Abstract
Background
Delayed gastric emptying (DGE) is a major cause of undernutrition that can be overcome using nasointestinal (NI) feeding, but tube placement often fails. We analyse which techniques enable successful NI tube placement.
Methods
Efficacy of tube technique was determined at each of six anatomical points: Nose, nasopharynx-oesophagus, stomach-upper and -lower, duodenum part-1 and intestine.
Results
In 913 first NI tube placements, significant associations with tube advancement were found in the pharynx (head tilt, jaw thrust, laryngoscopy), stomach_upper (air insufflation, 10 cm or 20–30 cm flexible tube tip ± reverse Seldinger manoeuvre), stomach_lower (air insufflation, possibly flexible tip and wire stiffener) and duodenum part-1 and beyond part-2 (flexible tip and combinations of micro-advance, slack removal, wire stiffener or prokinetic drugs).
Conclusion
This is the first study to show what techniques are associated with tube advancement and the alimentary tract level they are specific to.
Keywords: Delayed gastric emptying, manoeuvre, nasointestinal, nasojejunal, technique, tube advance
Introduction
Delayed gastric emptying (DGE) occurs in 30–46% of critically ill patients1–2 and is associated with prolonged ventilation, ICU and hospital stay and increased mortality.1,3 Although a causal link to these outcomes is not certain, DGE is associated with reduced feed and drug delivery. 1 However, early EN remains preferable to delayed nutrient intake or parenteral nutrition because it is associated with reduced mortality and infection. 4 Prokinetic drugs reduce DGE, 5 but even combined metoclopramide and erythromycin treatment is associated with tachyphylaxis. 6 Conversely, nasointestinal (NI) feeding, from duodenum part-1 to the jejunum, delivers more nutrition in patients with DGE refractory to metoclopramide treatment when compared with nasogastric (NG) feeding plus prokinetics. 7 However, aspiration risk appears to decline as NI placement becomes more distal. 8 In addition, NI feeding, rather than NG, was associated with less reflux, vomiting and ventilator-associated pneumonia 9–11
Endoscopy and fluoroscopy are highly successful in achieving intestinal tube placement, but increase clinical risk from their invasive nature, irradiation, off-ward location and exposure to infection. Guided bedside tube placement would minimise these risks and any delay to feeding. Unfortunately, published techniques for achieving intestinal placement are mostly limited to moving the tube through the pylorus. Using prokinetic drugs, combining air insufflation + right lateral decubitus position + a weighted tube or using tube rotation with a bent guide-wire, failed to reach the intestine in 8–17% and tubes only advanced beyond duodenum part-3 in 17–22%.12–14 Hawk and Valdivia 15 suggested operator skill as a reason for improved guided versus blind transpyloric tube placement.16,17 However, the success associated with guidance may only be achieved if the guidance prompts the use of techniques. 18 Manufacturer guidance for Cortrak-guided placement suggests use of IV metoclopramide, laying the patient flat (upright for a distended abdomen), an air bolus and slow tube insertion to prevent coiling. 19 However, this guidance was unsubstantiated by published citations. To address the lack of systematic evidence, we analysed techniques, tried or developed in clinical practice, to achieve tube advancement. To our knowledge, this is the first analysis of multiple techniques and their efficacy at different anatomical points.
Methods
Design and data collection
In a single UK ICU we retrospectively determined the success of our techniques for clinically required NI tube placements from 22.03.07 to 31.08.21. We acquired demographic data, tube position attained, problems of advancement, techniques and anatomical points at which they were used from a database of contemporaneous records of bedside NI tube placement. Anatomical points were cross-referenced with digital traces of the tube path. APACHE two scores were obtained from ICNARC (Intensive Care National Audit and Research Centre). All patient ID was removed and disease transformed into a general disease category prior to export to the statistical package for anonymised publication.
Techniques
All the techniques were developed and applied to specific anatomical points as part of clinical practice (Table 1). The safety of using ‘stiffener’ guide-wires was discussed with Interventional Radiology who use similar practice.
Table 1.
Techniques, order of use and their purpose as used at different levels of the alimentary tract.
