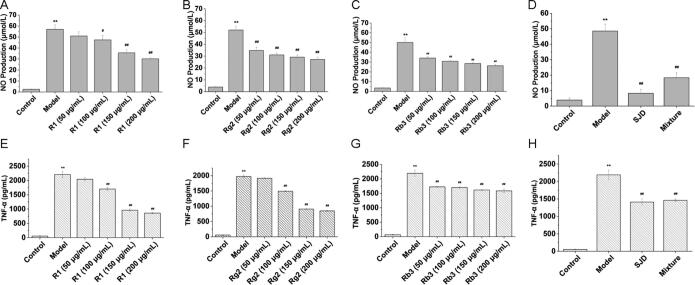
Macrophage inflammation also plays an important role in the course of the disease in the intestinal inflammation model (Lin et al., 2014, Chen et al., 2018), so we confirmed that anti-inflammatory effects of P1, P5 and P9 in the treatment of UC through detecting NO and TNF-α production in LPS-induced macrophage inflammation model. The results in Fig. 7 (A−C) illustrated that R1, Rg2 and Rb3 could inhibit NO production and the anti-inflammatory activity showed dose-dependence. In addition, R1, Rg2 and Rb3 were mixed in proportion to the corresponding content in model SJD as a mixture (Supplemental Table S2 and S3) to compare with the inhibitory effects of SJD on NO production. It can be seen from Fig. 7 (D), the inhibition of NO production of the mixture was close to SJD which demonstrated that three compositions in P. ginseng might play important roles in the anti-inflammatory activity of SJD.
Fig. 7.
Effects of R1, Rg2, Rb3, SJD and mixture on levels of NO (A − D) and TNF-α (E−H) in macrophage. Data are expressed as mean ± SD for three independent experiments. Data was analyzed by one-way ANOVA followed by post hoc Dunnett’s test. **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs model group.
From Fig. 7(E–G), it was observed that R1, Rg2 and Rb3 can inhibit the content of TNF-α in the LPS-induced macrophage and the inhibitory effect improved with the increase of concentration. The effect on the TNF-α in the mixture was quite close to SJD (Fig. 7H) which also pointed out that R1, Rg2 and Rb3 were the main anti-inflammatory ingredients in SJD.
4. Discussion
P. ginseng is a traditional herbal drug widely applied clinically with abundant pharmacological activities. The composition of P. ginseng varies with plant species, geographical origin and age which caused different medicinal properties and economic value. The existing quality control methods are mainly concerned with the establishment of ginsenoside detection method which strips the association between medicinal ingredients and pharmacological actions (Cheng et al., 2021, Xu et al., 2021). They are insufficient to assess P. ginseng in the herbal markets and clinical raw materials selection of P. ginseng. Spectrum-effect relationship can integrate the chemical fingerprints and efficacy indicators to disclose the efficacy components of TCM. It is particularly suitable for quality control of complex herbal medicine systems. Therefore, the UPLC fingerprints of 10 batches of SJD (S1–S10) from different resources of P. ginsengs were established for exploring the spectrum-effect relationship. Precision, repeatability and sample stability in method validation are crucial aspects for the quality of results. RSD values showed that they were always <3% during the analysis. Therefore, the detection method exhibited good performance with high precision and repeatability, and the samples were stable in the detection conditions. Considering the characteristics of multiple effects of P. ginseng, it is more reliable to investigate the pharmacodynamic components in the context of clinical application. Therefore, SJD with P. ginseng as the sovereign drug was selected to recreate the therapeutic environment of P. ginseng.
