Summary
Background
Recently we identified in patients with chronic cough a sensory dysregulation via which the urge-to-cough (UTC) or coughing are evoked mechanically from “somatic points for cough” (SPCs) in the neck and upper trunk. We investigated the prevalence and the clinical relevance of SPCs in an unselected population of patients with chronic cough.
Methods
From 2018 to 2021, symptoms of 317 consecutive patients with chronic cough (233 females) were collected on four visits (V1–V4) 2 months apart at the Cough Clinic of the University Hospital in Florence (I). Participants rated the disturbance caused by the cough (0–9 modified Borg Scale). We attempted to evoke coughing and/or UTC using mechanical actions in all participants who were subsequently categorised as responsive (somatic point for cough positive, SPC+) or unresponsive (SPC−) to these actions. An association was established between chronic cough and its commonest causes; treatments were administered accordingly.
Findings
169 patients were SPC+ and had a higher baseline cough score (p < 0.01). In most of the patients, the treatments reduced (p < 0.01) cough-associated symptoms. All patients reported a decrease (p < 0.01) in cough score at V2 (from 5.70 ± 1.4 to 3.43 ± 1.9 and from 5.01 ± 1.5 to 2.74 ± 1.7 for SPC+ and SPC− patients respectively). However, whilst in SPC− patients the cough score continued to decrease indicating virtually complete cough disappearance at V4 (0.97 ± 0.8), in SPC+ patients this variable remained close to V2 values during the entire follow-up.
Interpretation
Our study suggests that the assessment of SPCs may identify patients whose cough is unresponsive and are eligible for specific treatments.
Funding
This work was funded by an unrestricted grant from Merck (Italy).
Keywords: Chronic cough, Urge-to-Cough, Sensitisation, Refractory chronic cough, Unexplained chronic cough
Research in context.
Evidence before this study
Some patients with chronic cough also display an urge-to-cough or overt coughing evoked by mechanical stimulation of discrete body areas of the neck and upper trunk. We searched PubMed for articles using the terms “chronic cough” AND “somatic points for cough” OR “somatically evoked cough”, with no date restrictions and not limited to English-language publications. We found nine studies, none of which specifically investigated the clinical outcomes of patients with somatic points for cough. We set out to investigate the clinical outcomes of patients with chronic cough and somatic points for cough.
Added value of this study
In patients with chronic cough and somatic points for cough, guideline-based treatments improved cough-associated symptoms but not the patient-reported disturbance caused by the cough. Conversely, in chronic coughers without somatic points for cough, both coughing and the associated symptoms considerably improved with treatments.
Implications of all the available evidence
The assessment of somatic points for cough may help to more promptly identify patients whose cough is little responsive or unresponsive to guideline-based treatments; these patients may be those with refractory or unexplained chronic cough. Future studies, also involving functional brain imaging techniques, could be useful for characterising chronic coughers with somatic points for cough more precisely.
Introduction
Chronic cough, defined as a cough lasting longer than 8 weeks, is a common condition that affects 4–10% of adults worldwide.1 Patients with chronic cough who have never smoked are often diagnosed as having asthma, gastro-oesophageal reflux disease, or upper airway cough syndrome.2 Although the majority of patients can benefit from treatment of the associated conditions, it is increasingly recognised that cough does not improve with such treatments in a large percentage of patients; these patients are commonly classified as having refractory chronic cough.2,3 In a minority of patients with chronic cough, no clear cause can be established, and these patients are considered to be suffering from unexplained chronic cough.3
Patients with chronic cough typically report an irritating sensation in the throat and experience cough peals provoked by stimuli such as laughing, strong smells and changes in external temperature that are innocuous for the normal population.3 These clinical features point to a sensory dysregulation that some authors have considered as a separate clinical condition and termed “cough hypersensitivity syndrome”.4 Sensitisation of peripheral and/or central neural pathways involved in cough mediation may account for the diverse clinical presentations of chronic cough.4 Sensory dysregulation may also explain another clinical feature of chronic coughers which often makes patients complain of an urge-to-cough (UTC) or overt coughing in response to mechanical stimuli that operate on discrete areas of the neck and upper trunk.5, 6, 7 While this form of sensory dysregulation is frequent,5,6 to ascertain this, we set out to investigate, in an unselected population of chronic coughers, the prevalence and clinical relevance of somatically evoked cough,5,6 i.e., the cough and/or the sensation of an UTC triggered by mechanical stimuli, namely finger pressure, on regions of the upper chest and neck.5,6 We have hypothesised that chronic coughers with somatically evoked cough present different clinical outcomes compared to patients who are devoid of this sensory dysregulation.
