This systematic review and meta-analysis investigates the consistency of blood pressure reduction observed with ultrasound renal denervation compared with sham across studies of varying hypertension severity.
Key Points
Question
Is the magnitude of blood pressure (BP) reduction observed with ultrasound renal denervation (uRDN) compared with a sham procedure consistent across studies of varying hypertension severity?
Findings
In this patient-level pooled analysis of 3 randomized clinical trials including 506 patients with varying severities of hypertension, the reduction in daytime ambulatory systolic BP was significantly greater among patients receiving uRDN compared with sham at 2 months, with consistency across trials.
Meaning
uRDN reduces BP compared with a sham procedure.
Abstract
Importance
Ultrasound renal denervation (uRDN) was shown to lower blood pressure (BP) in patients with uncontrolled hypertension (HTN). Establishing the magnitude and consistency of the uRDN effect across the HTN spectrum is clinically important.
Objective
To characterize the effectiveness and safety of uRDN vs a sham procedure from individual patient-level pooled data across uRDN trials including either patients with mild to moderate HTN on a background of no medications or with HTN resistant to standardized triple-combination therapy.
Data Sources
A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN SOLO and TRIO) and A Study of the ReCor Medical Paradise System in Stage II Hypertension (RADIANCE II) trials.
Study Selection
Trials with similar designs, standardized operational implementation (medication standardization and blinding of both patients and physicians to treatment assignment), and follow-up.
Data Extraction and Synthesis
Pooled analysis using individual patient-level data using linear regression models to compare uRDN with sham across the trials.
Main Outcomes and Measures
The primary outcome was baseline-adjusted change in 2-month daytime ambulatory systolic BP (dASBP) between groups.
Results
A total of 506 patients were randomized in the 3 studies (uRDN, 293; sham, 213; mean [SD] age, 54.1 [9.3]; 354 male [70.0%]). After a 1-month medication stabilization period, dASBP was similar between the groups (mean [SD], uRDN, 150.3 [9.2] mm Hg; sham, 150.8 [10.5] mm Hg). At 2 months, dASBP decreased by 8.5 mm Hg to mean (SD) 141.8 (13.8) mm Hg among patients treated with uRDN and by 2.9 mm Hg to 147.9 (14.6) mm Hg among patients treated with a sham procedure (mean difference, −5.9; 95% CI, −8.1 to −3.8 mm Hg; P < .001 in favor of uRDN). BP decreases from baseline with uRDN vs sham were consistent across trials and across BP parameters (office SBP: −10.4 mm Hg vs −3.4 mm Hg; mean difference, −6.4 mm Hg; 95% CI, −9.1 to –3.6 mm Hg; home SBP: −8.4 mm Hg vs −1.4 mm Hg; mean difference, −6.8 mm Hg; 95% CI, −8.7 to −4.9 mm Hg, respectively). The BP reductions with uRDN vs sham were consistent across prespecified subgroups. Independent predictors of a larger BP response to uRDN were higher baseline BP and heart rate and the presence of orthostatic hypertension. No differences in early safety end points were observed between groups.
Conclusions and Relevance
Results of this patient-level pooled analysis suggest that BP reductions with uRDN were consistent across HTN severity in sham-controlled trials designed with a 2-month primary end point to standardize medications across randomized groups.
Trial Registration
ClinicalTrials.gov Identifier: NCT02649426 and NCT03614260
Introduction
Despite established treatment options, hypertension (HTN) remains a leading cause of cardiovascular morbidity and mortality. Rates of blood pressure (BP) control are static or falling, and reasons for this treatment gap are multifactorial.1,2 In this context, various device-based therapies that aim to lower BP are currently under investigation with the goal of controlling BP when used in conjunction with guideline-recommended approaches, including lifestyle modification and antihypertensive medications.
Endovascular renal denervation (RDN) has emerged as one such approach to lowering BP. Current RDN systems consist of specifically designed intra-arterial catheters that ablate both the efferent and afferent sympathetic nerves of the kidney, thereby decreasing sympathetic nerve activity. Following inconsistent results from earlier trials,3,4 these devices have been optimized to achieve more effective and reproducible circumferential nerve ablation of the kidney and, theoretically, a more consistent BP effect. Ultrasound-based RDN (uRDN) has been shown to safely reduce BP when compared with a sham comparator.5,6 Within these trials, the efficacy of uRDN in lowering BP over a sham procedure has been demonstrated among patients with mild-moderate HTN and among those with HTN resistant to a triple-combination antihypertensive therapy.
Leveraging a consistency of trial designs across differing populations, we sought to characterize the effectiveness and safety of uRDN compared with sham by pooling individual patient-level data from trials each powered to demonstrate BP-lowering efficacy, with similar end point ascertainment, operational implementation, and follow-up procedures (including blinding).
Methods
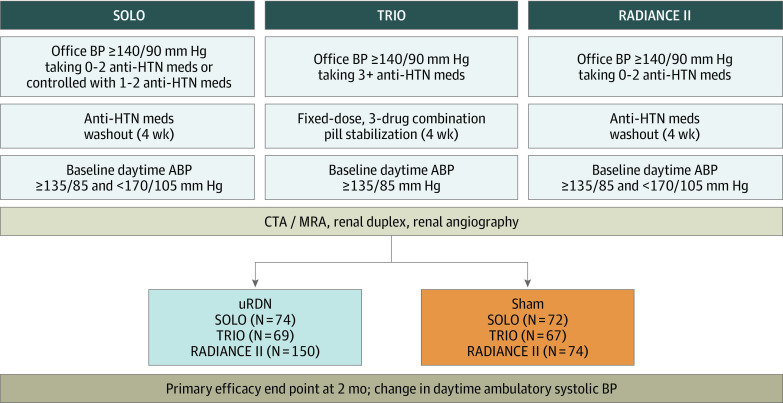
This patient-level analysis included pooled data from 3 prospective, individually powered, randomized, sham-controlled, international studies of RDN in patients with HTN: A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN SOLO and TRIO)5,6 and A Study of the ReCor Medical Paradise System in Stage II Hypertension (RADIANCE II)7 trials (Figure 1). Specifically, these are studies that adhered to the US Food and Drug Administration/American Society of Hypertension and European consensus on trial designs for RDN trials of HTN, including standardized design (prospective, randomized, sham controlled) with the same primary end point at 2 months (change in daytime ambulatory systolic BP from baseline), use of the same RDN technology and technique, and consistent study monitoring and data collection.8,9,10 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for each of the original randomized trials.