| GI level | Technique order | Detail | |
|---|---|---|---|
| Nose | −Nasal airway | Slit the airway along its lesser curvature, lubricate and insert into the nostril. Insert the tube through the airway. When the tube tip had reached the nasopharynx, withdraw airway, peeling the slit off the tube | |
| Pharynx-oesophagus | 1. Head tilt forward | This straightens the passage to the oesophagus, reducing both neck curvature and likelihood of tube deflection into the trachea | |
| 2. Jaw thrust | Lower jaw displacement pulls the tongue, endotracheal tube or tracheostomy cuff forward permitting easier tube entry into the oesophagus | ||
| 3. Laryngoscopy | Direct vision to place a tube into the oesophagus | ||
| STOMACH | Upper | 1. Flexible tip 10 cm | Guide-wire withdrawn 10 cm to make the tip flexible enough to navigate the gastric body flexure or folds |
| 2. Air insufflation | Initially 250 mL but up to 750 mL in increments to open a passage if the greater curvature or gastric folds have indented to block tube passage | ||
| 3. Flexible tip 20–30 cm ± reverse seldinger | When a tube tip has become stuck on the greater curvature or moves anti-clockwise, back towards the oesophagus, this technique facilitates tube entry into the lower stomach and orients the tip towards the pylorus. First withdraw the tube tip until just inside the stomach. Retract the guide-wire 20 cm then slowly advance the flexible tip of the tube until the guide-wire is just inside the stomach. Repeat after retracting the guide-wire another 10 cm. If the tip has dropped into the lower stomach, careful re-insertion of the guide-wire will make advance toward the pylorus possible. If the guide-wire meets resistance at the nadir of tube bulging into the lower stomach with the tip pointing towards the fundus, withdraw the tube and insert the guide-wire both in 1 cm increments, effectively pulling the tube back onto the guide-wire in a reverse seldinger manoeuvre. Repeat until the guide-wire is fully re-inserted into the tube and the tip is able to advance from within the lower stomach (see Figure 1(b) and (c) | ||
| 4. Prokinetics | 1 IV dose of either 250 mg erythromycin or 10 mg metoclopramide to increase peristalsis | ||
| 5. Wire stiffener | 1–3 extra 140 cm guide-wires (ie. The same type and length as the tube) were used to prevent dilation of the tube within a flaccid stomach. As the tube is advanced into the intestine the ‘stiffener wire(s)' are progressively withdrawn from the tube so as to remain within the stomach | ||
| Lower | 1. Air insufflation | As above | |
| 2. Flexible tip | |||
| 3. Wire stiffener | |||
| 4. Prokinetics | |||
| 5. Patient flat | Remove any gastric folding | ||
| Duodenum part-1 or -2 and beyond | 1. Flexible tip <12 cm | Incrementally withdraw the guide-wire (usually 3–7 cm) to make the tip flexible enough to go around the flexure. Alternately re-insert the guide-wire to check for when the tip has completely traversed the flexure | |
| Flexible tip + various combinations of | |||
| and/or | 2. Micro-advance | Advancing in mm’s, usually with an increasing length of flexible tip | |
| 3. Slack withdrawal | Gastric slack or coil reduces tube stiffness precluding forward advance. Its removal effectively stiffens the tube | ||
| 4. Wire stiffener | As above | ||
| 5. Prokinetics | As above | ||
| Last resort techniques | 6. Massage abdomen | Massaging the right upper quadrant in an inwards and upwards direction to move the tube tip over the superior flexure | |
| 7. NGT withdrawal | Pulling the NGT back into the upper stomach to reduce risk of tangling the NI tube or blocking the pylorus | ||
Patients and equipment
Patients were referred for NI tube placement when suffering delayed gastric emptying (DGE), defined as a gastric residual volume ≥250 mL in a 4-h period or vomiting, that was refractory to 24-h of treatment with 10 mg IV metoclopramide or, to avoid delayed feeding, if DGE occurred on Friday. Patients who were moribund, had anatomical contraindications or refused consent were declined tube placement. Criteria for patient referral and the equipment used for tube placement remained constant. Guided placement was done using a 140 cm 10FG Cortrak™ tube (Avanos Medical Inc). Cortrak produces a real-time computer trace of the path of an electromagnet within the tube. Anatomical points were interpreted from trace characteristics, previously described.20–22 This permitted the operator, an ICU dietitian or consultant, to guide tube placement and confirm final position. Tubes left in situ were used for feeding. There were no instances of undetected lung misplacement.
Analysis
Analysis was restricted to a patient’s first tube placement to avoid over-representation by repeat placements. Using ‘R Studio Version 1.1.463′ most parameters did not meet a normal distribution (Shapiro-Wilk test) so continuous data were analysed using the 2-sided Wilcox rank sum test and presented as median (inter-quartile range, IQR). Categorical variables were analysed using Fisher’s exact test. Significance was taken as a p <0.05. These tests were used to check for missing data bias, comparing baseline parameters for patients with versus those without 'techniques' data, and in univariate analysis of associations with tube advancement.
Difficulty in tube advancement and the techniques used to overcome it were analysed at six anatomical points:
(1) Nose,
(2) Pharynx when attempting to enter the oesophagus,
(3) Stomach_upper
(4) Stomach_lower,
(5) Duodenum part-1, particularly the superior flexure and
(6) Intestine from duodenum part-2 to jejunum, particularly the duodenuo-jejunal (DJ) flexure.
For each anatomical point, analysis:
(1) Only included difficult placements, based on operator comment and/or use of a technique to overcome difficulty and/or failure to advance;
(2) Omitted placements where an alternative technique had been used but;
(3) Coded as ‘failed placement’ when techniques, additional to the one being analysed, were later used.
Univariate analysis was conducted for each technique within its sub-set of placements. If a higher proportion of tube advancement was associated with use of the technique (p < 0.05) or the median or proportion of baseline parameters differed depending on use of the technique (p < 0.2) these variables were entered into a logistic regression model. Because techniques used at subsequent anatomical points might affect final tube position, these models were binary, reporting associations with advancement, or not, at a specific anatomical point. The exception was the use of ordinal logistic regression to analyse tube advancement from duodenum part-2 to parts -3, -4 or jejunum when using ≥3 techniques where further techniques would not be added. Small sample sizes and/or a zero value for an option sometimes caused logistic regression to fail to separate effects of independent variables and made statistical output unreliable. For this reason we present p-value, OR and 95%CI for univariate analysis, but note where LR failed or where the apparent association between technique and tube advance may be confounded. In all other analyses, even where baseline parameters showed a significant association to technique use, the association between technique and tube advance remained statistically significant. Co-linear variables (variance-inflation factor >5) were omitted from the model.
Baseline parameters included demography (age, estimated or actual height, weight and body mass index [BMI] and gender) and clinical parameters (APACHE two score, disease category, airway and consciousness). Analysis was done in the order techniques were used at a particular anatomical point.
Ethics
Data collection was done as part of a registered UK quality improvement project (QI71316), using standard practice, and therefore did not require ethics board approval.
