Abstract
Background and aims:
Low-grade inflammation is a mediator of muscle proteostasis. This study aimed to investigate the effects of isolated whey and soy proteins on inflammatory markers.
Methods:
We conducted a systematic literature search of randomised controlled trials (RCT) through MEDLINE, Web of Science, Scopus and Cochrane Library databases from inception until September 2021. To determine the effectiveness of isolated proteins on circulating levels of C-reactive protein (CRP), IL-6 and TNF-α, a meta-analysis using a random-effects model was used to calculate the pooled effects (CRD42021252603).
Results:
Thirty-one RCT met the inclusion criteria and were included in the systematic review and meta-analysis. A significant reduction of circulating IL-6 levels following whey protein [Mean Difference (MD): −0·79, 95 % CI: −1·15, −0·42, I2 = 96 %] and TNF-α levels following soy protein supplementation (MD: −0·16, 95 % CI: −0·26, −0·05, I2 = 68 %) was observed. The addition of soy isoflavones exerted a further decline in circulating TNF-α levels (MD: −0·20, 95 % CI: −0·31, −0·08, I2 = 34 %). According to subgroup analysis, whey protein led to a statistically significant decrease in circulating IL-6 levels in individuals with sarcopenia and pre-frailty (MD: −0·98, 95 % CI: −1·56, −0·39, I2 = 0 %). These findings may be dependent on participant characteristics and treatment duration.
Conclusions:
These data support that whey and soy protein supplementation elicit anti-inflammatory effects by reducing circulating IL-6 and TNF-α levels, respectively. This effect may be enhanced by soy isoflavones and may be more prominent in individuals with sarcopenia.
Keywords: Whey protein, Soy protein, Inflammation, IL-6, TNF-α, Sarcopenia
Ageing is associated with increased levels of circulating pro-inflammatory cytokines such as C-reactive protein (CRP), interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α)(1), which are forerunners of cellular senescence and muscle proteolysis(2).
Accruing adverse changes in muscle physiology across the lifespan may lead to reduced muscle mass and physical capacity, particularly after the fifth decade(3), known as sarcopenia(4). From the beginning of the fourth decade, muscle mass decreases by approximately 0·5 % every year. The multifactorial determinants of this phenomenon include reduced levels of anabolic hormones, chronic inflammation, degradation of the muscle contractile proteins, loss of regenerative capacity, altered neural activation, and mitochondrial dysfunction(5,6). Sarcopenia is associated with an increased circulating pro-inflammatory signalling (i.e., higher levels of TNF-α and IL-6)(7,8). In conjunction with sarcopenia, concomitant accumulation of adiposity has also been observed during ageing, representing sarcopenic obesity, which is also linked with elevated inflammatory markers(9,10). Accelerating age-related muscle wasting is partially explained through systemically and locally elevated oxidative stress and reactive oxygen species (ROS) accumulation(11–13). Excessive ROS levels may result in damaged muscle and DNA proteins, triggering the release of pro-inflammatory cytokines and leading to low-grade inflammation(14). Interestingly, antioxidative properties derived from nutrients may prevent excess ROS inflation that could alter muscle proteostasis(15). Hence, finding nutritional strategies to mitigate low-grade inflammation may be considered as a safe and effective strategy for the prevention and treatment of sarcopenia.
Albeit protein supplementation is associated with reduced circulating levels of pro-inflammatory cytokines(16,17), different protein sources may exert distinct anti-inflammatory effects(18). Specifically, soy food intake has been associated with lower circulating levels of IL-6 and TNF-α(19); however, the functional properties of whole foods may differ compared with nutrients in isolation(20). In this regard, previous systematic reviews have observed a reduction of serum CRP levels following intact whey and soy protein supplementation(21,22), while the addition of soy isoflavones has been linked with a decline in circulating IL-6 levels among postmenopausal women(23). Thus, isolated sources of protein may elicit promising isolated anti-inflammatory responses, although the most effective source of intact protein in alleviating circulating pro-inflammatory cytokine levels remains to be fully elucidated. To date, no previous meta-analysis has investigated the effects of intact whey and soy protein supplementation on multiple inflammatory markers in older adults. The aim of this systematic review and meta-analysis is to investigate the effects of intact whey and soy protein supplementation on serum CRP, IL-6 and TNF-α levels in older adults.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines(24). The protocol of this systematic review and meta-analysis was registered in the PROSPERO International prospective register of systematic reviews (CRD42021252603).
Search strategy
Two independent reviewers (KP and MI) searched the MEDLINE, Web of Science, Scopus and Cochrane Library databases from inception until September 2021, using the following search terms: ‘whey OR soy’ in combination with ‘older adults’ and ‘inflammation OR high sensitivity-C reactive protein OR C reactive protein OR IL-6 OR tumour necrosis factor-a’. The complete search strategy is presented in Supplementary Table 1. No restrictions in terms of geographical region were applied. Articles were written in English and discrepancies in the literature search process were resolved by a third investigator (MM).
Study selection
Studies in this systematic review and meta-analysis were included based on the following criteria: (1) they were RCT; (2) the intervention group received intact soy or whey protein supplements in oral form; (3) the comparator group received a placebo or a non-identical appropriate treatment; (4) circulating levels of CRP, IL-6 and/or TNF-α were assessed; (5) participants that took part in the intervention had a mean age ≥ 50 years old and (6) full text was written in English. Accordingly, studies were excluded if: (1) they were not randomised trials; (2) participants were institutionalised; (3) studies were missing the baseline and/or post-intervention outcome values; (4) whey and soy protein products were in peptide/whole-food form and (5) whey and soy protein supplements were consumed enterally (Supplementary Table 2). Finally, if studies were comprised of a comparator group of < 50 years of age, they were included in the analysis as long as the participant age was similar to the intervention group.
Data extraction and quality assessment
Two authors (KP and MI) extracted data independently on name of first author, date of publication, country of origin, study design, participant health status, gender, age, BMI, sample size, intervention type, dose and duration, daily energy and protein intake, serum high-sensitivity CRP (hs-CRP), CRP, IL-6 and TNF-α levels. CRP and hs-CRP units were converted to mg/l, while IL-6 and TNF-α values to pg/ml. Disagreements between authors on data eligibility were resolved by a third reviewer (MM). When studies contained multiple doses of protein supplementation, only the highest dose was considered as the intervention arm.
The quality of included studies was evaluated using the Risk-of-bias 2 tool and the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system approach. Risk-of-bias 2 is a detailed and comprehensive tool to assess the risk of bias in randomised trials included in Cochrane Reviews, focussing on (1) the evaluation of randomisation process, (2) deviations from intended interventions, (3) missing outcome data, and (4) measurement of the outcome and selection of the reported result(25). According to the Risk-of-bias 2 scoring system, study quality was defined as high, some concerns or low. Additionally, the GRADE approach involves the consideration of (1) within-study risk of bias, (2) directness of evidence, (3) study heterogeneity, and (4) precision of effect estimates and risk of publication bias, using four levels of quality (high, moderate, low and very low)(26).