Methods
Study design and participants
This is a 6-month observational cohort study performed from June 12th, 2018 to October 28th, 2021 at the Cough Clinic of the University Hospital of Florence (Italy). We enrolled all adult outpatients of both genders with chronic cough lasting at least 1 year (n = 338). The exclusion criteria included a history of recent (<4 weeks) respiratory infection and/or evidence of active lesions documented by a recent (within 2 months) chest X-ray, intake of protussive drugs, as well as reported psychiatric disturbances and/or intake of psychoactive drugs. Patients were also excluded if they were current smokers or had quit smoking at least 6 months before enrolment or had spirometric signs of bronchial obstruction (ratio of forced expiratory volume in 1 s to forced vital capacity of less than 70%). All procedures complied with the Helsinki Declaration. The study was approved by the local Institutional Review Board (OSS_14131), and followed the international guidelines for observational studies.8 A written informed consent of participants was obtained after detailed explanation of the purposes of the study.
Data collection
In each patient, data collection and routine clinical assessments were performed according to published diagnostic guidelines for chronic cough.9, 10, 11 In particular, a custom-designed, multi-item electronic questionnaire12 was used to collect symptoms at each visit. The questionnaire was devised to allow for mostly dichotomous (“yes/no”) responses, so that its administration was largely operator independent. At all visits, participants were also asked “How much has the cough bothered you during the last 2 weeks?”; participants responded by rating the magnitude of their disturbance using a 0–9 modified Borg Scale where 0 was “Not bothered at all” and 9 was “Worst disturbance I can possibly imagine”.12 The values obtained with this method were termed as “cough score”.12 In our experience, this rating method was the most readily understood by the majority of patients. Only patients who had a cough score of at least 3 at the first clinical assessment12 were included in the study.
Physical examination and treatments
In all patients, we attempted to evoke coughing and/or the sensation of an UTC via finger pressure on the previously described5,6 somatic points for cough (SPC), i.e., discrete body areas in the upper trunk or neck that can give rise to a UTC sensation or overt coughing in response to finger pressure, flexion or extension in chronic coughers.5,6 These stimulation techniques are described in more detail in the online supplement (Supplementary Fig. S1). Participants were subsequently categorised as being responsive (somatic point for cough positive, SPC+) or unresponsive (somatic point for cough negative, SPC−) to the stimuli. On each occasion, the assessment of SPCs was performed by one of four of the Authors (G.F., F.L., G.B., C.F.) and interrater reliability was determined by kappa statistics.6 Manoeuvres were repeated twice, and a cough or UTC response was taken as positive only if it appeared with both stimulations. The onset of cough was detected aurally; patients rated the intensity of the evoked UTC by using a visual analogue scale (VAS) ranging from 0 (no UTC at all) to 9 (maximum UTC). No attempt was made during these assessments to measure the intensity of the applied finger pressure or to establish a precise sequence of the areas to be stimulated.
The association between chronic cough and its commonest causes2 was established clinically and by using the results of recommended tests.2,9, 10, 11 Thus, classic asthma was suspected when patients reported wheezing or chest tightness or both, with and without reversible airway obstruction. Gastro-oesophageal reflux was suspected when at least one oesophageal symptom was reported, with or without one or more of the commonest extra-oesophageal manifestations.2,9, 10, 11 Finally, an upper airway cough syndrome was suspected when patients reported nasal obstruction and/or post-nasal drip episodes with or without throat clearing.2,9, 10, 11 In all instances, pharmacological treatments (Supplementary Table S1) were recommended according to current guidelines9, 10, 11; furthermore, patients received personalised lifestyle suggestions when appropriate. In brief, treatments also included acid-suppressing drugs (proton pump inhibitors and antacid drugs often associated with prokinetic drugs, n = 95), inhaled/oral steroids associated with long-acting bronchodilators (n = 38), nasal lavages, and steroids and/or antihistamines (n = 21). Combinations of drugs were prescribed in the remaining 151/317 patients due to the detection of multiple, putative cough-accompanying symptoms. In twelve patients (7 SPC+), cough was the sole clinically apparent disturbance and they were treated with cough suppressants such as Gabapentin, central (Codeine) or peripheral (Levodropropizine) antitussive medicines. For each patient, three follow-up visits were scheduled approximately 2 months apart; however, it was possible to anticipate the end of follow-up when the cough score was 1 (i.e., “cough bothers me very little”) or less.
