Abstract
Recent fossil discoveries suggest that the coevolution of insect pollinators and gymnosperms started long before the appearance of flowering plants. One of the keys to understanding the origins of pollination relationships is fossil insects with gymnosperm pollen attached to the body surface. Such fossils are exceedingly rare to find, especially from the Palaeozoic, a time when ambers with insect inclusions were absent. Here, we report compression fossils of Early Permian tillyardembiid insects (Polyneoptera) preserved with pollen on their heads, thoraces, legs and abdomens. This is the earliest finding of pollen-bearing insects, predating the previous oldest record from the Middle Jurassic by ca 120 Ma. Judging by the pollen composition, tillyardembiids visited a narrow range of host plants, including Rufloriaceae (Cordaitales). While it is impossible to say for certain whether tillyardembiids as pollen consumers contributed to pollination, a trophic specialization of this kind could be considered an evolutionary precursor of pollination mutualism.
Keywords: plant–insect interactions, pollination, Palaeozoic
1. Introduction
Mutualism between plants and insect pollinators is one of the most important symbiotic associations on Earth. However, fossil evidence for its evolution is mostly indirect, such as fossil cones and flowers seemingly adapted for entomophily and fossilized remains of insects with siphonate mouthparts thought to be suitable for nectar feeding [1,2]. By contrast, pollen attached to insects is a ‘smoking gun’ for their interactions with plant reproductive organs, but it does not often appear in the fossil record. Chances of being preserved with pollen on the body are higher for insects in fossil resins than for those in sedimentary rocks, since the latter fall into water prior to fossilization where adhering pollen could easily be washed away. Compression fossils of bees and fig wasps with a substantial amount of pollen are known from the Eocene [3,4], but more ancient pollen-carrying insects have been reported mostly from Cretaceous ambers [5–8]. Among the few exceptions are compression fossils of Jurassic kalligrammatids and Early Cretaceous brachyceran flies with sparse pollen grains adjacent to their heads and mouthparts [9,10]. The absence of ambers with macroscopic biological inclusions during the Palaeozoic poses a particular challenge for obtaining direct cues of insect involvement in plant reproduction. Previously, Palaeozoic insects have been found to contain pollen and spores only in the guts but not on the outer surfaces [11,12]. The only known Palaeozoic arthropod with pollen stuck to its body is a fragmentary specimen of Upper Carboniferous Arthropleura (Myriapoda) with a number of pollen grains along the base of its leg [13].
Here, we report Early Permian tillyardembiids with pollen on their thoraces, abdomens and legs from the Chekarda locality in European Russia. Tillyardembiids (Cnemidolestida: Tillyardembiidae) are a small family of Permian polyneopterous insects, somewhat resembling stoneflies in forewing venation [14]. Tillyardembiids are one of the most abundant insects in the Chekarda locality, comprising approximately 5% of all insect specimens collected there [14]. We used the taxonomic composition of the tillyardembiids' pollen loads to reveal their feeding strategies and host plant preferences. This is the earliest direct evidence of the involvement of insects in pollen transportation.
2. Materials and methods
(a) . Geological settings
Fossil specimens were collected in the Lower Permian continental deposits of the Koshelevka Formation, which are exposed on the left bank of the Sylva River 800 m to the northwest of the village of Chekarda (=Tchekarda) (Russia, Perm region, Cis-Urals). The Koshelevka Formation is lithostratigraphically assigned to the upper (Irenian) substage of the Kungurian stage [15,16], whose boundaries are currently dated at about 283–273 Ma [17] or 283–277 Ma [18]. The Chekarda locality, which has yielded more than 260 insect species in 91 families and 25 orders as well as diverse taxa of terrestrial plants, is one of the most important Permian Konservat-Lagerstätten [19]. All examined fossils are kept in the collection of the Arthropod Laboratory of Borissiak Paleontological Institute (Moscow, Russia) and accessible on request.
