This secondary analysis of a randomized clinical trial reports on the cognitive performance, risk of posttraumatic stress disorder and depression, and functional status 6 months after the administration of a treatment regimen consisting of antioxidants and hydrocortisone infusion in adults with sepsis.
Key Points
Question
Does early antioxidant and anti-inflammatory therapy improve the long-term cognitive, psychological, and functional outcomes in adults with sepsis?
Findings
In this secondary analysis of a randomized clinical trial involving 213 survivors of sepsis with respiratory and/or cardiovascular dysfunction, early treatment with vitamin C, thiamine, and hydrocortisone resulted in no improvement in many domains, worse immediate memory scores, and higher odds of posttraumatic stress disorder compared with placebo.
Meaning
Findings of this study suggest that antioxidant and anti-inflammatory therapy does not mitigate the development of long-term cognitive, psychological, and functional impairment in sepsis survivors.
Abstract
Importance
Sepsis is associated with long-term cognitive impairment and worse psychological and functional outcomes. Potential mechanisms include intracerebral oxidative stress and inflammation, yet little is known about the effects of early antioxidant and anti-inflammatory therapy on cognitive, psychological, and functional outcomes in sepsis survivors.
Objective
To describe observed differences in long-term cognitive, psychological, and functional outcomes of vitamin C, thiamine, and hydrocortisone between the intervention and control groups in the Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) randomized clinical trial.
Design, Setting, and Participants
This prespecified secondary analysis reports the 6-month outcomes of the multicenter, double-blind, placebo-controlled VICTAS randomized clinical trial, which was conducted between August 2018 and July 2019. Adult patients with sepsis-induced respiratory and/or cardiovascular dysfunction who survived to discharge or day 30 were recruited from 43 intensive care units in the US. Participants were randomized 1:1 to either the intervention or control group. Cognitive, psychological, and functional outcomes at 6 months after randomization were assessed via telephone through January 2020. Data analyses were conducted between February 2021 and December 2022.
Interventions
The intervention group received intravenous vitamin C (1.5 g), thiamine hydrochloride (100 mg), and hydrocortisone sodium succinate (50 mg) every 6 hours for 96 hours or until death or intensive care unit discharge. The control group received matching placebo.
Main Outcomes and Measures
Cognitive performance, risk of posttraumatic stress disorder and depression, and functional status were assessed using a battery of standardized instruments that were administered during a 1-hour telephone call 6 months after randomization.
Results
After exclusions, withdrawals, and deaths, the final sample included 213 participants (median [IQR] age, 57 [47-67] years; 112 males [52.6%]) who underwent long-term outcomes assessment and had been randomized to either the intervention group (n = 108) or control group (n = 105). The intervention group had lower immediate memory scores (adjusted OR [aOR], 0.49; 95% CI, 0.26-0.89), higher odds of posttraumatic stress disorder (aOR, 3.51; 95% CI, 1.18-10.40), and lower odds of receiving mental health care (aOR, 0.38; 95% CI, 0.16-0.89). No other statistically significant differences in cognitive, psychological, and functional outcomes were found between the 2 groups.
Conclusions and Relevance
In survivors of sepsis, treatment with vitamin C, thiamine, and hydrocortisone did not improve or had worse cognitive, psychological, and functional outcomes at 6 months compared with patients who received placebo. These findings challenge the hypothesis that antioxidant and anti-inflammatory therapy during critical illness mitigates the development of long-term cognitive, psychological, and functional impairment in sepsis survivors.