Data analysis
Continuous variables are reported as mean ± standard deviation (SD) or as median and interquartile range (IQR - 25th–75th percentile) for non-normal distributions and were compared between groups (i.e., patients with and without SPCs) with the generalized linear models (testing for homogeneity of variances through the Levene's test) or non-parametric tests, as appropriate. Categorical variables are reported as counts and percentages and were compared between groups with the Chi-squared test (or Fisher's exact test when appropriate). We used all observed data with no imputation for missing data in the analysis. For each patient, we calculated the cumulative number of cough-associated symptoms mentioned above (i.e., wheezing/chest tightness, heartburn, regurgitation, nasal obstruction, post-nasal drip). Thus, for a given patient at each visit, the calculated cumulative number of symptoms suggestive of one or more associated clinical conditions could be comprised between 0 and 5. Changes in the cumulative number of cough-associated symptoms and in cough score following personalized lifestyle modifications and pharmacological treatments in the two groups of patients were evaluated using mixed-effect models based on the presence of SPCs, number of visits, and their interaction, adjusted for the cough score at the first visit. Fixed effects included the patient subgroups (SPC+ vs SPC−), the different time of visits (1–4) and their interaction (patient subgroup × time). The random effects included random intercept and slope for times, thus allowing us to assess intraindividual differences in cough score at baseline and over time. Compound symmetry covariance structure and Tukey adjustment for multiple comparisons were considered. All statistical tests were two-tailed and statistical significance was assumed for p-value <0.05. The analyses were performed using SAS, V.9.4 (SAS Institute, Cary, NC, USA).
Role of the funding source
This work was funded by an unrestricted grant from MSD Italy. MSD Italy had no role in the study design, data collection, analysis and interpretation, as well as in the writing the report and in the decision to submit the paper. All Authors have access to the dataset and agreed to submit the paper for publication.
Results
Following the exclusion of 25 current smokers and 21 patients who failed to comply with the criteria for chronic cough - essentially short cough duration and ongoing infectious respiratory disorders - we studied 317 adult outpatients (Table 1, Supplementary Fig. S2) who were mostly female (233, 73.5%) with an average cough score of 5.4 ± 2.0. The reported median duration of cough was 3 years (range 1–10). Out of these 317 patients, 169 (53%) displayed at least one SPC for either UTC alone (n = 46) or cough (n = 123). The remaining 148 patients (99 females, 66.9%) were repeatedly shown to be unresponsive to mechanical stimulation of any SPCs. Of note, SPC+ patients were more likely to be female and to have a higher baseline cough score (p < 0.01) than SPC− patients (Table 1). In SPC+ patients, the mean (±SD) number of identified SPCs was 1.69 ± 1.5 and the mean intensity of the evoked UTC was 4.89 ± 1.05. The jugular notch was the most frequently identified SPC, whereas the spinous processes of the cervico-dorsal spinal trait was the least identified (Table 2). Induced coughing and UTC were reproducible (kappa test = 0.88; 95% CI, 0.80–0.94). Indeed, repeatability of the mechanically induced cough responses was taken as an index of a cause – effect relationship between stimulation and cough.
Table 1.
Demographic and clinical characteristics of the pooled chronic cough population and subgroups with (SPC+) and without (SPC−) somatic points for cough.
| Pooled (n = 317) | SPC+ (n = 169) | SPC− (n = 148) | p valuea | |
|---|---|---|---|---|
| Females, n (%) | 233 (73.5) | 134 (79.3) | 99 (66.9) | 0.012 |
| Age (years), mean ± SD | 59.0 ± 13.9 | 58.8 ± 13.2 | 59.40 ± 14.7 | 0.451 |
| Body Mass Index, mean ± SD | 25.56 ± 4.19 | 25.33 ± 4.27 | 25.83 ± 4.10 | 0.633 |
| Never smoked, n (%) | 237 (75) | 125 (74) | 112 (75) | |
| Ex-smoker, n (%) | 55 (17) | 31 (18) | 26 (17) | |
| Current smoker, n (%) | 25 (8) | 13 (8) | 10 (8) | |
| Cough duration, years (mean, IQ) | 3.00 (1, 10) | 3.1 (1, 9.5) | 3.05 (1, 10) | 0.425 |
| Cough score, mean ± SD | 5.4 ± 2.0 | 5.7 ± 1.4 | 5.01 ± 1.5 | 0.047 |
Participants had no missing data at visit 1.
IQ, interquartile range; n, number of patients; SD, standard deviation.
Comparing SPC+ and SPC− subgroups.
Table 2.
Patients (n, %) who were responsive (cough or UTC only) to stimulation of a somatic point for cough and number and intensity of evoked responses.
| Somatic point for cough | Cough n (%) |
UTC only n (%) |
Cough Mean ± SD number |
UTC Mean ± SD intensity |
|---|---|---|---|---|
| Head flexion or extension | 69 (40) | 43 (25) | 1.67 ± 0.78 | 5.24 ± 2.14 |
| Sternocleidomastoid muscle | 33 (19) | 20 (12) | 2.25 ± 1.50 | 4.83 ± 2.37 |
| Jugular notch | 80 (47) | 40 (24) | 2.06 ± 1.12 | 5.55 ± 2.12 |
| Cervico-dorsal spine | 14 (8) | 11 (6) | 2.60 ± 1.82 | 4.11 ± 2.15 |
| Sternum | 38 (24) | 24 (14) | 2.12 ± 0.64 | 5.02 ± 2.34 |
n, number of patients; UTC, urge-to-cough.