Data synthesis and statistical analysis
Our analysis reported on the differences among circulating inflammatory markers (hs-CRP, CRP, IL-6, and TNF-α) following whey and soy protein supplementation, when compared with individuals receiving placebo or a non-identical treatment. Quantitative data were treated as continuous measures and were combined by calculating the mean differences between outcomes from baseline and the follow-up period of each intervention. Statistical significance between the intervention and comparator groups was assessed using the random effects inverse-variance model. Missing standard deviations of outcomes were estimated depending on the availability of either CI, SE, t and P values or by calculating a correlation coefficient (Corr) from a known change from baseline standard deviation. A 95 % CI was used to calculate missing sd and considering the absence of studies with sd changes from baseline to follow-up, an extra analysis utilising a Corr value of 0·7 was performed(27).
The statistical heterogeneity between studies was assessed using the overlap of their 95 % CI and expressed as measures of Cochran’s Q (Chi-square test) and I2. Data classification as moderately heterogeneous was based on I2 from 50 % to 74 %, and highly heterogeneous from 75 % and above(28). Furthermore, sensitivity analysis was performed to evaluate the robustness of the reported statistical results by discounting the effect of confounding factors on outcome measures through a leave-one-out analysis. Studies with a high risk of bias and/or the study with the highest effect size were discounted through a leave-one-out sensitivity analysis. Publication bias was assessed using Begg’s funnel plots and Egger’s linear regression test(29) using R software. Data were meta-analysed and forest plots were drawn using Review Manager (RevMan 5.4.1). A P value of < 0·05 was considered statistically significant.
Subgroup and sensitivity analyses
Subgroup analyses were performed based on Corr equal to 0·7, age, BMI, treatment dose and duration, soy protein and isoflavone co-supplementation, soy protein supplementation during postmenopause, and whey protein supplementation in participants with sarcopenia and pre-frailty. Sensitivity analyses were performed using a leave-one-out analysis, excluding the study with the largest effect size and the study with the highest bias risk.
Results
Search results and study characteristics
The initial search generated 5432 records, in which 5220 were excluded due to ineligibility issues and study duplicates. Following a full-text review of the remaining 212 studies, 153 articles were removed and 59 articles were sought for retrieval. In total, 45 full-text reports were assessed for eligibility. Acute studies and articles with missing or incomplete data were excluded from the analysis. Overall, 31 studies were included in the systematic review and meta-analysis (Fig. 1).
Fig. 1.
PRISMA flowchart of literature search via databases and registers.
Study characteristics of the included trials using whey and soy protein supplementation are presented in Table 1 and Table 2, respectively. All trials utilising whey and soy protein supplementation as an intervention in males and females had a mean age between 50 and 80·8 years. Six studies contained additional nutrients alongside whey protein supplementation [one study contained vitamin D(30); one study contained vitamin C and Mg(31), one study contained vitamin D and vitamin E(32), one study contained Zn and Se(33), one study contained medium-chain saturated fatty acids(34), one study contained Ca and vitamin C(35)]. In studies providing soy protein supplements, nine out of 13 studies included isoflavones(35–44), one study included phytoestrogens(45) and one study included isoflavones with phytoestrogens(46).
Table 1.
Characteristics of whey protein supplementation included studies
| Study | Country | Study design | Number of participants (M/F) | Age range (years) | Study population | Experimental dose (g/d) | Comparator treatment | Treatment duration | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Biesek et al. 2021(35) | Brazil | Single-blind RCT | E: 11 (0/11) C: 9 (0/9) |
E: 73·1 ± 5·3 C: 70·4 ± 3·9 |
Pre-frailty | 21 and 224 mg Ca and 23 mg vitamin C |
Placebo | 12 weeks | IL-6 |
| Kirk et al. 2021(53) | UK | Unblinded RCT | E: 23 (11/12) C: 29 (12/17) |
E: 71·8 ± 6·5 C: 68·2 ± 5·9 |
Community-dwelling | 1·5 g/kg/body weight | Usual diet (no treatment) | 16 weeks | CRP IL-6 TNF-α |
| Mizubuti et al. 2021(60) | Brazil | Double-blind RCT |
E: 35 (22/13) C: 40 (28/12) |
E: 51·6 ± 9·5 C: 52·6 ± 11·4 |
Chronic liver disease | 40 | Casein protein | 2 weeks | IL-6 TNF-α |
| Ahmadi et al. 2020(31) | Iran | Single-blind RCT | E: 23 (23/0) C: 23 (23/0) |
E: 62·1 ± 7 C: 63·5 ± 7·2 |
COPD | 16 and vitamin C (685 mg) and Mg (275 mg) |
Dietary advice | 8 weeks | IL-6 TNF-α |
| Derosa et al. 2020(47) | Italy | Single-blind RCT | E: 59 (30/29) C: 58 (29/29) |
E: 59·7 ± 9·1 C: 58·6 ± 8·8 |
Type 2 diabetes | 5 | Casein protein | 3 months | hs-CRP IL-6 TNF-α |
| Bo et al. 2019(32) | China | Double-blind RCT | E: 30 (13/17) C: 30 (14/16) |
E: 73·2 ± 6·5 C: 74·8 ± 5·9 |
Sarcopenia | 44 and vitamin D (700 IU) and vitamin E (109 mg) |
Placebo | 6 months | CRP IL-6 TNF-α |
| Nabuco et al. 2019(55) | Brazil | Double-blind RCT | E: 13 (0/13) C: 13 (0/13) |
E: 68 ± 4·2 C: 70·1 ± 3·9 |
Sarcopenia | 35 | Placebo | 12 weeks | CRP IL-6 TNF-α |
| Rakvaag et al. 2019(48) | Denmark | Double-blind RCT | E: 15 (9/6) C: 16 (8/8) |
E: 67 (range: 60–69) C: 62 (range: 58–68) |
Abdominal Obesity | 72–87 | Placebo | 12 weeks | hs-CRP TNF-α |
| Bumrungpert et al. 2018(33) | Thailand | Double-blind RCT | E: 23 (7/16) C: 19 (3/16) |
E: 54·1 ± 9·3 C: 51·5 ± 9·6 |
Cancer | 40 and Zn (2·64 mg) and Se (0·76 mg) |
Placebo | 12 weeks | hs-CRP |
| Fernandes et al. 2018(52) | Brazil | Double-blind RCT | E: 16 (0/16) C: 16 (0/16) |
E: 67·3 ± 4·1 C: 67·8 ± 4·0 |
Healthy | 35 | Placebo | 12 weeks | CRP |
| Stojkovic et al. 2017(56) | USA | Double-blind RCT | E: 38 (0/38) C: 46 (0/46) |
E: 68·9 ± 0·9 C: 69·3 ± 0·9 |
Healthy | 20 | Placebo | 18 months | CRP IL-6 |
| Fekete et al. 2016(51) | Italy | Double-blind Crossover RCT | E: 38 (20/18) C:38 (20/18) |
E: 52·9 ± 2·1 C: 52·9 ± 2·1 |
Prehypertension | 56 | Placebo | 8 weeks | CRP IL-6 TNF-α |
| Sohrabi et al. 2016(49) | Iran | Single-blind RCT | E: 23 (10/13) C: 23 (10/13) |
E: 57 ± 9·6 C: 55 ± 6·5 |
Haemodialysis | 15 | No treatment | 8 weeks | hs-CRP IL-6 |
| Rondanelli et al. 2016(30) | Italy | Double-blind RCT | E: 69 (29/40) C: 61 (24/37) |
E: 80·8 ± 6·3 C: 80·2 ± 8·5 |
Sarcopenia | 22 and vitamin D (100 mg) |
Placebo | 12 weeks | CRP |
| Bohl et al. 2015(34) | Denmark | Double-blind RCT | E: 13 (5/8) C: 13 (7/6) |
E: 61·1 ± 6 C: 56·7 ± 10·6 |
Abdominal obesity | 60 and MC-SFA (6·9 g) |
Casein protein and MC-SFA (6·9 g) | 12 weeks | hs-CRP IL-6 |
| Duff et al. 2014(57) | Canada | Double-blind RCT | E: 21 (8/13) C: 19 (7/12) |
E: 57·5 ± 6·3 C: 61·8 ± 4·8 |
Abdominal obesity | 38 | Bovine colostrum (62 g/d) |
8 weeks | CRP |
| Weinheimer et al. 2012(59) | USA | Double-blind RCT | E: 30 C: 84 |
E: 50 ± 7·1 C: 49 ± 7 |
Obesity/ Overweight |
60 | Placebo | 18 weeks | CRP |
| Laviolette et al. 2010(58) | Canada | Double-blind RCT | E: 12 (10/2) C: 10 (4/6) |
E: 62·9 ± 10·1 C: 67·6 ± 4·4 |
COPD | 20 | Casein protein | 8 weeks | CRP IL-6 |
E, experimental group; C, comparator group; COPD, chronic obstructive pulmonary disease; CRP, c-reactive protein; F, females; hs-CRP; high sensitivity c-reactive protein; M, males; MC-SFA, medium-chain saturated fatty acids; RCT, randomised controlled trial.