SJD has a pharmacological effect on gastrointestinal function, immune system, ulcers, and tissue repair. It is often used clinically for peptic ulcers such as UC (Tian et al., 2021). UC, one of inflammatory bowel disease, is a chronic inflammatory state of the gastrointestinal tract. There is evidence that proinflammatory cytokines TNF-α, IL-1β, IL-6 and NO all are indispensable to the inflammatory response in the pathogenesis of UC (Dinarello, 2009, McAlindon et al., 1998, Li et al., 2010, Jahanshahi et al., 2004, Gillberg et al., 2012). In addition, TNF-α was confirmed to improve the expressions of IL-1β, IL-6 in UC (Clark, 2007, Sands and Kaplan, 2007, Bradley, 2008, Pugliese et al., 2017). For the sake of investigating the effective components of SJD in treating UC, the above four inflammatory factors were selected as effective indicators and correlated with the fingerprint of chemical components by GRA. It can be inferred that SJD reduced the expression of TNF-α, IL-1β, IL-6, NO and promoted the expressions of tight junction proteins such as occludin, and ZO-1 to maintain the intestinal mucosal barrier function for attenuating UC in mice. The anti-inflammatory effects of P. ginseng have been extensively studied and approven by purified ginsenosides (Chen et al., 2014, Agarwal et al., 2019, Wang et al., 2018, Huynh et al., 2020). In good agreement with previous results, GRA results indicate that R1, Rg2, Rb3 might explain the anti-inflammatory effect of P. ginseng in SJD. Meanwhile, the above spectrum-effect relationship results illustrated that R1, Rg2, Rb3 might be the main anti-inflammatory compounds in SJD. The anti-inflammatory activities of R1, Rg2, Rb3 were confirmed through LPS-stimulated RAW264.7 murine macrophages cell.
The pharmacodynamic components filtrated by biological activity will promote the establishment of quality standards and the variety selection of P. ginseng. Recent studies have proven its benefits in screening the activities of anti-lung cancer and diabetic retinopathy in P. ginseng (Zhou et al., 2021, Yu et al., 2022). Due to the abundant pharmacological activity of P. ginseng, more quality control markers are still needed to be explored.
5. Conclusion
This study focused on providing chemical components highly correlated with pharmacodynamic indexes by spectrum-effect relationship. The fingerprints of 10 batches of SJD were built by UPLC. According to the results of pharmacological experiments, SJD has a significant therapeutic effect on UC. Grey relational coefficient of GRA revealed that R1, Rg2 and Rb3 have the most significant effect on the anti-inflammatory action of P. ginseng. Therefore, the spectrum-effect relationship can be applicable for the detection of P. ginseng’s anti-inflammatory components in SJD.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by Science Foundation of Jilin Educational Committee (No. JJKH20200363KJ). Jilin Science & Technology Development Plan (No. 20190304009YY). Jilin Science & Technology Development Plan (No. 20200404090YY).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chmed.2022.04.003.
Contributor Information
Shuai Qu, Email: qushuai1027@163.com.
Yingping Wang, Email: yingpingw@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agarwal H., Nakara A., Shanmugam V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomedicine & Pharmacotherapy. 2019;109:2561–2572. doi: 10.1016/j.biopha.2018.11.116. [DOI] [PubMed] [Google Scholar]
- Bradley J.R. TNF-mediated inflammatory disease. The Journal of Pathology. 2008;214(2):149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- Cao Y.Y., Li S.H., Yuan S., Zhang Q.G., Liu L.P. Research progress on mechanism of antiarrhythmic action of Panax ginseng. Chinese Traditional and Herbal Drugs. 2021;52(10):3157–3166. [Google Scholar]
- Chen F., Huang G.L. Antioxidant activity of polysaccharides from different sources of ginseng. International Journal of Biological Macromolecules. 2019;125:906–908. doi: 10.1016/j.ijbiomac.2018.12.134. [DOI] [PubMed] [Google Scholar]