Trial Registration
ClinicalTrials.gov Identifier: NCT03509350
Introduction
Sepsis is a systemic inflammatory response to infection that frequently leads to multiorgan system failure, increased mortality, and long-term cognitive and functional impairment.1,2 Of the almost 2 million sepsis cases in the US annually, nearly 55% require intensive care unit (ICU) admission and 20% to 30% die.3 Sepsis survivors are at 3 to 4 times greater risk of moderate to severe cognitive impairment compared with patients who are hospitalized without sepsis.4 Sepsis survivors are also at greater risk of postintensive care syndrome (PICS), a collection of symptoms conferring long-term cognitive and psychological deterioration as well as worse quality of life.4,5,6,7 While early antibiotic administration, hemodynamic support, fluid resuscitation, and control of the infectious source have been associated with improved mortality,8,9 sepsis-associated cognitive, psychological, and functional decline remain a major public health problem.10,11
Postsepsis cognitive impairment is postulated to result from a combination of oxidative stress and prolonged neuroinflammation that results from the initial systemic inflammatory response. Increases in reactive oxygen species induce cellular damage, which, when combined with persistent activation of microglia, can contribute to blood-brain barrier dysfunction, loss of synapses, and neuronal cell death.12,13,14,15 Vitamin C is a potent antioxidant and may prevent cognitive decline after sepsis by protecting cells against oxidative lipid damage.16,17 Humans are among the few mammals that cannot synthesize vitamin C endogenously18 and are at risk for vitamin C depletion during sepsis.19,20,21 Both vitamin C and hydrocortisone have been associated with decreases in markers of acute inflammation in patients with sepsis22,23 and could act synergistically to prevent and repair endothelial cell dysfunction.24,25,26 The adverse cognitive and psychiatric effects of vitamin C deficiency in humans have been recognized for centuries.27 Thiamine, for its part, is also frequently deficient in sepsis.28 Thiamine deficiency increases inflammatory markers29 and is associated with cognitive impairment.30 These findings suggest that a combination of vitamin C, hydrocortisone, and thiamine may protect patients against postsepsis cognitive impairment. In addition, some studies have suggested a survival advantage from the same therapeutic approach.28 However, of 5 large randomized clinical trials,23,31,32,33,34 all but 1 failed to demonstrate a survival benefit from vitamin C–based therapy. Although 1 of these trials examined quality of life at 6 months,34 no study to date has evaluated the effects of these therapies on postsepsis cognitive and psychological outcomes.
The multicenter, double-blind, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) randomized clinical trial was conducted between August 2018 and July 2019 to test the hypothesis that this combination therapy would improve clinically important outcomes in patients with sepsis-induced respiratory and/or circulatory failure.35 The trial was designed a priori to evaluate the primary objective of effects on mortality and ventilator- and vasopressor-free days, which were shown to be unaffected by the treatment regimen.36 In addition, the trial was designed for a secondary objective of examining the effects of this combination therapy on the cognitive, psychological, and functional status at the 6-month follow-up of participants in the intervention and control groups, which was the focus of this secondary analysis.37 We hypothesized that, among survivors of sepsis requiring vasopressor or ventilator support, early treatment with high-dose vitamin C, thiamine, and hydrocortisone improves cognitive performance, psychological symptoms, and functional status 6 months after randomization.
Methods
Study Procedures
Details of the VICTAS trial protocol and analysis have been reported.35,36,37 Briefly, adult patients with acute respiratory and/or cardiac dysfunction due to sepsis who required ventilator or vasopressor support were recruited at 43 hospitals across the US. Participants were randomized 1:1 to either the intervention or control group. Participants, investigators, and study team personnel responsible for outcomes assessment were blinded to treatment allocation. The VICTAS trial, including this prespecified secondary analysis, was approved by the institutional review board of the Johns Hopkins Hospital. Participants or their legally authorized representatives provided written informed consent prior to enrollment and randomization. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
The intervention consisted of intravenous vitamin C (1.5 g), thiamine hydrochloride (100 mg), and hydrocortisone sodium succinate (50 mg) administered within 4 hours of randomization and every 6 hours thereafter for up to 96 hours or until death or ICU discharge (whichever occurred first). Participants in the control group received placebo injections of normal saline matched by volume at the same frequency. Participants could be treated with open-label corticosteroids as deemed appropriate by the clinicians; in cases of daily doses greater than or equal to 200 mg of hydrocortisone (or equivalent), investigational hydrocortisone or matching placebo was withheld by the pharmacy. Trained research personnel assessed participants for delirium daily during the intervention period using the Confusion Assessment Method for the ICU in conjunction with the Richmond Agitation-Sedation Scale. All other clinical management of participants, including enteral vitamin supplementation and nutrition, was at the discretion of the clinicians.
Measurement of Long-term Outcomes
At discharge or at 30 days, whichever occurred first, participants were invited to a follow-up telephone interview at 6 months after randomization. The invitation did not require separate informed consent from the main study but did require an expression of willingness by the participant or participant’s representative to be contacted at approximately 6 months. To facilitate long-term cohort retention, we used a follow-up window of 5 to 8 months. Follow-up assessments were conducted through January 2020.
