Abstract
Benincasae Exocarpium (BE, Dongguapi in Chinese), as the dried outer pericarp of Benincasa hispida (wax gourd) in Cucurbitaceae family, is one of traditional Chinese medicines with the same origin as medicine and food. Up to now, 43 compounds were isolated from BE, including flavonoids, alkaloids, tannins, phenolic acids, soluble fiber and carbohydrates. Modern pharmacological studies and clinical practice showed that BE has diuretic, hypolipidemic effects, hypoglycemic, antioxidant, antibacterial, and other effects. The folk uses, functional factors, pharmacological activities, patents and clinical applications of BE were reviewed in this paper. In addition, the paper also discussed the current problems for the further studies. The information summarized in this paper provides valuable clues for the comprehensive utilization of medicine and food resources and gives a scientific basis for the development of medicinal plants of BE.
Keywords: Benincasa hispida (Thunb.) Cogn., Benincasae Exocarpium, medicine and food homology, modern pharmacology, functional constituents
1. Introduction
Benincasa hispida (Thunb.) Cogn. (Wax gourd) is a plant of the Cucurbitaceae family, whose fruits have been used as food for thousands of years. B. hispida was originated in China and East India and later widely distributed in tropical, subtropical and temperate regions of Asia for now (Palamthodi, Kadam, & Lele, 2019). In addition to being used as vegetables, the fruits of B. hispida can also be dipped into various candies. The dried outer peels can be used as a medicinal, called Benincasae Exocarpium (BE, Dongguapi in Chinese), as traditional Chinese medicine (TCM) in China with a long history of use (Fig. 1). As a TCM, BE was first recorded in Materia Medica of the Kaibao Reign for removing lower abdomen water distension, diuresis and quenching thirst with taste sweet and slightly cold, authored by Han Liu and Zhi Ma in Song Dynasty. Now, BE, a kind of homology of medicine and food with some curative effects (Guo et al., 2022, Lee, Choi, & Kim, 2005), is documented in the first part of the 2020 edition of the Chinese Pharmacopoeia and its main functions are to dilute water to remove edema, relieve dysuria and heat (Commission, 2020). Phytochemistry analysis displayed that the main components of BE are flavonoids, alkaloids, tannins, trace elements and vitamins, which have pharmacological effects such as anti-oxidation, lipid-lowering, anti-cancer and bacteriostasis (Lee, Choi, & Kim, 2005, Shetty et al., 2008, Soliman et al., 2020). This review summarized the ethnopharmacology, functional constituents, modern pharmacology and applications of BE by searching the literatures over the years, with view to providing a trustworthy basis for the follow-up research on the homology of medicine and food of BE.
Fig. 1.
Photograph of Benincasae Exocarpium.
2. Ethnopharmacology of Benincasa Exocarpium
BE had been used in various countries for thousands of years as medicine and food. It not only appeared on the dining-table, but also often was made into medicinal food (Lan, Chen, & Yanagida, 2009, Yao et al., 2019). As food, it is a vegetable that is loved by people, especially in Asian regions such as China, India, Korea, and Japan. BE can be cooked on its own or eaten with meat or other vegetables, and it is mostly used as kimchi in Korea (Al-Snafi, 2013). As medicine, BE can be used as a single drug or in combination with other natural medicines to prevent or treat diseases such as poor urination, obesity and high blood sugar. In India, BE, known as Kushmanda, had been used to treat diabetes, diuretic diseases, urinary tract infections, and chronic inflammatory diseases under the guidance of traditional Ayurvedic medicine (Deeksha et al., 2021). A review, published in 2021, sorted out antiviral drugs or local plants commonly used in Thailand since ancient times, showing that BE has antiviral pharmacological activity (Julsrigival et al., 2021). Compared with the above countries, BE is more widely used in China. According to the guidance of traditional Chinese medicine theory, the nature of BE is slightly cool, and the taste is sweet (Li, 2020), mainly attributed to the spleen and small intestine meridians (Shih et al., 2001). BE has a long history of folk medicine to treat symptoms such as heat syndrome, dysuria, low back pain and urticaria. The ancient medical books about BE were arranged in Fig. 2. BE was recorded in various medical books, including Materia Medica of the Kaibao Reign published in A.D. 974, and recorded in books such as Illustrated Classic of Materia Medica and Compendium of Materia Medica latterly. In the Compendium of Materia Medica, BE is sweet, flat, non-toxic and mainly used to treat fall damage and low back pain. In 2020, BE was included in Chinese Pharmacopoeia as a traditional Chinese medicine.
Fig. 2.
Ancient medical books of Benincasae Exocarpium.
3. Functional constituents
In recent years, there had been many studies on the chemical constituents of the whole fruit of B. hispida, which mainly contain phenolic compounds, flavonoids, alkaloids, volatile compounds, and polysaccharides. However, there were few studies on the chemical constituents of BE, and only 43 compounds have been reported at present. The analysis of functional factors has demonstrated that BE contains flavonoids, alkaloids, tannins, trace elements and vitamins. Flavonoids and alkaloids are the main chemical components related to the biological activity and pharmacological properties of BE. The main compounds obtained from BE were exhibited in Table 1.
Table 1.
Functional factors isolated from Benincasae Exocarpium.
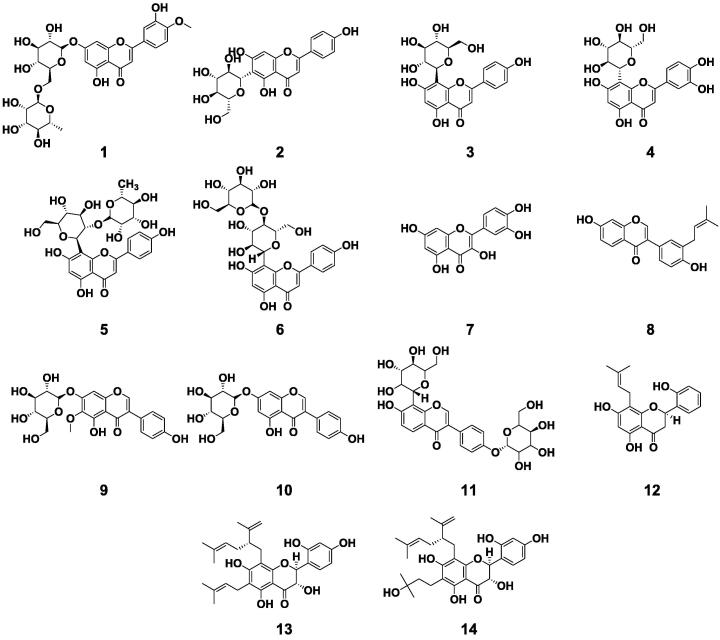
Flavonoids, a class of substances with a wide range of pharmacological activities, are one of the main chemical components in BE (Hakiki, Fauziyyah, & Wijanarti, 2021, Ryu, Lee, & Whang, 2021). A total of 14 flavonoids were isolated and identified from BE (1–14). These flavonoids were classified according to the structure, including six flavones (1–6), one flavonol (7), four isoflavones (8–11), one flavanone (12) and two flavanonols (13–14). The chemical structures of flavonoids in BE were shown in Fig. 3. There was a total of nine flavonoid glycosides in BE, among which there were five C8-glycosides and one C6-glycoside. Vitexin and orientin are both flavone C8-glycosides, while isovitexin belongs to flavone C6-glycoside. Flavonoids gained from medicinal and food homologous plants had special significance for the treatment of chronic diseases, due to the advantages of low cost, high safety, and high patient compliance (Hajiaghaalipour et al., 2015).