Figure 1. Design of the 3 Studies Included in the Pooled Analysis.
The studies included in the pooled analysis are A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN SOLO and TRIO) and A Study of the ReCor Medical Paradise System in Stage II Hypertension (RADIANCE II) trials. ABP indicates ambulatory blood pressure; BP, blood pressure; CTA, computed tomography angiography; HTN, hypertension; meds, medications; MRA, magnetic resonance angiography; uRDN, ultrasound-based renal denervation.
All 3 studies required patients to be between 18 and 75 years of age with an estimated glomerular filtration rate (eGFR) of at least 40 mL/min/1.73m2. For RADIANCE-HTN, the following categories were available: American Indian or Alaska Native; Asian; Black, African heritage; Black, Caribbean heritage; Black, other heritage, specify; Caucasian; Hispanic or Latino; multiple ethnicities, specify; Native Hawaiian or other Pacific Islander; or other ethnicity, specify. For RADIANCE II the following categories were available: American Indian or Alaska Native; Asian; Black or African American; Native Hawaiian or other Pacific Islander; White; or other/mixed race, with a separate question regarding Hispanic, Latino, or Spanish origin. This information was included in the study per guidance from the US Food and Drug Administration.11 The RADIANCE-HTN SOLO trial included patients with mild to moderate HTN whose BP was controlled with 1 to 2 medications or uncontrolled with 0 to 2 medications. All patients had medications withdrawn for 4 weeks before confirming a daytime ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg before a 1:1 randomization to uRDN vs sham procedure.
The RADIANCE-HTN TRIO trial included patients with resistant HTN whose BP was uncontrolled with 3 or more prescribed antihypertensive medications. All patients were switched to a 3-drug, fixed-dose, single combination pill including a calcium channel blocker, angiotensin receptor blocker, and a thiazide diuretic. Patients were eligible for randomization (1:1) if, after 4 weeks, their BP remained uncontrolled with a daytime ambulatory BP of at least 135/85 mm Hg while taking the single-pill triple-combination treatment.
The RADIANCE II trial included patients with uncontrolled hypertension who either currently or previously were prescribed up to 2 antihypertensive medications. All patients had medications withdrawn for 4 weeks before confirming a daytime ambulatory BP of at least 135/85 mm Hg and less than 170/105 mm Hg and a 2:1 randomization to uRDN vs sham procedure. A full listing of inclusion/exclusion criteria from each study as well as the description of the ambulatory, home, and office BP measurement methods is provided in eTables 1 and 2 in Supplement 1.
Within these studies, participants meeting all clinical and BP criteria after a 1-month medication stabilization phase (not taking medications for RADIANCE-HTN SOLO and RADIANCE II; taking a triple-combination treatment in a single pill for RADIANCE-HTN TRIO) who had qualifying anatomy as determined by computed tomography or magnetic resonance angiography then underwent invasive arterial angiography of the kidney. If angiography reconfirmed eligibility for uRDN, participants were randomly assigned (1:1 for the SOLO and TRIO trials; 2:1 for the RADIANCE II trial) to either uRDN with the Paradise Renal Denervation System (ReCor Medical) or a sham procedure (ie, arterial angiography of the kidney with subsequent time period in the laboratory to mimic an RDN procedure). The independent randomization process was also similar between studies. Eye covers, headphones, and sedation were used in all trials to maintain blinding of the patients. The patients underwent monthly follow-up, with ascertainment of both seated office and 7-day home BP and ascertainment of ambulatory BP at the 2-month follow-up visit. Of note, strict blinding of clinical assessors and patients was maintained throughout the follow-up phase, and participants and treating physicians were instructed to not change their HTN regimen during this period unless meeting prespecified BP escape criteria (eTable 2 in Supplement 1).
For this pooled analysis, the primary end point was the change from baseline in daytime ambulatory systolic BP at 2 months after the procedure for uRDN vs sham. Secondary end points included changes in additional BP parameters including 24-hour, home, office, and nighttime systolic BP as well as diastolic BP parameters. Randomization was established by each individual study, and intention-to-treat analyses were performed based on the original randomization assignment. For patients who met the protocol-defined criteria for medication changes, the last BP measurement before the medication change was used for the reduction in BP in the analysis, as was indicated in the original study designs. Any subsequent missing BP data were handled via multiple imputation, as previously described.7 Major adverse events were defined in the same manner as in RADIANCE II7 and included the following: all-cause mortality, renal failure, an embolic event, renal artery or vascular complications requiring intervention, hospitalization for hypertensive or hypotensive crisis or for major cardiovascular/cerebrovascular events within 30 days, and renal artery stenosis greater than 70% detected by noninvasive imaging at 6 months. All adverse events potentially meeting these definitions were sent to an independent clinical events committee for adjudication. We also assessed change in eGFR from baseline to 2 months.