At visit 1, the vast majority of patients (n = 305) had symptoms suggesting one or more of the commonest causes2 of chronic cough and symptom prevalence was similar in the SPC+ and SPC− subgroups (Table 3).
Table 3.
Baseline symptoms of the pooled chronic cough population and sub-groups with (SPC+) and without (SPC−) somatic points for cough.
| Pooled (n = 317) | SPC+ (n = 169) | SPC− (n = 148) | p valuea | |
|---|---|---|---|---|
| Wheezing and/or chest tightness, n (%) | 97 (30.6) | 46 (27.2) | 51 (34.5) | 0.1628 |
| Nasal obstruction, n (%) | 149 (47.0) | 79 (46.7) | 70 (41.4) | 0.4671 |
| Post-nasal drip, n (%) | 104 (33.1) | 61 (36.5) | 43 (29.3) | 0.1717 |
| Heartburn, n (%) | 151 (47.6) | 86 (50.1) | 65 (43.9) | 0.3923 |
| Regurgitation, n (%) | 146 (46.1) | 85 (50.2) | 61 (41.2) | 0.0632 |
n, number of patients.
Comparing SPC+ and SPC− subgroups.
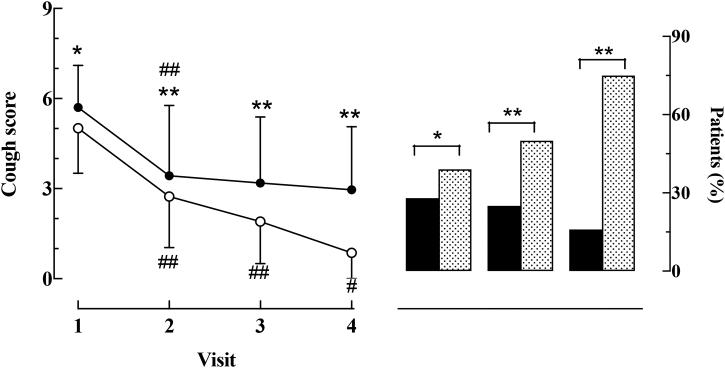
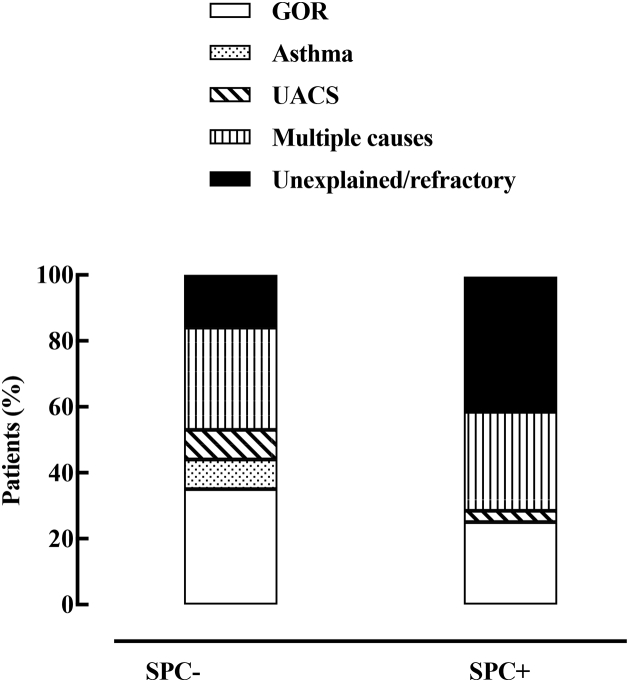
24 patients (13 SPC+) were lost to follow-up (Supplementary Fig. S2). Following attempts to obtain further information via a phone call, 8 out of 24 SPC− patients reported that discontinuation was due to the disappearance of their cough; in 5 SPC+ patients, discontinuation was mainly due to a lack of satisfactory results following treatment. In the remaining 293, the time course of changes in the cumulative number of cough-associated symptoms and cough score during follow-up are depicted in Figs. 1 and 2. As shown in Fig. 1, both SPC+ and SPC− patients displayed similar decreases (p < 0.01) in the cumulative number of symptoms at each follow-up visit. At visit 2 (Fig. 2A, Tables 4 and 5), the cough score decreased (p always < 0.01) in both SPC+ (from 5.70 ± 1.4 to 3.43 ± 1.9) and SPC− patients (from 5.01 ± 1.5 to 2.74 ± 1.7). However, whilst this variable continued to decrease during the remainder of the follow-up period in SPC− patients, the cough score virtually plateaued in SPC+ patients from visit 2 to the end of follow-up (Tables 4 and 5, Fig. 1A). Notably, in the vast majority of SPC+ patients (136, 87%), coughing or UTC could still be evoked at visit 4 with the same modalities as those of visit 1. Conversely, only 9 SPC− patients still coughed, although to a much lesser degree, after the completion of the follow up period. We never observed any SPC− patient become SPC+ during the entire course of this study. The number of patients who reported cough resolution (see methods) at each follow-up visit was significantly higher among the SPC− subgroup (Fig. 2B). In those SPC+ patients who had a favourable response (n = 23) to treatments, SPCs could no longer be detected. Retrospective analysis based on individual responses to symptom-driven treatment demonstrated an approximately 3-fold prevalence of non-responsive patients in the SPC+ subgroup (Fig. 3). Of note, out of the 12 patients with cough as the sole manifesting symptom, two were lost in follow-up. In the remaining 10 patients (7 SPC+), only one SPC+ patient significantly improved with gabapentin, whereas all the SPC− patients had a marked reduction in the cough score with either opioid (n = 1) or non-opioid (n = 2) antitussives.