Fig. 3.
Flavonoids compounds isolated from Benincasae Exocarpium.
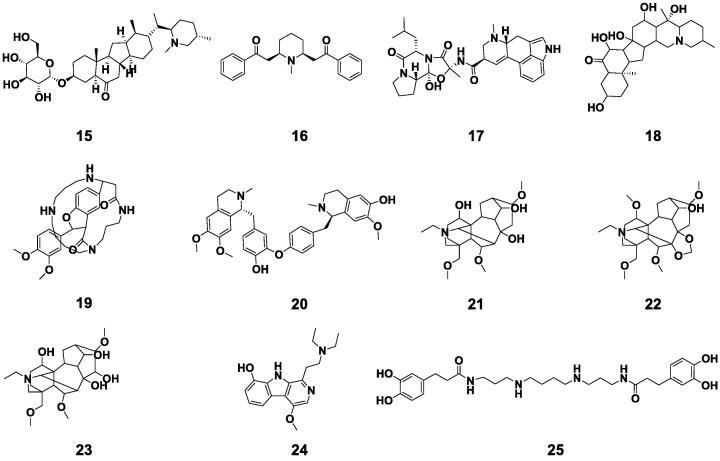
There were 11 alkaloids isolated and identified from BE (15–25), and their structures were exhibited in Fig. 4. These alkaloids were classified as heterocyclic alkaloids and non-heterocyclic alkaloids based on the position of nitrogen atoms. In 11 alkaloids of BE, nine compounds (15–24) were heterocyclic alkaloids and one (25) was non-heterocyclic alkaloid (Bhambhani, Kondhare, & Giri, 2021). According to the chemical structures, there were two piperidine alkaloids (15,16), four terpenoids alkaloids (17, 21–23), one steroid alkaloid (18), one macrocyclic spermine alkaloid (19), one tetrahydroisoquinoline alkaloid (20), one carboline alkaloid (24), and one spermidine alkaloid (25). Through combing and analyzing the pharmacological effects of alkaloids, it was exposed that they have preventive effects on obesity, diabetes, and oxidation and might be the functional constituents of BE (Dinda et al., 2020).
Fig. 4.
Alkaloids compounds isolated from Benincasae Exocarpium.
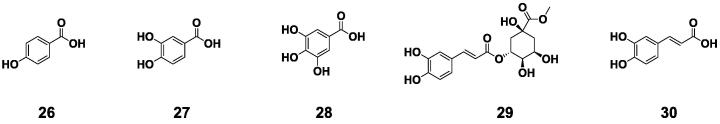
Phenolic acids are polyphenols with a carboxylic acid (–COOH) functional group. Phenolic acids are commonly classified as hydroxybenzoic acid and hydroxycinnamic acid based on the carbon framework (Tatipamula & Kukavica, 2021). Five phenolic acids, p-hydroxybenzoic acid (26), protocatechuic acid (27), gallic acid (28), methyl chlorogenate (29), and caffeic acid (30) were also discovered in BE and their structures were listed were listed in Fig. 5. Three phenolic acids (26–28) belong to hydroxybenzoic acid structure, and the other two (29–30) belong to hydroxycinnamic acid structure.
Fig. 5.
Small-molecule phenolic acids compounds isolated from Benincasae Exocarpium.
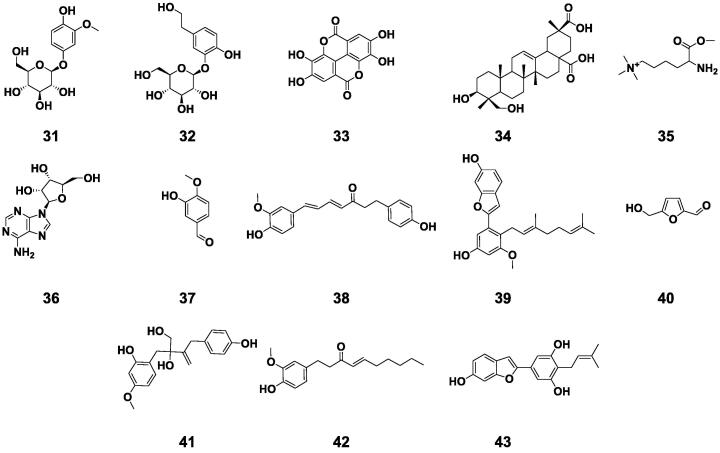
In addition, other types of components in BE, such as triterpenes, heptane, adenosine, soluble fibers, vitamins, trace elements and carbohydrates, were also identified. Some of these ingredients were listed in Table 1, and the structures were displayed in Fig. 6. Trace elements such as potassium and calcium may be diuretic components in BE (Kumar, Mythily, & Mythily, 2012). In addition, the study found that galactose, glucose, xylose and sorbose were identified in BE by thin-layer analysis (Kumar, Mythily, & Chandraju, 2012).
Fig. 6.
Other compounds isolated from Benincasae Exocarpium.
4. Modern pharmacology of Benincasa Exocarpium
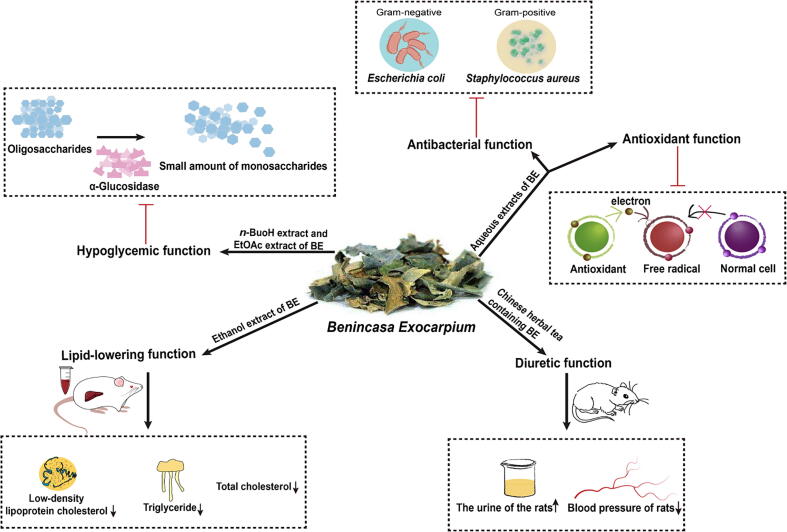
BE contains a variety of functional ingredients that are beneficial to the human body, and has high edible and medicinal value. Modern pharmacological studies have verified that BE showed many biological functions, such as lipid-lowering, hypoglycemic, anti-oxidation, antibacterial, diuretic and so on (Fig. 7).