Results
Study group
913 of 947 primary NI tube placements were analysed; all baseline parameters were similar to the 34 placements with missing data (Appendix 1), including tube placement day (p = 0.5) and operator (p = 0.1). The referral policy and contemporaneous records for tube placement remained constant during this period, but specific techniques were added over time. Most placements (83.7%) were undertaken for DGE refractory to 24 h of metoclopramide treatment; the remainder were placed for DGE where prokinetic drugs were contraindicated, previously failed or to permit peri-operative feeding.
Lead operator and tube position
Lead operators E and I placed most tubes: A 0.1%, B 2.9%, C 1.4%, D 0.9, E 24.0%, F 0.1%, G 2.4%, H 0.2%, I 67.9.%. Placements failed to go beyond the stomach in 9.4% and duodenum part-1 in 5.8%, but reached the late duodenum or jejunum (79%): In the table below the numbers and % columns need to be aligned for easy reading.
| ■Lung or pharynx 10 1.1% | ■Duodenum part: 2 25 2.7% |
| ■Stomach- upper 19 2.1% | ■ 3 28 3.1% |
| ■Stomach- lower 57 6.2% | ■ 4269 29.5% |
| ■Duodenum part: 1 53 5.8% | ■Jejunum 452 49.5% |
Techniques
Use of single and combined techniques (Table 2) increased over time. Although no placement failed at the level of the nose or mouth, 30 (3.3%) presented difficulty with advancement. A nasal airway was used to aid advancement in only 5 (0.5%), too few to analyse. In contrast advance from pharynx to oesophagus was difficult in 224 (24.5%) and 97 (10.6%) initially deviated into the respiratory tract before being removed; 10 (1.1%) ultimately failed to advance beyond the pharynx of which 5 had entered the respiratory tract. The preferred sequence of interventions, head tilt > jaw thrust > laryngoscopy, was often precluded by clinical condition. For example, neck trauma might indicate use of a jaw thrust instead of a head tilt. Because interventions did not follow a sequence it was impossible to analyse which intervention affected tube advancement. However, use of 1–3 of these interventions appeared to improve the chance of advancing the tube (p < 0.0001) independent of potential confounding associations (‘+' = positive, '-' = negative) from an artificial airway (+) or, separately, a conscious state (-).
Table 2.
Associations between technique and tube advancement beyond each anatomical point.
| Anatomical point& | N | % | OR | 95%CI | Confounders entered into LR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Technique | Used | Fail | Success | Fail | Success | p-value | — | 2.5 | 97.5 | — |
| Pharynx | ||||||||||
| Headtilt ± jaw thrust ±laryngoscopy | no | 9 | 48 | 15.8 | 84.2 | <0.0001 | 31.1 | 3.8 | 251.7 | Conscious (-: 0.006) |
| yes | 1 | 166 | 0.6 | 99.4 | ||||||
| Stomach_upper | ||||||||||
| Flexible tip 10 cm | no | 177 | 32 | 84.7 | 15.3 | <0.0001 | 4 | 2.3 | 7.1 | cm (ns) |
| yes | 50 | 36 | 58.1 | 41.9 | ||||||
| Air insufflation | no | 9 | 4 | 69.2 | 30.8 | 0.0003 | 7.8 | 1.9 | 31.4 | None: All (ns) |
| yes | 9 | 31 | 22.5 | 77.5 | ||||||
| Flexible tip 20–30 cm ± reverse seldinger | no | 5 | 5 | 50 | 50 | <0.0001 | 51 | 7.9 | 330.7 | BMI (0.06) |
| yes | 2 | 102 | 1.9 | 98.1 | ||||||
| Wire stiffener | no | 3 | 6 | 33.3 | 66.7 | 0.67 | 1.5 | 0.3 | 8.3 | Univariate ns |
| yes | 5 | 15 | 25 | 75 | ||||||
| Stomach_lower | ||||||||||
| Air insufflation | no | 69 | 3 | 95.8 | 4.2 | <0.0001 | 34.5 | 10.2 | 116.9 | APACHE 2 score (-, p=0.07), cm and disease (ns) |
| yes | 42 | 63 | 40 | 60 | ||||||
| Flexible tip | no | 34 | 0 | 100 | 0 | <0.0001 | 207 | 7.3 | 5888.5 | LR fails |
| yes | 1 | 4 | 20 | 80 | ||||||
| Wire stiffener | no | 9 | 0 | 100 | 0 | <0.0004 | 37.1 | 2 | 701.4 | LR fails: age (-:p=0.03). Others variables (ns) |
| yes | 10 | 20 | 33.3 | 66.7 | ||||||
| Lay flat | no | 15 | 20 | 42.9 | 57.1 | 0.047 | 0.1 | 0 | 2 | Only 4 interventions; unreliable analysis |
| yes | 4 | 0 | 100 | 0 | ||||||
| Duodenum part-1 | ||||||||||
| Slack removal | no | 14 | 2 | 87.5 | 12.5 | 0.03 | 8.4 | 1.3 | 56.1 | age and APACHE 2 score (ns) |
| yes | 5 | 6 | 45.5 | 54.5 | ||||||
| Flexible tip | no | 14 | 2 | 87.5 | 12.5 | <0.0001 | 13.3 | 3 | 59.1 | age and bmi (ns), tracheostomy (p=0.07) and trauma (-, p=0.007) |
| yes | 214 | 406 | 34.5 | 65.5 | ||||||