Table 2.
Characteristics of soy protein supplementation included studies
| Study | Country | Study design | N of participants (M/F) | Age range (years) | Health status | Experimental dose (g/d) | Comparator treatment | Treatment duration | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Sathyapalan et al. 2017(50) | Qatar | Double-blind RCT | E: 86 (86/0) C: 85 (85/0) |
E: 52 ± 2·5 C: 52 ± 2·5 |
Type 2 Diabetes | 15 | Soy protein and isoflavones (66 mg) | 3 months | hs-CRP |
| Liu et al. 2012(41) | Hong Kong | Double-blind RCT | E: 60 (0/60) C: 60 (0/60) |
E: 56·4 ± 4·7 C: 56 ± 4·4 |
Healthy | 15 & isoflavones (100 mg) |
Milk protein and isoflavones (100 mg) | 6 months | hs-CRP |
| Ma et al. 2011(54) | China | Single-blind RCT | E: 45 (14/31) C: 45 (12/33) |
E: 51·4 ± 1·7 C: 51·9 ± 1·5 |
Mild Hypercholesterolemia | 18 | Milk protein | 8 weeks | CRP TNF-α |
| Sathyapalan et al. 2011(45) | Qatar | Double-blind crossover RCT | E: 27 (6/21) C: 21 (2/19) |
E: 57·2 ± 13·8 C: 57·2 ± 13·8 |
Subclinical Hypothyroidism | 30 and phytoestrogens (2 mg) |
Soy protein and phytoestrogens (16 mg) | 8 weeks | hs-CRP |
| Napora et al. 2011(42) | USA | Double-blind RCT | E: 17 (17/0) C: 16 (16/0) |
E: 69·2 ± 2·5 C: 69 ± 2·2 |
Prostate Cancer | 20 and isoflavones (160 mg) |
Milk protein | 12 weeks | CRP IL-6 TNF-α |
| Christie et al. 2010(37) | USA | Double-blind RCT | E: 17 (0/17) C: 16 (0/16) |
E: 54·4 ± 3·3 C: 53·3 ± 4·9 |
Healthy | 20 and isoflavones (160 mg) |
Placebo | 12 weeks | CRP IL-6 TNF-α |
| Charles et al. 2009(36) | USA | Double-blind RCT | E: 32 (0/32) C: 43 (0/43) |
E: 57·3 ± 1·1 C: 56·1 ± 0·8 |
Healthy | 20 and isoflavones (160 mg) |
Whole-milk protein | 12 weeks | IL-6 TNF-α |
| Tormala et al. 2009(44) | Finland | Single-blind randomised crossover trial | E: 40 (0/40) | E: 57·7 ± 0·8 | Healthy | 52 & isoflavones (116 mg) |
Placebo | 8 weeks | CRP |
| Greany et al. 2008(39) | USA | Single-blind randomised crossover trial | E: 34 (0/34) | E: 57·7 ± 6 | Healthy | 26 ± 5 & isoflavones (44 ± 8 mg) |
Milk protein | 6 weeks | CRP |
| Fanti et al. 2006(38) | USA | Double-blind RCT | E: 15 (9/6) C: 10 (6/4) |
E: 60·7 ± 3·4 C: 61·6 ± 5·2 |
End Stage Renal Disease | 25 and isoflavones (56 mg) |
Milk protein | 8 weeks | CRP IL-6 TNF-α |
| Hanson et al. 2006(46) | USA | Double-blind RCT |
E: 14 (0/14) C: 14 (0/14) |
E: 56 (49–70) C: 58 (47–72) |
Mild Hypercholesterolemia during | 40 and isoflavones (1·2 mg) and phytate (0·22 g) |
Soy protein and isoflavones (86 mg) and phytate (0·22 g) | 6 weeks | CRP |
| Hermansen et al. 2005(40) | Denmark | Double-blind RCT | E: 49 (23/26) C: 51 (19/32) |
E: 60·6 ± 3·4 C: 58 ± 4·6 |
Hypercholesterolemia | 30 and isoflavones (100 mg) |
Casein protein | 24 weeks | TNF-α |
| Teede et al. 2004(43) | USA | Double-blind RCT | E: 30 (0/30) C: 20 (0/20) |
E: 61 ± 1 C: 62 ± 1 |
Healthy | 40 and isoflavones (118 mg) |
Casein protein | 3 months | CRP |
E, experimental group; C, comparator group; CRP, c-reactive protein; F, females; hs-CRP; high sensitivity c-reactive protein; M, males; RCT, randomised controlled trial.
Furthermore, seven studies measured serum hs-CRP(33,41,45,47–50), 18 studies serum CRP(30,32,37–39,42–44,46,51–59), 16 studies serum IL-6(31,32,34–38,42,47,49,51,53,55,56,58,60) and 14 studies serum TNF-α values(31,32,36–38,40,42,47,48,51,53–55,60). In total, 3274 individuals participated in both groups with 1611 individuals in the intervention group and 1663 individuals in the comparator group (online Supplementary Table 3a–c).