Fig. 7.
Modern pharmacological functions of Benincasae Exocarpium.
4.1. Diuretic function
The number of patients with hypertension, chronic kidney disease, and edema is increasing, and medicinal and food homologous substances with diuretic properties have been found to help urinate, reduce fluid retention, and assist in the treatment of cardiovascular disease (de Souza, Mariano, Cechinel-Zanchett, & Cechinel-Filho, 2021). The diuretic effect of BE had been reported since it was recorded for the first time in Materia Medica of the Kaibao Reign. There has been extensive clinical experience using BE to treat patients with systemic edema and dysuria, dating back to ancient times. A Chinese herbal tea containing BE has been shown to increase the urine production of rats (Chen et al., 2000). After drinking Chinese tea for two weeks, the urine volume of the control group was (20.7 ± 5.6) mL/d, and the urine volume of the administration group was (28.4 ± 8.9) mL/d, which indicated that there was a significant difference between the two groups of data (P < 0.001). At the same time, a decrease in blood pressure afterward was also observed, possibly due to the diuretic effect of the Chinese tea.
Although the diuretic effect of BE had a long history, there was little research on the active ingredient and mechanism of action with diuretic function. Therefore, the diuretic effect of BE still has great potential in modern pharmacological research.
4.2. Lipid-lowering function
Occasionally, medicinal and food homologous substances are referred to as “functional foods”. BE is one of the functional foods, which has a certain relieving effect on obesity and a controlling effect on weight (Choudhary & Grover, 2012). In the study on the prevention of obesity with BE, the weight gain of mice fed in the extract of BE (EBE), which was derived from 75% ethanol, was significantly inhibited (P < 0.05), by comparing with the normal high-fat diet group. Meanwhile, there was no significant difference in food consumption among each group, but the contents of low-density cholesterol, total protein, and triglycerides in serum and liver were reduced in C57BL/6 mice in EBE group. In the study of BE in treating obesity, mice were divided into normal HF group and HF with 1% EBE group, accompanied by two weeks of feeding. However, the weight of the administration group did not decrease significantly. These results suggested that the ethanolic extract of BE had the function of preventing obesity, but the treatment was not observed. It was indicated that the lipid-lowering effect of BE could originate from the inhibition of peroxisome proliferators-activated receptor (PPAR) and HMG-CoA reductase (HMGCR) signaling through studies at the cellular level and gene expression level (Gu et al., 2013).
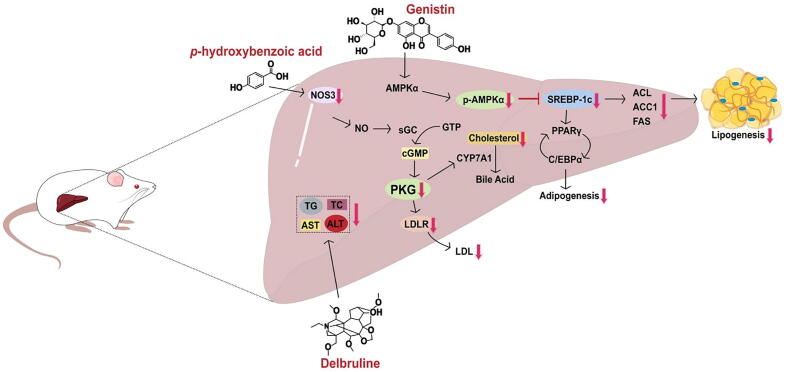
At present, lipid-lowering effects of drugs were mainly through the following four pathways: affecting total cholesterol (TC) pathway, triglyceride (TG) pathway, TC and TG pathway, and genomics intervention. Genistin is an isoflavone component of BE, which could inhibit lipid accumulation by adipocytes by affecting TC and TG pathways. The inhibition rate was 21.7% with the concentration of genistin 50 μmol/L and 69.2% with the concentration of 100 μmol/L. Further studies discovered that genistin can inhibit the production of specific proteins in adipocytes and the expression of genes responsible for these proteins, including adipocyte binding protein 2 (aP2)/fatty acid-binding protein 4 (FABP4), CCAAT-enhancer-binding protein α (C/EBPα), and peroxisome proliferator-activated receptor γ (PPARγ). At the same time, the production of lipase was also inhibited, such as fatty acid synthase (FAS), ATP citrate lyase (ACL), and acetyl-CoA carboxylase 1 (ACC1) (Choi, Shim, & Kim, 2020).
Delbruline is an alkaloid found in BE that exhibits bioactivity against lipid aggregation in vitro. The levels of TG, TC, alanine transaminase (ALT) and Aspartate transaminase (AST) in free fatty acid (FFA)-induced BRL cells were evaluated. Results indicated that Delbruline (1, 5, and 10 µmol/L) inhibited the levels of TG, ALT, and AST in a dose-dependent manner (Ma et al., 2022).
p-Hydroxybenzoic acid obtainedfrom BE also had the effect on lipid-lowering by affecting the TC pathway. After administration of p-hydroxybenzoic acid, the mRNA expression of stearoyl-CoA desaturase-1, ACC1, sterol regulatory element-binding protein 1c, peroxisome proliferator- activated receptor were decreased, compared with the comparison model group (Lin, Yang, Chen, & Yin, 2021). Through network pharmacology and molecular docking technology analysis, the total docking score of p-hydroxybenzoic acid in low density lipoprotein receptor (LDLR) was 5.75, indicating a good affinity with LDLR. LDLR is the receptor that encodes low density lipoprotein. Lan et al. speculated that p-hydroxybenzoic acid increased the expression of LDLR, enhanced low density lipoprotein (LDL) absorption and metabolism in the liver, and decreased the level of LDL in the serum. Therefore, p-hydroxybenzoic acid showed the activity of lipid-lowering (Lan et al., 2020). The lipid-lowering mechanisms of these components were shown in Fig. 8. The lipid-lowering effect of BE is widely used in the folk, and the mechanism of action could be the TC and TG pathway, which regulates lipid-metabolizing enzymes and other proteins such as PPAR.
Fig. 8.
Lipid-lowering function of some compositions of Benincasae Exocarpium.