| Flexible+Slack used in analyses below | yes | 138 | ||||||||
| Flexible tip + | ||||||||||
| micro-Advance | no | 10 | 12 | 45.5 | 54.5 | 0.053 | 4.4 | 1.1 | 17.1 | Airway (ns); conditional to inclusion, the technique became significant: p=0.011 |
| yes | 4 | 21 | 16 | 84 | ||||||
| slack removal | no | 10 | 12 | 45.5 | 54.5 | <0.0001 | 14.4 | 3.4 | 60.5 | Airway (ns) |
| yes | 3 | 52 | 5.5 | 94.5 | ||||||
| wire stiffener | no | 10 | 12 | 45.5 | 54.5 | 0.004 | 4.2 | 1.6 | 10.8 | None: All (ns) |
| yes | 25 | 127 | 16.4 | 83.6 | ||||||
| Flexible tip+wire stiffener + | ||||||||||
| micro-Advance | no | 16 | 14 | 53.3 | 46.7 | 0.054 | 4.6 | 1.1 | 19.7 | Airway (ETT), BMI, conscious, disease (ns) |
| yes | 3 | 12 | 20 | 80 | ||||||
| prokinetic drugs | no | 12 | 18 | 40 | 60 | 0.06 | 4.2 | 1 | 17.4 | None: All (ns) |
| yes | 3 | 19 | 13.6 | 86.4 | ||||||
| slack removal | no | 38 | 14 | 73.1 | 26.9 | <0.0001 | 10.6 | 4.3 | 26.1 | Age and conscious (ns) |
| yes | 11 | 43 | 20.4 | 79.6 | ||||||
| prokinetic+(micro±slack) | no | 19 | 3 | 86.4 | 13.6 | <0.0001 | 59.1 | 10.8 | 324.5 | None: All (ns) |
| yes | 3 | 28 | 9.7 | 90.3 | ||||||
| Duodenum part-2 and beyond | ||||||||||
| Slack removal | no | 36 | 0 | 100 | 0 | 0.07 | 15.9 | 0.7 | 355.8 | Univariate ns |
| yes | 11 | 2 | 84.6 | 15.4 | ||||||
| Flexible tip | no | 36 | 0 | 100 | 0 | <0.0001 | 45 | 2.7 | 736.9 | LR fails |
| yes | 362 | 223 | 61.9 | 38.1 | ||||||
| Flexible+Slack used in analyses below | yes | 127 | ||||||||
| Flexible tip + | ||||||||||
| micro-Advance | no | 139 | 8 | 94.6 | 5.4 | <0.0001 | 312.8 | 36.9 | 2648.2 | LR fails |
| yes | 1 | 18 | 5.3 | 94.7 | ||||||
| slack removal | no | 139 | 5 | 96.5 | 3.5 | <0.0001 | 23 | 8.3 | 63.7 | Airway (0.04), conscious (-, 0.04) |
| yes | 35 | 29 | 54.7 | 45.3 | ||||||
| wire stiffener | no | 139 | 5 | 96.5 | 3.5 | <0.0001 | 38.4 | 15 | 98.6 | APACHE 2 score, kg and airway (ns) |
| yes | 71 | 98 | 42 | 58 | ||||||
Cells showing the total number of difficult placements.
Of tubes reaching the upper stomach, advancement was difficult in 295 of 903 (32.7%) of placements; 2.1% failed. Sequential use of flexible tip (10 cm) or, where that failed, air insufflation and when that failed a 20–30 cm flexible tip ± reverse Seldinger manoeuvre were all significantly associated with tube advancement (p < 0.001) independent of BMI (trend) and other baseline parameters. Prokinetic drugs were not used and use of a wire stiffener was of marginal benefit to tube advancement.
Tubes reaching the lower stomach presented difficulty to advancement in 177 of 884 (20%) of placements; 6.2% failed. In univariate analysis, air insufflation, a flexible tip or stiffener wire were all associated with tube advancement. However, using logistic regression, only air insufflation was independent of the negative association with APACHE two score. Logistic regression including a flexible tip or wire stiffener failed due to small samples and zero successes when not using a technique; confounding is therefore possible for these variables. There were too few interventions of laying the patient flat or prokinetic drug use to analyse these techniques of last resort.
Of tubes reaching duodenum part-1, 785 of 827 (94.9%) of placements presented some difficulty to further advancement; 5.8% failed. Independent associations with tube advancement were found for slack removal (p = 0.03) and use of a flexible tip (p = 0.0001), after accounting for tracheostomy use (+: p = 0.07) and trauma (-: p = 0.007). In placements where a flexible tip failed, adding a secondary technique was associated with tube advancement: Micro-advance only reached a trend (p = 0.05) but use of slack removal (p < 0.0001) or a wire stiffener (p = 0.004) were independently associated with tube advancement. When combining a flexible tip and wire stiffener failed, tube advance was independently associated with adding a third technique: Micro-advance (p = 0.05) or slack removal (p < 0.0001). Addition of prokinetic drugs (erythromycin in all but one), after failure of two or three techniques, was independently associated with tube advancement (p < 0.0001). It may be noteworthy that erythromycin was used as a last resort and given as a 20 min IV infusion as advancement was re-attempted 1–2 h later.
