Abstract
Introduction
Multiple myeloma (MM) is a malignancy of plasma cells with characteristic bone disease. Despite recent great strides achieved in MM treatment owing to the implementation of new anti-MM agents, MM is still incurable and bone destruction remains a serious unmet issue in patients with MM.
Approach
In this review, we will summarize and discuss the mechanisms of the formation of bone disease in MM and the available preclinical and clinical evidence on the treatment for MM bone disease.
Conclusions
MM cells produce a variety of cytokines to stimulate receptor activator of nuclear factor-κB ligand-mediated osteoclastogenesis and suppress osteoblastic differentiation from bone marrow stromal cells, leading to extensive bone destruction with rapid loss of bone. MM cells alter the microenvironment through bone destruction where they colonize, which in turn favors tumor growth and survival, thereby forming a vicious cycle between tumor progression and bone destruction. Denosumab or zoledronic acid is currently recommended to be administered at the start of treatment in newly diagnosed patients with MM with bone disease. Proteasome inhibitors and the anti-CD38 monoclonal antibody daratumumab have been demonstrated to exert bone-modifying activity in responders. Besides their anti-tumor activity, the effects of new anti-MM agents on bone metabolism should be more precisely analyzed in patients with MM. Because prognosis in patients with MM has been significantly improved owing to the implementation of new agents, the therapeutic impact of bone-modifying agents should be re-estimated in the era of these new agents.
Keywords: Myeloma bone disease, Receptor activator of nuclear factor-κB ligand, Bone-modifying agents, Proteasome inhibitors, Daratumumab
Introduction
Multiple myeloma (MM) is a malignancy of plasma cells. It has a unique propensity to almost exclusively develop in the bone marrow and generates devastating bone destruction with enhanced osteoclastic bone resorption and concomitant suppression of bone formation. MM arises from its precancerous stage, monoclonal gammopathy of unknown significance (MGUS). In parallel with the progression of MGUS into MM, microenvironmental changes occur in the bone marrow, including increased osteoclastogenesis and angiogenesis and impaired immune function. This pathologically skewed bone marrow microenvironment in turn stimulates MM cell growth and survival to cause drug resistance.
Bone disease is a characteristic feature of MM. Various novel anti-MM agents have been developed, and recent combination treatments among them are able to exert prompt and deeper response in a greater portion of patients with relapsed/refractory MM as well as newly diagnosed MM. However, MM is still incurable and eventually relapses; and bone destruction after repeated relapses remains a serious unmet issue in patients with MM. This review summarizes the mechanisms of the formation of bone disease in MM and the available preclinical and clinical evidence on the treatment for MM bone disease.
Bone metabolism in MM
Imbalance between osteoclastogenesis and osteoblastogenesis in MM
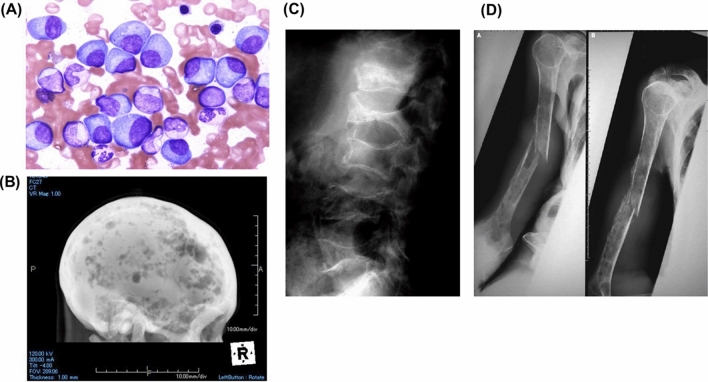
Image-documented bone lesions are observed in 80–90% of patients with MM during the course of their disease progression; 40% are reported to experience pathological fractures within the first year after diagnosis [1]. Typical images of bone disease in patients with MM are shown in Fig. 1. The pain and immobility caused by bone fractures can significantly reduce the quality of life (QoL) of patients with MM and may negatively affect treatment outcomes and thereby their life expectancy. Therefore, early detection of bone lesion(s) and therapeutic intervention are important in the treatment of MM. Bone metabolic markers can indicate ongoing bone metabolism and are widely used for the diagnosis and monitoring in osteoporosis and other disorders of bone metabolism. Levels of the bone resorption marker urinary deoxypyridinoline are increased in the majority of patients with MM, while levels of the bone formation marker serum osteocalcin are relatively decreased [2, 3], suggesting an imbalance of bone turnover with enhanced osteoclastic bone resorption and concomitantly suppressed bone formation.
Fig. 1.
Images of bone disease in patients with MM. A Myeloma tumor cells accumulate in the bone marrow. B Multiple compression fractures in lumbar vertebrae. C Multiple radiolucent lesions without ossification, known as “punched-out lesions” in a skull X-p, implying enhanced bone resorption along with impaired calcification. D Bone fractures in long bone occur in an advanced case
For osteoclastogenesis, the interaction between the receptor activator of nuclear factor-κB (RANK) and its ligand (RANKL) has been demonstrated to play a vital role. The expression of RANKL is induced in bone marrow stromal cells (BMSCs)/osteoblasts by various cytokines and physiologically active substances. Binding of RANK to RANKL stimulates osteoclastic differentiation and activation. Osteoprotegerin (OPG), a decoy receptor for RANKL, inhibits the binding of RANK to RANKL. OPG is produced from various types of cells, including T cells, megakaryocytes, and BMSCs/osteoblasts. These factors are also produced by osteocytes embedded in the bone matrix. Thus, the balance of RANKL expression and OPG production in the bone marrow determines the levels of osteoclastogenesis. In bone specimens from normal subjects, RANKL expression is low, but OPG expression is relatively high [4]. However, in those patients with MM, RANKL expression is increased in BMSCs, whereas OPG production is suppressed, indicating the predominance of RANKL activity in the MM bone marrow microenvironment.
Pathogenic factors and clinically relevant biomarkers for MM bone disease
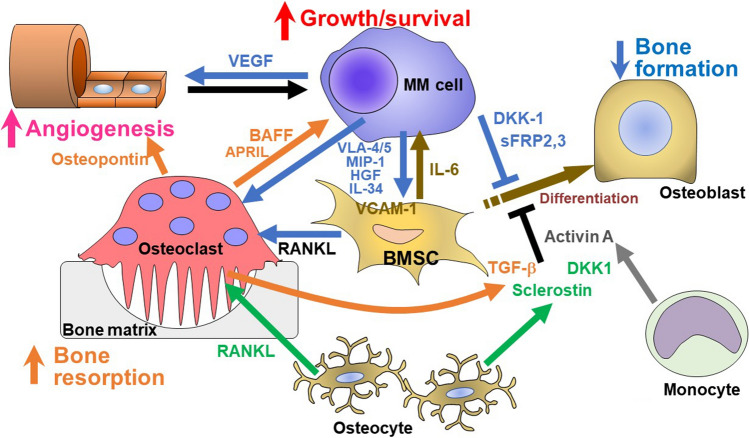
Cytokines aberrantly over-produced by MM cells, including macrophage inflammatory protein (MIP)-1α and interleukin (IL)-34 as well as MM cell adhesion up-regulate RANKL in BMSCs, which play a major role in the enhancement of osteoclastogenesis and bone resorption in MM [5–7]. In addition, factors over-produced by MM cells and/or their surrounding microenvironment in bone such as soluble Wnt inhibitors, IL-3, IL-7, tumor necrosis factor alfa (TNF-α), activin A, and transforming growth factor beta (TGF-β) have been demonstrated to suppress osteoblastic differentiation [8–13]. Therefore, multiple factors act together to eventually develop extensive bone destruction in MM (Fig. 2). These factors can be utilized as clinical biomarkers to detect bone disease and evaluate its severity. Some of them have been reported as follows:
Fig. 2.
Bone destruction by factors over-produced by MM cells and/or their surrounding microenvironment in bone in MM. MM cells enhance osteoclastogenesis and suppress osteoblastic differentiation from bone marrow stromal cells (BMSCs), leading to skewing of the cellular microenvironment in the bone marrow. Cytokines aberrantly over-produced by MM cells, including MIP-1, HGF and IL-34 as well as MM cell adhesion (VLA4/5-VCAM-1) up-regulate RANKL and IL-6 production in BMSCs to enhance osteoclastogenesis and MM cell growth/survival. Osteoclasts enhance MM cell growth/survival. MM cells enhance angiogenesis in concert with osteoclasts. In addition, factors over-produced by MM cells and/or their surrounding microenvironment in bone such as soluble Wnt inhibitors, IL-3, IL-7, TNF-α, activin A and TGF-β suppress osteoblastic differentiation. RANKL and sclerostin are over-produced by osteocytes. Therefore, multiple factors act together to eventually develop extensive bone destruction along with MM tumor expansion
Soluble RANKL
Serum levels of soluble RANKL (sRANKL) and OPG and sRANKL/OPG ratios have been reported to be indicators of osteoclastic activity in patients with MM [14]. The serum sRANKL/OPG ratios increase and correlate with the clinical severity of bone destruction in patients with MM. They were also reported to have a negative impact on overall survival in patients with MM [14]. However, the impact of such factors on prognosis should be re-estimated, because prognosis in patients with MM has been significantly improved by new anti-MM agents.
Macrophage inflammatory protein-1
MM cells from patients with multiple bone lesions secreted significantly higher amounts of MIP-1α and MIP-1β than those from patients with less advanced bone disease [7], suggesting the correlation between MM cell ability to produce MIP-1 and clinical severity of the bone disease. MIP-1α and MIP-1β as well as cocultures of MM cells enhanced in vitro osteoclast formation and activation from bone marrow cells. MIP-1α and MIP-1β induced RANKL expression in BMSCs in the presence of a physiologically low concentration of 1,25-dihydroxyvitamin D3. Addition of a surplus of OPG was able to inhibit RANKL activity and the effects of MIP-1α and MIP-1β and by MM cells, indicating a critical role of RANKL in osteoclast differentiation and activation in MM. Serum levels of MIP-1α positively correlated with the values of bone resorption markers in patients with MM with bone lesions [15].