4.3. Hypoglycemic function
BE, as food with hypoglycemic function, could not only play an important role in lowering blood sugar, slowing down the occurrence of complications caused, but also reduce the frequency of use of conventional drugs (Jia et al., 2003, Sharma, Chatterjee, Kumar, Variyar, & Sharma, 2010). In order to explore the hypoglycemic effect of BE, the experiment was conducted with the produced advanced glycation end products (AGE), α-glucosidase (α-G) and α-amylase (α-A) as indicators. The inhibitory effects of various extracts of BE (water, BuoH, ethyl acetate, n-hexane) on hypoglycemic indexes were determined. Among them, the comprehensive inhibitory effect of n-BuOH extract and ethyl acetate (EtOAc) extract of BE was better than others, showing significant differences with acarbose group (Ryu, Lee, & Whang, 2021). In another study, the water extract of BE had about three times the inhibitory effect on α-A than the methanol extract. The reason could be that the water extract of BE had the highest content of polyphenols (335.39 mg/g), the methanol extract had the highest content of flavonoids (0.58 mg/g), therefore, polyphenols may be the active ingredients in BE (Bellur Nagarajaiah & Prakash, 2014).
At present, the anti-diabetic mechanisms of natural products include the following: inhibition of α-glucosidase and α-amylase in the digestive tract; mediating glucose uptake and glucose transporter through the insulin pathway; promotion of insulin secretion and pancreatic β-cell proliferation; inhibition of protein tyrosine phosphatase 1B activity (Rios, Francini, & Schinella, 2015).
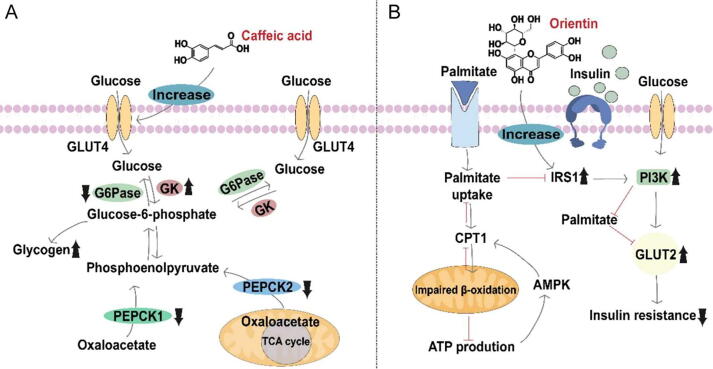
Caffeic acid, a small phenolic acid in BE, has insulin-mediated hypoglycemic effects. C57BL/ KsJ-db/db mice were given a 0.02% caffeic acid diet for five weeks. The caffeic acid group significantly reduced blood glucose levels compared to the control group. In the treated group, plasma insulin, c-peptide, and leptin levels were significantly higher than those in the control group, while plasma glucagon levels were significantly lower. Glucokinase activity and glycogen content were increased, while the activities of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase were decreased in the administration group. At the same time, glucose transporter 2 (GLUT2) expression was decreased in the liver, and glucose transporter 4 (GLUT4) expression was significantly increased in adipose cells. Caffeic acid works (Fig. 9A) by increasing insulin secretion and reducing glucose output from the liver in animals with type 2 diabetes while increasing glucose processing levels in fat cells (Espinoza-Hernandez et al., 2021).
Fig. 9.
Hypoglycemic function of caffeic acid and orientin from Benincasae Exocarpium.
The therapeutic effect of flavonoids with multiple phenolic hydroxyl groups on diabetes could be due to its inhibitory effect on α-A and α-G, and the strength of this effect may be related to the structure of the compound. Isovitexin and vitexin have hypoglycemic effects, and the mechanism of action could be the certain inhibitory effect on α-G and α-A. Isovitexin (IC50 value of 23.26 μmol/L) had a stronger inhibitory effect on α-G than vitexin (IC50 value of 25.11 μmol/L) (Li et al., 2009), which may be due to different sugar binding sites in their structures. Isovitexin is a C6 glycoside and vitexin is a C8 glycoside (Zhu et al., 2020). Vitexin-2″-O-rhamnoside, a C8 glycoside compound with multiple phenolic hydroxyl groups, was reported from n-BuOH and ethyl acetate extracts of BE (Hussain et al., 2022). It had inhibitory effects on both α-A and AGE of bovine serum albumin (BSA) -glucose and BSA-methylglyoxal (MGO) systems with IC50 values of (12.82 ± 1.18), (29.58 ± 1.02) and (12.49 ± 0.81) μmol/L, respectively (Ryu, Lee, & Whang, 2021).
Orientin had been shown to improve substrate utilization during insulin resistance. The basic mechanism of orientin could be through the regulation of insulin signaling and energy metabolism (Mazibuko-Mbeje et al., 2021). C3A liver cells were treated with palmitate at 0.75 mmol/L concentration for 16 h, and the model cells were treated with orientin at 10 μmol/L for 3 h to observe the effect of orientin. Enhancing palmitate and glucose uptake in cells by maintaining substrate utilization showed that orientin could effectively improve metabolic activity. Further studies had shown that the hypoglycemic effect of orientin may be associated with improved expression of genes related to insulin signal transduction (Irs1 and Pi3k), GLUT 2, and energy regulation (Ampk and Cpt1). The hypoglycemic mechanisms of orientin were shown in Fig. 9B.
4.4. Antioxidant function
Diseases caused by free radicals can be prevented by the use of antioxidants, which are used to eliminate over oxidizing free radicals (Abeyrathne, Nam, & Ahn, 2021, Munteanu & Apetrei, 2021). The DPPH free radical scavenging method was used to investigate the antioxidant effect of BE and its antioxidant components. The results showed that at the concentration range of 10–100 μg/ mL, 80 μg/ mL water extract had the highest scavenging ability, reaching 86.36%, while methanol extract had the highest antioxidant activity, reaching 88.02% at the concentration of 100 μg/mL (Rana & Suttee, 2012). In addition, it was also reported that the antioxidant capacity of BE 50% methanol extract was 83.85%, and the antioxidant capacity of BE 75% methanol extract was 82.30% (Huang, Huang, Tso, Tsai, & Chang, 2004).
Flavonoids are the main antioxidant components of BE. Orientin is a flavonoid glycoside of BE with certain antioxidant capacity. The antioxidant capacity was investigated by scavenging DPPH experiment, and the result displayed that the IC50 value of orientin was 0.84 μmol/L (Ma et al., 2020). In addition, the antioxidant activity of orientin was stronger than vitexin, and investigators speculated that the reason could be that the orientin has more O-phenolic hydroxyl structure (An et al., 2012, Xie et al., 2022).
Dietary plants have extensive antioxidant pharmacological effects. As a very popular vegetable in Asia, BE has been proved by experiments.