Statistical Analysis
The primary analysis was based on a maximum likelihood linear model for the change in daytime ambulatory systolic BP at 2 months after the procedure with fixed-effects terms including randomized study group (uRDN vs sham), baseline daytime ambulatory systolic BP, and study. A fixed-effects approach was used due to the limited number of studies; study poolability was tested using a treatment by arm interaction term as well as by calculation of I2 statistic in a 2-stage meta-analysis. Subgroup analyses for baseline characteristics were based on mean-centered values to prevent ecological bias.12 Sensitivity analyses were performed for missing data and for a per-protocol analysis cohort as defined in Supplement 2. Multivariable analyses using linear regression were performed to assess the independent effect of the randomization group on BP and to assess predictors of BP response to uRDN (eAppendix in Supplement 1 and Supplement 2). Continuous variables are expressed as mean (SD), unless otherwise specified, and between-group differences are expressed with their 2-sided 95% CIs. We used SAS software, version 9.4 (SAS Institute) and R (R Core Team). The R packages data.table, version 1.14.2 (Dowle & Srinivasan), metaphor,13 and meta14 were used to conduct the 2-stage meta-analyses.
Results
A total of 506 patients among 2830 patients screened for eligibility were randomized in the 3 included studies: (uRDN, 293; sham, 213; mean [SD] age, 54.1 [9.3]; 354 male [70.0%]; 152 female [30.0%]). Patients self-identified with the following race and ethnicity categories: 88 Black (17.4%), 378 White (74.7%), and 40 other or unknown (7.9%) (Table 1). Owing to the stringent exclusion criteria of these trials, only 34% of patients (173 of 506) had hyperlipidemia, and 12% (59 of 506) had diabetes across the 3 studies. Prior hospitalization for hypertensive crisis and prior cardiovascular or cerebrovascular events were rare (except in RADIANCE-HTN TRIO, which included patients with resistant HTN). The mean (SD) body mass index was 30.7 (5.5) (calculated as weight in kilograms divided by height in meters squared), and the mean (SD) office BP on initial screening for enrollment was 153.9/99.3 (15.6/10.1) mm Hg while receiving 1.8 (1.6) antihypertensive medications.
Table 1. Demographics and Clinical Characteristics at Enrollment and Baseline BP.
| Measure | No./total No. (%) | |
|---|---|---|
| uRDN (n = 293) | Sham (n = 213) | |
| Age, mean (SD), y | 54.2 (9.5) | 53.9 (9.0) |
| Sex | ||
| Female | 88/293 (30.0) | 64/213 (30.0) |
| Male | 205/293 (70.0) | 149/213 (70.0) |
| Race | ||
| Black | 47/293 (16.0) | 41/213 (19.2) |
| White | 219/293 (74.7) | 159/213 (74.6) |
| Other/unknowna | 27/293 (9.2) | 13/213 (6.1) |
| BMI, mean (SD)b | 30.7 (5.6) | 30.7 (5.4) |
| Abdominal obesityc | 185/289 (64.0) | 145/213 (68.1) |
| eGFR, mL/min/1.73 m2d | 83.3 (17.9) | 82.5 (16.6) |
| <60 | 16/292 (5.5) | 13/211 (6.2) |
| Type 2 diabetes | 32/293 (10.9) | 27/213 (12.7) |
| Sleep apnea syndrome | 47/293 (16.0) | 32/213 (15.0) |
| Prior hospitalization for hypertensive crisis | 26/293 (8.9) | 16/213 (7.5) |
| Prior myocardial infarction or cerebrovascular evente | 8/293 (2.7) | 8/213 (3.8) |
| History of heart failure | 2/293 (0.7) | 3/213 (1.4) |
| Office BP and heart rate at screening, mean (SD), mm Hg | ||
| Systolic | 153.9 (14.9) | 153.9 (16.4) |
| Diastolic | 99.9 (10.1) | 98.6 (10.0) |
| Heart rate, bpmf | 74.0 (11.8) | 74.7 (12.5) |
| No. of BP medications at screening, mean (SD) | 1.7 (1.5) | 2.0 (1.6) |
| BP medications at screening, No. | ||
| 0 | 67/293 (22.9) | 41/213 (19.2) |
| 1 | 87/293 (29.7) | 52/213 (24.4) |
| 2 | 70/293 (23.9) | 51/213 (23.9) |
| ≥3g | 69/293 (23.5) | 69/213 (32.4) |
| BP after 4-wk medication washout/standardization, mean (SD), mm Hg | ||
| Daytime ambulatory | ||
| Systolic | 150.3 (9.2) | 150.8 (10.5) |
| Diastolic | 93.6 (5.8) | 93.8 (6.9) |
| 24-h Ambulatoryh | ||
| Systolic | 143.3 (9.9) | 144.4 (11.2) |
| Diastolic | 88.3 (6.2) | 88.7 (7.1) |
| Nighttime ambulatoryh | ||
| Systolic | 132.3 (13.9) | 134.3 (15.3) |
| Diastolic | 79.8 (8.8) | 80.6 (9.6) |
| Homei | ||
| Systolic | 151.6 (11.5) | 150.3 (13.6) |
| Diastolic | 97.1 (7.9) | 95.8 (8.8) |
| Officej | ||
| Systolic | 155.9 (13.9) | 155.0 (15.1) |
| Diastolic | 101.4 (8.8) | 99.9 (9.3) |
Abbreviations: BMI, body mass index; BP, blood pressure; bpm, beats per minute; eGFR, estimated glomerular filtration rate; uRDN, ultrasound-based renal denervation.
Other/unknown race in the uRDN group included 2 Asian, 6 Hispanic or Latino, 18 other or mixed race, and 1 not reported. Other or unknown race in the sham group included 2 Asian, 4 Hispanic or Latino, 1 Native American or Alaska Native, 5 other or mixed race, and 1 not reported.
BMI is calculated as weight in kilograms divided by height in meters squared and is available for 291 patients in the uRDN group and 213 patients in the sham group.
Abdominal obesity was defined as a waist circumference greater than 102 cm for men and greater than 88 cm for women.
eGFR data were available for 292 patients in the uRDN group and 211 patients in the sham group.
A study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN TRIO) was the only study that permitted patients with prior cardiovascular and cerebrovascular events.
One patient in the uRDN group was missing heart rate.
The RADIANCE-HTN TRIO trial was only study that permitted patients taking 3 or more antihypertensive medications.