Hepatocyte growth factor
Serum levels of hepatocyte growth factor (HGF) are elevated in patients with MM with osteolytic lesions compared with patients with MGUS or normal subjects, and HGF levels correlated with the extent of osteolytic lesions [16, 17]. HGF is produced by MM cells and the bone marrow microenvironment and exerts diverse actions, including MM cell survival, homing, bone remodeling, and angiogenesis [18]. HGF induces RANKL expression in BMSCs/osteoblasts to promote osteoclastogenesis and suppresses their Runx2 and Osterix expression to impair osteoblastic differentiation [19].
Soluble Wnt inhibitors
Wnt/β-catenin signaling is essential for osteoblast differentiation, which is negatively regulated by multiple soluble inhibitors. A series of secreted Frizzled-related proteins (sFRPs) and dickkopf (DKK) family members as well as sclerostin have been identified as soluble inhibitors for Wnt/β-catenin signaling [9, 20, 21]. DKK1 is produced by osteoblasts and osteocytes along with MM cells [21, 22]. Serum DKK1 levels are increased in patients with MM with bone disease and decreased as MM responds to anti-MM treatment [22, 23]. Sclerostin is a glycoprotein with a cystine knot-like domain that is almost exclusively expressed and produced by osteocytes [24]. Serum sclerostin levels are increased in patients with MM with bone lesions and correlate with clinical stages and the severity of bone destruction [13]. Sclerostin expression was found in BMSCs/osteoblasts in addition to osteocytes in biopsy specimens from bone lesions in patients with MM [25]. The underlying mechanisms for the over-production of sclerostin in MM bone lesions remain to be clarified.
Other soluble factors
TNF-α, IL-7, and IL-3 have also been reported as inhibitory factors of osteoblast differentiation derived from MM cells [26–28]. TGF-β specifically inhibits the terminal differentiation (calcification) of osteoblasts [10]. TGF-β is stored as a latent form in the bone matrix and released from the bone by osteoclastic bone resorption and becomes an active form by acids and enzymes produced by osteoclasts. In osteoclastic bone lesions, activated TGF-β is abundantly released from bone tissues to suppress calcification. Activin A, another TGF-β family cytokine, is also overproduced from the bone marrow microenvironment in MM to suppress osteoblastogenesis [12, 29, 30]. In addition, other factors, including LIGHT [31], semaphorin 4D [32], and IL-34 [33] have been reported to be associated with the progression of MM bone lesions. These factors may become potential biomarkers for bone disease in MM.
MicroRNAs
MicroRNAs are short RNAs with about 21 bases that are produced when long RNAs transcribed from DNA are processed by DROSHA and DICER. MM cells and cells surrounding them in the bone marrow secrete exosomes that contain a wide variety of microRNAs [34]. As a potential biomarker for bone lesions, Hao et al. reported that serum miR-124 levels were higher in patients with MM with bone lesions and correlated with the extent of bone lesions [35]. Targets of miR-124 include the PTEN gene, which is involved in skeletal and muscle metabolic regulation via PI3K/AKT [36]. miR-135b has been implicated to inhibit the bone morphogenetic protein (BMP)-Smad pathway, and its increased production correlates with the severity of bone lesions [37]. miR-21-5p increases the RANKL/OPG ratio in BMSCs in patients with MM [38], whereas miR-342-3p, miR-363-5p and miR-203a-3p suppress osteoblastogeneis through targeting the Runx2, BMP-Smad and canonical Wnt-β-catenin pathways [39, 40]. A number of microRNAs have been demonstrated to be associated with MM pathogenesis.
Mutual interaction between MM cells and bone microenvironment
MM niche
MM cells enhance osteoclastogenesis and suppress osteoblastic differentiation from BMSCs, leading to skewing of the cellular microenvironment in the bone marrow (Fig. 2). Angiogenesis is also enhanced through these cellular interactions. These cells surrounding MM cells create a cellular microenvironment suitable for MM cell growth and survival to confer drug resistance, which can be called an “MM niche”.
Among cell components in the bone marrow in MM, the roles of BMSCs in MM cell growth and survival have been well studied. The interaction between MM cells and BMSCs confers MM cell homing, growth, survival, and resistance to chemotherapy [41]. MM cells stimulate BMSCs to produce various growth and anti-apoptotic factors for MM cells, including IL-6, IGF-1, SDF-1α, IL-21, B-cell-activating factor (BAFF), and vascular endothelial growth factor (VEGF) while inducing RANKL to enhance osteoclastogenesis. Notably, the adhesion of MM cells to BMSCs via VLA-4 and/or VLA-5 confers cell adhesion-mediated drug resistance (CAM-DR) in MM cells [42]. Autocrine activation of VLA-4 on MM cells by MM cell-derived MIP-1 and the up-regulation of MIP-1 production by MM cells through the VLA-4-VCAM-1 interaction appear to form a positive feedback loop between the adhesion of MM cells to BMSCs and MIP-1 production by MM cells [43]. In addition to osteoclastogenesis, MIP-1 has been suggested to promote MM cell homing to or colonization in the bone marrow, which further enhances CAM-DR in MM cells.
In normal trabecular bones, bone remodeling with bone resorption by osteoclasts followed by bone formation by osteoblasts occurs in the bone remodeling compartments covered by canopy cells [44]. However, canopy cells disappear, and bone remodeling compartments are disrupted in MM [45], and MM cells go into the bone remodeling compartments and directly interact with osteoclasts and osteoblastic lineage cells. When MM cells are isolated from patients with MM and cultured alone, MM cells soon die [46]. However, MM cells are alive and proliferating in the presence of osteoclasts, indicating that osteoclasts are not mere bone-resorbing cells but they support MM cell growth and survival. MM cells enhance osteoclastogenesis, and osteoclasts produce multiple growth and survival factors for MM cells, including TNF family cytokines, BAFF and APRIL [47, 48], thereby forming a vicious cycle between osteoclastic bone destruction and MM tumor progression. Lawson MA, et al. demonstrated that RANKL-driven osteoclastogenesis stimulates MM cell proliferation and reduces the percentage of a dormant MM cell fraction in mouse MM models [49], suggesting that osteoclasts make dormant myeloma cells divide and proliferate.
The TGF-β–activated kinase 1-PIM2 pathway
To effectively kill MM cells residing within the MM niche and improve the efficacy of treatment against MM, we looked for novel molecules to be targeted through comprehensive analysis using a DNA microarray. We found that the serine/threonine kinase PIM2 is constitutively over-expressed, and further up-regulated in MM cells in cocultures with BMSCs as well as osteoclasts [50–52]. Hematological cancers highly express PIM2; and MM expresses PIM2 at the highest level among hematological malignancies [53]. PIM2 expression is increased in plasma cells in MGUS and much more in MM cells [54]. IL-6 and the TNF family cytokines BAFF and APRIL were found to play a predominant role in the PIM2 up-regulation in MM cells by interaction with BMSCs and osteoclasts. A variety of factors responsible for growth and survival signaling pathways in cancer cells are substrates of PIM kinases [55]; these PIM substrates regulate cellular processes critical for tumor progression and therapeutic resistance, making PIM a promising target for cancer therapy. Importantly, we reported that the PIM inhibitor SMI-16a dose-dependently suppresses the viability of MM cells. Treatment with the PIM inhibitor markedly suppressed the phosphorylation of 4E-BP1 to inhibit translation, and reduces Mcl-1 and c-Myc levels in MM cells [50]. Therefore, PIM2 acts as an important pro-survival mediator in MM cells in the bone marrow microenvironment, and is suggested to be an important therapeutic target in MM. Notably, PIM2 is also upregulated in BMSCs by MM cells as well as factors over-produced in MM bone lesions known to suppress osteoblastogenesis, suggesting PIM2 as a common downstream mediator of these inhibitory factors [51]. Enforced expression of PIM2 suppresses mineralized nodule formation or osteoblastogenesis by BMP-2, demonstrating PIM2 in BMSCs as a negative regulator for bone formation. RANKL enhances PIM2 expression in osteoclastic lineage cells during their osteoclastogenesis along with the induction of osteoclastic differentiation markers, c-fos, NFATc1, and cathepsin K [52]. The PIM inhibitor SMI-16a suppresses TRAP-positive osteoclast formation by RANKL.