(b) . Pollen treatment
The pollen grains were obtained from the fossil specimens using a dissecting needle and a hair attached to a match. A drop of water was placed on treated areas to facilitate the process of pollen extraction. Most of the pollen associated with insects is embedded in the rock matrix and cannot be extracted without damaging the specimens. To avoid this, we sampled only easily detachable pollen grains. After extraction, the pollen grains were treated with a Codyson CD-4810 ultrasonic bath at 35 kHz in two 10 min cycles to remove non-organic particles. Additional cleaning was performed under a Leica M165c stereomicroscope with alcohol and distilled water. Finally, pollen grains were transferred into a glycerine drop on microscope slides.
(c) . Microscopy and imaging
The fossil specimens were studied and photographed using a Leica MZ9.5 stereomicroscope. SEM images of the insects were produced using a Tescan Vega II scanning electron microscope. Fluorescence photographs were taken with a Leica DM4 B microscope. The pollen grains were studied under light microscopy with a Zeiss Axioplan 2 microscope and photographed with a Zeiss Axiocam 105 colour camera.
3. Results
Tillyardembiids are represented in the Chekarda locality mostly by Tillyardembia G. Zalessky, 1937 with two species described, which are distinguishable only by the shape of the male forefemur [20]. In the present paper by tillyardembiids, we refer to the members of this genus. We checked 425 specimens of Tillyardembia from the collection of the Borissiak Paleontological Institute for pollen presence. Four of them were found to bear clusters of pollen grains on the body surface (figure 1a–l, figure 2h–j), and one was found to have a pollen mass partly extruded from the hindgut (figure 2d,f). One additional specimen of Tillyardembia from the same collection was previously reported to contain pollen in the gut [21]. However, re-examination of the specimen revealed that this insect, like four others, bears pollen on the outer surface of the body, not in the gut (figure 2a,b,e,c). First, it is evident from the distribution of the pollen, situated close to the margins of the meso- and metathorax, not along the middle line of the body, where the gut normally lies. Second, pollen grains on the specimen are fully intact, not chewed or partly ingested. In summary, six specimens bearing pollen grains were found, i.e. 1.4% of all examined tillyardembiids demonstrate direct evidence of pollen-eating behaviour (table 1). This small number is probably due to taphonomical reasons and does not reflect the actual frequency of visits by tillyardembiids to pollen-producing organs.
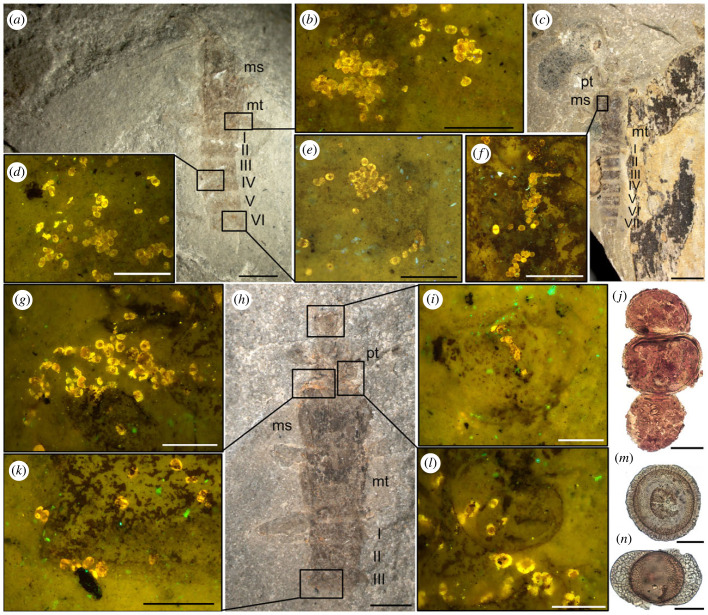
Figure 1.