- Chen H., Shi H.T., Liu Y.P., Ren X.Y., He S.X., Chang X.M., Yin Y. Activation of corticotropin-releasing factor receptor 1 aggravates dextran sodium sulphate-induced colitis in mice by promoting M1 macrophage polarization. Molecular Medicine Reports. 2018;17(1):234–242. doi: 10.3892/mmr.2017.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.Q., Rong L., Qiao J.O. Anti-inflammatory effects of Panax notoginseng saponins ameliorate acute lung injury induced by oleic acid and lipopolysaccharide in rats. Molecular Medicine Reports. 2014;10(3):1400–1408. doi: 10.3892/mmr.2014.2328. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Peng S.H., Li F.Y., Cui P.D., Zhao C.X., Yan X.H.…Li Z. Quality distinguish of Red Ginseng from different origins by HPLC–ELSD/PDA combined with HPSEC–MALLS–RID, focus on the Sugar-Markers. Separations. 2021;8(11):198. [Google Scholar]
- Clark I.A. How TNF was recognized as a key mechanism of disease. Cytokine & Growth Factor Reviews. 2007;18(3–4):335–343. doi: 10.1016/j.cytogfr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Dai J., Liu X., Hu F. Research and application for grey relational analysis in multigranularity based on normality grey number. The Scientific World Journal. 2014;2014 doi: 10.1155/2014/312645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Interleukin-1beta and the autoinflammatory diseases. The New England Journal of Medicine. 2009;360(23):2467–2470. doi: 10.1056/NEJMe0811014. [DOI] [PubMed] [Google Scholar]
- Dokladny, K., Zuhl, M. N., Moseley, P. L. (2016). Intestinal epithelial barrier function and tight junction proteins with heat and exercise. Journal of Applied Physiology, 120(6), 692-701. [DOI] [PMC free article] [PubMed]
- Gao B.B., Peng Y., Li X.B. Research progress on intestinal immunoregulation and mechanism of Sijunzi Decoction polysaccharide. Chinese Traditional and Herbal Drugs. 2018;49(2):462–467. [Google Scholar]
- Gillberg L., Varsanyi M., Sjöström M., Lördal M., Lindholm J., Hellstrom P.M. Nitric oxide pathway-related gene alterations in inflammatory bowel disease. Scandinavian Journal of Gastroenterology. 2012;47(11):1283–1297. doi: 10.3109/00365521.2012.706830. [DOI] [PubMed] [Google Scholar]
- Guo Y.L., Wu X.X., Wu Q., Lu Y., Shi J., Chen X. Dihydrotanshinone I, a natural product, ameliorates DSS-induced experimental ulcerative colitis in mice. Toxicology and Applied Pharmacology. 2018;344:35–45. doi: 10.1016/j.taap.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Hao J.J., Hu H.W.Y., Liu J., Wang X., Liu X.Y., Wang J.B.…Xiao X.H. Integrated metabolomics and network pharmacology study on immunoregulation mechanisms of Panax ginseng through macrophages. Evidence-Based Complementary and Alternative Medicine. 2019;2019:3630260. doi: 10.1155/2019/3630260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Zhou Y. How to identify “Material basis–Quality markers” more accurately in Chinese herbal medicines from modern chromatography-mass spectrometry data-sets: Opportunities and challenges of chemometric tools. Chinese Herbal Medicines. 2021;13(1):2–16. doi: 10.1016/j.chmed.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh D.T.N., Baek N., Sim S., Myung C.S., Heo K.S. Minor ginsenoside Rg2 and Rh1 attenuates LPS-induced acute liver and kidney damages via downregulating activation of TLR4-STAT1 and inflammatory cytokine production in macrophages. International Journal of Molecular Sciences. 2020;21(18):6656. doi: 10.3390/ijms21186656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi G., Motavasel V., Rezaie A., Hashtroudi A.A., Daryani N.E., Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Digestive Diseases and Sciences. 2004;49(11–12):1752–1757. doi: 10.1007/s10620-004-9564-5. [DOI] [PubMed] [Google Scholar]
- Kadier A., Abdeshahian P., Simayi Y., Ismail M., Hamid A.A., Kalil M.S. Grey relational analysis for comparative assessment of different cathode materials in microbial electrolysis cells. Energy. 2015;90:1556–1562. [Google Scholar]
- Kang J., Lee S., Kang S., Kwon H.N., Park J.H., Kwon S.W., Park S. NMR-based metabolomics approach for the differentiation of ginseng (Panax ginseng) roots from different origins. Archives of Pharmacal Research. 2008;31(3):330–336. doi: 10.1007/s12272-001-1160-2. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Shajib M.S., Manocha M.M., Khan W.I. Investigating intestinal inflammation in DSS-induced model of IBD. Jove-Journal of Visualized Experiments. 2012;60:e3678. doi: 10.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Kwon J.E., Cho M.L. Immunological pathogenesis of inflammatory bowel disease. Intestinal Research. 2018;16(1):26–42. doi: 10.5217/ir.2018.16.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.Y., Saba E., Irfan M., Kim M., Chan J.Y., Jeon B.S.…Rhee M.H. The anti-inflammatory and anti-nociceptive effects of Korean black ginseng. Phytomedicine. 2019;54:169–181. doi: 10.1016/j.phymed.2018.09.186. [DOI] [PubMed] [Google Scholar]