There was some difficulty in advancement from duodenum part-2 onwards in 761 of 774 (98.3%); and 2.7% failed to advance from duodenum part-2. Placements involving prokinetic drug use was analysed separately from other techniques because it was started when the tube was in duodenum part-1 in 28 of 32 placements reaching duodenum part-2 or beyond. Univariate analysis showed that slack removal (p = 0.07) or use of a flexible tip (p < 0.0001) were associated with tube advancement (Table 3), but only 15.4% and 38.1% of tubes, respectively, reached the jejunum. Logistic regression failed to compute so confounding may exist. When single techniques failed, using a second technique (micro-advance, slack removal, wire stiffener) alongside a flexible tip was significantly associated with tube advancement (p < 0.0001). Logistic regression failed to compute for micro-advance, so confounding may exist, but confirmed independent associations for slack removal and use of a wire stiffener. When a minimum of two techniques had failed, adding micro-advance or slack removal to use of a flexible tip and a wire stiffener or a prokinetic drug to a flexible tip + 1–3 other techniques, were all independently associated with tube advancement from duodenum part-2 (to part-3, part-4 or jejunum) (p < 0.0001). Finally, in the sub-group of placements where a flexible tip and wire stiffener fail, addition of two more techniques out of micro-advancement, prokinetic drug use or slack removal was independently associated with tube advancement (p < 0.0001).
Table 3.
Association between technique and the distance the tube advances.
| Technique | Used | Intestinal position a | p-value | OR b | 95%CI | Confounders entered into LR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | 2.5 | 97.5 | |||||||||||
| Flexible tip+ | 2 | 3 | 4 | 5 | 2 | 3 | 4 | 5 | ||||||
| wire stiffener+rmicro-advance | No | 8 | 7 | 60 | 0 | 10.7 | 9.3 | 80 | 0 | <0.0001 | 357.7 | 45.5 | 2811 | APACHE 2 score and conscious (ns) |
| Yes | 0 | 0 | 1 | 13 | 0 | 0 | 7.1 | 92.9 | ||||||
| prokinetic drugs+ (micro±slack±wire) | No | 9 | 8 | 114 | 62 | 4.7 | 4.1 | 59.1 | 32.1 | <0.0001 | 13.7 | 4.6 | 41 | general surgery (-, p=0.009) |
| Yes | 0 | 0 | 4 | 26 | 0 | 0 | 13.3 | 86.7 | ||||||
| wire stiffener+slack removal | No | 8 | 7 | 63 | 1 | 10.1 | 8.9 | 79.7 | 1.3 | <0.0001 | 112.1 | 14.1 | 891 | Sex (male) (p=0.1), APACHE 2 score (ns) |
| Yes | 0 | 1 | 15 | 23 | 0 | 2.6 | 38.5 | 59 | ||||||
| wire stiffener+2 of: micro/prokinetic/slack | No | 8 | 8 | 83 | 60 | 5.4 | 5.4 | 56.1 | 33.1 | <0.0001 | 14.9 | 1.8 | 121 | APACHE 2 score (- p=0.02) |
| Yes | 0 | 0 | 1 | 9 | 0 | 0 | 4.8 | 95.2 | ||||||
aIntestinal position: 2–4 = duodenum parts-2, three or four and 5= jejunum.
bOR (95%CI) is based on binary analysis of whether the technique succeeds in jejunal placement.
Discussion
Main findings
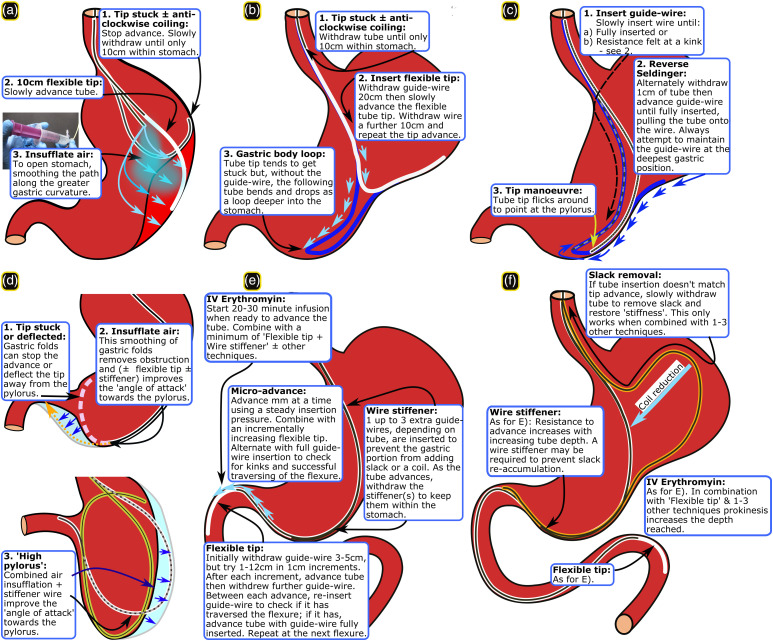
Successful tube advancement is highly associated with use of certain techniques. Baseline parameters were similar between placements analysed and the 3.6% for which data were missing. Techniques that may aid tube advancement were analysed only for placements that were difficult: Nose (3.3%), pharynx (24.5%), stomach_upper (32.7%), stomach_lower (20%), duodenum part-1 (94.9%), intestine (98.3%). There were too few techniques used and placement failures to analyse technique efficacy at the level of the nose. However, advancing from the pharynx to the oesophagus appeared to be aided by use of a head tilt, jaw thrust, laryngoscopy or combinations of these. Specific techniques were associated with tube advancement in the stomach_upper (10 cm flexible tip, air insufflation and 20–30 cm flexible tip ± reverse Seldinger manoeuvre), stomach_lower (air insufflation, possibly a flexible tip and wire stiffener) (Figure 1(a)–(d)) and for duodenum part-1 or beyond duodenum part-2 (flexible tip alone or combined with 1–3 techniques: micro-advance, slack removal, wire stiffener and prokinetic drugs when previous techniques failed) (Figure 1(e) and (f).
Figure 1.
