Summary:
Low-hypodiploid acute lymphoblastic leukemia (LH-ALL) in both children and adults is characterized by biallelic TP53 alterations in virtually all cases. However, in contrast to a common germline origin of the TP53 mutations in pediatric cases, those in adult cases are mostly somatic and are derived from age-related clonal hematopoiesis (ARCH), highlighting the role of TP53-mutant ARCH in the development not only of myeloid leukemogenesis but also of LH-ALL in aged populations.
B-cell precursor acute lymphoblastic leukemia (B-ALL) is the predominant type of pediatric leukemia, whose clinical outcome has dramatically been improved during the past 60 years (1). By contrast, being much less common than myeloid leukemia, adult ALL is still associated with a dismal prognosis (2). This has been explained by poor tolerability to intensive therapies in adult cases and also by intrinsically different biology of leukemic cells between adult and childhood leukemia, as anticipated by distinct patterns of genetic abnormalities therein, an increasing overrepresentation of high-risk diseases in adult cases with aging, such as Ph+ and Ph-like and KMT2A-rearranged diseases, as well as low-hypodiploid ALL (LH-ALL; refs. 1–3). However, the genetic basis of this increasing overrepresentation of high-risk diseases in adult cases is poorly understood. In this issue of Blood Cancer Discovery, through the analysis of a large cohort of adult cases with LH-ALL using paired diagnosis and remission samples and advanced single-cell immune-genotyping sequencing technologies, Kim and colleagues provide a clue to answer the question by demonstrating the frequent presence of TP53-mutated clonal hematopoiesis from which LH-ALL evolved in the elderly (4).
LH-ALL is a distinct subtype of B-ALL defined by a modal number of chromosomes ranging from 32–39 with nonrandom losses of chromosomes and discriminated from other hypodiploid ALL, including near-haploid and high-hypodiploid ALL carrying 24–31 and 42–44 chromosomes, respectively (5). Other cases having a near-triploid genome (64–78 chromosomes) as a result of duplication of the LH genome are known as “masked low hypodiploidy” and are thought to share the same pathogenesis with LH-ALL (6). LH-ALL is highly enriched in adult cases with increasing age, associated with a uniformly dismal prognosis even in pediatric cases who were treated with the most intensive regimens (5). A cardinal genetic feature of LH-ALL is a very high frequency of TP53 mutations (91.2%). Of note, nearly half of the TP53 mutations in pediatric cases are also found in nontumor compartments (3), suggesting that they are inherited or acquired as de novo in the germline or hematopoietic compartments in pediatric cases indicating Li–Fraumeni cancer predisposition.
To investigate the genetic origins of adult LH-ALL, Kim and colleagues first screened 591 Ph-negative adult ALL cases using karyotype and targeted capture sequencing designed to detect copy-number alterations (CNA) and loss-of-heterozygosity (LOH) in addition to common mutations in B-cell ALL. On the basis of the pattern of chromosomal losses and LOH, they found 40 LH-ALL cases together with an additional 40 cases with masked LH-ALL cases with a near-triploid genome. The pattern of chromosomal losses and other LOH was highly uniform and recapitulates that in pediatric cases, almost invariably involving chromosomes 3, 7, 16, and 17, and to a lesser extent, chromosomes 13 and 15 (3). The importance of sequencing-based analysis of CNA/LOH is underscored because conventional karyotyping had failed in 26 out of the 80 cases and only a few masked cases had ancestral cells having low-hypodiploid genomes that allows to discriminate between masked cases from other hyperploid cases. Accounting for 13.5% (80/591) of their consecutively collected prospective cohort, LH-ALL represents a major subtype of adult B-ALL. The strong enrichment of this subtype in older patients suggested in previous studies was confirmed in the current cohort (32% in patients over 55 years vs. only 3% in those below the age of 40) and so were other features of LH-ALL, including a lower white blood cell count and a lower marrow blast percentage in LH-ALL than in other ALL cases.