Tillyardembiids from the Lower Permian of Chekarda, general views (a,c,h), close-ups of body areas covered with pollen, taken under a fluorescence microscope (b,d–g,i,k,l) and pollen grains obtained from the specimens (j,m,n). (a,b,d,e) PIN 1700/3727; (c,f) PIN 1700/1164; (g–i,k,l) PIN 1700/4286; (j) Cladaitina pollen (from PIN 1700/1164); (m) Cordaitina pollen (from PIN 1700/4286); (n) Striatites pollen (from PIN 1700/4286). Scale bars = 2 mm (a,c), 1 mm (h), 400 µm (e,d), 250 µm (b,i,l,g,f), 300 µm (k), 20 µm (j,m,n). Roman numbers indicate abdominal segments; pt, prothorax; ms, mesothorax; mt, metathorax.
Figure 2.
Tillyardembiids from the Lower Permian of Chekarda. (a,b,c,e) PIN 1700/666, general view (b) and fluorescent photographs of body areas with pollen (a,e,c); (d,f) PIN 1700/984, general view and end of abdomen with semi-ejected gut content consisting of pollen; (g–j) PIN 4987/669, general view (i), fluorescent photographs of body areas with pollen (h,j) and SEM photograph of tergal area showing rows of hairs (g); (k) artistic reconstruction of female Tillyardembia feeding on Pechorostrobus pollen organ (Rufloriaceae), with another female on Bardocarpus cone in the background, artist A. Atuchin. Scale bars = 5 mm (b), 2 mm (i,d), 1 mm (f), 200 μm (a,c,g,j), 100 µm (h). Roman numbers indicate abdominal segments; pt, prothorax; ms, mesothorax; mt, metathorax.
Table 1.
Specimens of Tillyardembiidae with pollen from the Lower Permian of Chekarda.
| specimen | position of pollen | pollen taxa and number of identified pollen grains |
||
|---|---|---|---|---|
| Luberisaccites | Cladaitina | Striatites | ||
| PIN 1700/4286 | on head, thorax, abdomen | 6 | — | 9 |
| PIN 1700/3727 | on thorax, legs, abdomen | 1 | — | 2 |
| PIN 1700/1164 | on thorax | — | 18 | — |
| PIN 4987/669 | on abdomen | — | — | 3 |
| PIN 1700/984 | in gut, at end of abdomen | — | — | 6 |
| PIN 1700/666 | on thorax | — | ca 60a | — |
aData from [21].
Two of the tillyardembiids, PIN 1700/4286 and PIN 1700/3727, have pollen scattered all over their bodies. Pollen is present on the head, thorax and first three preserved abdominal segments of PIN 1700/4286 and is especially abundant in the intersegment area between the prothorax and mesothorax (figure 1g–i,k,l). Specimen PIN 1700/3727 carries a pollen load on the thorax, on the base of the legs and on I–VI preserved abdominal segments (figure 1a,b,d,e). The other two tillyardembiids, PIN 4987/669 and PIN 1700/1164, are covered with pollen to a lesser degree. Specimen PIN 1700/1164 bears pollen clusters laterally on the mesothorax, with some pollen grains on the 1st abdominal segment (figure 1c,f), while PIN 4987/669 has several pollen grains attached to the lateral edge of the 1st abdominal segment and a small cluster of pollen on the 7th abdominal segment (figure 1h–j). Finally, a partly ejected gut content consisting of chewed and half-digested pollen grains is visible near the tip of the abdomen of PIN 1700/984 (figure 2d,f). Importantly, groups of pollen grains never occur on stone slabs beyond the insect bodies; at most, only rare isolated pollen grains could be spotted on the surface of the adjacent rock matrix.