Techniques: (a–c) Upper stomach, (d) Lower stomach, (e) Duodenum part-1, and (f) Intestine. [© Stephen Taylor-with permission].
Confounding variables
Baseline parameters that were associated with technique use (p < 0.2) in one or more analysis were BMI, and presence of an ETT or tracheostomy. Past study has shown that placement can be particularly difficult at GI flexures when a patient’s BMI is low, hence a higher BMI may favour easier placement, 23 possibly because flexures are less acute. In addition, presence of an ETT or tracheostomy may be surrogates of deep sedation which improves patient tolerance during prolonged tube placement. Age, APACHE II score, being conscious and trauma were negatively associated with tube advance. APACHE II score was previously associated with advancement failure24,25 potentially paralleling its association with DGE. 3 In DGE the fundus is typically distended and flaccid causing tube advancement to stall or move anti-clockwise towards the oesophagus. Being conscious reduced patient tolerance while age and trauma may pre-dispose to poor gastric tone and reduced peristalsis.
Technique efficacy by GI level
Stomach_upper
Air insufflation13,26 and use of a 10 cm or 20–30 cm flexible tip with or without a reverse Seldinger manoeuvre, widen the stomach and permit the flexible tube to deflect past any gastric indentation, respectively. This facilitates movement of the tube tip into the lower stomach.
Stomach_lower
Again, air insufflation appears to help tube advancement by opening a collapsed stomach. Numbers were small, but a flexible tip or wire stiffener may aid tube advancement by deflecting past obstruction or changing the ‘angle of attack’ towards the pylorus, respectively. We did not employ the right lateral decubitus position or a cork-screwing (tube rotation) manoeuvre with a bent guide-wire.13-14 This was because a Cortrak receiver unit’s position would be difficult to maintain and the electromagnetic wire easily breaks, respectively. These techniques require testing using different guidance equipment. Too few patients were lay flat or given prokinetic drugs to know their effect.
Duodenum part-1
It appears that use of a flexible tip facilitates tube advance through duodenum part-1 and specifically enabled the tube to slide over the, often acute, superior flexure. When this fails adding one or more of micro-advance, slack removal or wire stiffener appears to aid advance. Micro-advance enables the flexible tip to move around the flexure without kinking and, along with adding one or more wire stiffeners up to the level of the lower stomach, reduces the risk of accumulating a slack loop in the stomach. Removing slack restores the guide-wire rigidity to facilitate forward pressure. Erythromycin infusion started when re-attempting passage of the superior flexure initiates increased peristalsis. 27 Use of 3–4 of the above techniques appear to succeed when single or dual techniques fail. Use of abdominal massage or NG tube removal were too rare to analyse. However, when NG tube insertion was >70 cm, its withdrawal to 50 cm immediately led to NI tube advancement on a few occasions, suggesting that it was blocking duodenum part-1.
Intestine
Successful tube advancement into the jejunum appeared to be aided by the same single, dual and triple techniques as for duodenum part-1 with the exception that slack removal alone only reached a trend. The latter may be due to small numbers. In addition, resistance to advance increases the deeper the tube moves into the intestine. Hence, slack removal alone may not restore enough rigidity to the tube within the stomach to prevent repeated collapse into a coil. Combinations of 3–4 techniques or prokinetic drug use with two or more other techniques was associated with tube advance further into the intestine, regardless of whether the tube reached the jejunum.
Limitations
Tube placement results were from a single hospital, mostly by two operators, with differing experience, over different time periods. It was therefore not possible to exclude the effect of subtle operator-specific differences of technique. However, patient referral criteria and placement equipment were constant, mitigating temporal bias. Most important, except where small sample size or zero values prevented analysis, specific techniques were highly significantly associated with placement success, independent of baseline parameters. These results do not guarantee success or failure of different techniques at specific levels of the alimentary trace, even on the same patient. Rather, the associations are a ‘try list’ guide for operators. There will be exceptions and techniques often require several attempts even after previous failure. Most of this guidance applies to active tube advancement, not to ‘peristaltic’ tube placement where prokinetic use may be essential. 28 The predominant use of in-procedure IV erythromycin but not metoclopramide related to metoclopramide use and tachyphylaxis prior to tube placement; others found similar efficacy for these drugs regarding transpyloric migration. 29 Aside from patient position, all discussed techniques could be used in a prone position with two cautions: (a) Head tilt downwards and jaw thrust are more difficult when aiding tube movement into the oesophagus; (b) If using Cortrak™ electromagnetic guidance (EMG), the anterior and lateral traces must be interpreted as mirror and inverted images, respectively; ENvue® EMG doesn’t require this. Lastly, the techniques were tested using a 10FG, 140 cm Cortrak tube and may require adaptation where tube characteristics differ. For example, traversing flexures may be more difficult with a wider-bore or stiffer IRIS (Kangaroo™) feeding tube but easier with the more pliant ENvue guide-wire. Conversely lack of stiffness at the level of the stomach more often necessitated stiffening with extra guide-wires. Good internal tube lubrication is essential to manoeuvre the guide-wire. Real-time guidance is needed for timely application of these techniques and has also been used with an IRIS direct vision tube 30 but ENvue is not yet available or tested within the UK.
Description of placement techniques, especially manoeuvres, is largely absent from manufacturer guidance. Operators therefore require clinical permissions to use these techniques within their healthcare settings. However, similar techniques are used during endoscopy. Substitution of the manufacturer guide-wire with a specialist guide-wire, often of different stiffness, is common during fluoroscopic feeding tube placement. Specifically, moving a ‘stiffener wire’ within a tube would be similar to re-tracing tube position using a near identical Cortrak guide-wire, something that is part of manufacturer guidance.