During the follow-up telephone interview, we used a previously validated38 battery of cognitive assessment instruments, including the following: Telephone Confusion Assessment Method39 to assess for delirium; Telephone Interview for Cognitive Status40 to assess global cognitive function; Wechsler Adult Intelligence Scale, Fourth Edition, Digit Span Subtest to assess attention and working memory capacity; Weschler Memory Scale, Fourth Edition, Logical Memory I and II to examine immediate and delayed memory, respectively; Wechsler Adult Intelligence Scale, Fourth Edition, Similarities Subtest to assess language conceptualization and verbal abstraction; Controlled Oral Word Association Test to assess verbal fluency; and Hayling Sentence Completion Test to assess response inhibition as a form of executive function. Higher scores on these instruments indicate better outcomes. Complete descriptions of these assessments, their respective scoring systems, and score interpretation are provided in eAppendix 1 in Supplement 1.
To assess psychological status, we used the Posttraumatic Stress Disorder 8-item questionnaire (PTSD-8)41 and the Patient-Reported Outcomes Measurement Information System Depression 6-item Short Form (PROMIS-6).42 Posttraumatic stress disorder (PTSD) screening was anchored to the ICU experience, and participants were considered to have PTSD if they reported a PTSD-8 score of 3 or higher on a 4-point Likert scale in 3 of 4 symptom categories.41 Participants were considered to have depression if the total symptom burden on the PROMIS-6 exceeded a T score of 60, which corresponds to moderate depression on commonly used depression measures.43 Higher scores on the PTSD-8 and PROMIS-6 indicate worse outcomes. These instruments and their scoring criteria are described in further detail in eAppendix 1 in Supplement 1.
We used the Katz Activities of Daily Living Scale44 and the Functional Activities Questionnaire45 to assess basic and instrumental activities of daily living, respectively. Higher scores on these instruments indicate worse outcomes. We used the EuroQoL 5-Dimensions 3-Level (EQ-5D-3L)46 to assess overall health-related quality of life. A higher score on the EQ-5D-3L reflects better outcomes. We included additional standardized questions to assess health care use since discharge47 (eAppendix 1 in Supplement 1).
Telephone interviews lasted approximately 60 minutes. In cases where the participant was unable or unwilling to undergo formal cognitive and psychological assessment by telephone (eg, due to illness, excessive fatigue, or cognitive deterioration), functional status and health care use data were gathered from a designated surrogate. Reasons for inability to participate in individual assessments were recorded.
Statistical Analysis
Categorical variables were reported using frequencies and proportions. Continuous variables were reported as means with SDs or medians with IQRs. Participants were analyzed according to the group to which they were randomized (ie, intervention vs control). Comparisons between groups, with 2-tailed P values, used a multivariable proportional odds logistic regression for ordinal outcomes and a binary logistic regression for dichotomous outcomes. We adjusted for the following covariates: age, sex, race and ethnicity (identified by self-report or researcher observation), educational level, Acute Physiology and Chronic Health Evaluation version II score, presence of diabetes, neurological or cardiovascular comorbidity, number of days requiring mechanical ventilation, and ICU length of stay. P < .05 was considered to be statistically significant. For standardized cognitive assessments, scaled scores were used in the statistical analysis.
This secondary analysis reported on the key secondary objective for the VICTAS trial. The trial was explicitly powered for the primary objective and was administratively terminated after recruiting 501 participants. The secondary analysis was specified a priori with the intent to describe the magnitude of differences between groups rather than to focus on hypothesis tests. Therefore, no adjustments for multiplicity were made, and adjusted effect sizes (ie, odds ratios [ORs]) with 95% CIs were reported. Multiple imputation based on predictive mean matching was used to overcome missing covariate data.
Approximately one-third of participants who were interviewed at follow-up were unable or unwilling to complete the full battery of cognitive and psychological assessments (eAppendix 2 and eTable 2 in Supplement 1 describe the completion rates for individual assessments). In less than 7% of included participants who were unable to complete a given assessment due to cognitive impairment, the lowest possible score was assigned. All other missing data were assumed to be missing completely at random.
Sensitivity analysis was performed with a complete case approach. All analyses were conducted between February 2021 and December 2022 using R, version 3.4.3 (R Foundation for Statistical Computing).