Twenty-seven microscope slides with pollen were prepared for five specimens, and 45 individual pollen grains proved to be intact enough for taxonomic identification (electronic supplementary material, figures S1–S3). Three pollen genera were identified: Cladaitina sp., Luberisaccites sp. and Striatites sp. (Figure 1j,m,n). Cladaitina are oval to subcircular pollen grains with a thin monosaccus, a slit-like structure, and a verrucate surface [22]. Luberisaccites, also assignable to Cordaitina s.l., are circular monosaccate pollen grains, with saccus covering the distal side and subequatorially attaching to the proximal side [23,24]. Striatites are haploxyponoid or slightly diploxylonoid bisaccate striate pollen grains with a slit-like structure on the proximal side [25]. The pollen loads of two insects (PIN 1700/4286 and PIN 1700/3727) are mixed, consisting of Luberisaccites and Striatites; the other three have yielded only one pollen genus each: Cladaitina (PIN 1700/1164) and Striatites (PIN 1700/984 and PIN 4987/669) (table 1). The pollen from the thorax of the specimen examined by Afonin also belongs to Cladaitina [21].
4. Discussion
While the botanical affinity of Striatites is unclear, Cladaitina and Luberisaccites pollen was previously extracted from Pechorostrobus and Cladostrobus pollen organs from the Upper Permian of European Russia [26]. Based on their association with Rufloria leaves, these pollen organs are considered to belong to Rufloriaceae, an Angaran family of cordaitanthalean gymnosperms [27]. Hence, it could be said that tillyardembiids, as pollen feeders, showed a preference for a narrow range of host plants, that is, Rufloriaceae and Striatites pollen-producing plants. Interestingly, the composition of insect coprolites from the Upper Carboniferous of North America is also suggestive of feeding preferences towards cordaitanthalean pollen [12].
The conclusion about trophic specialization of tillyardembiids, although based on a small sample, is supported by striking differences in pollen composition between them and other insects from the same locality. In addition to Tillyardembia, five insect species in four orders (Hypoperlida, Permopsocida, Miomoptera and Grylloblatida) from Chekarda with ingested pollen were reported and dissected, but unlike tillyardembiids, none of them was found to contain Striatites and ruflorian pollen in their guts [28–30]. In these studies, however, only one specimen per species was examined, so further research is needed to confirm niche partitioning among Chekarda pollinivorous insects.
The non-generalist feeding strategy of tillyardembiids could also be inferred from palynology. While Rufloriaceae is well represented in the Chekarda locality by Rufloria leaves and Bardocarpus seeds and seed-bearing organs [31], their pollen is not abundant there, with Luberisaccites (=Cordaitina) and Cladaitina amounting to only 5% and 2.2% of the Chekarda pollen spectrum, respectively [32]. Striatites, also favoured by tillyardembiids, is absent in the Chekarda pollen spectrum altogether [32]. This could be taken as an indication that tillyardembiids preferably fed on pollen produced or dispersed in small amounts, possibly favouring it because of its superior nutritive value. By contrast, pollen consumed by other insect species from Chekarda usually includes the most common pollen taxa, such as Protohaploxypinus and Vittatina (17.6% and 24.3% in the pollen spectrum, respectively [32]). As a rule, wind-pollinated plants tend to produce significantly more grains per ovule than animal-pollinated plants [33], but they apparently did not attract tillyardembiids.
The short legs and elongated fusiform bodies of tillyardembiids suggest that they could have spent most of their time under stones, fallen logs and in leaf litter on the forest floor, similar to extant earwigs and rove beetles [20]. While these ground-dwelling insects do not fit the stereotype of flower visitors, nonetheless, they are known to add pollen and nectar to their diet, sometimes acting as pollinators [34,35]. It is possible to hypothesize that tillyardembiids could have done the same, pollinating ruflorian gymnosperms and some other Palaeozoic plants. Short legs and elongated spindle-shaped bodies probably made it easy for tillyardembiids to creep between scales of megastrobili in seeking sugary fluids or a resting place. While pollen organs of Rufloriaceae were 15–20 mm long [26], ruflorian female cones Bardocarpus were sizable enough (at least 50 mm long and 20 mm wide [31]) to shelter tillyardembiids (body length ca 15 mm). Dusted with pollen, tillyardembiids could easily have transferred it to the ovules if they visited megastrobili for any reason (figure 2k).