Data collection for whey protein supplementation was performed on three studies in participants with abdominal obesity(34,48,57), three studies with sarcopenia(30,32,55) according to the European Working Group on Sarcopenia in Older People consensus(61), two studies with COPD(31,58), one study with type 2 diabetes(47), pre-frailty(35) based on Fried’s frailty phenotype(62), chronic liver disease(60), cancer(33), haemodialysis(49), prehypertension(51) and obesity(59), while in three studies participants were community-dwelling(53), healthy(52) and on postmenopause(56). Additionally, data collection for soy protein supplementation was performed on seven studies during postmenopause(36,37,39,41,43,44,46), two studies with hypercholesterolaemia(40,54), one study with type 2 diabetes(50), subclinical hypothyroidism(45), prostate cancer(42) and end stage renal disease(38).
Risk of bias and quality of evidence assessment
Out of 18 studies utilising whey protein supplements, 11 studies had an overall low risk of bias(20,30,32,35,47–49,51,52,55,57,60), five studies had some concerns(33,34,53,56,58) and two studies had a high risk of bias(31,59). Specifically, one study was unblinded(53) and six studies did not provide any details on allocation treatment(33,34,52,56,58,59), whereas although one study claimed there was allocation concealment, no further details were provided(48). In addition, one study had a high risk of trial personnel being aware of participants’ assigned intervention(31). In two studies, there were some concerns regarding missing outcome data(35,59). Finally, in two studies, the outcome assessment could have been influenced by knowledge of the intervention received(31,53).
Out of 13 studies utilising soy protein supplements, nine studies had an overall low risk of bias(36–38,40–43,45,50), one study had some concerns(46) and three studies had a high risk of bias(39,44,54). Particularly, three studies did not provide details on allocation concealment(39,44,46), while two studies claimed there was allocation concealment; however, no further details were provided(45,50). Furthermore, three studies had a high risk of trial personnel being aware of participants’ assigned intervention(39,44,54) and likewise, in three studies, the outcome assessment could have been influenced by knowledge of intervention received(39,44,54).
Traffic light plots were created using robvis visualisation tool. A detailed description of Risk-of-bias 2 traffic light plots for whey and soy protein supplementation studies are presented in Supplementary Tables 4 and 5, respectively. Finally, the GRADE system approach showed that the quality of evidence for the primary outcomes was moderate (Supplementary Tables 6a–d and 7a–d).
Effect of whey protein supplementation on circulating inflammatory markers analysis
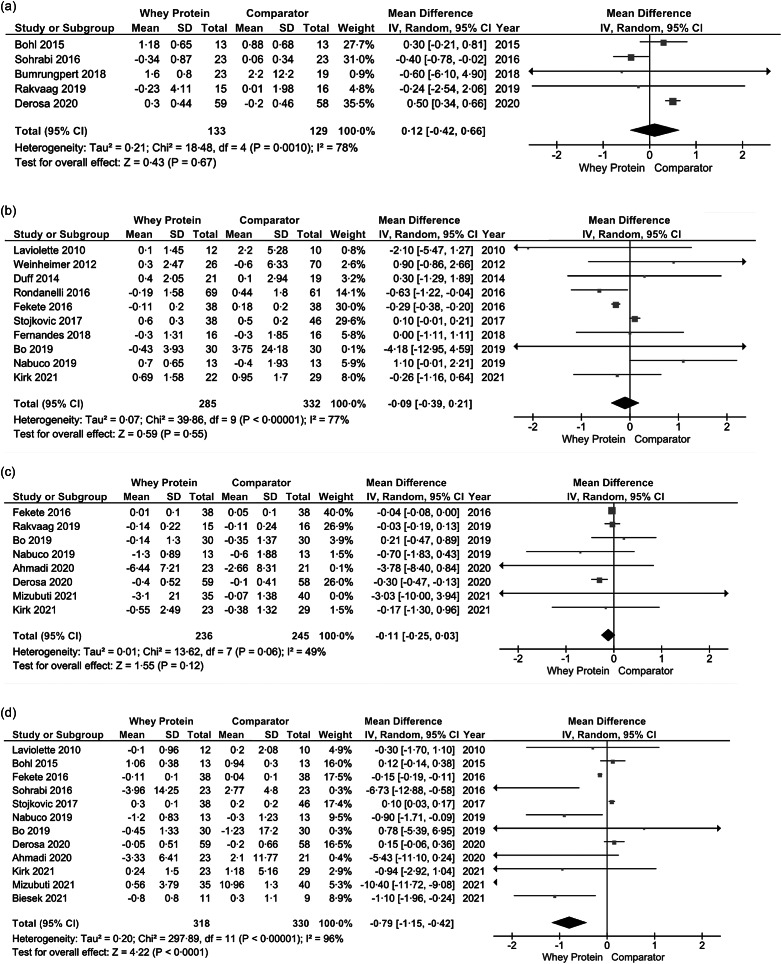
Following whey protein supplementation, no changes were observed on serum hs-CRP (k = 5, MD: 0·12, 95 % CI: −0·42, 0·66, I2 = 78 %) (Fig. 2a), serum CRP (k = 10, MD: −0·09, 95 % CI: −0·39, 0·21, I2 = 77 %) (Fig. 2b), and serum TNF-α levels (k = 8, MD: −0·11, 95 % CI: −0·25, 0·03, I2 = 49 %) (Fig. 2c). Interestingly, whey protein supplementation reduced serum IL-6 levels significantly (k = 12, MD: −0·79, 95 % CI: −1·15, −0·42) (Fig. 2d); however, a high heterogeneity among studies was observed (I2 = 96 %). Using Corr equal to 0·7 did not demonstrate any significant changes compared with the main analysis (online Supplementary Fig. 1a–d).
Fig. 2.
Effects of whey protein supplementation on (a) hs-CRP, (b) CRP, (c) TNF-α and (d) IL-6. CRP, C-reactive protein.
Subgroup analysis of whey protein supplementation trials
Subgroup analysis based on age revealed no significant changes in serum hs-CRP, CRP, TNF-α and IL-6 in adults < 60 and ≥ 60 years of age (online Supplementary Fig. 3a, d).
A subgroup analysis revealed no benefits of whey protein supplementation in individuals with sarcopenia and pre-frailty on serum CRP (k = 3, MD: 0·02, 95 % CI: –1·60, 1·65, I2 = 75 %) (online Supplementary Fig. 13a) and TNF-α levels (k = 2, MD: –0·13 95 % CI: -0·99, 0·73, I2 = 45 %) (online Supplementary Fig. 13b); however, whey protein displayed a significant reduction of serum IL-6 levels (k = 3, MD: –0·98, 95 % CI: –1·56, –0·39, I2 = 0 %) (Supplementary Fig. 13c).
Based on treatment duration, whey protein supplementation ≤ 8 weeks showed a significant reduction in serum CRP levels (k = 4, MD: –0·30, 95 % CI: –0·39, –0·21, I2 = 0 %) compared with a treatment duration of > 8 weeks (k = 6, MD: 0·13, 95 % CI: –0·13, 0·40, I2 = 9 %) (online Supplementary Fig. 9a), whereas serum TNF-α and IL-6 concentrations remained unaltered (online Supplementary Fig. 9b, c).