4.5. Antibacterial function
The bacteriostatic effect of BE has been concerned. The experimenters used the disc diffusion method to determine that the water extract of BE exhibited inhibitory effect on Staphylococcus epidermidis (the inhibition zone was 6.6 ± 0.07 mm) and Proteus vulgaris (the inhibition zone was 6.5 ± 0.08 mm) (Noriham Abdullah et al., 2012). In addition, the antibacterial effect of different extracts of BE was studied by pore diffusion method. The results showed that methanol extract and ethyl acetate extract had stronger antibacterial activity than chloroform extract. The MIC of methanol extract against Bifidobacterium subtilis, Sarcina lutea, Xanthomonas campestris, Escherichia coli and Pseudomonas denitrificans were 510, 260, 120, 510 and 120 µg/mL, respectively (Md et al., 2021). Gold nanoparticles (GNPs) was synthesized by the aqueous extract of BE. The effects of BE nanoparticles on the viability of Klebsiella pneumonia, Salmonella abony, Gram-positive Staphylococcus aureus and Gram-negative E. coli were investigated. In addition, the minimum bactericidal concentration (MBC) values of the GNPs assayed against bacteria were 65.5, 84.5, 111.5 and 80.8 μg/mL, respectively. The above experimental results showed that BE had antibacterial effect, and the antibacterial effect was improved when BE was made into nanoparticles (Al Saqr et al., 2021, Devi & Ahmaruzzaman, 2016).
4.6. Other functions
In addition, BE had anti-inflammatory and analgesic effects. Methanol extract of BE inhibited hot plate pain in mice, acetic acid-induced writhing, and formalin-induced pain in a dose-dependent manner (0.25–1.5 g/kg). Moreover, the extract could inhibit the inflammatory response induced by fresh egg albumin in rats and the convulsion response induced by pentetetrazole in mice. These inhibitions were statistically significant (Parida, Sahu, Debata, & Panda, 2010).
Moreover, some scholars synthesized gold nanoparticles, namely GNPs, using water extract of BE as raw material. The MTT method was used to investigate the toxicity of BE Gold Nanoparticles on cancer cells (Hela) and normal cells (osteoblasts). The results presented that when the concentration of Nanoparticles was 0.62–20 µg/mL, cancer cells lost their viability quickly, and the IC50 value was 2.25 µg/mL. Interestingly, the nanoemulsion was less toxic to normal cells, and at a concentration of 20 µg/mL, the inhibition rate against normal cells was still<30% (Al Saqr et al., 2021). This means that GNPs have certain selectivity for tumor cells and have significance for targeted drug delivery of tumor cells.
5. Application
5.1. Patents of Benincasae Exocarpium
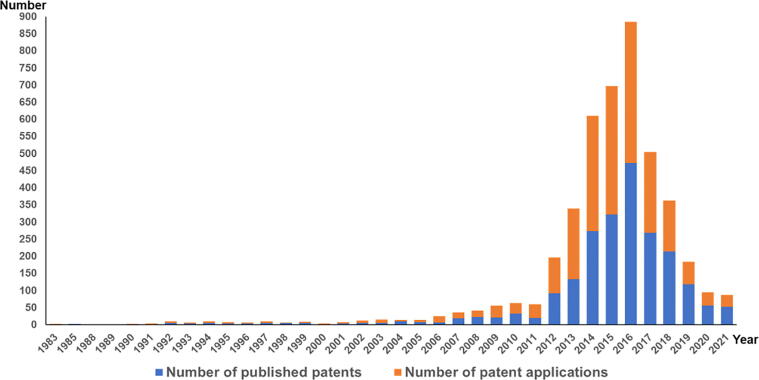
As “medicine and food homologous” food has received growing attention in the world, the research and product development of medicine and food homologous food have been become the focus of studying (Wang et al., 2022). The Baiten website (https://www1.baiten.cn/) can be used to find patent information (Ji et al., 2020). As shown in Fig. 10, the patent application began in 1983. Till now, there are 2188 patents related to BE, of which 2057 published patents were applied for in China at most. And the number of applications reached a staged peak (4 1 3) and set a new record high in 2016. The number of patent applications in China was the top one, indicating that the region had a high degree of activity in technological innovation and fierce market competition. After technical classification analysis, it was found that most of these patents were developed around the necessities of human life, accounting for 91.91% of the total number of patents. Further data analysis revealed that most of the patents were related to drugs and food. The number of pharmaceutical and food patents accounted for a large proportion, accounting for 47.8% and 38.2% of the total number of patents respectively. Among them, food-related patents mainly include the production of tea, noodles, beverages, biscuits and other food products. In addition, there was also a patent for designing the waxy surface of BE as a fruit preservation layer, which is for the preservation of strawberries (Sreenivas, Chaudhari, & Lele, 2011).
Fig. 10.
Annual patent quantity of Benincasae Exocarpium.
5.2. Food application
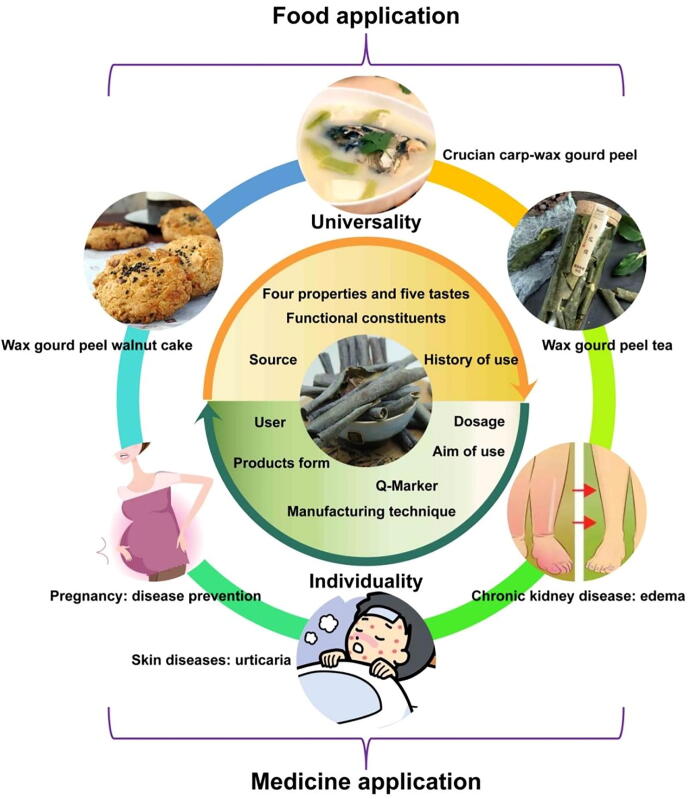
The fruits of B. hispida can be used as vegetables and fruits, and the peel is also used as food in daily life. The application about BE as food were arranged in Fig. 11. The nutritious compositions of peel and pulp are similar, but the content of each component is different (Deeksha et al., 2021). Interestingly, BE can also be used alone to make medicinal diet and tea. Wax gourd peel-black bean soup is the medicinal food using BE as the raw material with the effect of clearing heat and removing dampness, diuresis and detoxification. In addition, crucian carp-wax gourd peel soup is also a delicacy that was often eaten on the table. Soup can be used to relieve body edema and relieve bloating caused by irregular diet (Tan & Zhang, 2017).