Results
Enrollment and Patient Characteristics
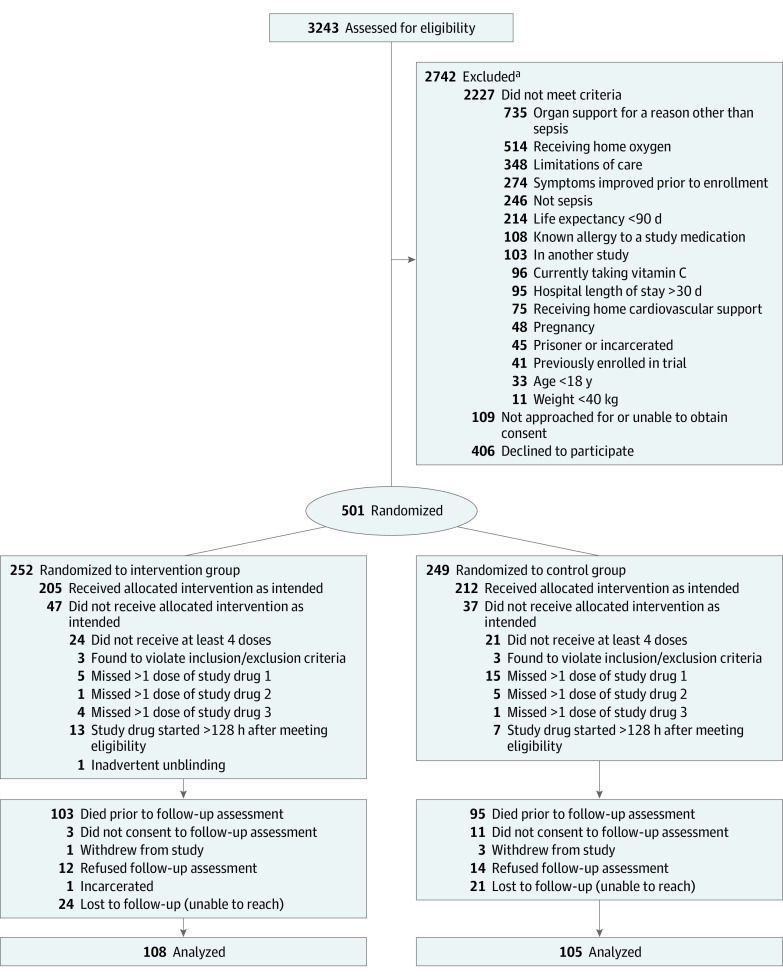
The flowchart of enrollment, randomization, and follow-up of VICTAS trial participants is depicted in Figure 1. Of the 501 participants randomized, 198 (39.5%) died prior to undergoing long-term outcome assessment (196 died within 180 days of randomization and 2 died after 180 days of randomization but before their 5- to 8-month follow-up window ended). Of the remaining 303 participants, 4 (1.3%) withdrew from the study and 40 (13.2%) did not agree to follow-up assessment. Of the 285 participants available for follow-up, 32 (11.2%) could not be reached within their 5- to 8-month follow-up window and 13 (4.6%) had a truncated follow-up window due to administrative termination of the trial. One participant was administratively withdrawn due to incarceration, and 26 (9.1%) refused assessment at follow-up.
Figure 1. Recruitment, Randomization, and Flow of Participants in the VICTAS Study.
aParticipants could meet multiple criteria; thus, exclusions were not mutually exclusive.
The final sample included 213 participants, of whom 108 were randomized to the intervention group and 105 were randomized to the control group. The characteristics of the included participants are described in Table 1 and eTable 1 in Supplement 1, along with participants who died and those for whom outcomes were unavailable (eg, due to withdrawal or loss to follow-up). The included participants had a median (IQR) age of 57 (47-67) years and comprised 101 females (47.4%) and 112 males (52.6%). Data were reported by participant representatives only in 39 cases (18.3%). The intervention and control groups had comparable characteristics (Table 2) except that the intervention group included more White participants than the control group (72 [66.7%] vs 57 [54.3%]). One hundred fifty-seven participants (73.7%) in the follow-up cohort, 72 (68.6%) in the control group, and 85 (78.7%) in the intervention group received parenteral norepinephrine. Sixty-six patients (31.0%) in the follow-up cohort (32 patients [30.5%] from the control group and 34 [31.5%] from the intervention group) received open-label corticosteriods while in the ICU.