Significant reductions of serum CRP levels were revealed in participants with BMI < 25 kg/m2 (k = 2, MD: –0·65, 95 % CI: –1·23, 0·06, I2 = 0 %) vs. BMI ≥ 25 kg/m2 (k = 8, MD: 0·00, 95 % CI: –0·32, 0·32, I2 = 80 %) (online Supplementary Fig. 5a), whereas a significant decline was observed in serum IL-6 levels in participants with BMI ≥ 25 kg/m2 (k = 7, MD: –1·00, 95 % CI: –1·14, –0·58, I2 = 97 %) (online Supplementary Fig. 5c).
In addition, an intervention dose of ≥ 30 g/d led to significant decreases in serum IL-6 levels (k = 6, MD: −2·15, 95 % CI: −3·41, 0·89, I2 = 96 %) (online Supplementary Fig. 7c), while serum CRP and TNF-α concentrations compared with the comparator group remained statistically unchanged (online Supplementary Fig. 7a, b). All available information regarding subgroup analyses and whey protein supplementation are detailed in Supplementary Table 8a, b.
Effect of soy protein supplementation on circulating inflammatory markers analysis
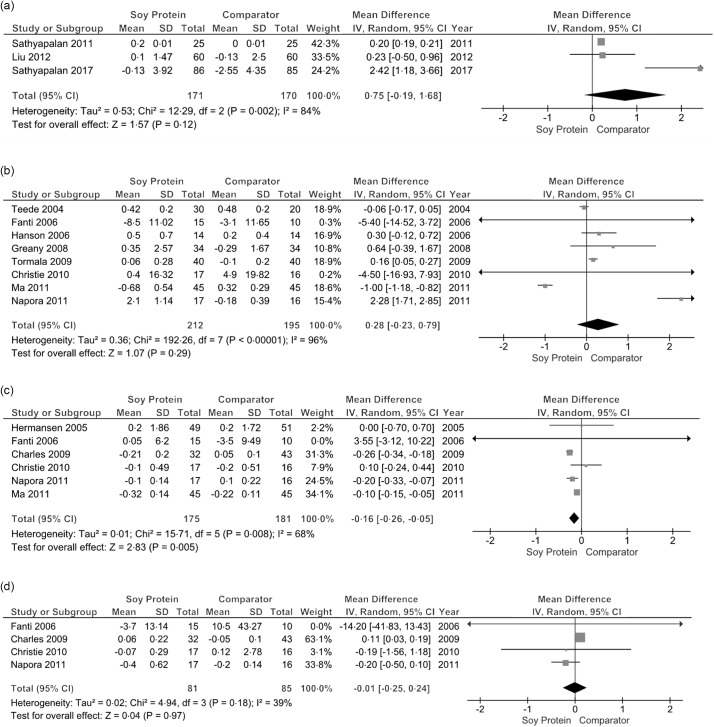
Following soy protein supplementation, no changes were observed on serum hs-CRP (k = 3, MD: 0·75, 95 % CI: −0·19, 0·66, P = 0·12, I2 = 84 %) (Fig. 3a), serum CRP (k = 8, MD: 0·28, 95 % CI: −0·23, 0·79, I2 = 96 %) (Fig. 3b) and serum IL-6 levels (k = 4, MD: −0·01, 95 % CI: −0·25, 0·24, I2 = 39 %) (Fig. 3d). Soy protein supplementation however displayed a significant reduction in serum TNF-α (k = 6, MD: −0·16, 95 % CI: −0·26, −0·05) (Fig. 3c), which was accompanied by a moderate homogeneity among studies (I2 = 68 %).
Fig. 3.
Effects of soy protein supplementation on (a) hs-CRP, (b) CRP, (c) TNF-α and (d) IL-6. CRP, C-reactive protein.
Subgroup analysis of soy protein supplementation trials
Using Corr equal to 0·7 did not demonstrate any significant changes compared with the main analysis (online Supplementary Figures 2a–d).
Subgroup analysis based on age revealed a significant reduction of serum TNF-α levels in older adults ≥ 60 years (k = 3, MD: –0·19, 95 % CI: –0·32, –0·07, I2 = 0 %) as opposed to older adults < 60 years (k = 3, MD: –0·14, 95 % CI: –0·29, 0·01, I2 = 85 %) (online Supplementary Fig. 4c).
Subgroup analyses showed a significant decline of serum TNF-α levels in older adults with BMI ≥ 25 kg/m2 (k = 5, MD: –0·20, 95 % CI: –0·31, –0·08, I2 = 34 %) (online Supplementary Fig. 6c), soy protein dose < 30 g/d (k = 6, MD: –0·16, 95 % CI: –0·26, –0·05, I2 = 68 %) (online Supplementary Fig. 8b), treatment duration > 8 weeks (k = 4, MD: –0·20, 95 % CI: –0·31, –0·09, I2 = 38 %) (online Supplementary Fig. 10c) and addition of isoflavones (k = 5, MD: –0·20, 95 % CI: –0·31, –0·08, I2 = 34 %) (online Supplementary Fig. 11c). Notably, the addition of isoflavones also demonstrated a significant increase in serum CRP levels (k = 7, MD: 0·53, 95 % CI: 0·12, 0·94) (online Supplementary Fig. 11b), although there was a high heterogeneity among trials (I2 = 91 %).
There were not enough number of studies for treatment duration and sarcopenia status subgroup analysis with the soy protein supplementation.
Sensitivity analysis based on effect size and bias risk
Sensitivity analyses using a leave-one-out strategy based on the effect size of whey protein (online Supplementary Fig. 14a–d) and soy protein supplementation studies (online Supplementary Fig. 15a–d) did not alter outcome measures. Likewise, sensitivity analyses using a leave-one-out strategy for bias risk did not reveal any changes compared with the results from the main analysis (whey protein studies: Supplementary Fig. 16a–d; soy protein studies, Supplementary Fig. 17a, b).
Publication bias
Visual examination to test for asymmetry among studies for serum IL-6 and CRP levels using Begg’s funnel plots are illustrated in Supplementary Fig. 18a, b and Supplement Fig. 18c, d, respectively. Egger’s linear regression test revealed no evidence for publication bias in both the intervention (z = −0·6174, P = 0·5369) and the comparator group (z = −0·0367, P = 0·9708) for serum IL-6 levels following whey protein supplementation based on twelve RCT in this meta-analysis. Additionally, Egger’s linear regression test also revealed no evidence for publication bias in the intervention group for serum CRP levels (z = −0·0043, P = 0·9966); however, an increased risk for publication bias was observed in the comparator group (z = 2·5193, P = 0·0118).
Discussion
This meta-analysis showed a significant decline in circulating IL-6 and TNF-α levels following whey and soy protein supplementation, respectively. Subgroup analysis based on age (< 60 years) revealed a significant reduction of serum TNF-α following whey protein consumption, while subgroup analysis accounting for sarcopenia and pre-frailty status also exhibited a significant reduction of serum IL-6. In addition, a decline in serum CRP levels was observed following a treatment duration of ≤ 8 weeks and in participants with BMI ≤ 25 kg/m2. Similarly, subgroup analyses based on age (≥ 60 years) and treatment duration of > 8 weeks showed a significant reduction of serum TNF-α following soy protein supplementation, while the addition of isoflavones exhibited further benefits by reducing serum CRP levels. Overall, these findings suggest that whey and soy protein supplementation may exert distinct anti-inflammatory properties, which are dependent on participant physiological characteristics, treatment duration, and addition of isoflavones.