Dense rows of hairs on abdominal tergites could have facilitated attachment of pollen to the body of tillyardembiids (figure 2g). The effectiveness of tillyardembiids as pollen vectors was possibly also enhanced by the presence of unreduced membranous wings, though their long cerci and weakly sclerotized wing venation indicate that they were poor and occasional flyers. The abundance of tillyardembiids in the Chekarda locality could be explained by their swarming behaviour in the vicinity of the paleolagoon. It is worth noting that stoneflies proposed as a sister-group to tillyardembiids [14] have been observed participating in pollination in riparian areas [36].
However, it is no less likely that tillyardembiids simply robbed specific plants of their pollen, contributing little or nothing to their pollination, as many extant flower-visiting insects do. Like many pollen-eating insects, tillyardembiids had largely unmodified mandibulate mouthparts [20] (electronic supplementary material, figure S4). In any case, it is clear from these results that plant–insect interactions had already reached a considerable complexity by the Triassic, when a new phase of the development of palynivory and nectarivory began, marked by the appearance of Bennettitales and other entomophilous gymnosperm lineages [10].
Acknowledgements
We are grateful to Roman Rakitov (Borissial Paleontological Institute) and Jolanta Brożek (University of Silesia in Katowice) for assisting with the SEM. We also would like to thank two anonymous reviewers for their helpful comments.
Data accessibility
The additional data are provided in the electronic supplementary material [37]. All fossils discussed in the paper are reposited in publicly accessible institutions.
Authors' contributions
A.V.K.: conceptualization, investigation, visualization, writing—original draft and writing—review and editing; T.F.: conceptualization, investigation, visualization and writing—original draft; P.W.: conceptualization, funding acquisition, project administration, resources, supervision and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The research was supported by the Russian Science Foundation (project 21-14-00284) (for A.V.K.) and the Ulam Program of the Polish National Agency for Academic Exchange (NAWA), project no. PPN/ULM/2020/1/00159/U/00001.
References
- 1.Labandeira CC. 2010. The pollination of mid Mesozoic seed plants and the early history of long-proboscid insects. Ann. Missouri Bot. Gard. 97, 469-513. ( 10.3417/2010037) [DOI] [Google Scholar]
- 2.Khramov AV, Bashkuev AS, Lukashevich ED. 2020. The fossil record of long-proboscid nectarivorous insects. Entomol. Rev. 100, 881-968. ( 10.1134/S0013873820070015) [DOI] [Google Scholar]
- 3.Compton SG, Ball AD, Collinson ME, Hayes P, Rasnitsyn AP, Ross AJ. 2010. Ancient fig wasps indicate at least 34 myr of stasis in their mutualism with fig trees. Biol. Lett. 6, 838-842. ( 10.1098/rsbl.2010.0389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wappler T, Labandeira CC, Engel MS, Zetter R, Grimsson F. 2015. Specialized and generalized pollen-collection strategies in an ancient bee lineage. Curr. Biol. 25, 3092-3098. ( 10.1016/j.cub.2015.09.