- Li S., Li X.R., Wang G.L., Nie L.X., Yang Y.J., Wu H.Z.…Lin R.C. Rapid discrimination of Chinese red ginseng and Korean ginseng using an electronic nose coupled with chemometrics. Journal of Pharmaceutical and Biomedical Analysis. 2012;70:605–608. doi: 10.1016/j.jpba.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Li X.D., Chen H.Y., Jia W., Xie G.X. A metabolomics-based strategy for the quality control of traditional Chinese medicine: Shengmai injection as a case study. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/836179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., de Haar C., Chen M., Deuring J., Gerrits M.M., Smits R.…van der Woude C.J. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59(2):227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- Lin Y., Yang X.G., Yue W.J., Xu X.F., Li B.J., Zou L.L., He R. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cellular & Molecular Immunology. 2014;11(4):355–366. doi: 10.1038/cmi.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.X., Xiao P.G. Recent advances on ginseng research in China. Journal of Ethnopharmacology. 1992;36(1):27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- Liu H.Y., Zhu S., Liu Q., Zhang Y.Q. Spectrum-effect relationship study between HPLC fingerprints and antioxidant of honeysuckle extract. Biomedical Chromatography. 2019;33(10):e4583. doi: 10.1002/bmc.4583. [DOI] [PubMed] [Google Scholar]
- Lu Y., Lin H.J., Zhang J.W., Wei J.A., Sun J., Han L. Sijunzi Decoction attenuates 2, 4, 6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats and ameliorates TNBS-induced claudin-2 damage via NF-κB pathway in Caco2 cells. BMC Complementary and Alternative Medicine. 2017;17(1):35. doi: 10.1186/s12906-016-1549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlindon M.E., Hawkey C.J., Mahida Y.R. Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut. 1998;42(2):214–219. doi: 10.1136/gut.42.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelabhotla D.M., Jayaraman T.V., Asghar K., Das D. The optimization of chemical mechanical planarization process-parameters of c-plane gallium-nitride using Taguchi method and grey relational analysis. Materials & Design. 2016;104:392–403. [Google Scholar]
- Nunes N.S., Chandran P., Sundby M., Visioli F., Gonçalves F., Burks S.R.…Frank J.A. Therapeutic ultrasound attenuates DSS-induced colitis through the cholinergic anti-inflammatory pathway. EBioMedicine. 2019;45:495–510. doi: 10.1016/j.ebiom.2019.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier C., Rutgeerts P. Cytokine blockade in inflammatory bowel diseases. Immunotherapy. 2011;3(11):1341–1352. doi: 10.2217/imt.11.122. [DOI] [PubMed] [Google Scholar]
- Pugliese D., Felice C., Papa A., Gasbarrini A., Rapaccini G.L., Guidi L., Armuzzi A. Anti TNF-α therapy for ulcerative colitis: Current status and prospects for the future. Expert Review of Clinical Immunology. 2017;13(3):223–233. doi: 10.1080/1744666X.2017.1243468. [DOI] [PubMed] [Google Scholar]
- Qiao C., Wan J., Zhang L., Luo B., Liu P.L., Di A.…Zhao G. Astragaloside II alleviates the symptoms of experimental ulcerative colitis in vitro and in vivo. American Journal of Translational Research. 2019;11(11):7074–7083. [PMC free article] [PubMed] [Google Scholar]
- Sands B.E., Kaplan G.G. The role of TNFalpha in ulcerative colitis. Journal of Clinical Pharmacology. 2007;47(8):930–941. doi: 10.1177/0091270007301623. [DOI] [PubMed] [Google Scholar]