Conclusion
This is the first study to specify the anatomical level at which single or combined placement techniques may facilitate NI tube placement. Future investigation may examine the efficacy of patient position, flexible tip and wire stiffener use in lower stomach and abdominal massage close to the pyloric, superior duodenal and DJ flexures.
Impact
(1) Delayed gastric emptying (DGE) is common, can be overcome by NI feeding, but tube placement often fails.
(2) Nurses, dietitians, radiographers and medics require expertise to succeed in NI tube placement.
(3) To our knowledge, this is the first paper to determine the efficacy of NI tube placement techniques for each stage of the placement and explicitly describe them in order to disseminate expertise and encourage wider use.
(4) We identify single or combined techniques that may significantly increase the likelihood of tube advancement at each anatomical level.
Acknowledgements
We thank other members of the NJ team (Rowan Clemente, Danielle Milne, Francis Greer) and ICU nursing and medical staff for advice and support without which this work could not have been done. Thank you to Danielle Milne and Katie Williams for critiquing the MS.
Appendix 1. Demography, disease, treatment characteristics and NI day: Technique data missing vs obtained.
| Parameter | Detail | Technique data | p-value | |||
|---|---|---|---|---|---|---|
| Missing | Obtained | |||||
| Median or n | *IQR or % | Median or n | *IQR or % | |||
| Number | (n) | 34 | 3.6 | 913 | 96.4 | - |
| Age | Years | 56 | 40–68 | 53 | 37–68 | 0.8 |
| Sex | Male | 24 | 75 | 655 | 72 | 0.5 |
| BMI | kg/m2 | 25 | 23–28 | 25 | 23–29 | 0.9 |
| Height | Cm | 174 | 164–180 | 175 | 167–180 | 0.2 |
| Weight | Kg | 76 | 63–84 | 78 | 68–89 | 0.3 |
| APACHE 2 | Score | 17 | 9–23 | 15 | 9–21 | 0.5 |
| Disease* | Medical | 10 | 29 | 259 | 28 | 0.3 |
| Neurosurgical (non-trauma) | 1 | 2.9 | 121 | 13 | — | |
| Surgery (general) | 9 | 27 | 223 | 24 | — | |
| Trauma | 14 | 41 | 310 | 34 | — | |
| Conscious | — | 2 | 6.1 | 138 | 15 | 0.2 |
| Airway | Normal | 4 | 12 | 129 | 14 | 0.9 |
| Endotracheal | 24 | 71 | 639 | 70 | — | |
| Tracheostomy | 6 | 18 | 145 | 16 | — | |
| NI day | — | 5 | 4–7.3 | 5 | 3–8 | 0.5 |
Footnotes
Author contributions: S.J. Taylor equally contributed to the conception and design of the research; S.J. Taylor and K. Sayer contributed to the acquisition of the data; P. White and S.J. Taylor contributed to the analysis and interpretation of the data; S.J. Taylor drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final manuscript.
ST served on a Corpak consultation committee once in 2007 and directed a lecture fee to the Tear Fund Syrian charity 2014.2. ST and KS undertook studies sponsored by Cortrak (now Avanos Medical Inc, 2012–14) and Cardinal Health (2020- current) through North Bristol NHS Trust, but these companies had no part in the planning, execution or publication of the projects.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
IMPORTANT: The UK Health Research Authority recognise this study as an audit not requiring Ethics Board approval. Their email is below and they are willing to be contacted. However, if ICCN still require IRB, please decline the submission and inform me. Because of COVID it would take many months to obtain an IRB approval.
ORCID iDs
Stephen J Taylor https://orcid.org/0000-0003-3970-1819
Paul White https://orcid.org/0000-0002-7503-9896
References
- 1.Gungabissoon U, Hacquoil K, Bains C, et al. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr 2015; 39: 441–448. [DOI] [PubMed] [Google Scholar]
- 2.Mentec H, Dupont H, Bocchetti M, et al. Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit Care Med 2001; 29: 1955–1961. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NQ, Ng MP, Chapman M, et al. The impact of admission diagnosis on gastric emptying in critically ill patients. Crit Care 2007; 11: R16. DOI: 10.1186/cc5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CCN (Critical Care Nutrition) . 1.0 The Use of Enteral Nutrition vs. Parenteral Nutrition & 2.0 Early vs. Delayed Nutrient Intake. Atlanta, GA: CCN, 2021, https://www.criticalcarenutrition.com/systematic-reviews. [Google Scholar]
- 5.Lewis K, Alqahtani Z, Mcintyre L, et al. The efficacy and safety of prokinetic agents in critically ill patients receiving enteral nutrition: a systematic review and meta-analysis of randomized trials. Crit Care 2016; 20: 259. DOI: 10.1186/s13054-016-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen NQ, Chapman MJ, Fraser RJ, et al. Erythromycin is more effective than metoclopramide in the treatment of feed intolerance in critical illness. Crit Care Med 2007; 35: 483–489. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SJ, Allan K, McWilliam H, et al. A randomised controlled feasibility and proof-of-concept trial in delayed gastric emptying when metoclopramide fails: We should revisit <nasointestinal> feeding versus dual prokinetic treatment: Achieving goal nutrition in critical illness and delayed gastric emptying: Trial of feeding versus nasogastric feeding plus prokinetics. Clin Nutr ESPEN 2016; 14: 1–8. DOI: 10.1016/j.clnesp.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Metheny NA, Stewart BJ, McClave SA. Relationship between feeding tube site and respiratory outcomes. JPEN J Parenter Enteral Nutr 2011; 35: 346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CW, Sun SF, Lin SL, et al. Duodenal versus gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med 2009; 37: 1866–1872. [DOI] [PubMed] [Google Scholar]