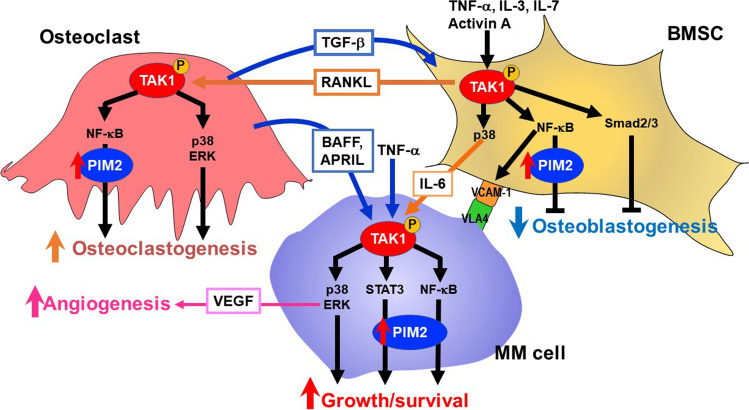
We further looked into the molecular mechanism to upregulate PIM2 expression in MM cells and found the critical role of TGF-β–activated kinase 1 (TAK1) [56]. TAK1 inhibition reduced PIM2 expression along with suppression of the phosphorylation of 4E-BP1, a substrate of PIM2, in MM cells. TAK1 is constitutively overexpressed and phosphorylated in MM cell lines and primary MM cells from patients; and TAK1 inhibition suppresses the viability of MM cells. Cell–cell contact between MM cells and BMSCs via the VLA-4-VCAM-1 interaction is important for MM cell growth and drug resistance and osteoclastogenesis. VCAM-1 expression is upregulated in BMSCs when co-cultured with MM cells or cultured in the presence of TNF-α. However, TAK1 inhibition abrogates the upregulation of VCAM-1 expression, thereby suppressing MM cell adhesion to BMSCs and MM cell growth enhancement. Cocultures with MM cells enhance RANKL and IL-6 expression in BMSCs, which is also inhibited by TAK1 inhibition. Besides, TAK1 inhibition markedly suppressed the secretion of VEGF from MM cells. MM cell conditioned media as well as inhibitory factors for osteoblastogenesis overproduced in MM induce phosphorylation of TAK1 in BMSCs and suppress their osteoblastogenesis. In addition, RANKL-induced phosphorylation of TAK1 in preosteoclastic cells in parallel with degradation of IκB and nuclear localization of the NF-κB subunit p65 and phosphorylation of p38MAPK and ERK. Therefore, MM cells interact with BMSCs and osteoclasts in bone lesions to activate TAK1-mediated signaling in these cells to enhance MM tumor progression and osteoclastogenesis whereas suppressing osteoblastogenesis (Fig. 3). However, TAK1 inhibition directly and/or indirectly suppresses MM growth and resumes bone formation while suppressing osteoclastogenesis. Therefore, TAK1 inhibition may become a promising therapeutic option, targeting the interaction between MM cells and their surrounding micro-environment in MM bone lesions.
Fig. 3.
The TAK1-PIM2 pathway in the mutual interaction between MM cells and the bone microenvironment. PIM2 is a novel pro-survival mediator for MM cells. Interaction with the MM bone marrow microenvironment potentiates PIM2 expression in MM cells through activation of the JAK2/STAT3 pathway by IL-6 and the NF-κB pathway by TNF family cytokines, TNF-α, BAFF, and APRIL, to promote MM cell growth and survival. At the same time, PIM2 is induced in osteoclasts and bone marrow stromal cells (BMSCs) though the interaction with MM cells to cause bone destruction. TAK1 is overexpressed and activated upstream of PIM2 in MM cells, BMSCs and osteoclasts through mutual interaction between these cells in the bone marrow. Besides PIM2 upregulation, TAK1 mediates a wide range of intracellular signaling pathways, including VEGF production via ERK in MM cells and the expression of VCAM-1 and RANKL in BMSCs. Therefore, TAK1 activation is vital for MM cell growth and survival and bone destruction
Osteocytes
Osteocytes are the most abundant cells in bone. Osteocytes are derived from osteoblasts and embedded in bone. Most osteoblasts undergo apoptosis after forming bone, but a portion of osteoblasts differentiate into osteocytes in the bone matrix [57, 58]. Osteocytes reside in the lacunae and connect with each other via their dendrites and sense and transmit mechanical signals via the lacuno-canalicular networks [59, 60]. In addition to being a sensor of mechanical stress, osteocytes act as a master regulatory cell of bone remodeling [61].
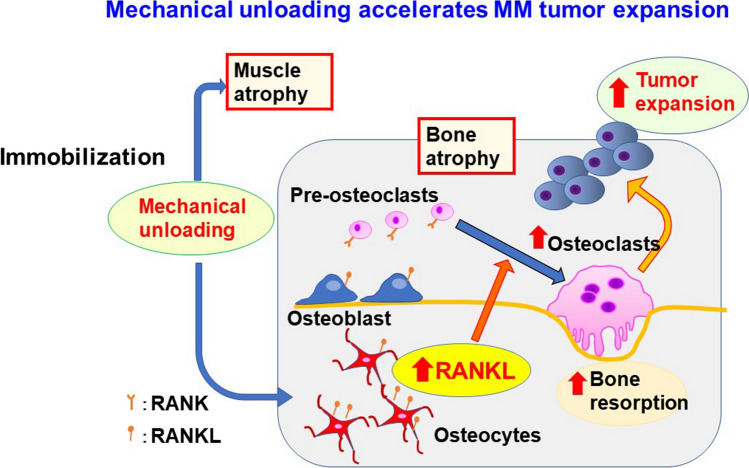
Under mechanical loading, the production of Wnt inhibitors sclerostin and DKK1 by osteocytes is decreased to increase bone formation [62, 63]. In contrast, mechanical unloading induces RANKL expression by osteocytes to enhance osteoclast formation and activity and thereby decreasing bone mass. Rummler et al. reported an interesting experiment with mechanical loading in MM animal models [64]. The knee and ankle were fixated and mechanical loading with repeated forced compression was applied to tibiae into which MM cells were inoculated. Bone resorption area was increased in MM cell-inoculated tibiae more than in non-loaded control mice. However, mechanical loading suppressed bone resorption and instead increased bone formation. Notably, MM tumor growth was suppressed in mice that underwent mechanical loading compared with control mice. We conducted an experiment with mechanical unloading in MM-bearing mice [65]. Right hind legs of mice were immobilized and exposed to mechanical unloading by sciatic denervation or casting with an adhesive bandage. RANKL expression was upregulated in osteocytes that experienced immobilization or mechanical unloading. Mechanical unloading reduced trabecular bone volume, and bone morphometric analyses indicated an increase in osteoclast number and activity. To investigate the effects of mechanical unloading on tumor growth, after sciatic denervation in the right hind legs, we inoculated the same MM cell line with different fluorescein colors, namely green fluorescent protein (GFP) or red fluorescent protein, simultaneously into right and left tibiae, respectively. More MM tumor growth was recorded in the immobilized legs than the intact ones. In addition, extraosseous tumors developed and tumorous lesions outside of the bone were composed of MM cells expressing GFP. In addition, GFP-expressing MM cells were predominantly observed in peripheral blood, indicating that mechanical unloading accelerated MM tumor expansion in bone and egress of cancer cells into the circulation and dissemination outside of the bone (Fig. 4).
Fig. 4.
Mechanical unloading accelerates MM tumor expansion. The mechanical unloading not only induce muscle atrophy but also bone loss with up-regulation of RANKL expression in osteocytes and thereby osteoclastogenesis in the bone marrow. The increased and activated osteoclasts then enhance MM growth and dissemination in and outside of the bone. These results suggest the importance of mechanical loading in maintaining bone mass and suppression of tumor expansion in MM, and also the importance of inhibition of RANKL activity for immobilized patients in a bed-ridden state or paralysis
Patients with MM often suffer from bone pain and fractures, leading to immobilization or a bed-redden state with mechanical unloading. Mechanical unloading not only induces muscle atrophy but also bone loss with up-regulation of RANKL expression in osteocytes and thereby osteoclastogenesis in the bone marrow (Fig. 4). The increased and activated osteoclasts then enhance MM growth and dissemination in and outside of the bone. These results suggested the importance of mechanical loading for maintaining bone mass and suppression of tumor expansion in MM.
Paradigm shift in MM treatment
Although MM is a heterogeneous disease in terms of MM cell- and patient-related risk factors, major improvements in clinical outcomes of patients with MM in terms of overall survival (OS) has occurred since 2000 owing to the implementation of new agents. Additionally, high-quality responses with minimal residual disease (MRD) negativity can be used as a surrogate of OS and should be achieved [66]. Proteasome inhibitors (PIs) and immunomodulatory drugs are currently the mainstay of MM treatment. However, most patients eventually relapse with drug resistance. To overcome this issue, new combination regimens with therapeutic antibodies have been explored. Adding anti-CD38 monoclonal antibodies, daratumumab or isatuximab, as well as the anti-SLAMF7 antibody elotuzumab offer better results for patients with MM. Furthermore, immune-based therapies, including antibody–drug conjugates, autologous chimeric antigen receptor (CAR) T-cell-based therapies, and bispecific antibodies, have shown promising activity for relapsed disease even with high-risk cytogenetic abnormality.
Elderly patients with poor performance status are often excluded from clinical studies. Establishment of effective treatment for elderly frail patients with MM, for example, those over 90 years old, remains an important issue in the era of longevity.
Treatment for MM bone disease with bone-modifying agents
Bone destruction and renal impairment are common clinical consequences in patients with MM. The MRC Myeloma IX trial, an important study comparing the effect of zoledronic acid intravenous injection every 3–4 weeks and oral daily clodronate from the start of MM treatment for newly diagnosed patients with MM irrespective of image-documented bone lesion(s) [67]. Zoledronic acid effectively suppressed the occurrence of skeletal-related events (SREs) and extended OS by 5.5 months compared with oral clodronate, although the survival benefit was preferentially observed in patients with bone disease at presentation. As such, some guidelines recommend initiating bone therapy with zoledronic acid concurrently with anti-MM therapy in all patients with symptomatic MM (Table 5).
Table 5.