Fig. 11.
Application of Benincasae Exocarpium. in food and medicine.
Tea was the first Chinese herbal medicine used by the Chinese. Currently, tea has become an attractive complementary and alternative medicine (Pan et al., 2022). However, for people with weak spleen and stomach, long-term use of BE has certain sub-health risks, because the essence of BE is cool. Therefore, people tend to add warm ingredients to neutralize the cold of BE. As a material of tea, BE was often used with lotus leaf, cassia seed, adlay and tuckahoe. Wax gourd peels tea and wax gourd peel-lotus leaf tea had a great sales volume on Chinese shopping applications. These teas can be used daily, with a certain diuretic and detumescent effect, and they are benefit for edema and weight loss as one of the choices of daily drinks.
Furthermore, the dried and crushed BE was good food ingredient, which was added to the walnut cake to help reduce the content of oil and sucrose, and could decrease the risk of obesity and high blood sugar caused by long-term consumption (Li, Li, & Zhao, 2015). BE powder with an added amount of 4.6% was made into steamed bread together with strong flour. The final sensory evaluation was 95 points (Li, Li, & Zhang, 2015).
5.3. Clinical application
The diuresis and swelling effect of BE had been used by experts since ancient times. BE can be used as a single prescription or as a traditional Chinese medicine (TCM) compound with other TCM for the treatment of edema symptoms in all ages. A total of 100 patients with acute nephritis from Daye County People's Hospital in Hubei Province received the compound TCM containing BE, which reduced facial swelling and increased urination. The formula consists of 10 TCMs, among which the amount of BE was the largest. The 68% of patients were cured, and other patients supplied signs of improvement (Hu, 1991). Decoction of BE and red bean was recorded in Modern Practical Chinese Medicine, published in 1956, which can reduce swelling and diuresis. A total of 120 patients with primary nephrotic syndrome in the Department of Nephrology of the First Affiliated Hospital and the Second Affiliated Hospital of the Hunan University of Chinese Medicine from June 2016 to June 2017 were selected as clinical samples. A total of 60 patients were treated with conventional western medicine, and the other 60 patients were treated with BE decoction. The time of edema resolution was (13.6 ± 4.0) d in the BE group and (22.9 ± 5.0) d in the western medicine group. The time of edema resolution in BE group was significantly shorter than that in western medicine group (P < 0.05) (Cai et al., 2019). The experimental group used BE concentrate, and in the control group, the 11 patients were given the same amount of water (Antian Wang, 1964). The study found that patients in BE group urinated faster and had higher urine output in the first 2 h by compared with the two groups (P < 0.01). Professor Nie, a famous TCM expert, attributed kidney disease to edema of TCM. The frequency of BE used by Nie in the treatment of kidney diseases was 65.37% (Li, 2012).
In particular, BE can be used to treat urticaria, both acute and chronic. Dr. Chen had 495 prescriptions for urticaria, including 379 cases (76.57%) of BE (Xiao, 2021). BE treatment of acute urticaria in Guangdong folk has a long history. Later, under the guidance of folk experience, BE was designed as internal and external washing prescriptions, for the treatment of wind-heat and wind-cold acute urticaria (Huang, 1995).
Due to the reasons for pregnancy, the range of treatment options is greatly limited, but clinically, some doctors used BE and corn silk to prevent the occurrence of pregnancy poisoning. As wax gourd and corn are commonly used foods, they have less toxic and side effects, and are inexpensive and simple to prepare. Therefore, it is suitable for most pregnant women to prevent the occurrence of diseases at an early stage (Liu, 2017). Other doctors boiled BE and Cyperi Rhizoma (Xiangfu in Chinese), seasoned with honey, and relieved the swelling of the limbs and face of pregnant women (Guo, 2021). BE was widely used in clinical and was shown in Fig. 11.
6. Conclusion and prospect
BE has been used as a diuretic and dampness drug for thousands of years of clinical experience in the treatment of diseases. With practice and improvement, it gradually became a well-known medicine and food homologous substance. Current studies indicated that BE mainly contains flavonoids, alkaloids, tannins, trace elements, vitamins and other functional factors. Modern pharmacology of BE extract showed that BE had diuretic, hypoglycemic, lipid-lowering and antioxidant effects, but its specific active components were not clear. Guided by the theory of TCM, the effects of BE are detumescence, diuresis and antipyretic, which is a common drug in the treatment of nephrotic syndrome, diabetes and skin disease in TCM. At the same time, BE is also widely welcomed as a food. According to the history of medicine and food homology and modern pharmacological research of BE, it can be seen that it has very objective potential. Whereas studies on the efficacy of BE are mostly carried out on the extract, and its pharmacodynamic material basis and mechanism of action still need to be further studied. It is hoped that in future study, the quality evaluation, active fractionation and drug design of BE will be paid attention to by researchers.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (No. 81673693) and Tianjin Intelligent Manufacturing Special Project of China (No. 20201178) for financial support.
Contributor Information
Jingze Zhang, Email: zhangjingze1977@163.com.
Dailin Liu, Email: dailinlch@163.com.