- 10.Sajid MS, Harper A, Hussain Q, et al. An integrated systematic review and meta-analysis of published randomized controlled trials evaluating nasogastric against postpyloris (nasoduodenal and nasojejunal) feeding in critically ill patients admitted in intensive care unit. Eur J Clin Nutr 2014; 68: 424–432. [DOI] [PubMed] [Google Scholar]
- 11.Wan B, Fu H, Yin J. Early jejunal feeding by bedside placement of a <nasointestinal> tube significantly improves nutritional status and reduces complications in critically ill patients versus enteral nutrition by a nasogastric tube. Asia Pac J Clin Nutr 2015; 24: 51–57. [DOI] [PubMed] [Google Scholar]
- 12.Schulz MA, Santanello SA, Monk J, et al. An improved method for transpyloric placement of nasoenteric feeding tubes. Int Surg 1993; 78: 79–82. [PubMed] [Google Scholar]
- 13.Ugo PJ, Mohler PA, Wilson GL. Bedside postpyloric placement of weighted feeding tubes. Nutr Clin Pract 1992; 7: 284–287. [DOI] [PubMed] [Google Scholar]
- 14.Zaloga GP. Bedside method for placing small bowel feeding tubes in critically ill patients. A prospective study. Chest 1991; 100: 1643–1646. [DOI] [PubMed] [Google Scholar]
- 15.Hawk H, Valdivia H. Bedside methods for transpyloric feeding tube insertion in hospitalized children: a systematic review of randomized and non-randomized trials. J Pediatr Nurs 2021; 60: 238–246. [DOI] [PubMed] [Google Scholar]
- 16.Brown AM, Perebzak C, Handwork C, et al. Use of electromagnetic device to insert postpyloric feeding tubes in a pediatric intensive care unit. Am J Crit Care 2017; 26: 248–254. [DOI] [PubMed] [Google Scholar]
- 17.Goggans M, Pickard S, West AN, et al. Transpyloric feeding tube placement using electromagnetic placement device in children. Nutr Clin Pract 2017; 32: 233–237. DOI: 10.1177/0884533616682683. [DOI] [PubMed] [Google Scholar]
- 18.October TW, Hardart GE. Successful placement of postpyloric enteral tubes using electromagnetic guidance in critically ill children. Pediatr Crit Care Med 2009; 10: 196–200. [DOI] [PubMed] [Google Scholar]
- 19.Avanos Medical Inc . Trainee Booklet: Selection, Insertion and Ongoing Safe Use of Nasogastric (NG) Tubes in Adults with the Cortrak 2 Enteral Access System (EAS). Alpharetta, GA: Avanos Medical Inc, 2019. [Google Scholar]
- 20.Taylor SJ, Clemente R, Allan K, et al. Cortrak tube placement part 1: confirming by quadrant may be unsafe. Br J Nurs 2017; 26: 751–755. [DOI] [PubMed] [Google Scholar]
- 21.Taylor SJ, Clemente R, Allan K, et al. Cortrak tube placement part 2: guidance to avoid misplacement is inadequate. Br J Nurs 2017; 26: 876–881. [DOI] [PubMed] [Google Scholar]
- 22.Taylor S, Manara A, Brown J, et al. Cortrak feeding tube placement: accuracy of the 'GI flexure system' versus manufacturer guidance. Br J Nurs 2020; 29(29): 1277–1281. DOI: 10.12968/bjon.2020.29.21.1277. PMID: 33242271. [DOI] [PubMed] [Google Scholar]
- 23.Holzinger U, Brunner R, Miehsler W, et al. Jejunal tube placement in critically ill patients: A prospective, randomized trial comparing the endoscopic technique with the electromagnetically visualized method. Crit Care Med 2011; 39: 73–77. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Sun C, Wei R, et al. Establishing decision trees for predicting successful postpyloric nasoenteric tube placement in critically ill patients. JPEN J Parenter Enteral Nutr 2018; 42: 132–138. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Xuan Y, Liu C, et al. Blind placement of postpyloric feeding tubes at the bedside in intensive care. Crit Care 2021; 25: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deane AM, Fraser RJ, Young RJ, et al. Evaluation of a bedside technique for postpyloric placement of feeding catheters. Crit Care Resusc 2009; 11: 180–183. [PubMed] [Google Scholar]
- 27.Shaikh N, Nainthramveetil MM, Nawaz S, et al. Optimal dose and duration of enteral erythromycin as a prokinetic: a surgical intensive care experience. Qatar Med J 2020; 2020: 36, DOI: 10.5339/qmj.2020.36 10.5339/qmj.2020.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puiggròs C, Molinos R, Ortiz MD, et al. Experience in bedside placement, clinical validity, and cost-efficacy of a self-propelled nasojejunal feeding tube. Nutr Clin Pract 2015; 30: 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu B, Ouyang X, Lei L, et al. Erythromycin versus metoclopramide for post-pyloric spiral nasoenteric tube placement: a randomized non-inferiority trial. Intensive Care Med 2018; 44: 2174–2182, DOI: 10.1007/s00134-018-5466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor S, Sayer K, Milne D, et al. Integrated real-time imaging system, 'IRIS', Kangaroo feeding tube: a guide to placement and image interpretation. BMJ Open Gastroenterol 2021; 8: e000768. DOI: 10.1136/bmjgast-2021-000768. [DOI] [PMC free article] [PubMed] [Google Scholar]