Twenty-four–hour and nighttime ambulatory systolic and diastolic BPs are available for 293 patients in the uRDN group and 212 patients in the sham group.
Home systolic and diastolic BPs are available for 289 patients in the uRDN group and 212 patients in the sham group.
Average of last 2 office BP among 3 measures in the seated position.
After the 1-month medication stabilization period, the mean (SD) daytime ambulatory systolic values were similar between the groups (systolic: uRDN, 150.3 [9.2] mm Hg vs sham, 150.8 [10.5] mm Hg). Mean (SD) home systolic BP values in the uRDN and sham groups were 151.6 (11.5) mm Hg and 150.3 (13.6) mm Hg, respectively. The mean (SD) office systolic BP values at baseline were similar between the groups (uRDN, 155.9 [13.9] mm Hg vs sham, 155.0 [15.1] mm Hg). Baseline BP measurements compared across treatment groups are shown in Table 1.
Mean (SD) total procedural duration including the preprocedural renal angiogram and the randomization process was 78.1 (25.8) minutes for uRDN vs 41.8 (15.7) minutes for sham (P < .001), and conscious sedation or monitored anesthesia was used for most patients (eTable 3 in Supplement 1). Among patients randomly assigned to uRDN, the mean (SD) device procedural time was 35.3 (20.1) minutes, and 5.6 (1.1) sonications of main and accessory arteries (diameter of at least 3 mm) were performed with a mean (SD) total emission time of 38.9 (7.8) seconds. Successful bilateral ablation was performed in all but 7 patients (286 of 293 [97.6%]) (eTable 3 in Supplement 1).
Efficacy Outcomes
At 2-month follow-up, mean (SD) daytime ambulatory systolic BP decreased from the prerandomization baseline by 8.5 mm Hg to 141.8 (13.8) mm Hg after uRDN vs a decrease of 2.9 mm Hg to 147.9 (14.6) mm Hg after sham (mean difference, −5.9; 95% CI, −8.1 to −3.8 mm Hg; P < .001 in favor of uRDN) (Figure 2; eTable 4 in Supplement 1). Reductions were consistent across the 3 included trials, and there was no heterogeneity when tested using a study by treatment arm interaction term or by I2 statistic (eFigure 1 in Supplement 1). Similarly, greater reductions in BP were observed with uRDN compared with sham for 24-hour ambulatory systolic BP (mean [SD], −7.9 [11.1] mm Hg vs −3.1 [12.2] mm Hg; mean difference, −5.3; 95% CI, −7.4 to −3.3 mm Hg; P < .001), home systolic BP (mean [SD], −8.4 [11.0] mm Hg vs −1.4 [9.4] mm Hg; mean difference, −6.8; 95% CI, −8.7 to −4.9 mm Hg; P < .001), and office systolic BP (mean [SD], −10.4 [15.0] mm Hg vs −3.4 [16.7] mm Hg; mean difference, −6.4; 95% CI, −9.1 to −3.6 mm Hg; P < .001) (eTable 4 in Supplement 1). Decreases in daytime and nighttime systolic BP were greater with uRDN compared with sham, with a similar magnitude of BP decrease over the 24-hour circadian cycle (Figure 2; eTable 4 in Supplement 1). In addition, decreases in diastolic BP were greater with uRDN compared with sham irrespective of whether measured with ambulatory BP monitoring, at home, or in the office (eTable 4 in Supplement 1). Per-protocol analyses as well as analyses conducted only using available data (without imputation) paralleled the primary intent-to-treat analyses (eTables 5 and 6 in Supplement 1).
Figure 2. Pooled Results for Systolic Blood Pressure (SBP) Readings and SBP Curves Over a 24-Hour Period .

A, Patient-level pooled results for SBP readings including daytime ambulatory (primary end point), 24-hour ambulatory, home, and office. Results include available data for each group (daytime ambulatory: ultrasound-based renal denervation [uRDN], 281; sham, 211; home: uRDN, 271; sham, 205; office: uRDN, 275; sham, 209). Between-group comparisons include multiple imputation for missing data. B, Ambulatory systolic BP curves over the 24-hour period at baseline and 2 months for uRDN and sham groups. Curves include available data (uRDN, 280; sham, 209) and impute for patients meeting escape criteria.
Not only was the overall magnitude of BP reduction greater among uRDN compared with patients in the sham group, but a greater proportion of patients achieved large reductions, of 10 mm Hg or greater, with uRDN compared with sham (46.3% [130 of 281] vs 19.9% [42 of 211]; P < .001) (eTable 7 in Supplement 1). Despite a greater proportion of patients randomly assigned to sham receiving additional BP medications before the ascertainment of the 2-month BP assessment (14.6% [31 of 213] vs 6.8% [20 of 293]; P = .004), rates of BP control were still greater among patients randomized to uRDN compared with sham (eTable 8 in Supplement 1). At a target threshold of daytime ambulatory BP less than 135/85 mm Hg, 24.2% of patients (68 of 281) after uRDN were controlled, compared with 12.3% (26 of 211) after sham (P < .001). A similarly greater rate of control in favor of uRDN was achieved using a home BP threshold less than 135/85 mm Hg.