Guidelines for the treatment and prevention of MM bone disease
| IMWG2021 | ESMO2020 | NCCN2023 ver.1 | ASCO2018 | |
|---|---|---|---|---|
| Patient selection | BTAs should be administered to all patients with active MM, regardless of the presence or absence of MM-related bone disease on imaging studies | BTAs should be initiated at MM diagnosis | BTAs should be given in all patients receiving primary MM therapy | BTAs are recommended for patients with active symptomatic MM that requires systemic chemotherapy |
| Choice of BTAs |
Bone disease is present: 1st option, D-mab or ZOL; 2nd option, pamidronate Bone disease is absent: 1st option, ZOL; 2nd option, pamidronate |
1st option, D-mab or ZOL; 2nd option, pamidronate | D-mab or ZOL or pamidronate | ZOL or pamidronate; D-mab as an alternative option to ZOL |
| Dose, and treatment schedule |
ZOL 4 mg iv every 3–4 weeks Pamidronate 30 mg (45 min) or 90 mg (120 min) administered every 3–4 weeks |
D-mab every 4 weeks ZOL every 4 weeks for 3–6 months, then every 12 weeks |
D-mab 120 mg SC every 4 weeks ZOL 4 mg iv every 3–4 weeks Pamidronate 90 mg iv every 3–4 weeks |
ZOL 4 mg iv every 3–4 weeks Pamidronate 90 mg iv every 3–4 weeks D-mab 120 mg sc every 4 weeks |
| Duration | ZOL should be administered monthly for at least 12 months. If patients achieve VGPR or better, physicians can consider decreasing the dosing frequency to every 3–6 months, or ZOL discontinuation | If patients achieve VGPR or better, physicians consider interrupting bone modifying agents after 24 months |
Up to 2 years The frequency of dosing (monthly vs. every 3 months) would depend on the individual patient criteria and response to therapy. Continue beyond 2 years based on clinical judgment |
Up to 2 years. Less-frequent dosing should be considered in patients with responsive or stable disease |
| Notes | D-mab might be preferred in patients with renal impairment (Ccr < 30 mL/min) | D-mab is the agent of choice in MM patients with renal impairment (Ccr < 60 mL/min) | D-mab is preferred in patients with renal disease | D-mab may be preferred in patients with impaired renal function |
BTA bone targeting agent, D-mab denosumab, ZOL zoledronic acid, CR complete response, VGPR very good partial response, Ccr creatinine clearance, iv intravenous, sc subcutaneous injection
Denosumab is an anti-RANKL neutralizing, fully human monoclonal IgG2 antibody [68]. Dose adjustments are not required for denosumab administration, because denosumab is not excreted from the kidney, nor metabolized in the liver [69–71]. A single subcutaneous injection of denosumab exerts long-term efficacy [72]. A randomized, double-blind, multicenter phase 3 study of denosumab compared with zoledronic acid in the treatment of bone disease in patients with newly diagnosed MM with at least one image-documented bone lesion [73]. Denosumab met the primary endpoint of non-inferiority to zoledronic acid for the prevention of the occurrence of on-study SREs. Because most on-study SREs occurred within the first 3 months, a post-hoc, landmark superiority analysis of time to first on-study SREs was done at 15 months. Denosumab was more effective in terms of suppression of SRE occurrence. Notably, denosumab extended median progression-free survival (PFS) by 10.7 months in an exploratory analysis, although OS was superimposed. The majority (79%) of patients were treated with PI-containing regimens in this study, implying that denosumab may prolong the efficacy of induction treatment with PIs.
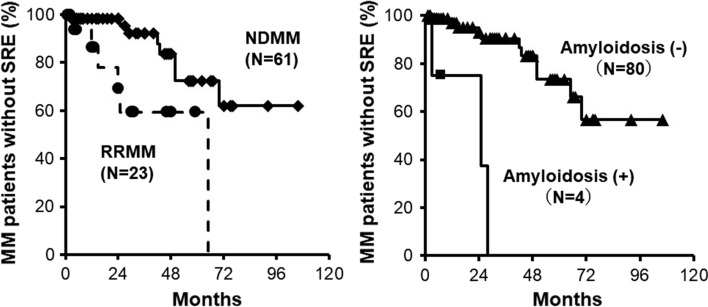
We retrospectively analyzed the efficacy of denosumab with PI-based regimens and updated the previously reported data [74]. Patient characteristics are listed in Table 1. All patients were treated with PI-based regimens with denosumab. At the median follow-up of 24 months (interquartile range 1–106), SRE occurred in 8 out of 61 patients with newly diagnosed MM (NDMM) and 6 out of 23 with relapsed/refractory MM (RRMM) (Fig. 5, left). The proportion of patients without SRE at 3 years was 92.3% in the NDMM group and 59.3% in the RRMM group. Factors contributing to the SRE occurrence were MM progression and AL amyloidosis. Bone fractures occurred by falling in 3 out of 4 cases with AL amyloidosis (Fig. 5, right). Balance loss and falling were caused by orthostatic hypotension and muscle weakness at the time of fracture in most cases (Table 2), indicating the importance of retaining physical function to prevent SREs. Bone fractures further deteriorate patients’ physical function, and resultant immobilization may accelerate bone loss with the tumor spreading within and outside of the bone.
Table 1.
Patient characteristics
| Sex (male/female) | 45/39 |
| Median age (range), years | 69 (41–88) |
| Newly diagnosed | 61 (73%) |
| Relapsed/refractory | 23 (27%) |
| Immunoglobulin class | |
| IgG | 49 (58%) |
| IgA | 15 (18%) |
| IgD | 3 (4%) |
| Light chain only | 16 (19%) |
| Non-secretory | 1 (1%) |
| PS (ECOG) | |
| 0 | 29 (35%) |
| 1 | 26 (31%) |
| 2 | 11 (13%) |
| 3 | 11 (13%) |
| 4 | 7 (8%) |
| Durie and Salmon stage | |
| I | 0 (0%) |
| II | 26 (31%) |
| III | 58 (69%) |
| A | 67 (80%) |
| B | 17 (20%) |
| ISS stage | |
| 1 | 26 (31%) |
| 2 | 31 (37%) |
| 3 | 27 (32%) |
| Bone scale | |
| 1 | 14 (17%) |
| 2 | 47 (56%) |
| 3 | 23 (27%) |
| AL amyloidosis | 4 (5%) |
| Anti-myeloma treatment | |
| Proteasome inhibitors | 84 (100%) |
| IMiDs | 59 (70%) |
| ASCT | 26 (31%) |
PS performance status, ECOG Eastern Cooperative Oncology Group, ISS International Staging System, SRE skeletal-related event, IMiD immunomodulatory drug, ASCT autologous stem cell transplantation
Fig. 5.
Proportion of MM patients without SRE. Time to first SRE on denosumab was retrospectively analyzed in 84 MM patients treated with proteasome inhibitor-based regimens between June 2012 and August 2022 in Tokushima University Hospital. The present study was approved by the Institutional Review Board of Tokushima University (permission number 3086-2)
Table 2.
Physical function and triggering factors for SRE occurrence
| No. | Age/Sex | Amyloidosis | Details of SRE | Treatment response | Triggers | Complications/comorbidities | PS ECOG |
|---|---|---|---|---|---|---|---|
| 1 | 76/M | None | Femoral fracture | SD |
Loss of balance Falling down |
Peripheral neuropathy | 2 |
| 2 | 47/M | None |
Rib fracture Spinal cord compression |
PD | None | None | 2 |
| 3 | 56/M | None | Pelvic fracture | PD |
Loss of balance Falling down |
DM type 2 Orthostatic hypotension |
4 |
| 4 | 55/M | None |
Vertebral fracture Spinal cord compression |
PD | None | Peripheral neuropathy | 4 |
| 5 | 78/M | None |
Sacral fracture Spinal cord compression |
PD | None | None | 3 |
| 6 | 77/M | None |
Vertebral fracture Rib fracture |
PD | None |
DM type 2 Orthostatic hypotension |
1 |
| 7 | 74/F | None | Vertebral fracture | PD | Loss of balance | Peripheral neuropathy | 3 |
| 8 | 80/F | None | Vertebral fracture | PD | None |
DM type 2 Muscle weakness |
3 |
| 9 | 73/F | None | Femoral fracture | SD |
Loss of balance Falling down |
None | 1 |
| 10 | 85/F | None |
Vertebral fracture Spinal cord compression |
PD | Falling down | None | 1 |
| 11 | 59/F | None | Vertebral fracture | PD | None | None | 1 |
| 12 | 73/F | Heart, tongue, skin | Femoral fracture | VGPR | Falling down |
Muscle weakness Orthostatic hypotension |
2 |
| 13 | 78/F | GI tract, skin, muscle | Vertebral fracture | VGPR |
Loss of balance Falling down |
Muscle weakness | 1 |
| 14 | 70/M | Heart, kidney, GI tract | Rib fracture | VGPR | Falling down |
DM type 2 Peripheral neuropathy Orthostatic hypotension |
0 |
M male, F female, PS performance status, ECOG Eastern Cooperative Oncology Group, VGPR very good partial response, SD stable disease, PD progressive disease, GI gastrointestinal, DM diabetes mellitus
The characteristics of zoledronic acid and denosumab are listed in Table 3. Zoledronic acid is administered by intravenous drip injection over 15 min or more. Denosumab is given subcutaneously. Zoledronic acid takes 3–4 days to exert its activity, whereas denosumab acts immediately after injection. Zoledronic acid acts on bone-resorbing mature osteoclasts but denosumab can act on immature and mature osteoclasts. Renal toxicity is less with denosumab. The incidence of osteonecrosis of the jaw was similar in both drug arms around 4% per year. Because of its potent activity, patients treated with denosumab have hypocalcemia more often than those treated with zoledronic acid. Risk factors for hypocalcemia in cancer patients after receiving denosumab are listed in Table 4. To prevent severe hypocalcemia with denosumab, corrected serum calcium levels should be monitored during treatment, especially in the first cycle of denosumab. Additionally, adequate calcium and vitamin D intake is recommended. Because patients with MM often have renal failure and vitamin D is activated in the kidney, patients with MM with renal failure should take active forms of VD3 (25-hydroxyvitamin D3 or 1,25-dihydroxyvitamin D3) rather than natural vitamin D.
Table 3.
Characteristics of zoledronic acid and denosumab
| Zoledronic acid | Denosumab | |
|---|---|---|
| Injection route | iv | sc |
| Emergence of effects | 3–4 days | < 1 day |
| Target to | Mature OCs | Immature and mature OCs |
| Hypocalcemia | Sometimes | More often |
| Renal impairment | + | − |
| Acute reaction | + | Rare |
| Bone deposition | + (cumulative) | − |
| ONJ | + | + |
| γδT cell induction | + | − |
iv intravenous, sc subcutaneous, OC osteoclast, ONJ osteonecrosis of the jaw
Table 4.