Mutation analysis recapitulated core findings in pediatric LH-ALL (3). Among others, a high frequency of TP53 mutations was conspicuous and found in virtually all LH-ALL cases (78/80, 98%). Wild-type TP53 allele was lost in all mutated cases either by allelic deletion or copy-neutral LOH involving chromosome 17p or by multiple mutations, leading to biallelic TP53 alterations. TP53 mutations were also present in other B-ALL subtypes but much less common as a whole (<5%, 24/511). Thus, together with the uniform pattern of chromosomal loss, biallelic TP53 mutations is another hallmark of LH-ALL. The pattern of additional mutations was also similar to that in pediatric cases and characterized by mutually exclusive mutations and focal deletions in CDKN2A (26%) and RB1 (23%), together with those of IKZF2 (19%). Recurrent alterations of genes implicated in cell signaling, such as NF1 (22%), FLT3, and NRAS, were also identified, whereas IKZF1-targeted alterations were rare in LH-ALL, even though common in other B-ALL. These findings support that pediatric and adult LH-ALL cases share the same pathophysiology and define a distinct subtype of B-ALL characterized by biallelic TP53 alteration, unique pattern of chromosomal losses and comutations, poor outcomes, and unique hematologic features. Nevertheless, the authors addressed an important difference that discriminates between the origin of adult and pediatric diseases, which could explain why LH-ALL is highly enriched in elderly patients.
Unlike an initial report including a small cohort of adult LH-ALL cases (3), identical TP53 mutations that were present in the diagnostic samples were detected in the postremission bone marrow samples in 25 of 76 (34%) patients analyzed. Although widely distributed from 2.6% to 51.2%, their variant-allele frequencies (VAF) in most cases were small enough to support the somatic origin of the TP53 mutations in the remission sample. In 3 of 5 cases with large VAF size (>40%), the possibility of a germline origin could be excluded by analysis of sorted T cells. Because minimal residual disease (MRD) as measured by clonal rearrangements of immunoglobulin or T-cell receptor genes was undetectable in all samples or detected, if ever, only at much lower levels compared with those of the mutant TP53, the TP53 mutations did not represent residual leukemic cells but nonleukemic clones harboring somatic TP53 mutations. Overall, these findings suggest that the persistence of TP53 mutations found in postremission samples suggests the presence of a TP53-mutated somatic clone contributing to the reconstitution of hematopoiesis after intensive chemotherapy, most likely representing age-related clonal hematopoiesis (ARCH).
On the basis of the above hypothesis, the authors investigated the lineage contribution of TP53-mutated somatic clones in three cases, using simultaneous genotyping and immunotyping of postremission samples containing TP53-mutant clones at a single-cell level, which was enabled by antibody-derived tag sequencing and custom single-cell DNA sequencing (scDNA-seq) based on the Tapestri platform (Mission Bio). Based on immunophenotyping data, their single-cell platform confidently identified 7 distinct clusters that conformed to T-cell, B-cell, monocyte and dendritic cell (DC), myeloid and erythroid cell fractions, as well as other poorly defined cell clusters on Uniform Manifold Approximation and Projection (UMAP) analysis. Projecting TP53 mutation data on the protein UMAP representation, the authors depicted the architecture of TP53-mutant clones. In all three cases, TP53-mutated cells showed multilineage differentiation into myeloid, erythroid, and monocyte/dendritic cells. Although two cases had heterozygous mutations, in the remaining case, almost all myeloid/erythroid fractions were replaced by cells harboring biallelic TP53 alterations with a minor T-cell fraction carrying heterozygous TP53 mutants. Moreover, these TP53-mutated myeloid/erythroid cells also had losses of 5q and 16q arms that were distinct from CNAs in the diagnostic sample but lacked other CNA lesions obligatory to LH-ALL, such as losses of whole chromosomes 3, 7, 16, and 17. Given the lack of leukemia-specific rearrangement, these populations may represent a clone related to myelodysplastic syndromes (MDS) that was distinct from the LH-ALL clone at diagnosis. Thus, these data suggest the presence of TP53-mutated hematopoietic stem/progenitor clone(s) before chemotherapy that can repopulate apparently normal hematopoiesis after chemotherapy. The authors further analyzed diagnostic samples from three patients including two paired samples with remission samples using the same single-cell platform. Of interest, these diagnostic samples were shown to harbor small but discrete myeloid and NK-cell populations that carried heterozygous TP53 mutation but were devoid of tumor-specific Ig rearrangement. Thus, the multilineage expansion of TP53-mutant clones in postremission samples was already present at the time of diagnosis, but not as a result of a clonal selection related to cytotoxic chemotherapy. Taken together, these single-cell data strongly support the model that LH-ALL evolves from clonal hematopoiesis carrying a heterozygous TP53 mutation that contributes to multilineage cell fractions and repopulates hematopoiesis after remission. Of interest in this regard is the presence of multiple clonal hematopoiesis-related mutations and their chronological behaviors. In some cases, although the TP53 mutation was the initial driver mutation to initiate LH-ALL, an independent JAK2-mutant clone evolved parallelly, which, together with the TP53-mutated clone, repopulated the patient's hematopoiesis after remission. In another case, the TP53-mutated clone emerged from within the DNMT3A-mutated population and further acquired hypodiploidy and del(5q) independently to give rise to LH-ALL and MDS-like clones, respectively, of which the latter finally replaced the LH-ALL clone after remission.