Fig. 1.
Mean (±SD) cumulative number of cough-associated symptoms in SPC+ (filled circles) and SPC− patients at each visit. ∗p < 0.05 comparing visits 2–4 with visit 1.
Fig. 2.
Time course of changes in cough score (left panel) and percent of patients showing cough resolution during follow-up. Left panel: Mean (±SD) values of cough score recorded at each visit in patients with (filled circles) and without (empty circles) somatic points for cough. Right panel: Percent of patients with (black columns) or without (dotted columns) somatic points for cough who reported cough resolution at each follow-up. ∗p < 0.05 and ∗∗p < 0.01 between-group comparisons of cough scores. #p < 0.05 and ##p < 0.01 within group comparisons of cough scores. See also Tables 4 and 5 for raw values.
Table 4.
Cough scores and corresponding between-groups mean differences at visit 1–4 in patients with (SPC+) or without (SPC−) somatic points for cough.
| Cough score |
||||
|---|---|---|---|---|
| SPC+ | SPC− | Mean difference | p value | |
| Visit 1 | 5.70 (5.28, 5.80) | 5.01 (4.90, 5.64) | 0.65 (−0.21, 0.81) | 0.044 |
| Visit 2 | 3.43 (3.17, 3.70) | 2.74 (2.47, 3.05) | 0.69 (0.07, 1.28) | 0.0149 |
| Visit 3 | 3.19 (2.83, 3.55) | 1.91 (1.54, 2.28) | 1.28 (0.48, 2.08) | <0.0001 |
| Visit 4 | 2.94 (2.44, 3.44) | 0.97 (0.39, 1.56) | 1.97 (0.78, 3.16) | <0.0001 |
Values are estimated means and 95% Confidence Interval (CI) from mixed-model repeated measures analyses adjusted for cough score at V1. Including the sex as an additional covariate in the models did not change the outcomes.
Table 5.
Within-group mean differences in cough score in patients with (SPC+) or without (SPC−) somatic points for cough.
| Cough score |
||||
|---|---|---|---|---|
| SPC+ | p value | SPC− | p value | |
| V2 vs V1 | −2.27 (−2.62, −1.60) | <0.0001 | −2.32 (2.06, 3.15) | <0.0001 |
| V3 vs V2 | −0.24 (−0.87, 0.39) | 0.9445 | −0.83 (−1.51, −0.18) | 0.0042 |
| V4 vs V3 | −0.25 (−1.13, 0.63) | 0.9890 | −0.94 (−1.95, 0.08) | 0.0943 |
Values are mean differences and 95% Confidence Interval (CI) from mixed-model repeated measures analyses adjusted for cough score at V1. Including the sex as an additional covariate in the models did not change the outcomes.
Fig. 3.
Relative percent distribution of causes of chronic cough established after symptom-driven treatments in patients with (SPC+) and without (SPC−) somatic points for cough. GOR, gastro-oesophageal reflux; UACS, upper airway cough syndrome.
Discussion
To our knowledge, this is the first study to investigate the clinical relevance of somatically evoked cough or UTC in a real-life population of adult patients with chronic cough referred to a tertiary care centre in Italy. These results confirm previous observations5, 6, 7 regarding a high percentage of SPC+ individuals among chronic coughers (particularly females), the anatomical distribution of SPCs in the upper trunk and neck, and the characteristics of the evoked responses. More importantly, symptom-driven treatments of cough-associated symptoms almost invariably resulted in a marked reduction of symptoms other than cough itself in both SPC+ and SPC− patients. However, SPC+ patients failed to display a consistent reduction in cough score over the 6-month follow-up period. Furthermore, in the latter the percentage of non-responsive patients turned out to be almost three times higher than in the SPC− subgroup.