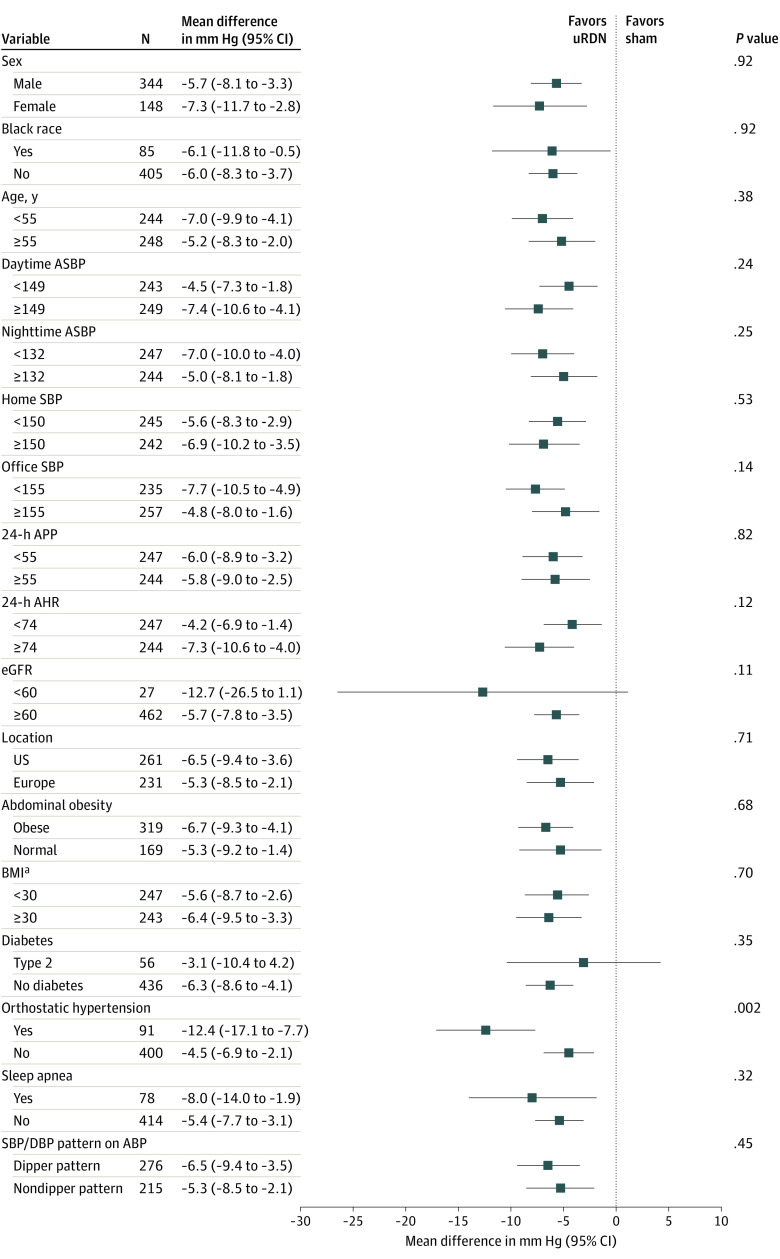
Subgroup and Multivariable Analyses
Consistently greater reductions in BP with uRDN compared with sham were observed across prespecified subgroups (Figure 3). The only significant (quantitative) treatment interaction observed was among patients with orthostatic hypertension at baseline (defined as an increase in office BP when standing as compared with sitting of ≥20 mm Hg systolic and/or ≥10 mm Hg diastolic). Patients with orthostatic hypertension had a greater treatment effect (mean difference in daytime ambulatory systolic BP between uRDN and sham, −12.4 mm Hg; 95% CI, −17.1 to −7.7 mm Hg) compared with those without orthostatic hypertension (mean difference between uRDN and sham, −4.5 mm Hg; 95% CI, −6.9 to −2.1 mm Hg; interaction P = .002).
Figure 3. Between-Group Differences in 2-Month Daytime Ambulatory Systolic Blood Pressure (ASBP) Change for Prespecified Subgroups.
AHR, indicates ambulatory heart rate; APP, ambulatory pulse pressure; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; uRDN, ultrasound-based renal denervation.
aCalculated as weight in kilograms divided by height in meters squared.
Although the effect of uRDN vs sham was consistent across tertiles of baseline BP, the overall magnitude of the reduction in daytime ambulatory systolic BP was associated with baseline BP. Among patients who did not start additional medications, greater reductions in daytime ambulatory systolic BP were observed among patients with higher starting BP (eFigure 2 in Supplement 1) with smaller reductions observed among those with lower baseline BP. However, among patients in the lowest tertile of starting BP, 42.6% of patients (40 of 94) in the uRDN group vs 16.7% of patients (11 of 66) in the sham group (P < .001) had BP controlled at 2 months, with lower rates of control achieved with higher baseline BP (P < .001 for trend) (eFigure 2 in Supplement 1). The effect of uRDN vs sham was robust in multivariable analysis. In an exploratory analysis among patients treated with uRDN, independent correlates of 2-month daytime ambulatory systolic BP were higher baseline BP, greater heart rate, and the presence of orthostatic hypertension (eTable 9 in Supplement 1).
Safety Outcomes
The uRDN procedure was well-tolerated with follow-up to 2 months, with 287 of 291 patients (98.6%) being discharged the same day or the following day. eGFR level remained stable through 2 months (eTable 10 in Supplement 1), and the rate of adverse safety end points was low, with 1 periprocedural vasovagal event and a vascular access complication with sequelae observed in the uRDN arm and deemed to be related to the procedure (Table 2). There was additionally 1 case of a sudden death at 21 days after the procedure in a uRDN-treated patient with resistant HTN that was adjudicated as unrelated to device or procedure.
Table 2. Safety Outcomes: Major Adverse Event Rates (Patient-Level, Nonhierarchical)a.
| Events | No. (%) | |
|---|---|---|
| uRDN (n = 293) | Sham (n = 213) | |
| 30-d Events | ||
| All-cause mortalityb | 1 (0.3) | 0 (0) |
| New-onset ESKD (eGFR<15 mL/min/m2 or need for kidney replacement therapy) | 0 (0) | 0 (0) |
| Significant embolic event resulting in end-organ damage | 0 (0) | 0 (0) |
| Kidney artery perforation or dissection requiring an invasive intervention | 0 (0) | 0 (0) |
| Major vascular complications requiring surgical repair, interventional procedure, thrombin injection, or blood transfusionc | 1 (0.3) | 0 (0) |
| Hospitalization for hypertensive or hypotensive crisisc | 1 (0.3) | 0 (0) |
| Hospitalization for major cardiovascular or hemodynamic-related eventsd | 1 (0.3) | 0 (0) |
| New-onset stroke | 0 (0) | 0 (0) |
| New-onset myocardial infarction | 0 (0) | 0 (0) |
| 6-mo Events | ||
| New-onset kidney artery stenosis >70% | 0 (0) | 0 (0) |
Abbreviations: eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; IV, intravenous; uRDN, ultrasound-based renal denervation.