References
- Al-Snafi A.E. The pharmacological importance of Benincasa hispida. A review. International Journal of Pharma Sciences and Research. 2013;4:165–170. [Google Scholar]
- Abeyrathne E., Nam K., Ahn D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants (Basel) 2021;10(10):1587. doi: 10.3390/antiox10101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Saqr A., Khafagy E.S., Alalaiwe A., Aldawsari M.F., Alshahrani S.M., Anwer M.K.…Hegazy W.A. H. Synthesis of Gold Nanoparticles by using green machinery: Characterization and in vitro toxicity. Nanomaterials (Basel) 2021;11(3):808. doi: 10.3390/nano11030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F., Yang G., Tian J., Wang S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regeneration Research. 2012;7(33):2565–2575. doi: 10.3969/j.issn.1673-5374.2012.33.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antian Wang F.S. Experimental observation on diuretic effect of Wax gourd peel. Jiangsu Journal of Traditional Chinese Medicine. 1964;10:10–12. [Google Scholar]
- Bellur Nagarajaiah S., Prakash J. Chemical composition and bioactive potential of dehydrated peels of Benincasa hispida, Luffa acutangula, and Sechium edule. Journal of Herbs, Spices & Medicinal Plants. 2014;21(2):193–202. [Google Scholar]
- Bhambhani S., Kondhare K.R., Giri A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules. 2021;26(11):3374. doi: 10.3390/molecules26113374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Chen X., Feng J., Liu Q., Ai M., Yang N. Clinical observation on improving edema and renal function of patients with nephrotic syndrome by Jiyu Dongguangpi decoction. Journal of Hunan University of Chinese Medicine. 2019;05:631–634. [Google Scholar]
- Chen X., Qian X., Huang Z. Effects of Chinese herbal decoction on blood pressure and diuresis in rats. Negative. 2000;12:1563–1564. [Google Scholar]
- Choi Y.R., Shim J., Kim M.J. Genistin: A novel potent anti-adipogenic and anti-lipogenic agent. Molecules. 2020;25(9):2042. doi: 10.3390/molecules25092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary M., Grover K. Development of functional food products in relation to obesity. Functional Foods in Health and Disease. 2012;2(6):188–197. [Google Scholar]
- Commission C.P. 2020 edition. China Medical Science Press; 2020. Pharmacopoeia of the People’s Republic of China. [Google Scholar]
- de Souza P., Mariano L.N.B., Cechinel-Zanchett C.C., Cechinel-Filho V. Promising medicinal plants with diuretic potential used in brazil: State of the art, challenges, and prospects. Planta Medica. 2021;87(1–2):24–37. doi: 10.1055/a-1257-0887. [DOI] [PubMed] [Google Scholar]
- Devi T.B., Ahmaruzzaman M. Bio-inspired sustainable and green synthesis of plasmonic Ag/AgCl nanoparticles for enhanced degradation of organic compound from aqueous phase. Environmental Science and Pollution Research. 2016;23(17):17702–17714. doi: 10.1007/s11356-016-6945-1. [DOI] [PubMed] [Google Scholar]
- Dinda B., Dinda M., Roy A., Dinda S. Dietary plant flavonoids in prevention of obesity and diabetes. Advances in Protein Chemistry and Structural Biology. 2020;120:159–235. doi: 10.1016/bs.apcsb.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Espinoza-Hernandez F., Andrade-Cetto A., Escandon-Rivera S., Mata-Torres G., Mata R. Contribution of fasting and postprandial glucose-lowering mechanisms to the acute hypoglycemic effect of traditionally used Eryngium cymosum F. Delaroche. Journal of Ethnopharmacology. 2021;279 doi: 10.1016/j.jep.2021.114339. [DOI] [PubMed] [Google Scholar]
- Gu M., Fan S., Liu G., Guo L., Ding X., Lu Y.…Huang C. Extract of Wax Gourd peel prevents high-fat diet-induced hyperlipidemia in C57BL/6 mice via the inhibition of the PPARgamma Pathway. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/342561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P., Zhang B., Zhao J., Wang C., Wang Z., Liu A., Du G. Medicine-Food herbs against Alzheimer's disease: A review of their traditional functional features, substance basis, clinical practices and mechanisms of action. Molecules. 2022;27(3):901. doi: 10.3390/molecules27030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. Decoction of honey and wax gourd peel Corydalis to eliminate pregnancy edema. Journal of Bee. 2021;5:57. [Google Scholar]
- Hajiaghaalipour F., Khalilpourfarshbafi M., Arya A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. International Journal of Biological Sciences. 2015;11(5):508–524. doi: 10.7150/ijbs.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakiki D.N., Fauziyyah A., Wijanarti S. Aktivitas Antioksidan dan screening Fitokimia Kulit Bligo (Benincasa hispida) ALCHEMY Jurnal Penelitian Kimia. 2021;17(1):27. [Google Scholar]
- Huang H.Y., Huang J.J., Tso T.K., Tsai Y.C., Chang C.K. Antioxidant and angiotension-converting enzyme inhibition capacities of various parts of Benincasa hispida (wax gourd) Nahrung. 2004;48(3):230–233. doi: 10.1002/food.200300428. [DOI] [PubMed] [Google Scholar]
- Huang J. 50 cases of acute urticaria treated with Donggua peel soup. Shandong Journal of Traditional Chinese. 1995;6:252–253. [Google Scholar]
- Hussain H., Mamadalieva N.Z., Hussain A., Hassan U., Rabnawaz A., Ahmed I., Green I.R. Fruit peels: Food waste as a valuable source of bioactive natural products for drug discovery. Current Issues in Molecular Biology. 2022;44(5):1960–1994. doi: 10.3390/cimb44050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji M.Y., Bo A., Yang M., Xu J.F., Jiang L.L., Zhou B.C., Li M.H. The pharmacological effects and health benefits of platycodon grandiflorus-A medicine food homology species. Foods. 2020;9(2):142. doi: 10.3390/foods9020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Gao W., Tang L. Antidiabetic herbal drugs officially approved in China. Phytotherapy Research. 2003;17(10):1127–1134. doi: 10.1002/ptr.1398. [DOI] [PubMed] [Google Scholar]
- Julsrigival J., Sirisa-Ard P., Julsrigival S., Akarchariya N. Antiviral medicinal plants found in Lanna traditional medicine. Chinese Herbal Medicines. 2021;13(4):494–501. doi: 10.1016/j.chmed.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar C.S.C., Mythily R., Chandraju S. Extraction and mass characterization of sugars from ash gourd peels (Benincasa hispida) Rasayan Journal of Chemistry. 2012;5:280–285. [Google Scholar]
- Kumar C.S.C., Mythily R., Mythily R. Extraction and mass characterization of sugars from ash gourd peels (Benincasa hispida) Rasayan Journal of Chemistry. 2012;5:280–285. [Google Scholar]
- Lan T., Li Q., Chang M., Yin C., Zhu D., Wu Z.…Guo H. Lei-gong-gen formula granule attenuates hyperlipidemia in rats via cGMP-PKG signaling pathway. Journal of Ethnopharmacology. 2020;260 doi: 10.1016/j.jep.2020.112989. [DOI] [PubMed] [Google Scholar]
- Lan W.T., Chen Y.S., Yanagida F. Isolation and characterization of lactic acid bacteria from Yan-dong-gua (fermented wax gourd), a traditional fermented food in Taiwan. Journal of Bioscience and Bioengineering. 2009;108(6):484–487. doi: 10.1016/j.jbiosc.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Choi H.R., Kim C.H. Anti-angiogenic effect of the seed extract of Benincasa hispida Cogniaux. Journal of Ethnopharmacology. 2005;97(3):509–513. doi: 10.1016/j.jep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Li A. China Academy of Chinese Medical Sciences; 2012. The study of professor Nie Lifeng's treatment experience of primary nephrotic syndrome based on complex network and mutual infoemation. Thesis of Master Degree. [Google Scholar]