Sensitisation of peripheral and central neural pathways mediating cough is considered the fundamental mechanism underlying the cough hypersensitivity syndrome.3,4 Previous clinical studies in patients with chronic cough have implicated sensitisation of the vagal sensory pathway for coughing evoked by maximal expulsive respiratory efforts13, 14, 15 and probing of the external acoustic meatus.16 Our finding of a large prevalence of SPCs among patients with chronic cough suggests the role of a central sensitisation mechanism also facilitating the cough reflex.
Epidemiological studies have demonstrated a preponderance of females complaining from chronic cough in the general population.17 In addition, females have a heightened sensitivity of the cough reflex in response to tussigenic challenges with variable chemical agents.17 Objective recordings of cough frequency in outpatients also point to a lower threshold for the cough reflex in females. In addition, females also display lower pain thresholds and are more likely to develop chronic pain than males.18,19 In keeping with the findings, recent magnetic resonance imaging studies have shown greater activation of the somatosensory cortex in response to low-intensity tussigenic stimuli in healthy females.17 We found an even more marked prevalence of females among patients who are SPC, suggesting higher degrees of central cough facilitation in this subgroup of female chronic coughers. We also observed a high proportion of patients who reported reflux and nasal symptoms; of note, these were similarly distributed in both the SPC+ and SPC− subgroups.
The skin and skeletal muscles are richly innervated by mechano-sensitive fibres20 originating from neurons in the dorsal roots and eventually projecting into the nucleus tractus solitarii (NTS); once stimulated, these sensory fibres may locally release tachykinins, including substance P.21 It has been shown that substance P microinjected into the commissural sub-nucleus of the NTS, where cough-related afferents terminate,22 potentiates the cough responses induced by mechanical airway stimulation in anaesthetized rabbits and guinea-pigs.23,24 Therefore, the phenomenon of somatically evoked cough may share similarities with the cough reflex sensitisation promoted by central convergence and synergy of different types of terminal fibres.24 In SPC+ patients, activation of mechanically sensitive endings via finger pressure or functionally equivalent manoeuvres might increase tachykinin concentration at the NTS level, thus facilitating the perception of an UTC or overt coughing by otherwise ineffective stimuli such as talking and laughing25 or even, in some instances, the chest wall and respiratory muscle movements of breathing. In addition to central cough-facilitating mechanisms, studies in animal models have implicated hyper-excitable airway second-order neurons following airway exposure to environmental pollutants, possibly owing to a dysregulation of neuropeptide expression.26,27 The role of chronic exposure to environmental irritants in the genesis of SPCs remains to be elucidated. Cough triggered in response to non-tussive stimuli including talking,28 exposure to cold air26 and, presumably, the cough responses described here, has been termed “allotussia”.28 Interestingly, allotussia, is a key characteristic of central sensitization.28
Even though the symptom-driven treatment of cough-associated symptoms almost invariably resulted in a marked decrease of symptoms other than cough itself in both SPC+ and SPC− patients, the cough scores only improved in SPC− patients. To explain this, it can be hypothesised that in SPC+ patients peripheral stimuli activating the cough reflex also generate a state of central hypersensitivity that may persist over time irrespective of the disappearance of the peripheral stimulus initiating cough. It has been shown that synaptic plasticity taking place in sensory pathways, from the spinal dorsal horn to cortical areas, contributes to chronic pain.29 Potentiation at synapses of nociceptive nerve fibres constitutes a powerful model for pain amplification following trauma, inflammation and nerve injury.30 Since cough can be considered a defensive response to nociceptive stimulation, it is not surprising that mechanisms underlying nociception and cough may share similar features leading to the development of pain and cough hypersensitivities.31, 32, 33 These mechanisms might constitute the neurophysiological basis of clinical forms of chronic cough - conventionally referred to as refractory or unexplained chronic cough - of which SPCs may represent a clinical hallmark.