Five major adverse events occurred in 3 of 293 patients (1.0%) in the uRDN arm. Multiple events occurred in a single patient (2 vascular complications and a hospitalization for hypotension).
The patient was well at 1 week outpatient follow-up; on postprocedure day 13, the patient was diagnosed with prostate carcinoma. On day 21, patient was found deceased at home.
The patient was diagnosed with a pseudoaneurysm treated with IV thrombin injection; the patient also received IV fluids for symptomatic hypotension. On postprocedure day 15, the patient was readmitted and was diagnosed with a deep venous thrombosis that was treated.
The patient had a vasovagal response postprocedure reversed after administration of fluids and atropine, and the patient was admitted for monitoring.
Discussion
This pooled analysis of individual patient data from the RADIANCE RDN clinical trial program sought to combine the results of 3 independently powered, randomized, sham-controlled, clinical trials of uRDN to increase the precision of the estimate of BP lowering while examining the consistency of this effect across differing populations of patients with HTN. Notably, the primary end points of these 3 trials were ascertained at 2 months to ensure medication standardization across randomized groups. There were several key findings. First, there was a reduction in daytime ambulatory systolic BP of 5.9 mm Hg with uRDN in comparison with sham with an overall reduction in daytime ambulatory systolic BP of 8.5 mm Hg from baseline after uRDN (vs a reduction of 2.9 mm Hg with sham), although both the magnitude of BP reduction from baseline as well as rates of BP control varied based on baseline BP. Second, reductions in BP were consistent across the 3 included trials and for all BP parameters irrespective of measurement method. Third, greater reductions in BP observed with uRDN compared with sham were consistent across prespecified subgroups, with greater response to uRDN observed in patients with orthostatic hypertension. Fourth, no appreciable differences in early periprocedural safety end points were observed between randomized treatment groups.
After the neutral primary efficacy results of the Renal Denervation in Patients with Uncontrolled Hypertension (SYMPLICITY-HTN 3) trial, a critical reappraisal of RDN devices and trial designs was conducted.8,15,16 Devices were iterated and refined to achieve more complete ablation patterns and thereby more consistent reductions in sympathetic nerve activity.17,18,19 The circumferential ablations at a depth of 1 to 6 mm achieved with uRDN represent 1 way of achieving a more consistent RDN effect. In addition, trial designs with serial BP measurements before randomization and establishment of a stable baseline were developed to avoid regression to the mean after treatment assignment. These newer trials further used medication washout or stabilization phases to allow for better control of the confounding effect of medications (and/or behavioral changes) that could further influence BP. In addition, ambulatory and home BP monitoring were used to reduce within-patient variability in BP measurements, along with the strict blinding of patients and assessors in conjunction with urine ascertainment of medication adherence for trials where participants were taking medication.
The 3 included trials within this prespecified pooled analysis all represent this newer generation of RDN trial design while enrolling differing populations of patients with HTN. For RADIANCE II, a greater range of catheter sizes (balloon diameters) was used than originally available in SOLO and TRIO, but the core catheter technology itself (ultrasound transducer) was unchanged. All 3 trials established a stable baseline either without medications (SOLO and RADIANCE II) or with receipt of a single combination pill (TRIO), with serial elevated BP measurements required prior to randomization. Ambulatory BP monitoring was used for both the confirmation of the hypertensive phenotype after 4 weeks of medication washout (SOLO and RADIANCE II) or medication stabilization (TRIO) as well as for assessment of the primary end point at 2 months. Despite the logistical challenges entailed, both patients and treating clinicians/assessors were blinded to the initial randomization assignment. As such, combining these 3 clinical trials allowed for a more precise estimate of overall effect of uRDN compared with sham, even while assessing different gradations of hypertension severity. We notably did not include the Renal Denervation on Quality of 24-hour BP Control by Ultrasound in Resistant Hypertension (REQUIRE) trial conducted in Japan and South Korea using the same uRDN catheter within this analysis, in part due to the lack of medication standardization (or assessment for medication adherence) within the trial as well as the lack of blinding of treating physicians and study coordinators, which could have influenced the unexpectedly large BP-lowering effect within the sham group of the trial.20
The observed reductions in BP within the SOLO, TRIO, and RADIANCE II trials were consistent across these 3 trials, each of which met its powered primary study end point assumption at 2 months, the time point of this pooled analysis. The observed BP reductions were present irrespective of how BP was measured (ambulatory, home, and office). As compared with sham, BP was lowered throughout the 24-hour circadian cycle, which may represent differentiation of mechanism from conventional medical therapies for hypertension. The consistency of effect across various prespecified subgroups is also notable, and the amplified effect among patients with orthostatic hypertension should be viewed as hypothesis generating. However, orthostatic hypertension is thought to be a sympathetically mediated phenomenon,21 and as such, further study of whether it can be used as a potential marker of more pronounced (or consistent) response to uRDN seems indicated. The finding of a greater lowering of BP among uRDN-treated patients with greater heart rate is also potentially indicative of a marker of sympathetic activity and consistent with a prior report of the same finding.22 Notably, only a minority of patients were able to achieve control with uRDN alone, reemphasizing that in clinical practice, the optimal role of uRDN is as an adjunct to lifestyle modification and medications, rather than a replacement for them.