Risk factors for hypocalcemia in patients with cancer after receiving denosumab
| The first cycle of denosumab treatment |
| Renal insufficiency |
| Hypercalcemia before treatment |
| Aberrantly high baseline serum ALP |
| Higher baseline bone turnover markers of NTx and BSAP |
| Potential vitamin D deficiency |
ALP alkaline phosphatase, NTx N-terminal cross-linking telopeptide of type 1 collagen, BSAP bone-specific alkaline phosphatase
In the guideline issued by the Japanese Society of Hematology [75], it is recommended to administer denosumab or zoledronic acid at the start of treatment in NDMM patients with bone lesion(s). Denosumab is more strongly recommended in patients with renal impairment due to its low renal toxicity. Current recommendations for the use of bone‐modifying agents for patients with MM are listed in Table 5 [76–78]. Most guidelines say that these bone‐modifying agents should be given every month for up to 2 years and stopped if the disease is controlled or continued when the disease is active. During the current COVID-19 pandemic, zoledronic acid can be given every 3 months for bone disease prophylaxis. These bone‐modifying agents can be discontinued or changed to oral bisphosphonates for patients achieving a good durable response. Oral bisphosphonates may prevent a rebound effect by denosumab discontinuation.
Effects of new anti-MM agents on bone metabolism in patients with MM
MM cells express both constitutive proteasomes and immunoproteasomes. The inhibition of proteasome action results in the accumulation of misfolded proteins and functional proteins to be degraded by proteasomes in the endoplasmic reticulum lumen and cytosol, which facilitates several stresses, including endoplasmic reticulum overload, generation of excess reactive oxygen species, and functional disorder of intracellular proteins, eventually leading to apoptosis. Bortezomib and oral ixazomib are reversible PIs. Carfilzomib irreversibly inhibits both β5 and β5i proteasome subunits at low concentrations to induce more potent and prolonged suppression of proteasome activity in MM cells, compared with the reversible inhibitors bortezomib and ixazomib [79, 80]. Functionally, carfilzomib has been demonstrated to overcome resistance to the first-in-class PI bortezomib [81].
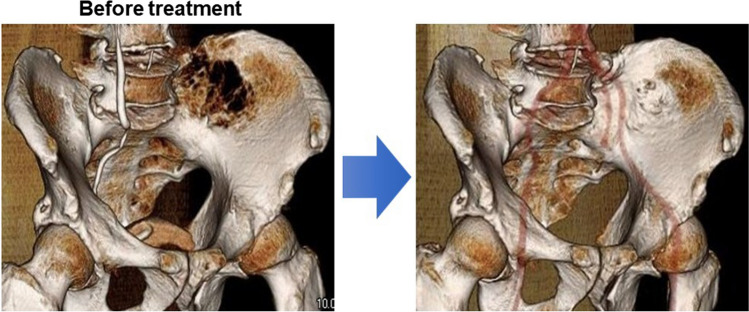
Besides their anti-tumor activity, PIs exerts bone anabolic actions [82–86]. During treatment with PIs, bone formation is restored preferentially in bone-destructive lesions in good responders (Fig. 6) [87]. Patients exhibiting bone formation in bone lesions tend to show a better and prolonged reduction of tumors [88–95]. Therefore, tumor reduction appears to trigger the anabolic effects of bortezomib. PIs induce MM cell death, which reduces the production of anti-anabolic mediators by MM cells and from bone lesions. PIs also suppress DKK1 production by BMSCs and sclerostin mainly by osteocytes [25, 96, 97]. With enough reduction of tumor cells, PIs are able to directly induce critical transcription factor for osteoblastogenesis, including Runx2 and ATF4, and activate osteoblasts to form bone [82, 83, 98, 99].
Fig. 6.
Bone recovery in responders to bortezomib. A newly diagnosed patient was treated with bortezomib and dexamethasone and achieved a very good partial response after 2 cycles of the treatment. Bone formation appeared in bone defective lesions in the iliac bone (right)
The REBUILD trial is a notable clinical study with daratumumab monotherapy to evaluate the role of daratumumab in bone remodeling among patients with RRMM [100]. Daratumumab monotherapy did not show statistically significant differences in serum levels of indicators of bone resorption, C-terminal cross-linking telopeptide of type 1 collagen and tartrate resistant acid phosphatase 5b (TRACP-5b), nor changes in the RANKL/OPG ratios over time. In contrast to the weak or marginal suppression of osteoclastic bone resorption, daratumumab showed a positive effect on bone formation with increasing serum levels of bone formation markers, including procollagen type-I N-pro-peptide, bone-specific alkaline phosphatase, and osteocalcin. The anabolic benefit was greater among responders and those with a prolonged duration of treatment. Such anabolic effects were gradually observed even after 4 months in patients on daratumumab monotherapy in contrast to those observed in responders to PIs at early treatment cycles. The anabolic effect of daratumumab was associated with a significant decrease in serum DKK1 and C–C motif ligand-3 levels. Although MM cells substantially influence bone remodeling to skew towards bone resorption, daratumumab can improve bone turnover towards bone formation in responders.
Perspectives
To maintain bone health in patients with MM, controlling MM tumors is required. We can now reduce MM tumors in the majority of patients using induction therapy, but MM regrows in most cases even after achieving a very good response with MRD negative state. New immunotherapies and/or new drug classes are expected for RRMM, especially for functional high-risk or triple-class refractory MM. To contain MM tumors without a relapse, however, the underlying mechanism of relapse needs to be further clarified. In this regard, we should elucidate the alteration of immune function and the tumor microenvironment in response to different therapeutic modalities. Cytotoxic agents often cause immune dysfunction, which may allow tumor cells to regrow. Additionally, bone marrow microenvironment with increased osteoclasts as well as BMSCs with defective osteoblastic differentiation provide a niche to support MM cell growth and induce tumor drug resistance. Therefore, reshaping of the bone marrow niche-environment and immune system is needed to suppress a relapse.
Risk-stratified therapy should be taken into account with different new agents. To better stratify prognosis, a second revision of the international staging system (R2-ISS) has been recently established [101]. The R2-ISS assigns a prognostic value to each baseline risk feature: ISS stage II (1 point), ISS stage III (1.5 points), del(17p) (1 point), high lactate dehydrogenase (1 point), t(4;14) (1 point), and 1q copy number alterations (0.5 point). Patients are stratified into four risk groups according to the total additive scores: low (0 points), low-intermediate (0.5–1 points), intermediate high (1.5–2.5 points), and high (3–5 points). However, a portion of standard-risk patients has a dismal prognosis, whereas high-risk patients do not always show poor prognosis, indicating that other factors responsible for predicting a relapse should be incorporated into prognostic assessments before treatment and response assessments during or after treatment. Indicators of immune function and bone marrow microenvironment surrounding MM cells may be such factors to be included.
To treat functional high-risk patients, new treatment modalities will play an important role. Among others, immunotherapies, including autologous CAR T-cell-based therapies and bispecific antibodies, are drawing considerable attention. However, we are still quite behind in our understanding of the heterogeneous biology of MM and its implications for therapy. Therefore, we need to further elucidate the efficacy of new agents especially in combinatory treatments with forthcoming treatment modalities such as immunotherapies with CAR T cells and bispecific antibodies to make the best use of these important agents and obtain better and more beneficial therapeutic outcomes in patients with MM.
Acknowledgements
This work was supported in part by JSPS KAKENHI Grants Nos. JP21H03111 and JP22K19626 to J.T., JP20K08712 to H.M., JP19K08839 to S.N., JP22K08455 to H.T. and JP22H03104 to M.A., a Japanese Society of Hematology Research Grant (No. 20247 to J.T.), a Research Clusters Program of Tokushima University Grant (No. 1803003 to M.A.), and a Research Clusters Program of Tokushima University Grant (No. 2202003 to T.H.). The funders had no role in the study design, data collection and analysis, decision to publish, and manuscript preparation.
Declarations
Conflict of interest
M.A. received research funding from Chugai Pharmaceutical, GlaxoSmithKline, Sanofi K.K., Kyowa Kirin, Nippon Shinyaku, Teijin Pharma, and Ono Pharmaceutical, and honoraria from Daiichi Sankyo, Janssen Pharmaceutical K.K., Takeda Pharmaceutical, Sanofi K.K., Bristol-Myers Squibb, and Ono Pharmaceutical. Other authors have no competing financial interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jumpei Teramachi, Email: jumptera@okayama-u.ac.jp.
Masahiro Abe, Email: masabe@tokushima-u.ac.jp.