Inferred only indirectly from skewed X-chromosome inactivation in early studies, the presence of positively selected clones in otherwise healthy individuals has been reproducibly substantiated by detecting somatic mutations and/or mosaic chromosomal abnormalities (mCA) commonly found in hematologic malignancies (7, 8). Frequently designated as ARCH, these clones are associated with an elevated risk of developing leukemia and other hematologic malignancies. Although the association has been shown for both myeloid and lymphoid neoplasms, the vast majority of ARCH-related mutations and mCA have been more implicated in myeloid than lymphoid neoplasms, and the focus in most studies so far has been on the association with myeloid neoplasms; in particular, its association with ALL has rarely been addressed. Through the advanced molecular analysis of the largest cohort of adult LH-ALL ever published, the authors have now shed new light on the pathogenesis of adult LH-ALL, which might intrinsically be linked to ARCH. Their data unequivocally support the presence of TP53-mutated ARCH clones, from which adult LH-ALL and its preleukemic clones should have evolved, and are in agreement with another recent report also describing preleukemic TP53-mutated clones that persisted in multilineage blood compartments after remission (Fig. 1; ref. 9). Given that the strong age-dependent nature of ARCH, including mutated TP53-associated ARCH (7, 10), parallels the progressive enrichment of LH-ALL in increasing age groups, their findings not only clarify the origin of adult LH-ALL but also open a new link between ARCH and lymphoid neoplasms in the elderly.
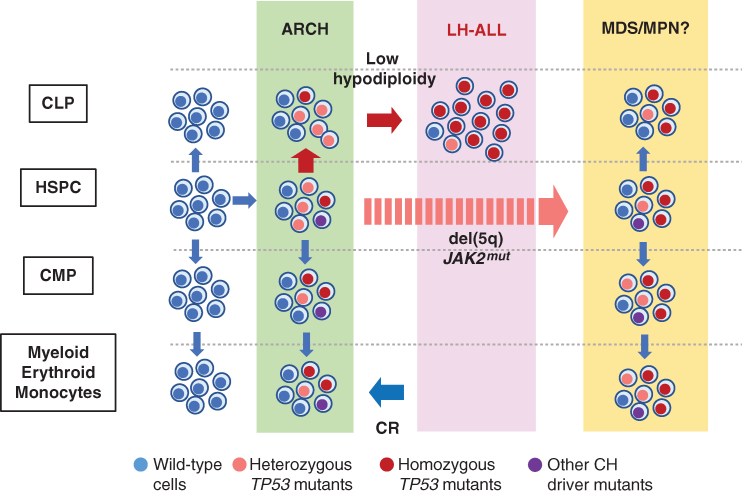
Figure 1.
ARCH-derived evolution of LH-ALL. With aging those HSPCs that acquire driver mutations, including TP53 mutations, are positively selected to establish ARCH, multilineage hematopoiesis of mutant clones. Among these, TP53-mutated hematopoietic stem and progenitor cells (HSPC) give rise to common lymphoid progenitors (CLP) and B-cell precursors, in addition to common myeloid progenitors (CMP). TP53 alteration further acquires hypodiploidy to develop LH-ALL. TP53-mutated ARCH cells survive and reconstitute hematopoiesis after remission induction. In some cases, the bone marrow is reconstituted by ARCH clones after remission induction. In other cases, the reconstitution was made by MDS or MDS-related clones.