A previous meta-analysis has demonstrated that whey protein may mitigate low-grade inflammation by decreasing serum CRP levels; however, the increased heterogeneity among studies may have influenced such findings(22). Although a high degree of heterogeneity among studies was detected, our analysis revealed a significant effect of whey protein supplementation in reducing serum IL-6 levels. Noteworthy that insignificant results were found in the subgroup analyses on serum TNF-α according to age (< 60 vs. ≥ 60 years), BMI (< 25 vs. ≥ 25 kg/m2) and treatment duration (≤ 8 vs. > 8 weeks) on serum CRP levels, our findings should be treated with caution due to the small number of studies. Interestingly, our subgroup analysis revealed significant benefits of whey protein supplementation on sarcopenia and pre-frailty, highlighting a significant decline in circulating IL-6 levels. The combination of these two populations was based on their identical characteristics in relation to muscle mass and strength, displaying a low degree of study heterogeneity. In this context, hospitalised patients with frailty have elicited a beneficial effect on reducing serum IL-6 following whey protein supplementation(63), which may be explained by a concomitant increase in glutathione concentrations and a decrease in ROS accumulation(64). Moreover, reduced serum IL-6 levels have also been demonstrated in individuals with sarcopenia by comparing a whey protein-based product to placebo; however, its nutrient content may have masked the effectiveness of whey protein in isolation(65). Particularly, the combination of carotenoids, choline, vitamin A and E and Fe may exert anti-inflammatory effects(21,66,67) and act as confounders in assessing the efficacy of whey protein in alleviating low-grade inflammation. In a subgroup analysis, one study combined whey protein with vitamin D, which may be partially responsible for serum IL-6 level reduction(68). However, research is conflicting regarding the effects of vitamin D on reducing serum inflammatory markers in older adults(9,69,70). Our findings suggest that a –0·98 pg/ml mean reduction in serum IL-6 concentrations of individuals with sarcopenia and pre-frailty may be of clinical relevance given a 0·7 pg/ml mean difference between younger and older populations based on cross-sectional data(71). Therefore, whey protein supplementation may be a valuable dietary strategy to attenuate the progression of low-grade inflammation and exacerbation of sarcopenia and frailty risk. Considering the increased baseline pro-inflammatory profile in people with sarcopenia, the effects of intact protein supplementation may be more prevalent in these populations. However, due to the limited number of studies and their heterogeneous designs, our results regarding the effectiveness of whey protein in reducing circulating inflammatory markers in individuals with sarcopenia and frailty should be treated with caution.
Previous meta-analyses have revealed that soy-based protein foods and supplements may not alter serum inflammatory status(72,73). However, these findings were based on flavonoid-enriched foods(65) and postmenopausal women from which only serum CRP levels were measured(72). Additionally, experimental studies have not observed a significant effect of soy food consumption on serum CRP levels(74) that may be attributed to the interaction of multiple nutrients contained in whole soy foods(75) compared with isolated sources(76). Our analysis revealed a significant effect of soy protein supplementation in reducing serum TNF-α levels, which are in line with previous research(77,78), although, insignificant reductions of serum IL-6 levels were displayed as reported previously(23). Furthermore, subgroup analysis showed that the addition of isoflavones did not decrease serum CRP and IL-6 levels; however a significant reduction of serum TNF-α was observed. These results may be attributed to the bioactive substances in soy isoflavones (i.e. phenolic compounds, daidzein, and genistein) that exert antioxidant effects(79,80) through glutathione peroxidase regulation and reduction of ROS and malondialdehyde infiltration(81). Although several soy isoflavone doses were administered in this systematic review, subgroup analysis based on dose was not feasible due to the low number of studies. Therefore, whether greater isoflavone quantities correspond to higher decreases of circulating inflammatory cytokines is currently unclear.
Limitations
Our study was prone to limitations. High variability regarding participant health status, isoflavone dose, and study sample size potentially accounted for the increased heterogeneity in multiple subgroup analyses. The sample size of studies did not allow for subgroup analyses based on healthy populations and individuals with comorbidities. Hence, definitive conclusions around specific conditions and healthy older populations cannot be extrapolated. In addition, the quality of evidence was moderate according to GRADE system approach, while several studies did not use a placebo group as a comparator. Finally, nutrient intake was not controlled in multiple studies, which may have influenced the participants’ inflammatory profile. More importantly, the effects of vitamins, minerals, alcohol, and energy intake may be pivotal contributors in regulating pro-inflammatory cytokine status; hence, the scarcity of data on these parameters should be considered in future studies.
Conclusions
Systemic low-grade inflammation is a critical contributor to muscle proteolysis during ageing. Our study found a significant reduction of circulating IL-6 and TNF-α levels following whey and soy protein supplementation, respectively. These effects were particularly augmented with the addition of soy isoflavones and populations with sarcopenia and pre-frailty. Whey and soy protein supplementation may serve as a valuable dietary intervention in reducing serum inflammatory cytokine levels, however, more homogeneous studies are required to provide more reliable results on healthy populations and individuals with comorbidities.
Acknowledgements
This study received no funding/sponsorship.
K. P., M. I. and M. M. developed the method and constructed the framework for this study. K. P. and M. I. wrote the manuscript. A. M., B. T., M. I., M. M. and R. S. revised the manuscript.
The authors declare no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114522001787.