References
- 1.Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management (in eng) J Bone Oncol. 2013;2:59–69. doi: 10.1016/j.jbo.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pecherstorfer M, Seibel MJ, Woitge HW, Horn E, Schuster J, Neuda J, Sagaster P, Köhn H, Bayer P, Thiébaud D, Ludwig H. Bone resorption in multiple myeloma and in monoclonal gammopathy of undetermined significance: quantification by urinary pyridinium cross-links of collagen (in eng) Blood. 1997;90:3743–3750. doi: 10.1182/blood.V90.9.3743. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto T, Abe M, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T. Ability of myeloma cells to secrete macrophage inflammatory protein (MIP)-1alpha and MIP-1beta correlates with lytic bone lesions in patients with multiple myeloma (in eng) Br J Haematol. 2004;125:38–41. doi: 10.1111/j.1365-2141.2004.04864.x. [DOI] [PubMed] [Google Scholar]
- 4.Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression (in eng) Proc Natl Acad Sci USA. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SJ, Cruz JC, Craig F, Chung H, Devlin RD, Roodman GD, Alsina M. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma (in eng) Blood. 2000;96:671–675. doi: 10.1182/blood.V96.2.671. [DOI] [PubMed] [Google Scholar]
- 6.Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand (in eng) Blood. 2001;97:3349–3353. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- 7.Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, Ozaki S, Wakatsuki S, Kosaka M, Kido S, Inoue D, Matsumoto T. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma (in eng) Blood. 2002;100:2195–2202. doi: 10.1182/blood.V100.6.2195. [DOI] [PubMed] [Google Scholar]
- 8.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma (in eng) N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 9.Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2 (in eng) Blood. 2005;106:3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi K, Abe M, Hiasa M, Oda A, Amou H, Kido S, Harada T, Tanaka O, Miki H, Nakamura S, Nakano A, Kagawa K, Yata K, Ozaki S, Matsumoto T. Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth (in eng) PLoS ONE. 2010;5:e9870. doi: 10.1371/journal.pone.0009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Specchia G, Rinaldi E, Curci P, Liso V, Passeri G, Zallone A, Rizzi R, Grano M. Myeloma cells suppress osteoblasts through sclerostin secretion (in eng) Blood Cancer J. 2011;1:e27. doi: 10.1038/bcj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S, Santo L, Cirstea D, Patel K, Sohani AR, Guimaraes A, Xie W, Chauhan D, Schoonmaker JA, Attar E, Churchill M, Weller E, Munshi N, Seehra JS, Weissleder R, Anderson KC, Scadden DT, Raje N. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease (in eng) Proc Natl Acad Sci USA. 2010;107:5124–5129. doi: 10.1073/pnas.0911929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, Delimpasi S, Pouli A, Meletis J, Kastritis E, Zervas K, Dimopoulos MA. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy (in eng) Int J Cancer. 2012;131:1466–1471. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- 14.Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, Viniou N, Yataganas X, Goldman JM, Rahemtulla A. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index (in eng) Blood. 2003;102:1064–1069. doi: 10.1182/blood-2003-02-0380. [DOI] [PubMed] [Google Scholar]
- 15.Terpos E, Politou M, Szydlo R, Goldman JM, Apperley JF, Rahemtulla A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma (in eng) Br J Haematol. 2003;123:106–109. doi: 10.1046/j.1365-2141.2003.04561.x. [DOI] [PubMed] [Google Scholar]
- 16.Andrews RE, Brown JE, Lawson MA, Chantry AD. Myeloma bone disease: the osteoblast in the spotlight (in eng) J Clin Med. 2021 doi: 10.3390/jcm10173973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristensen IB, Christensen JH, Lyng MB, Møller MB, Pedersen L, Rasmussen LM, Ditzel HJ, Abildgaard N. Hepatocyte growth factor pathway upregulation in the bone marrow microenvironment in multiple myeloma is associated with lytic bone disease (in eng) Br J Haematol. 2013;161:373–382. doi: 10.1111/bjh.12270. [DOI] [PubMed] [Google Scholar]
- 18.Derksen PW, de Gorter DJ, Meijer HP, Bende RJ, van Dijk M, Lokhorst HM, Bloem AC, Spaargaren M, Pals ST. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma (in eng) Leukemia. 2003;17:764–774. doi: 10.1038/sj.leu.2402875. [DOI] [PubMed] [Google Scholar]
- 19.Standal T, Abildgaard N, Fagerli UM, Stordal B, Hjertner O, Borset M, Sundan A. HGF inhibits BMP-induced osteoblastogenesis: possible implications for the bone disease of multiple myeloma (in eng) Blood. 2007;109:3024–3030. doi: 10.1182/blood-2006-07-034884. [DOI] [PubMed] [Google Scholar]
- 20.Wang FS, Lin CL, Chen YJ, Wang CJ, Yang KD, Huang YT, Sun YC, Huang HC. Secreted frizzled-related protein 1 modulates glucocorticoid attenuation of osteogenic activities and bone mass (in eng) Endocrinology. 2005;146:2415–2423. doi: 10.1210/en.2004-1050. [DOI] [PubMed] [Google Scholar]
- 21.Auziņa D, Beinaroviča I, Janicka-Kupra B, Lejniece S, Lejnieks A, Groma V. Dickkopf-related protein 1 expression in bone marrow of multiple myeloma patients: correlation with bone disease and plasma cell malignancy type (in eng) Exp Oncol. 2020;42:285–288. doi: 10.32471/exp-oncology.2312-8852.vol-42-no-4.15289. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser M, Mieth M, Liebisch P, Oberländer R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, Stover D, Sezer O, Heider U. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma (in eng) Eur J Haematol. 2008;80:490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 23.Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation (in eng) Int J Cancer. 2006;119:1728–1731. doi: 10.1002/ijc.22033. [DOI] [PubMed] [Google Scholar]
- 24.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist (in eng) J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eda H, Santo L, Wein MN, Hu DZ, Cirstea DD, Nemani N, Tai YT, Raines SE, Kuhstoss SA, Munshi NC, Kronenberg HM, Raje NS. Regulation of sclerostin expression in multiple myeloma by Dkk-1: a potential therapeutic strategy for myeloma bone disease (in eng) J Bone Miner Res. 2016;31:1225–1234. doi: 10.1002/jbmr.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Shi M, Li J, Zhang H, Chen B, Chen L, Gao W, Giuliani N, Zhao RC. Elevated tumor necrosis factor-alpha suppresses TAZ expression and impairs osteogenic potential of Flk-1+ mesenchymal stem cells in patients with multiple myeloma (in eng) Stem Cells Dev. 2007;16:921–930. doi: 10.1089/scd.2007.0074. [DOI] [PubMed] [Google Scholar]
- 27.Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation (in eng) Blood. 2005;106:2472–2483. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S, Rizzoli V, Roodman GD, Giuliani N. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma (in eng) Blood. 2005;106:1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- 29.Terpos E, Kastritis E, Christoulas D, Gkotzamanidou M, Eleutherakis-Papaiakovou E, Kanellias N, Papatheodorou A, Dimopoulos MA. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy (in eng) Ann Oncol. 2012;23:2681–2686. doi: 10.1093/annonc/mds068. [DOI] [PubMed] [Google Scholar]
- 30.Abe M, Shintani Y, Eto Y, Harada K, Kosaka M, Matsumoto T. Potent induction of activin A secretion from monocytes and bone marrow stromal fibroblasts by cognate interaction with activated T cells (in eng) J Leukoc Biol. 2002;72:347–352. doi: 10.1189/jlb.72.2.347. [DOI] [PubMed] [Google Scholar]
- 31.Brunetti G, Rizzi R, Storlino G, Bortolotti S, Colaianni G, Sanesi L, Lippo L, Faienza MF, Mestice A, Curci P, Specchia G, Grano M, Colucci S. LIGHT/TNFSF14 as a new biomarker of bone disease in multiple myeloma patients experiencing therapeutic regimens (in eng) Front Immunol. 2018;9:2459. doi: 10.3389/fimmu.2018.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terpos E, Ntanasis-Stathopoulos I, Christoulas D, Bagratuni T, Bakogeorgos M, Gavriatopoulou M, Eleutherakis-Papaiakovou E, Kanellias N, Kastritis E, Dimopoulos MA. Semaphorin 4D correlates with increased bone resorption, hypercalcemia, and disease stage in newly diagnosed patients with multiple myeloma (in eng) Blood Cancer J. 2018;8:42. doi: 10.1038/s41408-018-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baghdadi M, Ishikawa K, Nakanishi S, Murata T, Umeyama Y, Kobayashi T, Kameda Y, Endo H, Wada H, Bogen B, Yamamoto S, Yamaguchi K, Kasahara I, Iwasaki H, Takahata M, Ibata M, Takahashi S, Goto H, Teshima T, Seino KI. A role for IL-34 in osteolytic disease of multiple myeloma (in eng) Blood Adv. 2019;3:541–551. doi: 10.1182/bloodadvances.2018020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papanota AM, Karousi P, Kontos CK, Ntanasis-Stathopoulos I, Scorilas A, Terpos E. Multiple myeloma bone disease: implication of micrornas in its molecular background (in eng) Int J Mol Sci. 2021 doi: 10.3390/ijms22052375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao M, Zang M, Zhao L, Deng S, Xu Y, Qi F, An G, Qin Y, Sui W, Li F, Yang W, Li Z, Yi S, Zou D, Zhan F, Qiu L. Serum high expression of miR-214 and miR-135b as novel predictor for myeloma bone disease development and prognosis (in eng) Oncotarget. 2016;7:19589–19600. doi: 10.18632/oncotarget.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Kuek V, Liu Y, Tickner J, Yuan Y, Chen L, Zeng Z, Shao M, He W, Xu J. MiR-214 is an important regulator of the musculoskeletal metabolism and disease (in eng) J Cell Physiol. 2018;234:231–245. doi: 10.1002/jcp.26856. [DOI] [PubMed] [Google Scholar]