This study has some limitations. First, the mechanical actions of our experiments involved body areas that are anatomically and functionally different.6,7 For instance, finger pressure on flabby areas of the neck might have stimulated the sensory endings of the underlying larynx, thereby provoking cough. Conversely, pressure on the sternum or cervico-dorsal spine is unlikely to have stimulated a sensory pathway for cough. Nonetheless, different SPCs often coexisted in the same patient and their mechanical stimulation evoked identical clinical responses,6 suggesting a common underlying mechanism. Second, this was a single-centre study and the observed responses might be a specific feature of the studied population. However, the fact that the demographic and clinical characteristics of the patients included in this study are comparable to those of studies performed in other world areas17 points to the possibility of the characteristics of patients with chronic cough being similar globally. Third, the study lacks a more objective evaluation of the burden of cough in our patients. Common methods for the quantitative analysis of cough severity include objective recordings of cough frequency and administration of cough-related quality-of-life questionnaires, such as the Leicester Cough Questionnaire (LCQ).9 In real-life settings, objective cough frequency recording techniques are difficult due to the lack of availability of validated devices on the market. On the other hand, administration of the LCQ would be difficult since the tool has not been validated in Italy and would therefore prolong the duration of each consultation somewhat beyond the acceptable time limits. Recent guidelines9,11 have recommended the use of “simple measures” such as the cough score used here to record the impact of cough. Admittedly, the use of a VAS scale in rating the UTC may be not totally appropriate.34 The Florence Cough Centre was established in the late 1990's when the VAS scale was the most widely used rating method for sensations. For this reason, we favoured methodological consistency. Last, adherence to treatments was not formally assessed, as is often the case in observational studies. Nevertheless, the finding of obvious improvements in cough-associated symptoms strongly suggests high levels of adherence in all patients.
In conclusion, we have shown how SPCs reflect a condition that is commonest among patients whose cough is little responsive or unresponsive to symptom-driven treatments, i.e., those who are currently diagnosed as suffering from difficult-to-treat forms of chronic cough, such as refractory or unexplained chronic cough.3 Consequently, the assessment of SPCs could help to identify patients eligible for treatments specifically conceived for these forms of chronic cough. Future studies, also involving brain function imaging techniques, would help to clarify the neural substrates implicated in the genesis of the somatic points for cough.
Contributors
F.L.: study conceptualization and coordination, data collection and analysis, interpretation, drafting and revision of the manuscript. G.A.F.: study conceptualization and coordination, data collection and analysis, interpretation, drafting and revision of the manuscript. G.B.: participants’ enrolment, interpretation, revision of the manuscript. C.F.: statistical analysis, interpretation, revision of the manuscript. M.N.: statistical analysis, interpretation, revision of the manuscript. S.M.: statistical analysis, interpretation, revision of the manuscript. D.M.: study conceptualization, interpretation, revision of the manuscript. E.C.: study conceptualization, interpretation, revision of the manuscript. F.L., G.A.F., G.B. and M.N. have verified the underlying data. All authors had full access to all the data in the study and accept responsibility to submit the paper for publication. Both F.L. and G.A.F. were responsible for manuscript preparation.
Data sharing statement
According with the Italian Law on privacy and sensible data sharing, restrictions apply to the availability of these data and they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the Careggi University Hospital in Florence.
Declaration of interests
F.L. and G.A.F. received grant for research and fees for speaking by MSD Italy. S.M. received grant for research by MSD Italy. G.B., C.F., D.M. and E.C. have no conflict to declare.
Acknowledgments
This work was supported by an unrestricted grant by MSD Italy.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101869.
Appendix A. Supplementary data
References
- 1.Song W.S., Chang Y.S., Faruqi S., et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45:1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 2.Dicpinigaitis P. Understanding the foundations of chronic cough. Am J Manag Care. 2020;26:S232–S238. doi: 10.37765/ajmc.2020.88514. [DOI] [PubMed] [Google Scholar]
- 3.Mazzone S.B., Chung K.F., McGarvey L. The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity. Lancet Respir Med. 2018;6:636–646. doi: 10.1016/S2213-2600(18)30150-4. [DOI] [PubMed] [Google Scholar]
- 4.Song W.J., Morice A.H. Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol Res. 2017;9:394–402. doi: 10.4168/aair.2017.9.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavorini F., Fontana G.A. A 57-year-old chronic cougher with somatically evoked cough. Pulm Pharmacol Ther. 2017;47:56–58. doi: 10.1016/j.pupt.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Mannini C., Bernacchi G., Bonti V., et al. Somatic points for cough and urge to cough in chronic coughers. Respir Med. 2022;200:106929. doi: 10.1016/j.rmed.2022.106929. [DOI] [PubMed] [Google Scholar]