TP53 mutation and 17pLOH are among the most common alterations implicated in ARCH. In a recent study describing the risk of different hematologic malignancies associated with ARCH that were detected by both leukemia mutations and allelic imbalances (7), TP53-mutated ARCH was identified in 161 of 11,153 Japanese individuals ages ≥60 years without a known history of hematologic malignancies, including 49 cases suspected for biallelic alterations on the basis of cooccurring 17pLOH or multiple mutations. In line with other studies, TP53-mutated ARCH cases, particularly those with 17pLOH, are at risk for the development of hematologic malignancies. Among these 161 TP53-mutated cases, 45 developed and died of hematologic malignancies during a median of 10.4 years of the observation period. The most common tumor type associated with TP53-mutant ARCH was MDS (n = 20), followed by non-Hodgkin lymphoma (NHL; n = 10), and acute myeloid leukemia (AML; n = 4), with only 1 case who developed ALL, although the TP53 mutation status at the time of tumor diagnosis is unknown. Thus, TP53-mutated ARCH seems to be predisposed much less common to ALL than to MDS/AML and NHL, as anticipated from the relative prevalence of these cancers in the elderly. However, considering the very high incidence of tumor development overall (45/161), adult LH-ALL patients might have another risk of developing AML/MDS and NHL. In this regard, two cases that showed expansion of del(5q) and JAK2 mutation–positive clones suggestive of MDS or MPN after remission are of interest.
Collectively, the findings by Kim and colleagues (4) and Chitadze and colleagues (9) have important clinical implications for leukemia treatment, MRD monitoring, treatment selection, and prediction of the treatment outcome. First, the positivity for TP53 mutations in clinical remission may not necessarily predict MRD, in which a careful evaluation combining immunoglobulin rearrangement status is needed to correctly estimate the risk of residual disease. Second, the risk of the development of independent tumors sharing the identical TP53 mutation might be kept in mind, and surveillance for additional mutations should be monitored using next-generation sequencing. Meanwhile, the mechanism of TP53-mutated ARCH for developing different tumor types is unclear, although the type and the cell target of the secondary hits seem to play a role. Further studies should be warranted.
Acknowledgments
This work is supported by JSPS KAKENHI grant JP19H05656, the Core Research for Evolutional Science and Technology, Moonshot Research and Development Program, and Biobank Japan. We used the questionnaire information of the Vital Statistics of Japan.
Authors’ Disclosures
S. Ogawa reports grants from the Japan Agency for Medical Research and Development, the Japan Society for the Promotion of Science, the Ministry of Education, Culture, Sports, Science and Technology, and Takeda Science Foundation during the conduct of the study; grants from Japan Agency for Medical Research and Development, Chordia Therapeutics, Japan Science and Technology Agency, personal fees from Novartis Pharmaceuticals and Astellas Pharmaceuticals outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Pui CH, Nichols KE, Yang JJ. Somatic and germline genomics in paediatric acute lymphoblastic leukaemia. Nat Rev Clin Oncol 2019;16:227–40. [DOI] [PubMed] [Google Scholar]
- 2. Jabbour E, Pui CH, Kantarjian H. Progress and innovations in the management of adult acute lymphoblastic leukemia. JAMA Oncol 2018;4:1413–20. [DOI] [PubMed] [Google Scholar]
- 3. Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L, et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 2013;45:242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim R, Bergugnat H, Larcher L, Duchmann M, Passet M, Gachet S, et al. Adult low-hypodiploid acute lymphoblastic leukemia emerges from preleukemic TP53-mutant clonal hematopoiesis. Blood Cancer Discov 2023;4:134–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harrison CJ, Moorman AV, Broadfield ZJ, Cheung KL, Harris RL, Jalali GR, et al. Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol 2004;125:552–9. [DOI] [PubMed] [Google Scholar]
- 6. Charrin C, Thomas X, Ffrench M, Le QH, Andrieux J, Mozziconacci MJ, et al. A report from the LALA-94 and LALA-SA groups on hypodiploidy with 30 to 39 chromosomes and near-triploidy: 2 possible expressions of a sole entity conferring poor prognosis in adult acute lymphoblastic leukemia (ALL). Blood 2004;104:2444–51. [DOI] [PubMed] [Google Scholar]
- 7. Saiki R, Momozawa Y, Nannya Y, Nakagawa MM, Ochi Y, Yoshizato T, et al. Combined landscape of single-nucleotide variants and copy number alterations in clonal hematopoiesis. Nat Med 2021;27:1239–49. [DOI] [PubMed] [Google Scholar]
- 8. Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science 2019;366:eaan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chitadze G, Stengel A, John-Klaua C, Bruckmuller J, Trautmann H, Kotrova M, et al. Somatic TP53 mutations are pre-leukemic events in acute lymphoblastic leukemia. Blood 2022. Nov 30 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Kar SP, Quiros PM, Gu M, Jiang T, Mitchell J, Langdon R, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat Genet 2022;54:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]