click here to view supplementary material
References
- 1. Chung HY, Kim DH, Lee EK, et al. (2019) Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis 10, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Londhe P & Guttridge DC (2015) Inflammation induced loss of skeletal muscle. Bone 80, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw S, Dennison E & Cooper C (2017) Epidemiology of sarcopenia: determinants throughout the lifecourse. Calcified Tissue Int 101, 229–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Agine 48, 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell WK, Atherton PJ, Williams J, et al. (2012) Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venturelli M, Reggiani C, Richardson RS, et al. (2018) Skeletal muscle function in the oldest-old: the role of intrinsic and extrinsic factors. Exerc Sport Sci Rev 46, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li CW, Yu K, Shyh-Chang N, et al. (2019) Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia, Sarcopenia Muscle 10, 586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rong Y-D, Bian A-L, Hu H-Y, et al. (2018) Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr 18, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Cunha Nascimento D, da Cunha Oliveira S, Vieira DCL, et al. (2018) The impact of sarcopenic obesity on inflammation, lean body mass, and muscle strength in elderly women. Int J Gen Med 11, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schrager MA, Metter EJ, Simonsick E, et al. (2007) Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 102, 919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson MJ & McArdle A (2011) Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol 589, 2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vasilaki A, Richardson A, Van Remmen H, et al. (2017) Role of nerve–muscle interactions and reactive oxygen species in regulation of muscle proteostasis with ageing. J Physiol 595, 6409–6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venturelli M, Morgan GR, Donato AJ, et al. (2014) Cellular aging of skeletal muscle: telomeric and free radical evidence that physical inactivity is responsible and not age. Clin Sci 127, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalle S, Rossmeislova L & Koppo K (2017) The role of inflammation in age-related sarcopenia. Front Physiol 8, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powers SK (2014) Can antioxidants protect against disuse muscle atrophy? Sports Med 44, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mose M, Brodersen K, Rittig N, et al. (2021) Anabolic effects of oral leucine-rich protein with and without β-hydroxybutyrate on muscle protein metabolism in a novel clinical model of systemic inflammation—a randomized crossover trial. Am J Clin Nutr 114, 1159–1172. [DOI] [PubMed] [Google Scholar]
- 17. Rittig N, Bach E, Thomsen H, et al. (2016) Amino acid supplementation is anabolic during the acute phase of endotoxin-induced inflammation: a human randomized crossover trial. Clin Nutr 35, 322–330. [DOI] [PubMed] [Google Scholar]
- 18. Draganidis D, Chondrogianni N, Chatzinikolaou A, et al. (2017) Protein ingestion preserves proteasome activity during intense aseptic inflammation and facilitates skeletal muscle recovery in humans. Br J Nutr 118, 189–200. [DOI] [PubMed] [Google Scholar]
- 19. Wu SH, Shu XO, Chow W-H, et al. (2012) Soy food intake and circulating levels of inflammatory markers in Chinese women. J Acad Nutr Diet 112, 996–1004.e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aguilera JM (2019) The food matrix: Implications in processing, nutrition and health. Crit Rev Food Sci Nutr 59, 3612–3629. [DOI] [PubMed] [Google Scholar]
- 21. Asbaghi O, Sadeghian M, Nazarian B, et al. (2020) The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomized clinical trials. Sci Rep 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou L-M, Xu J-Y, Rao C-P, et al. (2015) Effect of whey supplementation on circulating C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients 7, 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gholami A, Baradaran HR & Hariri M (2021) Can soy isoflavones plus soy protein change serum levels of interlukin-6? A systematic review and meta-analysis of randomized controlled trials. Phytother Res 35, 1147–1162. [DOI] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88, 105906. [DOI] [PubMed] [Google Scholar]
- 25. Farrah K, Young K, Tunis MC, et al. (2019) Risk of bias tools in systematic reviews of health interventions: an analysis of PROSPERO-registered protocols. Syst Rev 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Altman DG, Gøtzsche PC, et al. (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borenstein M, Hedges LV, Higgins JP, et al. (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Meth 1, 97–111. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egger M, Smith GD, Schneider M, et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rondanelli M, Klersy C, Terracol G, et al. (2016) Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 103, 830–840. [DOI] [PubMed] [Google Scholar]
- 31. Ahmadi A, Eftekhari MH, Mazloom Z, et al. (2020) Fortified whey beverage for improving muscle mass in chronic obstructive pulmonary disease: a single-blind, randomized clinical trial. Respir Res 21, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bo Y, Liu C, Ji Z, et al. (2019) A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: a double-blind randomized controlled trial. Clin Nutr 38, 159–164. [DOI] [PubMed] [Google Scholar]
- 33. Bumrungpert A, Pavadhgul P, Nunthanawanich P, et al. (2018) Whey protein supplementation improves nutritional status, glutathione levels, and immune function in cancer patients: a randomized, double-blind controlled trial. J Med Food 21, 612–616. [DOI] [PubMed] [Google Scholar]
- 34. Bohl M, Bjørnshave A, Gregersen S, et al. (2016) Whey and casein proteins and medium-chain saturated fatty acids from milk do not increase low-grade inflammation in abdominally obese adults. Review Diabet Stud: RDS 13, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biesek S, Vojciechowski AS, Ferreira AC, et al. (2021) Effects of exergames and protein supplementation on body composition and musculoskeletal function of prefrail community-dwelling older women: a randomized, controlled clinical trial. Int J Environ Res Public Health 18, 9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Charles C, Yuskavage J, Carlson O, et al. (2009) Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause 16, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Christie DR, Grant J, Darnell BE, et al. (2010) Metabolic effects of soy supplementation in postmenopausal Caucasian and African American women: a randomized, placebo-controlled trial. Am J Obstet Gynecol 203, 153. e151–153. e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fanti P, Asmis R, Stephenson TJ, et al. (2006) Positive effect of dietary soy in ESRD patients with systemic inflammation—correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol Dialysis Transplant 21, 2239–2246. [DOI] [PubMed] [Google Scholar]
- 39. Greany K, Nettleton J, Wangen K, et al. (2008) Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur J Clin Nutr 62, 1419–1425. [DOI] [PubMed] [Google Scholar]
- 40. Hermansen K, Hansen B, Jacobsen R, et al. (2005) Effects of soy supplementation on blood lipids and arterial function in hypercholesterolaemic subjects. Eur J Clin Nutr 59, 843–850. [DOI] [PubMed] [Google Scholar]
- 41. Liu Z-M, Ho S, Chen Y-M, et al. (2012) The effects of isoflavones combined with soy protein on lipid profiles, C-reactive protein and cardiovascular risk among postmenopausal Chinese women. Nutr Metab Cardiovasc Dis 22, 712–719. [DOI] [PubMed] [Google Scholar]
- 42. Napora JK, Short RG, Muller DC, et al. (2011) High-dose isoflavones do not improve metabolic and inflammatory parameters in androgen-deprived men with prostate cancer. J Androl 32, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Teede HJ, Dalais FS & McGrath BP (2004) Dietary soy containing phytoestrogens does not have detectable estrogenic effects on hepatic protein synthesis in postmenopausal women. Am J Clin Nutr 79, 396–401. [DOI] [PubMed] [Google Scholar]
- 44. Törmälä R, Appt S, Clarkson TB, et al. (2008) Impact of soy supplementation on sex steroids and vascular inflammation markers in postmenopausal women using tibolone: role of equol production capability. Climacteric 11, 409–415. [DOI] [PubMed] [Google Scholar]