- 7.Kamimura M., Mouri A., Takayama K., et al. Cough challenge tests involving mechanical stimulation of the cervical trachea in patients with cough as a leading symptom. Respirology. 2010;15:1244–1251. doi: 10.1111/j.1440-1843.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- 8.Von Elm E., Altman D.G., Egger M., et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwin R.S., Fench C.L., Chang A.B., Altman K.W., on behalf of the Chest Expert Panel Classification of cough as a symptom in adults and management algorithms. Chest. 2018;153:196–209. doi: 10.1016/j.chest.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morice A.H., Fontana G.A., Sovijarvi A.R., et al. The diagnosis and management of chronic cough. Eur Respir J. 2004;24:481–492. doi: 10.1183/09031936.04.00027804. [DOI] [PubMed] [Google Scholar]
- 11.Morice A.H., Millqvist E., Bieksiene K., et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55:1901136. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campi G., Noale M., Fabbrizzi A., Lavorini F., Maggi S., Fontana G. The demographic and clinical characteristics of an Italian population of adult outpatients with chronic cough. Aging Clin Exp Res. 2020;32:741–746. doi: 10.1007/s40520-019-01464-4. [DOI] [PubMed] [Google Scholar]
- 13.Lavorini F., Fontana G.A., Chellini E., Magni C., Pistolesi M., Widdicombe J. Respiratory expulsive efforts evoked by maximal lung emptying. Chest. 2011;140:690–696. doi: 10.1378/chest.10-1084. [DOI] [PubMed] [Google Scholar]
- 14.Lavorini F., Fontana G., Chellini E., Magni C., Pistolesi M., Widdicombe J. The Fontana paradoxical reflex? Chest. 2011;140:586–588. doi: 10.1378/chest.11-0262. [DOI] [PubMed] [Google Scholar]
- 15.Lavorini F., Chellini E., Bigazzi F., Surrenti E., Fontana G.A. The clinical value of deflation cough in chronic coughers with reflux symptoms. Chest. 2016;149:1467–1472. doi: 10.1016/j.chest.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Dicpinigaitis P.V., Enilari O., Cleven K.L. Prevalence of Arnold nerve reflex in subjects with and without chronic cough: relevance to cough hypersensitivity syndrome. Pulm Pharmacol Ther. 2019;54:22–24. doi: 10.1016/j.pupt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Morice A.H., Jakes A.D., Faruqi S. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014;44:1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- 18.Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racine M., Tousignant-Laflamme Y., Kloda L.A., et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012;153:602–618. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman M.P., Longhurst J.C., Rybicki K.J., Wallach J.H., Mitchell J.H. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 21.Potts J.T., Fuchs I.E., Li J., Leshnower B., Mitchell J.H. Skeletal muscle afferent fibres release substance P in the nucleus tractus solitarii of anaesthetized cats. J Physiol. 1999;514(Pt 3):829–841. doi: 10.1111/j.1469-7793.1999.829ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubin L., Alheid G.F., Zuperku E.J., McCrimmon D.R. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutolo D., Bongianni F., Fontana G.A., Pantaleo T. The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull. 2007;74:284–293. doi: 10.1016/j.brainresbull.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Mazzone S.B., Mori N., Canning B.J. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in Guinea-pigs. J Physiol. 2005;569:559–573. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacheco A., Domingo C. Airway reflux: an emerging topic in respiratory medicine. Lancet Respir Med. 2018;6:810–812. doi: 10.1016/S2213-2600(18)30376-X. [DOI] [PubMed] [Google Scholar]
- 26.Sekizawa C.Y., Chen A.G., Bechtold J.M., et al. Extended second-hand tobacco smoke exposure induces plasticity in nucleus tractus solitarius second-order lung afferent neurons in young Guinea pigs. Eur J Neurosci. 2008;28:771–781. doi: 10.1111/j.1460-9568.2008.06378.x. [DOI] [PubMed] [Google Scholar]
- 27.Chounlamountry k, Boyer B., Penalba V., et al. Remodeling of glial coverage of glutamatergic synapses in the rat nucleus tractus solitarii after ozone inhalation. J Neurochem. 2015;134:857–864. doi: 10.1111/jnc.13193. [DOI] [PubMed] [Google Scholar]
- 28.Vertigan A.E., Gibson P.J. Management of chronic refractory cough. BMJ. 2015;351:h5590. doi: 10.1136/bmj.h5590. [DOI] [PubMed] [Google Scholar]
- 29.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130146. doi: 10.1098/rstb.2013.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandkühler J., Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol. 2012;12:18–27. doi: 10.1016/j.coph.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Driessen Ak, McGovern A.E., Narula M., et al. Central mechanisms of airway sensation and cough hypersensitivity. Pulm Pharmacol Ther. 2017;47:9–15. doi: 10.1016/j.pupt.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Mutolo D. Brainstem mechanisms underlying the cough reflex and its regulation. Respir Physiol Neurobiol. 2017;243:60–76. doi: 10.1016/j.resp.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Ji R.R. Neuroimmune interactions in itch: do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm Pharmacol Ther. 2015;35:81–86. doi: 10.1016/j.pupt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davenport P.W., Sapienza C.M., Bolser D.C. Psychophysical assessment of the urge-to-cough. Eur Respir J. 2002;12:249–253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.