- 37.Xu S, Cecilia Santini G, De Veirman K, Vande Broek I, Leleu X, De Becker A, Van Camp B, Vanderkerken K, Van Riet I. Upregulation of miR-135b is involved in the impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients (in eng) PLoS ONE. 2013;8:e79752. doi: 10.1371/journal.pone.0079752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitari MR, Rossi M, Amodio N, Botta C, Morelli E, Federico C, Gullà A, Caracciolo D, Di Martino MT, Arbitrio M, Giordano A, Tagliaferri P, Tassone P. Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts (in eng) Oncotarget. 2015;6:27343–27358. doi: 10.18632/oncotarget.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gowda PS, Wildman BJ, Trotter TN, Xu X, Hao X, Hassan MQ, Yang Y. Runx2 suppression by miR-342 and miR-363 inhibits multiple myeloma progression (in eng) Mol Cancer Res. 2018;16:1138–1148. doi: 10.1158/1541-7786.Mcr-17-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan FY, Deng R, Qiu L, Wen Q, Zeng Y, Gao L, Zhang C, Kong P, Zhong J, Zeng N, Li Z, Su Y, Zhang X. miR-203a-3p.1 is involved in the regulation of osteogenic differentiation by directly targeting Smad9 in MM-MSCs (in eng) Oncol Lett. 2019;18:6339–6346. doi: 10.3892/ol.2019.10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landowski TH, Olashaw NE, Agrawal D, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-kappa B (RelB/p50) in myeloma cells (in eng) Oncogene. 2003;22:2417–2421. doi: 10.1038/sj.onc.1206315. [DOI] [PubMed] [Google Scholar]
- 42.Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ, Mundy GR, Yoneda T. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis (in eng) Blood. 2004;104:2149–2154. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 43.Abe M, Hiura K, Ozaki S, Kido S, Matsumoto T. Vicious cycle between myeloma cell binding to bone marrow stromal cells via VLA-4-VCAM-1 adhesion and macrophage inflammatory protein-1alpha and MIP-1beta production (in eng) J Bone Miner Metab. 2009;27:16–23. doi: 10.1007/s00774-008-0012-z. [DOI] [PubMed] [Google Scholar]
- 44.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit (in eng) Bonekey Rep. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado-Calle J, Bellido T, Roodman GD. Role of osteocytes in multiple myeloma bone disease (in eng) Curr Opin Support Palliat Care. 2014;8:407–413. doi: 10.1097/spc.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion (in eng) Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 47.Moreaux J, Cremer FW, Reme T, Raab M, Mahtouk K, Kaukel P, Pantesco V, De Vos J, Jourdan E, Jauch A, Legouffe E, Moos M, Fiol G, Goldschmidt H, Rossi JF, Hose D, Klein B. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature (in eng) Blood. 2005;106:1021–1030. doi: 10.1182/blood-2004-11-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abe M, Kido S, Hiasa M, Nakano A, Oda A, Amou H, Matsumoto T. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma (in eng) Leukemia. 2006;20:1313–1315. doi: 10.1038/sj.leu.2404228. [DOI] [PubMed] [Google Scholar]
- 49.Lawson MA, McDonald MM, Kovacic N, Hua Khoo W, Terry RL, Down J, Kaplan W, Paton-Hough J, Fellows C, Pettitt JA, Neil Dear T, Van Valckenborgh E, Baldock PA, Rogers MJ, Eaton CL, Vanderkerken K, Pettit AR, Quinn JM, Zannettino AC, Phan TG, Croucher PI. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche (in eng) Nat Commun. 2015;6:8983. doi: 10.1038/ncomms9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asano J, Nakano A, Oda A, Amou H, Hiasa M, Takeuchi K, Miki H, Nakamura S, Harada T, Fujii S, Kagawa K, Endo I, Yata K, Sakai A, Ozaki S, Matsumoto T, Abe M. The serine/threonine kinase Pim-2 is a novel anti-apoptotic mediator in myeloma cells (in eng) Leukemia. 2011;25:1182–1188. doi: 10.1038/leu.2011.60. [DOI] [PubMed] [Google Scholar]
- 51.Hiasa M, Teramachi J, Oda A, Amachi R, Harada T, Nakamura S, Miki H, Fujii S, Kagawa K, Watanabe K, Endo I, Kuroda Y, Yoneda T, Tsuji D, Nakao M, Tanaka E, Hamada K, Sano S, Itoh K, Matsumoto T, Abe M. Pim-2 kinase is an important target of treatment for tumor progression and bone loss in myeloma (in eng) Leukemia. 2015;29:207–217. doi: 10.1038/leu.2014.147. [DOI] [PubMed] [Google Scholar]
- 52.Teramachi J, Hiasa M, Oda A, Harada T, Nakamura S, Amachi R, Tenshin H, Iwasa M, Fujii S, Kagawa K, Miki H, Kurahashi K, Yoshida S, Endo I, Haneji T, Matsumoto T, Abe M. Pim-2 is a critical target for treatment of osteoclastogenesis enhanced in myeloma (in eng) Br J Haematol. 2018;180:581–585. doi: 10.1111/bjh.14388. [DOI] [PubMed] [Google Scholar]
- 53.Lu J, Zavorotinskaya T, Dai Y, Niu XH, Castillo J, Sim J, Yu J, Wang Y, Langowski JL, Holash J, Shannon K, Garcia PD. Pim2 is required for maintaining multiple myeloma cell growth through modulating TSC2 phosphorylation (in eng) Blood. 2013;122:1610–1620. doi: 10.1182/blood-2013-01-481457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jöhrer K, Obkircher M, Neureiter D, Parteli J, Zelle-Rieser C, Maizner E, Kern J, Hermann M, Hamacher F, Merkel O, Wacht N, Zidorn C, Scheideler M, Greil R. Antimyeloma activity of the sesquiterpene lactone cnicin: impact on Pim-2 kinase as a novel therapeutic target (in eng) J Mol Med (Berl) 2012;90:681–693. doi: 10.1007/s00109-011-0848-x. [DOI] [PubMed] [Google Scholar]
- 55.Warfel NA, Kraft AS. PIM kinase (and Akt) biology and signaling in tumors (in eng) Pharmacol Ther. 2015;151:41–49. doi: 10.1016/j.pharmthera.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teramachi J, Tenshin H, Hiasa M, Oda A, Bat-Erdene A, Harada T, Nakamura S, Ashtar M, Shimizu S, Iwasa M, Sogabe K, Oura M, Fujii S, Kagawa K, Miki H, Endo I, Haneji T, Matsumoto T, Abe M. TAK1 is a pivotal therapeutic target for tumor progression and bone destruction in myeloma (in eng) Haematologica. 2021;106:1401–1413. doi: 10.3324/haematol.2019.234476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonewald LF, Kiel DP, Clemens TL, Esser K, Orwoll ES, O'Keefe RJ, Fielding RA. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop (in eng) J Bone Miner Res. 2013;28:1857–1865. doi: 10.1002/jbmr.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: games without frontiers (in eng) Arch Biochem Biophys. 2014;561:3–12. doi: 10.1016/j.abb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Chen H, Senda T, Kubo KY. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis (in eng) Med Mol Morphol. 2015;48:61–68. doi: 10.1007/s00795-015-0099-y. [DOI] [PubMed] [Google Scholar]
- 60.Tiede-Lewis LM, Dallas SL. Changes in the osteocyte lacunocanalicular network with aging (in eng) Bone. 2019;122:101–113. doi: 10.1016/j.bone.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakashima T, Hayashi M, Takayanagi H. New insights into osteoclastogenic signaling mechanisms (in eng) Trends Endocrinol Metab. 2012;23:582–590. doi: 10.1016/j.tem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Goldring SR. The osteocyte: key player in regulating bone turnover (in eng) RMD Open. 2015;1:e000049. doi: 10.1136/rmdopen-2015-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi RB, Robling AG. The Wnt pathway: an important control mechanism in bone’s response to mechanical loading (in eng) Bone. 2021;153:116087. doi: 10.1016/j.bone.2021.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rummler M, Ziouti F, Bouchard AL, Brandl A, Duda GN, Bogen B, Beilhack A, Lynch ME, Jundt F, Willie BM. Mechanical loading prevents bone destruction and exerts anti-tumor effects in the MOPC315.BM.Luc model of myeloma bone disease (in eng) Acta Biomater. 2021;119:247–258. doi: 10.1016/j.actbio.2020.10.041. [DOI] [PubMed] [Google Scholar]
- 65.Tanimoto K, Hiasa M, Tenshin H, Teramachi J, Oda A, Harada T, Higa Y, Sogabe K, Oura M, Sumitani R, Hara T, Endo I, Matsumoto T, Tanaka E, Abe M. Mechanical unloading aggravates bone destruction and tumor expansion in myeloma (in eng) Haematologica. 2022;107:744–749. doi: 10.3324/haematol.2021.278295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munshi NC, Avet-Loiseau H, Anderson KC, Neri P, Paiva B, Samur M, Dimopoulos M, Kulakova M, Lam A, Hashim M, He J, Heeg B, Ukropec J, Vermeulen J, Cote S, Bahlis N. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma (in eng) Blood Adv. 2020;4:5988–5999. doi: 10.1182/bloodadvances.2020002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial (in eng) Lancet. 2010;376:1989–1999. doi: 10.1016/s0140-6736(10)62051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ. Denosumab in postmenopausal women with low bone mineral density (in eng) N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 69.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, Holmes GB, Dunstan CR, DePaoli AM. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women (in eng) J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/jbmr.040305. [DOI] [PubMed] [Google Scholar]
- 70.Lewiecki EM. Denosumab: an investigational drug for the management of postmenopausal osteoporosis (in eng) Biologics. 2008;2:645–653. doi: 10.2147/btt.s2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development (in eng) BioDrugs. 2010;24:23–39. doi: 10.2165/11530560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 72.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, Holloway D, Peterson MC, Bekker PJ. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer (in eng) Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.Ccr-05-1933. [DOI] [PubMed] [Google Scholar]