- Li H., Song F., Xing J., Tsao R., Liu Z., Liu S. Screening and structural characterization of alpha-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MS(n) and SORI-CID FTICR MS. Journal of the American Society for Mass Spectrometry. 2009;20(8):1496–1503. doi: 10.1016/j.jasms.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Li N., Li Y., Zhang Y. Using response surface methodology to optimization of steam bread made from exocarpium benincasae powder. Grain Processing. 2015;3:44–48. [Google Scholar]
- Li N., Li Y., Zhao G. Preparation of low sugar and low fat wax melon peel powder and peach cake. Packaging and Food. Machinery. 2015;33 [Google Scholar]
- Li X. Nanjing University of Chinese Medicine; 2020. The research on cognition and development of the efficacy of water-disinhibiting damp-percolationg medicine on the basis of ancient and modern literature. Thesis of Master Degree. [Google Scholar]
- Lin C.C., Yang Y.C., Chen C.Y., Yin M.C. Combination of s-methyl cysteine and protocatechuic acid provided greater lipid-lowering and anti-inflammatory effects in mice liver against chronic alcohol consumption. Iranian Journal of Basic Medical Sciences. 2021;24(8):1146–1152. doi: 10.22038/ijbms.2021.56705.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. Winter melon rind and corn silk boiled in water to prevent pregnancy poisoning. Electronic Journal of Clinical Medical Literature. 2017;4:16801. [Google Scholar]
- Ma H., Ma Y., Dawa Z., Yao Y., Wang M., Zhang K.…Lin C. Diterpenoid alkaloids isolated from Delphinium brunonianum and their inhibitory effects on hepatocytes lipid accumulation. Molecules. 2022;27(7):2257. doi: 10.3390/molecules27072257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N.H., Guo J., Xu Chen S.H., Yuan X.R., Zhang T., Ding Y. Antioxidant and compositional HPLC analysis of three common bamboo leaves. Molecules. 2020;25(2):409. doi: 10.3390/molecules25020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munteanu I.G., Apetrei C. Analytical methods used in determining antioxidant activity: A Review. International Journal of Molecular Sciences. 2021;22(7):3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamthodi S., Kadam D., Lele S.S. Physicochemical and functional properties of ash gourd/bottle gourd beverages blended with jamun. Journal of Food Science and Technology. 2019;56(1):473–482. doi: 10.1007/s13197-018-3509-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S.Y., Nie Q., Tai H.C., Song X.L., Tong Y.F., Zhang L.J.…Liang C. Tea and tea drinking: China’s outstanding contributions to the mankind. Chinese Medicine. 2022;17(1):27. doi: 10.1186/s13020-022-00571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida N.K., Sahu M.R., Debata P.C., Panda P.K. Antinociceptive and antiinflammatory effects of methanolic extract of Benincasa hispida fruit peel in Rodents. Asian Journal of Chemistry. 2010;22:7573–7579. [Google Scholar]
- Rana S., Suttee A. Phytochemical investigation and evaluation of free radical scavenging potential of Benincasa hispida peel extracts. International Journal of Current Pharmaceutical Review and Research. 2012;3:43–46. [Google Scholar]
- Rios J.L., Francini F., Schinella G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Medica. 2015;81(12–13):975–994. doi: 10.1055/s-0035-1546131. [DOI] [PubMed] [Google Scholar]
- Ryu H.S., Lee S.J., Whang W.K. Isolation of anti-diabetic active compounds from Benincasae Exocarpium and development of simultaneous analysis by HPLC-PDA. Molecules. 2021;27(1):9. doi: 10.3390/molecules27010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J., Chatterjee S., Kumar V., Variyar P.S., Sharma A. Analysis of free and glycosidically bound compounds of ash gourd (Benincasa hispida): Identification of key odorants. Food Chemistry. 2010;122(4):1327–1332. [Google Scholar]
- Shetty B.V., Arjuman A., Jorapur A., Samanth R., Yadav S.K., Valliammai N.…Rao G.M. Effect of extract of Benincasa hispida on oxidative stress in rats with indomethacin induced gastric ulcers. Indian Journal of Physiology and Pharmacology. 2008;52(2):178–182. [PubMed] [Google Scholar]
- Shih C.T., Wu J., Jia S., Khan A.A., Ting K.H., Shih D.S. Purification of an osmotin-like protein from the seeds of Benincasa hispida and cloning of the gene encoding this protein. Plant Science. 2001;160(5):817–826. doi: 10.1016/s0168-9452(00)00450-7. [DOI] [PubMed] [Google Scholar]
- Soliman W.E., Khan S., Rizvi S.M.D., Moin A., Elsewedy H.S., Abulila A.S., Shehata T.M. Therapeutic applications of Biostable Silver Nanoparticles synthesized using peel extract of Benincasa hispida: Antibacterial and anticancer activities. Nanomaterials (Basel) 2020;10(10):1954. doi: 10.3390/nano10101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivas K.M., Chaudhari K., Lele S.S. Ash gourd peel wax: Extraction, characterization, and application as an edible coat for fruits. Food Science and Biotechnology. 2011;20(2):383–387. [Google Scholar]
- Tan L., Zhang Q. Crucian Carp-Wax Gourd Peel Soup relieve abdominal distension. Friends of the Farmhouse. 2017;08:45. [Google Scholar]
- Tatipamula V.B., Kukavica B. Phenolic compounds as antidiabetic, anti-inflammatory, and anticancer agents and improvement of their bioavailability by liposomes. Cell Biochemistry and Function. 2021;39(8):926–944. doi: 10.1002/cbf.3667. [DOI] [PubMed] [Google Scholar]
- Upadhyaya Deeksha K.G., Konety Rucha, Shruthi S.D. Comparative phytochemical and antimicrobial analysis of extracts from different parts of ash gourd. International Journal of Current Science. 2021;11(4):274–285. [Google Scholar]
- Wang Y., Zhao Y., Liu X., Li J., Zhang J., Liu D. Chemical constituents and pharmacological activities of medicinal plants from Rosa genus. Chinese Herbal Medicines. 2022;14(2):187–209. doi: 10.1016/j.chmed.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X. Beijing university of Chinese Medicine; 2021. Summary and analysis of professor Chen Tongyun's experience in treating chronic urticaria. Thesis of Master Degree. [Google Scholar]
- Xie L., Deng Z., Zhang J., Dong H., Wang W., Xing B., Liu X. Comparison of flavonoid O-glycoside, C-glycoside and their aglycones on antioxidant capacity and metabolism during in vitro digestion and in vivo. Foods. 2022;11(6):882. doi: 10.3390/foods11060882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Wu D., Dong N., Ouyang P., Pu J., Hu Q.…Huang J. Moracin C, A phenolic compound isolated from Artocarpus heterophyllus, suppresses lipopolysaccharide-activated inflammatory responses in murine Raw264.7 macrophages. International Journal of Molecular Sciences. 2016;17(8):1199. doi: 10.3390/ijms17081199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Liu W., Zhou H., Zhang D., Li R., Li C., Wang S. The relations between minor components and antioxidant capacity of five fruits and vegetables seed oils in China. Journal of Oleo Science. 2019;68(7):625–635. doi: 10.5650/jos.ess19005. [DOI] [PubMed] [Google Scholar]
- Zhu J., Chen C., Zhang B., Huang Q. The inhibitory effects of flavonoids on alpha-amylase and alpha-glucosidase. Critical Reviews in Food Science and Nutrition. 2020;60(4):695–708. doi: 10.1080/10408398.2018.1548428. [DOI] [PubMed] [Google Scholar]