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao T, Wang B, Li J, Dilcher D. 2019. Pollination of Cretaceous flowers. Proc. Natl Acad. Sci. USA 116, 24 707-24 711. ( 10.1073/pnas.1916186116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peñalver E, Labandeira CC, Barrón E, Delclòs X, Nel P, Nel A, Tafforeau P, Soriano C. 2012. Thrips pollination of Mesozoic gymnosperms. Proc. Natl Acad. Sci. USA 109, 8623-8628. ( 10.1073/pnas.1120499109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peñalver E, Arillo A, Pérez-de la Fuente R, Riccio ML, Delclòs X, Barrón E, Grimaldi DA. 2015. Long-proboscid flies as pollinators of Cretaceous gymnosperms. Curr. Biol. 25, 1917-1923. ( 10.1016/j.cub.2015.05.062) [DOI] [PubMed] [Google Scholar]
- 8.Peris D, Labandeira CC, Barrón E, Delclòs X, Rust J, Wang B. 2020. Generalist pollen-feeding beetles during the mid-Cretaceous. iScience 23, 100913. ( 10.1016/j.isci.2020.100913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labandeira CC, et al. 2016. The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proc. R. Soc. B 283, 20152893. ( 10.1098/rspb.2015.2893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labandeira CC, Kvaček J, Mostovski MB. 2007. Pollination drops, pollen, and insect pollination of mesozoic gymnosperms. Taxon 56, 663-695. ( 10.2307/25065852) [DOI] [Google Scholar]
- 11.Krassilov VA, Rasnitsyn AP, Afonin SA. 2007. Pollen eaters and pollen morphology: co-evolution through the Permian and Mesozoic. Afr. Invertebr. 48, 3-11. [Google Scholar]
- 12.Labandeira CC. 2000. The paleobiology of pollination and its precursors. Paleontol. Soc. Pap. 6, 233-269. ( 10.1017/S1089332600000784) [DOI] [Google Scholar]
- 13.Scott AC, Taylor TN. 1983. Plant/animal interactions during the Upper Carboniferous. Bot. Rev. 49, 259-307. ( 10.1007/BF02861089) [DOI] [Google Scholar]
- 14.Aristov DS, Rasnitsyn AP. 2009. The family Tillyardembiidae Zalessky, 1938 and the system of the plecopteroid insects. Russian Entomol. J. 18, 257-264. [Google Scholar]
- 15.Chuvashov BI. 1986. The main types of carbonate rocks of the Kungurian evaporite basin of the Urals. Geol. Soc. Spec. Publ. 22, 225-239. ( 10.1144/GSL.SP.1986.022.01.22) [DOI] [Google Scholar]
- 16.Naugolnykh SV, Kerp H. 1996. Aspects of Permian palaeobotany and palynology. XV. On the oldest known peltasperms with radially symmetrical ovuliferous discs from the Kungurian (uppermost Lower Permian) of the Fore-Urals (Russia). Rev. Palaeobot. Palynol. 91, 35-62. ( 10.1016/0034-6667(95)00066-6) [DOI] [Google Scholar]
- 17.Lucas SG, Shen SZ. 2018. The Permian timescale: an introduction. Geol. Soc. Spec. Publ. 450, 1-19. ( 10.1144/SP450.15) [DOI] [Google Scholar]
- 18.Davydov VI, Biakov AV, Schmitz MD, Silantiev VV. 2018. Radioisotopic calibration of the Guadalupian (middle Permian) series: review and updates. Earth-Sci. Rev. 176, 222-240. ( 10.1016/j.earscirev.2017.10.011) [DOI] [Google Scholar]
- 19.Zhuzhgova LV, Ponomareva GY, Aristov DS, Naugolnykh SV. 2015. Chekarda is a location of fossil insects and plants from the Permian period. Monograph on the geology, paleobotany and paleoentomology of Chekarda. Perm, Russia: Perm State University. (In Russian) [Google Scholar]
- 20.Vilesov AP, Novokshonov VG. 1993. Permian Tillyardembiidae (Insecta, Grylloblattida). Paleontol. J. 27, 71-82. [Google Scholar]
- 21.Afonin SA. 2000. Pollen grains of the genus Cladaitina extracted from the gut of the Early Permian insect Tillyardembia (Grylloblattida). Paleontol. J. 34, 575-579. [Google Scholar]