Finally, the pooled safety data from these 3 included trials is additionally reassuring regarding the acute/subacute safety of uRDN. As an invasive procedure, the up-front risk of RDN is of course greater than that of initiation of medications or lifestyle modification. As such, all efforts to ensure the safety of the procedure are paramount. Based on these pooled results, the primary early safety concerns surrounding the procedure are largely associated with the site of vascular access. It is likely that with modest procedural refinements, uRDN could be performed via transradial access in ambulatory settings, further limiting the overall risks and resource use involved with the procedure. Of course, longer-term safety and durability of effect need to be established, but based on initial data from SOLO, TRIO, and the ACHIEVE registry23,24 as well as data from radiofrequency-based RDN,25,26 safety and efficacy findings have been reassuring.
Limitations
The primary limitation of this analysis is that its findings are restricted to 2-month follow-up, corresponding to ascertainment of the primary study end points. Notably, this was the time frame in which there were to be no changes to the patient’s medications (other than for escape purposes) allowing for a pure evaluation of the BP-lowering effect relative to sham while minimizing the confounding effect of a changing background of antihypertensive medications. Additional follow-up from the included trials will be required to examine durability of effect, especially in conjunction with additional antihypertensive medications.27,28 Similarly, although adverse events were rare, longer follow-up will provide additional safety data. In addition, minor adverse events (events other than the specified major adverse events) were not adjudicated. The 3 included trials used strict inclusion and exclusion criteria, especially with respect to clinical comorbid conditions. As such, broader application of uRDN will require additional studies, some of which are currently enrolling. At present and to our knowledge, there is still no reliable procedural marker of successful uRDN, and variability in treatment effect may influence the results of a uRDN procedure in an individual patient.
Conclusions
This was a patient-level pooled analysis of data from the RADIANCE-HTN SOLO, RADIANCE-HTN TRIO, and RADIANCE II trials. The reductions in BP observed with uRDN compared with sham were consistent across trials enrolling patients with varying severities of HTN.
eAppendix
eFigure 1. Two-Stage Meta-analysis Forest Plots Depicting 2-Month Mean Differences (MDs) and 95% CIs Results by Study and in Aggregate for Systolic BPs for Daytime Ambulatory, 24-Hour Ambulatory, Nighttime Ambulatory, Home, and Office Readings
eFigure 2. Daytime Ambulatory BP Control Rates and Daytime Systolic Ambulatory BP Change at 2 Months Stratified by Tertile of Starting Daytime Ambulatory Systolic BP Excluding Patients That Had Additional Antihypertension Medications Prior to 2 Months
eTable 1. Inclusion/Exclusion Criteria in Each Trial
eTable 2. Blood Pressure Measurement Methods, Safety Escape Criteria, and Procedural Methods Used in All Studies
eTable 3. Pooled Procedural Data Across Studies
eTable 4. Change in Ambulatory, Office, and Home Blood Pressure at 2 Months in the Ultrasound Renal Denervation Group (uRDN) and in the Sham Group in the Intention to Treat Population
eTable 5. Change in Ambulatory, Office, and Home Blood Pressure at 2 Months in the Ultrasound Renal Denervation Group (uRDN) and in the Sham Group in the Per-Protocol Population
eTable 6. Change in Ambulatory, Office, and Home Blood Pressure at 2 Months in the Ultrasound Renal Denervation Group (uRDN) and in the Sham Group in the Complete Ambulatory Blood Pressure Population
eTable 7. Proportion of Patients with Drops in Daytime Ambulatory, 24-Hour Ambulatory, Nighttime Ambulatory, and Home Systolic BP of ≥5, ≥10, ≥15 and ≥20 mm Hg in the Intention-to-Treat Population
eTable 8. Hypertension Control Rates at 2 Months
eTable 9. Multivariable Analysis of Daytime Ambulatory Systolic Blood Pressure in the Ultrasound Renal Denervation Group Only in the Intention-to-Treat Population
eTable 10. Changes in Kidney Function at 2 Months in Patients With Matched Data at Baseline and 2 Months
eReferences
Statistical Analysis Plan
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190-1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau KH, Azizi M, Kirtane AJ. What we know and don’t know about renal denervation to lower blood pressure. JAMA Cardiol. 2022;7(5):471-472. doi: 10.1001/jamacardio.2022.0169 [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Kandzari DE, O’Neill WW, et al. ; SYMPLICITY HTN-3 Investigators . A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401. doi: 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 4.Azizi M, Sapoval M, Gosse P, et al. ; Renal Denervation for Hypertension (DENERHTN) investigators . Optimum and stepped care andomizedd antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, andomized controlled trial. Lancet. 2015;385(9981):1957-1965. doi: 10.1016/S0140-6736(14)61942-5 [DOI] [PubMed] [Google Scholar]
- 5.Azizi M, Schmieder RE, Mahfoud F, et al. ; RADIANCE-HTN Investigators . Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, andomized, sham-controlled trial. Lancet. 2018;391(10137):2335-2345. doi: 10.1016/S0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 6.Azizi M, Sanghvi K, Saxena M, et al. ; RADIANCE-HTN investigators . Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a andomized, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476-2486. doi: 10.1016/S0140-6736(21)00788-1 [DOI] [PubMed] [Google Scholar]