- 73.Raje N, Terpos E, Willenbacher W, Shimizu K, García-Sanz R, Durie B, Legieć W, Krejčí M, Laribi K, Zhu L, Cheng P, Warner D, Roodman GD. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study (in eng) Lancet Oncol. 2018;19:370–381. doi: 10.1016/s1470-2045(18)30072-x. [DOI] [PubMed] [Google Scholar]
- 74.Miki H, Nakamura S, Oura M, Nakamura M, Sumitani R, Sogabe K, Takahashi M, Maruhashi T, Harada T, Fujii S, Hamano H, Kondo M, Okada N, Endo I, Abe M. The importance of retaining physical functions to prevent skeletal-related events in multiple myeloma patients with bone disease (in eng) EJHaem. 2022;3:480–483. doi: 10.1002/jha2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iida S, Shimazaki C, Abe M, Nakaseko C. JSH practical guidelines for hematological malignancies, 2018: III. Myeloma-2. Related disorders of multiple myeloma (in eng) Int J Hematol. 2019;109:633–640. doi: 10.1007/s12185-019-02640-y. [DOI] [PubMed] [Google Scholar]
- 76.Coleman R, Hadji P, Body JJ, Santini D, Chow E, Terpos E, Oudard S, Bruland Ø, Flamen P, Kurth A, Van Poznak C, Aapro M, Jordan K. Bone health in cancer: ESMO clinical practice guidelines (in eng) Ann Oncol. 2020;31:1650–1663. doi: 10.1016/j.annonc.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 77.Anderson K, Ismaila N, Flynn PJ, Halabi S, Jagannath S, Ogaily MS, Omel J, Raje N, Roodman GD, Yee GC, Kyle RA. Role of bone-modifying agents in multiple Myeloma: american society of clinical oncology clinical practice guideline update (in eng) J Clin Oncol. 2018;36:812–818. doi: 10.1200/jco.2017.76.6402. [DOI] [PubMed] [Google Scholar]
- 78.Terpos E, Zamagni E, Lentzsch S, Drake MT, García-Sanz R, Abildgaard N, Ntanasis-Stathopoulos I, Schjesvold F, de la Rubia J, Kyriakou C, Hillengass J, Zweegman S, Cavo M, Moreau P, San-Miguel J, Dimopoulos MA, Munshi N, Durie BGM, Raje N. Treatment of multiple myeloma-related bone disease: recommendations from the bone working group of the international Myeloma working group (in eng) Lancet Oncol. 2021;22:e119–e130. doi: 10.1016/s1470-2045(20)30559-3. [DOI] [PubMed] [Google Scholar]
- 79.Andreu-Vieyra C, Berenson JR. Carfilzomib in multiple myeloma (in eng) Expert Opin Biol Ther. 2014;14:1685–1699. doi: 10.1517/14712598.2014.953050. [DOI] [PubMed] [Google Scholar]
- 80.Jayaweera SPE, Wanigasinghe Kanakanamge SP, Rajalingam D, Silva GN. Carfilzomib: a promising proteasome inhibitor for the treatment of relapsed and refractory multiple myeloma (in eng) Front Oncol. 2021;11:740796. doi: 10.3389/fonc.2021.740796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruschak AM, Slassi M, Kay LE, Schimmer AD. Novel proteasome inhibitors to overcome bortezomib resistance (in eng) J Natl Cancer Inst. 2011;103:1007–1017. doi: 10.1093/jnci/djr160. [DOI] [PubMed] [Google Scholar]
- 82.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, Mancini C, Martella E, Ferrari L, Tabilio A, Rizzoli V. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients (in eng) Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 83.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice (in eng) J Clin Invest. 2008;118:491–504. doi: 10.1172/jci33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B, Shaughnessy JD., Jr Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling (in eng) Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hurchla MA, Garcia-Gomez A, Hornick MC, Ocio EM, Li A, Blanco JF, Collins L, Kirk CJ, Piwnica-Worms D, Vij R, Tomasson MH, Pandiella A, San Miguel JF, Garayoa M, Weilbaecher KN. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects (in eng) Leukemia. 2013;27:430–440. doi: 10.1038/leu.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia-Gomez A, Quwaider D, Canavese M, Ocio EM, Tian Z, Blanco JF, Berger AJ, Ortiz-de-Solorzano C, Hernández-Iglesias T, Martens AC, Groen RW, Mateo-Urdiales J, Fraile S, Galarraga M, Chauhan D, San Miguel JF, Raje N, Garayoa M. Preclinical activity of the oral proteasome inhibitor MLN9708 in myeloma bone disease (in eng) Clin Cancer Res. 2014;20:1542–1554. doi: 10.1158/1078-0432.Ccr-13-1657. [DOI] [PubMed] [Google Scholar]
- 87.Ozaki S, Tanaka O, Fujii S, Shigekiyo Y, Miki H, Choraku M, Kagawa K, Asano J, Takeuchi K, Kitazoe K, Hashimoto T, Abe M, Matsumoto T. Therapy with bortezomib plus dexamethasone induces osteoblast activation in responsive patients with multiple myeloma (in eng) Int J Hematol. 2007;86:180–185. doi: 10.1532/ijh97.07030. [DOI] [PubMed] [Google Scholar]
- 88.Zangari M, Esseltine D, Lee CK, Barlogie B, Elice F, Burns MJ, Kang SH, Yaccoby S, Najarian K, Richardson P, Sonneveld P, Tricot G. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma (in eng) Br J Haematol. 2005;131:71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 89.Zangari M, Yaccoby S, Pappas L, Cavallo F, Kumar NS, Ranganathan S, Suva LJ, Gruenwald JM, Kern S, Zhan F, Esseltine D, Tricot G. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients (in eng) Haematologica. 2011;96:333–336. doi: 10.3324/haematol.2010.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zangari M, Aujay M, Zhan F, Hetherington KL, Berno T, Vij R, Jagannath S, Siegel D, Keith Stewart A, Wang L, Orlowski RZ, Belch A, Jakubowiak A, Somlo G, Trudel S, Bahlis N, Lonial S, Singhal S, Kukreti V, Tricot G. Alkaline phosphatase variation during carfilzomib treatment is associated with best response in multiple myeloma patients (in eng) Eur J Haematol. 2011;86:484–487. doi: 10.1111/j.1600-0609.2011.01602.x. [DOI] [PubMed] [Google Scholar]
- 91.Terpos E, Sezer O, Croucher P, Dimopoulos MA. Myeloma bone disease and proteasome inhibition therapies (in eng) Blood. 2007;110:1098–1104. doi: 10.1182/blood-2007-03-067710. [DOI] [PubMed] [Google Scholar]
- 92.Zavrski I, Krebbel H, Wildemann B, Heider U, Kaiser M, Possinger K, Sezer O. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function (in eng) Biochem Biophys Res Commun. 2005;333:200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 93.Accardi F, Toscani D, Bolzoni M, Dalla Palma B, Aversa F, Giuliani N. Mechanism of action of Bortezomib and the new proteasome inhibitors on myeloma cells and the bone microenvironment: impact on myeloma-induced alterations of bone remodeling (in eng) Biomed Res Int. 2015;2015:172458. doi: 10.1155/2015/172458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zangari M, Suva LJ. The effects of proteasome inhibitors on bone remodeling in multiple myeloma (in eng) Bone. 2016;86:131–138. doi: 10.1016/j.bone.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Accardi F, Toscani D, Costa F, Aversa F, Giuliani N. The proteasome and myeloma-associated bone disease (in eng) Calcif Tissue Int. 2018;102:210–226. doi: 10.1007/s00223-017-0349-1. [DOI] [PubMed] [Google Scholar]
- 96.Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B, Bonomini S, Martella E, Agnelli L, Neri A, Ceccarelli F, Palumbo C. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation (in eng) Leukemia. 2012;26:1391–1401. doi: 10.1038/leu.2011.381. [DOI] [PubMed] [Google Scholar]
- 97.Toscani D, Palumbo C, Dalla Palma B, Ferretti M, Bolzoni M, Marchica V, Sena P, Martella E, Mancini C, Ferri V, Costa F, Accardi F, Craviotto L, Aversa F, Giuliani N. The proteasome inhibitor Bortezomib maintains osteocyte viability in multiple myeloma patients by reducing both apoptosis and autophagy: a new function for proteasome inhibitors (in eng) J Bone Miner Res. 2016;31:815–827. doi: 10.1002/jbmr.2741. [DOI] [PubMed] [Google Scholar]
- 98.Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, Harris SE, Gallwitz W, Kim KB, Hu S, Crews CM, Mundy GR. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro (in eng) J Clin Invest. 2003;111:1771–1782. doi: 10.1172/jci16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura S, Miki H, Kido S, Nakano A, Hiasa M, Oda A, Amou H, Watanabe K, Harada T, Fujii S, Takeuchi K, Kagawa K, Ozaki S, Matsumoto T, Abe M. Activating transcription factor 4, an ER stress mediator, is required for, but excessive ER stress suppresses osteoblastogenesis by bortezomib (in eng) Int J Hematol. 2013;98:66–73. doi: 10.1007/s12185-013-1367-z. [DOI] [PubMed] [Google Scholar]
- 100.Terpos E, Ntanasis-Stathopoulos I, Kastritis E, Hatjiharissi E, Katodritou E, Eleutherakis-Papaiakovou E, Verrou E, Gavriatopoulou M, Leonidakis A, Manousou K, Delimpasi S, Malandrakis P, Kyrtsonis MC, Papaioannou M, Symeonidis A, Dimopoulos MA. Daratumumab improves bone turnover in relapsed/refractory multiple myeloma; phase 2 study “REBUILD” (in eng) Cancers (Basel) 2022 doi: 10.3390/cancers14112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.D'Agostino M, Cairns DA, Lahuerta JJ, Wester R, Bertsch U, Waage A, Zamagni E, Mateos MV, Dall'Olio D, van de Donk NWCJ, Jackson G, Rocchi S, Salwender H, Bladé Creixenti J, van der Holt B, Castellani G, Bonello F, Capra A, Mai EK, Dürig J, Gay F, Zweegman S, Cavo M, Kaiser MF, Goldschmidt H, Hernández Rivas JM, Larocca A, Cook G, San-Miguel JF, Boccadoro M, Sonneveld P. Second revision of the international staging system (R2-ISS) for overall survival in multiple myeloma: a european myeloma network (EMN) report within the harmony project (in eng) J Clin Oncol. 2022;40:3406–3418. doi: 10.1200/jco.21.02614. [DOI] [PubMed] [Google Scholar]