- 22.Maheshwari HK, Meyen SV. 1975. Cladostrobus and the systematics of cordaitalean leaves. Lethaia 8, 103-123. ( 10.1111/j.1502-3931.1975.tb01306.x) [DOI] [Google Scholar]
- 23.Dibner AF. 1971. Pollen of cordaiteans of Angaraland. Uch. Zap. NIIGA, Paleontol. Biostrat. 32, 5-66. (In Russian) [Google Scholar]
- 24.Oshurkova MV. 2003. Morphology, classification and description of form genera of Late Paleozoic miospores. St Petersburg, FL: VSEGEI Press. (In Russian) [Google Scholar]
- 25.Klaus W. 1963. Sporen aus dem südalpinen Perm (Vergleichsstudie für die Gliederung nordalpiner Salzserien). Jb. Geol. B. A 106, 229-361. [Google Scholar]
- 26.Meyen SV. 1982. Fructifications of Upper Paleozoic Cordaitanthales from the Angaran region. Paleontol. J. 2, 109-120. [Google Scholar]
- 27.Meyen SV. 1984. Basic features of gymnosperm systematics and phylogeny as evidenced by the fossil record. Bot. Rev. 50, 1-112. ( 10.1007/BF02874305) [DOI] [Google Scholar]
- 28.Krassilov VA, Rasnitsyn AP, Afonin SA. 1999. Pollen morphotypes from the intestine of a Permian booklouse. Rev. Palaeobot. Palynol. 106, 89-96. ( 10.1016/S0034-6667(99)00002-0) [DOI] [Google Scholar]
- 29.Krassilov VA, Rasnitsyn AP. 1997. Pollen in the guts of Permian insects: first evidence of pollinivory and its evolutionary significance. Lethaia 29, 369-372. ( 10.1111/j.1502-3931.1996.tb01672.x) [DOI] [Google Scholar]
- 30.Rasnitsyn AP, Krassilov VA. 1996. First find of pollen grains in the gut of Permian insects. Paleontol. J. 30, 484-490. [Google Scholar]
- 31.Naugolnykh SV. 1998. Kungurian flora of the Middle Cis-Urals. Moscow, Russia: Geos. (In Russian) [Google Scholar]
- 32.Naugolnykh SV. 2014. Fossil flora and stratigraphy of the terrigenous Kungurian Beds (Lower Permian) of the basin of the Barda River (Urals, Perm Krai). Stratigr. Geol. Correl. 22, 680-707. ( 10.1134/S0869593814070041) [DOI] [Google Scholar]
- 33.Cruden RW. 2000. Pollen grains: why so many? Plant Syst. Evol. 222, 143-165. ( 10.1007/BF00984100) [DOI] [Google Scholar]
- 34.Wright MG, Wright GEP, Smith P. 1989. Entomophily and seed predation of Witsenia maura (Iridaceae), a rare fynbos species. S. Afr. J. Bot. 55, 273-277. ( 10.1016/S0254-6299(16)31175-9) [DOI] [Google Scholar]
- 35.Sayers TDJ, Steinbauer MJ, Miller RE. 2019. Visitor or vector? The extent of rove beetle (Coleoptera: Staphylinidae) pollination and floral interactions. Arthropod-Plant Interact. 13, 685-701. ( 10.1007/s11829-019-09698-9) [DOI] [Google Scholar]
- 36.Wong Sato AA, Kato M. 2017. Pollination system of Corylopsis gotoana (Hamamelidaceae) and its stonefly (Plecoptera) co-pollinator. Plant Species Biol. 32, 440-447. ( 10.1111/1442-1984.12178) [DOI] [Google Scholar]
- 37.Khramov AV, Foraponova T, Węgierek P. 2023. The earliest pollen-loaded insects from the Lower Permian of Russia. Figshare. ( 10.6084/m9.figshare.c.6430258) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Khramov AV, Foraponova T, Węgierek P. 2023. The earliest pollen-loaded insects from the Lower Permian of Russia. Figshare. ( 10.6084/m9.figshare.c.6430258) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The additional data are provided in the electronic supplementary material [37]. All fossils discussed in the paper are reposited in publicly accessible institutions.