- 7.Azizi M, Saxena M, Wang Y, et al. ; RADIANCE II Investigators and Collaborators . Endovascular ultrasound renal denervation to treat hypertension: the RADIANCE II randomized clinical trial. JAMA. Published online February 28, 2023. doi: 10.1001/jama.2023.0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White WB, Galis ZS, Henegar J, et al. Renal denervation therapy for hypertension: pathways for moving development forward. J Am Soc Hypertens. 2015;9(5):341-350. doi: 10.1016/j.jash.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 9.Mahfoud F, Azizi M, Ewen S, et al. Proceedings from the 3rd European Clinical Consensus Conference for clinical trials in device-based hypertension therapies. Eur Heart J. 2020;41(16):1588-1599. doi: 10.1093/eurheartj/ehaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandzari DE, Mahfoud F, Weber MA, et al. Clinical trial design principles and outcomes definitions for device-based therapies for hypertension: a consensus document from the Hypertension Academic Research Consortium. Circulation. 2022;145(11):847-863. doi: 10.1161/CIRCULATIONAHA.121.057687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Collection of race and ethnicity data in clinical trials. Accessed October 15, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials
- 12.Hua H, Burke DL, Crowther MJ, Ensor J, Tudur Smith C, Riley RD. One-stage individual participant data meta-analysis models: estimation of treatment-covariate interactions must avoid ecological bias by separating out within-trial and across-trial information. Stat Med. 2017;36(5):772-789. doi: 10.1002/sim.7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3)1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 14.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahfoud F, Böhm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J. 2015;36(33):2219-2227. doi: 10.1093/eurheartj/ehv192 [DOI] [PubMed] [Google Scholar]
- 16.Weber MA, Kirtane A, Mauri L, Townsend RR, Kandzari DE, Leon MB. Renal denervation for the treatment of hypertension: making a new start, getting it right. J Clin Hypertens (Greenwich). 2015;17(10):743-750. doi: 10.1111/jch.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzafriri AR, Mahfoud F, Keating JH, et al. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64(11):1079-1087. doi: 10.1016/j.jacc.2014.07.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64(7):635-643. doi: 10.1016/j.jacc.2014.03.059 [DOI] [PubMed] [Google Scholar]
- 19.Mahfoud F, Edelman ER, Böhm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol. 2014;64(7):644-646. doi: 10.1016/j.jacc.2014.05.037 [DOI] [PubMed] [Google Scholar]
- 20.Kario K, Yokoi Y, Okamura K, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45(2):221-231. doi: 10.1038/s41440-021-00754-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kario K. Orthostatic hypertension-a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9(12):726-738. doi: 10.1038/nrneph.2013.224 [DOI] [PubMed] [Google Scholar]
- 22.Böhm M, Tsioufis K, Kandzari DE, et al. Effect of heart rate on the outcome of renal denervation in patients with uncontrolled hypertension. J Am Coll Cardiol. 2021;78(10):1028-1038. doi: 10.1016/j.jacc.2021.06.044 [DOI] [PubMed] [Google Scholar]
- 23.Rader F, Kirtane AJ, Wang Y, et al. Durability of blood pressure reduction after ultrasound renal denervation: three-year follow-up of the treatment arm of the andomized RADIANCE-HTN SOLO trial. EuroIntervention. 2022;18(8):e677-e685. Published online 2022. doi: 10.4244/EIJ-D-22-00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daemen J, Mahfoud F, Kuck K-H, et al. Safety and efficacy of endovascular ultrasound renal denervation in resistant hypertension: 12-month results from the ACHIEVE study. J Hypertens. 2019;37(9):1906-1912. doi: 10.1097/HJH.0000000000002120 [DOI] [PubMed] [Google Scholar]
- 25.Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40(42):3474-3482. doi: 10.1093/eurheartj/ehz118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahfoud F, Kandzari DE, Kario K, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a andomized, sham-controlled trial. Lancet. 2022;399(10333):1401-1410. doi: 10.1016/S0140-6736(22)00455-X [DOI] [PubMed] [Google Scholar]
- 27.Azizi M, Schmieder RE, Mahfoud F, et al. ; RADIANCE-HTN Investigators . Six-month results of treatment-blinded medication titration for hypertension control following randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO trial. Circulation. 2019;139(22):2542-2553. doi: 10.1161/CIRCULATIONAHA.119.040451 [DOI] [PubMed] [Google Scholar]
- 28.Azizi M, Daemen J, Lobo MD, et al. ; RADIANCE-HTN Investigators . 12-month results from the unblinded phase of the RADIANCE-HTN SOLO trial of ultrasound renal denervation. JACC Cardiovasc Interv. 2020;13(24):2922-2933. doi: 10.1016/j.jcin.2020.09.054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix
eFigure 1. Two-Stage Meta-analysis Forest Plots Depicting 2-Month Mean Differences (MDs) and 95% CIs Results by Study and in Aggregate for Systolic BPs for Daytime Ambulatory, 24-Hour Ambulatory, Nighttime Ambulatory, Home, and Office Readings
eFigure 2. Daytime Ambulatory BP Control Rates and Daytime Systolic Ambulatory BP Change at 2 Months Stratified by Tertile of Starting Daytime Ambulatory Systolic BP Excluding Patients That Had Additional Antihypertension Medications Prior to 2 Months
eTable 1. Inclusion/Exclusion Criteria in Each Trial
eTable 2. Blood Pressure Measurement Methods, Safety Escape Criteria, and Procedural Methods Used in All Studies
eTable 3. Pooled Procedural Data Across Studies
eTable 4. Change in Ambulatory, Office, and Home Blood Pressure at 2 Months in the Ultrasound Renal Denervation Group (uRDN) and in the Sham Group in the Intention to Treat Population
eTable 5. Change in Ambulatory, Office, and Home Blood Pressure at 2 Months in the Ultrasound Renal Denervation Group (uRDN) and in the Sham Group in the Per-Protocol Population
eTable 6. Change in Ambulatory, Office, and Home Blood Pressure at 2 Months in the Ultrasound Renal Denervation Group (uRDN) and in the Sham Group in the Complete Ambulatory Blood Pressure Population
eTable 7. Proportion of Patients with Drops in Daytime Ambulatory, 24-Hour Ambulatory, Nighttime Ambulatory, and Home Systolic BP of ≥5, ≥10, ≥15 and ≥20 mm Hg in the Intention-to-Treat Population
eTable 8. Hypertension Control Rates at 2 Months
eTable 9. Multivariable Analysis of Daytime Ambulatory Systolic Blood Pressure in the Ultrasound Renal Denervation Group Only in the Intention-to-Treat Population
eTable 10. Changes in Kidney Function at 2 Months in Patients With Matched Data at Baseline and 2 Months
eReferences
Statistical Analysis Plan
Nonauthor Collaborators
Data Sharing Statement