- 45. Sathyapalan T, Manuchehri AM, Thatcher NJ, et al. (2011) The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab 96, 1442–1449. [DOI] [PubMed] [Google Scholar]
- 46. Hanson LN, Engelman HM, Alekel DL, et al. (2006) Effects of soy isoflavones and phytate on homocysteine, C-reactive protein, and iron status in postmenopausal women. Am J Clin Nutr 84, 774–780. [DOI] [PubMed] [Google Scholar]
- 47. Derosa G, D’Angelo A & Maffioli P (2020) Change of some oxidative stress parameters after supplementation with whey protein isolate in patients with type 2 diabetes. Nutrition 73, 110700. [DOI] [PubMed] [Google Scholar]
- 48. Rakvaag E, Fuglsang-Nielsen R, Bach Knudsen KE, et al. (2019) The combination of whey protein and dietary fiber does not alter low-grade inflammation or adipose tissue gene expression in adults with abdominal obesity. Rev Diabetic Stud 15, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sohrabi Z, Eftekhari MH, Eskandari MH, et al. (2016) Intradialytic oral protein supplementation and nutritional and inflammation outcomes in hemodialysis: a randomized controlled trial. Am J Kidney Dis 68, 122–130. [DOI] [PubMed] [Google Scholar]
- 50. Sathyapalan T, Rigby AS, Bhasin S, et al. (2017) Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: a randomized controlled study. J Clin Endocrinol Metab 102, 425–433. [DOI] [PubMed] [Google Scholar]
- 51. Fekete AA, Giromini C, Chatzidiakou Y, et al. (2016) Whey protein lowers blood pressure and improves endothelial function and lipid biomarkers in adults with prehypertension and mild hypertension: results from the chronic Whey2Go randomized controlled trial. Am J Clin Nutr 104, 1534–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fernandes RR, Nabuco HC, Junior PS, et al. (2018) Effect of protein intake beyond habitual intakes following resistance training on cardiometabolic risk disease parameters in pre-conditioned older women. Exp Gerontol 110, 9–14. [DOI] [PubMed] [Google Scholar]
- 53. Kirk B, Mooney K, Vogrin S, et al. (2021) Leucine-enriched whey protein supplementation, resistance-based exercise, and cardiometabolic health in older adults: a randomized controlled trial. J Cachexia, Sarcopenia Muscle 12, 2022–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma L, Grann K, Li M, et al. (2011) A pilot study to evaluate the effect of soy isolate protein on the serum lipid profile and other potential cardiovascular risk markers in moderately hypercholesterolemic Chinese adults. Ecol Food Nutr 50, 473–485. [DOI] [PubMed] [Google Scholar]
- 55. Nabuco HC, Tomeleri CM, Fernandes RR, et al. (2019) Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: A randomized, double-blind, placebo-controlled trial. Clin Nutr ESPEN 32, 88–95. [DOI] [PubMed] [Google Scholar]
- 56. Stojkovic V, Simpson CA, Sullivan RR, et al. (2017) The effect of dietary glycemic properties on markers of inflammation, insulin resistance, and body composition in postmenopausal American women: an ancillary study from a multicenter protein supplementation trial. Nutrients 9, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duff WR, Chilibeck PD, Rooke JJ, et al. (2014) The effect of bovine colostrum supplementation in older adults during resistance training. Int J Sport Nutr Exerc Metab 24, 276–285. [DOI] [PubMed] [Google Scholar]
- 58. Laviolette L, Lands LC, Dauletbaev N, et al. (2010) Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: a randomized, controlled, double-blind pilot study. J Med Food 13, 589–598. [DOI] [PubMed] [Google Scholar]
- 59. Weinheimer EM, Conley TB, Kobza VM, et al. (2012) Whey protein supplementation does not affect exercise training-induced changes in body composition and indices of metabolic syndrome in middle-aged overweight and obese adults. J Nutr 142, 1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mizubuti Y, Vieira E, Silva T, et al. (2021) Comparing the effects of whey and casein supplementation on nutritional status and immune parameters in patients with chronic liver disease: a randomised double-blind controlled trial. Br J Nutr 125, 768–779. [DOI] [PubMed] [Google Scholar]
- 61. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing 39, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fried LP, Tangen CM, Walston J, et al. (2001) Frailty in older adults: evidence for a phenotype. J Gerontol Ser A: Biol Sci Med Sci 56, M146–M157. [DOI] [PubMed] [Google Scholar]
- 63. Niccoli S, Kolobov A, Bon T, et al. (2017) Whey protein supplementation improves rehabilitation outcomes in hospitalized geriatric patients: a double blinded, randomized controlled trial. J Nutr Gerontol Geriatr 36, 149–165. [DOI] [PubMed] [Google Scholar]
- 64. Kerasioti E, Stagos D, Priftis A, et al. (2014) Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem 155, 271–278. [DOI] [PubMed] [Google Scholar]
- 65. Liberman K, Njemini R, Luiking Y, et al. (2019) Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: the PROVIDE study. Aging Clin Exp Res 31, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaulmann A & Bohn T (2014) Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res 34, 907–929. [DOI] [PubMed] [Google Scholar]
- 67. Mehta AK, Singh BP, Arora N, et al. (2010) Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology 215, 527–534. [DOI] [PubMed] [Google Scholar]
- 68. Elenkova M, Tipton DA, Karydis A, et al. (2019) Vitamin D attenuates human gingival fibroblast inflammatory cytokine production following advanced glycation end product interaction with receptors for AGE. J Periodontal Res 54, 154–163. [DOI] [PubMed] [Google Scholar]
- 69. Agbalalah T, Hughes SF, Freeborn EJ, et al. (2017) Impact of vitamin D supplementation on endothelial and inflammatory markers in adults: a systematic review. J Steroid Biochem Mol Biol 173, 292–300. [DOI] [PubMed] [Google Scholar]
- 70. Jamka M, Woźniewicz M, Walkowiak J, et al. (2016) The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: a systematic review with meta-analysis. Eur J Nutr 55, 2163–2176. [DOI] [PubMed] [Google Scholar]
- 71. Calder PC, Bosco N, Bourdet-Sicard R, et al. (2017) Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev 40, 95–119. [DOI] [PubMed] [Google Scholar]
- 72. Hariri M, Ghasemi A, Baradaran HR, et al. (2021) Beneficial effect of soy isoflavones and soy isoflavones plus soy protein on serum concentration of C-reactive protein among postmenopausal women: an updated systematic review and meta-analysis of randomized controlled trials. Complementary Ther Med 59, 102715. [DOI] [PubMed] [Google Scholar]
- 73. Peluso I, Raguzzini A & Serafini M (2013) Effect of flavonoids on circulating levels of TNF-α and IL-6 in humans: a systematic review and meta-analysis. Mol Nutr Food Res 57, 784–801. [DOI] [PubMed] [Google Scholar]
- 74. Khodarahmi M, Jafarabadi MA, Moludi J, et al. (2019) A systematic review and meta-analysis of the effects of soy on serum hs-CRP. Clin Nutr 38, 996–1011. [DOI] [PubMed] [Google Scholar]
- 75. Cassidy A, Brown JE, Hawdon A, et al. (2006) Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr 136, 45–51. [DOI] [PubMed] [Google Scholar]
- 76. Reinwald S, Akabas SR & Weaver CM (2010) Whole v. the piecemeal approach to evaluating soy. J Nutr 140, 2335S–2343S. [DOI] [PubMed] [Google Scholar]
- 77. Khodarahmi M, Foroumandi E & Asghari Jafarabadi M (2021) Effects of soy intake on circulating levels of TNF-α and interleukin-6: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr 60, 581–601. [DOI] [PubMed] [Google Scholar]
- 78. Rezazadegan M, Mirjalili F, Clark CC, et al. (2021) The effect of soya consumption on inflammatory biomarkers: a systematic review and meta-analysis of clinical trials. Br J Nutr 125, 780–791. [DOI] [PubMed] [Google Scholar]
- 79. Patel RP, Boersma BJ, Crawford JH, et al. (2001) Antioxidant mechanisms of isoflavones in lipid systems: paradoxical effects of peroxyl radical scavenging. Free Radical Biol Med 31, 1570–1581. [DOI] [PubMed] [Google Scholar]
- 80. Rodríguez-Roque MJ, Rojas-Graü MA, Elez-Martínez P, et al. (2013) Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem 136, 206–212. [DOI] [PubMed] [Google Scholar]
- 81. Jin L, Zhao X, Qin Y, et al. (2015) Soy isoflavones protect against H2O2-induced injury in human umbilical vein endothelial cells. Mol Med Rep 12, 4476–4482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114522001787.
click here to view supplementary material