- Shan S.M., Luo J.G., Huang F., Kong L.Y. Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng C.A. Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. Journal of Pharmaceutical and Biomedical Analysis. 2014;89:76–82. doi: 10.1016/j.jpba.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Shen C.H., Liu C.T., Song X.J., Zeng W.Y., Lu X.Y., Zheng Z.L.…Yan P. Evaluation of analgesic and anti-inflammatory activities of Rubia cordifolia L. by spectrum-effect relationships. Journal of Chromatography B. 2018;1090:73–80. doi: 10.1016/j.jchromb.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Shi W., Wang Y.T., Li J., Zhang H.Q., Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chemistry. 2007;102(3):664–668. [Google Scholar]
- Song H.P., Li R.Y., Huang H.Y., Cai X., Yuan Z.Y., Liu P.A., Zhou C. Sijunzi Decoction promote the repair of intestinal epithelial cell injury via activating TLR-2/My D88 signaling pathway. Journal of Chinese Medicinal Materials. 2016;39(9):2081–2085. [PubMed] [Google Scholar]
- Tan Y., Zheng C.Q. Effects of alpinetin on intestinal barrier function, inflammation and oxidative stress in dextran sulfate sodium-induced ulcerative colitis mice. American Journal of the Medical Sciences. 2018;355(4):377–386. doi: 10.1016/j.amjms.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Tian S.W., Zhang Y.L., Wang B., Liu J.P., Wang C., Zhang J. Clinical efficacy and safety of modified Sijunzi decoction for the treatment of ulcerative colitis. Medicine. 2021;100(4):e23703. doi: 10.1097/MD.0000000000023703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.Y., Chen X.N., Jin W.Q., Xu X.H., Li X.Y., Sun L.W. Ginsenoside Rb3 exerts protective properties against cigarette smoke extract-induced cell injury by inhibiting the p38 MAPK/NF-kappaB and TGF-beta1/VEGF pathways in fibroblasts and epithelial cells. Biomedicine & Pharmacotherapy. 2018;108:1751–1758. doi: 10.1016/j.biopha.2018.10.018. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., He S., Cheng X.C., Lu Y.X., Zou Y.P., Zhang Q.L. UPLC–Q-TOF-MS/MS fingerprinting of traditional Chinese formula SiJunZiTang. Journal of Pharmaceutical and Biomedical Analysis. 2013;80:24–33. doi: 10.1016/j.jpba.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Xie P.S., Chen S.B., Liang Y.Z., Wang X.H., Tian R.T., Upton R. Chromatographic fingerprint analysis–a rational approach for quality assessment of traditional Chinese herbal medicine. Journal of Chromatography A. 2006;1112(1–2):171–180. doi: 10.1016/j.chroma.2005.12.091. [DOI] [PubMed] [Google Scholar]
- Xu X.Y., Wang S.M., Wang H.M., Hu W.D., Han L.F., Chen B.X.…Yang W.Z. Simultaneous quantitative assays of 15 ginsenosides from 119 batches of ginseng samples representing 12 traditional Chinese medicines by ultra-high performance liquid chromatography coupled with charged aerosol detector. Journal of Chromatography A. 2021;1655(11) doi: 10.1016/j.chroma.2021.462504. [DOI] [PubMed] [Google Scholar]
- Yu W.G., Lu B., Zhang H.W., Zhang Y.X., Yan J. Effects of the Sijunzi decoction on the immunological function in rats with dextran sulfate-induced ulcerative colitis. Biomedical Reports. 2016;5(1):83–86. doi: 10.3892/br.2016.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.T., Zhu Z.Y., Xie M.J., Deng L.P., Xie X.J., Zhang M. Investigation on the Q-markers of Bushen Huoxue Prescriptions for DR treatment based on chemometric methods and spectrum-effect relationship. Journal of Ethnopharmacology. 2022;285 doi: 10.1016/j.jep.2021.114800. [DOI] [PubMed] [Google Scholar]
- Zhang J.D., Chen T., Li K., Xu H.Y., Liang R.X., Wang W.H.…Yang B. Screening active ingredients of rosemary based on spectrum-effect relationships between UPLC fingerprint and vasorelaxant activity using three chemometrics. Journal of Chromatography B. 2019;1134–1135 doi: 10.1016/j.jchromb.2019.121854. [DOI] [PubMed] [Google Scholar]
- Zhang T., Han M., Yang L.M., Han Z.M., Cheng L., Sun Z., Yang L. The effects of environmental factors on ginsenoside biosynthetic enzyme gene expression and saponin abundance. Molecules. 2018;24(1):14. doi: 10.3390/molecules24010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Zhou L.W., Yu L.T., Liu S.C., Luo Q.M., Sun P.J., Wu J.K. Monitoring chip fatigue in an IGBT module based on grey relational analysis. Microelectronics Reliability. 2016;56:49–52. [Google Scholar]
- Zhou X.W., Liu H.Y., Zhang M.Y., Li C.Y., Li G.H. Spectrum-effect relationship between UPLC fingerprints and anti-lung cancer effect of Panax ginseng. Phytochemical Analysis. 2021;32:339–346. doi: 10.1002/pca.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.