Table 1. Patient Characteristics Stratified by Follow-up Status.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Follow-up | No follow-up | Overall | ||
| Died | Ineligible or lost to follow-upa | |||
| No. of patients | 213 | 198b | 90 | 501 |
| Age, median (IQR), y | 57 (47-67) | 65 (55-75) | 60 (48-70) | 62 (50-70) |
| Sex | ||||
| Male | 112 (52.6) | 117(59.1) | 44 (48.9) | 273 (54.5) |
| Female | 101 (47.4) | 81 (40.9) | 46 (51.1) | 228 (45.5) |
| Race and ethnicityc | ||||
| Black | 68 (31.9) | 56 (28.3) | 26 (28.9) | 150 (29.9) |
| Hispanic or Latino | 13 (6.1) | 28 (14.1) | 15 (16.7) | 56 (11.2) |
| White | 129 (60.6) | 107 (54.0) | 48 (53.3) | 284 (56.7) |
| Otherd | 16 (7.5) | 35 (17.7) | 16 (17.8) | 67 (13.4) |
| Educational levele | ||||
| <High school | 41 (19.2) | 19 (9.6) | 11 (12.2) | 71 (14.2) |
| High school diploma or GED | 55 (25.8) | 28 (14.1) | 14 (15.6) | 97 (19.4) |
| Some college | 112 (52.6) | 36 (18.2) | 23 (25.6) | 171 (34.1) |
| Unknown | 5 (2.3) | 115 (58.1) | 42 (46.7) | 162 (32.3) |
| Medical history at enrollment | ||||
| BMI, median (IQR) | 28 (23-33) | 27 (23-33) | 27 (23-33) | 27 (23-33) |
| Diabetes | 64 (30.0) | 61 (30.8) | 37 (41.1) | 162 (32.3) |
| Cardiovascular disease | 98 (46.0) | 117 (59.1) | 40 (44.4) | 255 (50.9) |
| Respiratory disease | 49 (23.0) | 46 (23.2) | 16 (17.8) | 111 (22.2) |
| Current cancer | 28 (13.1) | 54 (27.3) | 14 (15.6) | 96 (19.2) |
| Neurological disease | 41 (19.2) | 38 (19.2) | 15 (16.7) | 94 (18.8) |
| APACHE II score, median (IQR)f | 25 (19-31) | 30 (25-35) | 24.5 (18-33) | 27 (21-33) |
| SOFA score, median (IQR)g | 8 (6-10) | 10 (8-13) | 8 (6-11) | 9 (7-12) |
| Organ support at enrollment | ||||
| Vasopressor | 93 (43.7) | 55 (27.8) | 42 (46.7) | 190 (37.9) |
| Ventilator | 48 (22.5) | 35 (17.7) | 20 (22.2) | 103 (20.6) |
| Both | 72 (33.8) | 108 (54.5) | 27 (30.0) | 207 (41.3) |
| Days on mechanical ventilation, median (IQR) | 0 (0-3) | 1 (0-9) | 0 (0-2) | 0 (0-4) |
| ICU admission source | ||||
| Emergency | 145 (68.1) | 132 (66.7) | 76 (84.4) | 353 (70.5) |
| Hospital floor | 34 (16.0) | 45 (22.7) | 8 (8.9) | 87 (17.4) |
| Step-down unit | 13 (6.1) | 3 (1.5) | 1 (1.1) | 17 (3.4) |
| Intermediate care | 1 (0.5) | 3 (1.5) | 0 | 4 (0.8) |
| Otherh | 20 (9.4) | 15 (7.6) | 5 (5.6) | 40 (8.0) |
| Length of ICU stay, d | 3.00 (2.00-6.00) | 5.00 (2.00-12.00) | 3.00 (2.00-6.00) | 4.00 (2.00-8.00) |
| Admission reason | ||||
| Sepsis | 149 (70.0) | 137 (69.2) | 70 (77.8) | 356 (71.1) |
| Other medical | 53 (24.9) | 59 (29.8) | 18 (20.0) | 130 (25.9) |
| Other surgical | 11 (5.2) | 2 (1.0) | 2 (2.2) | 15 (3.0) |
| Coma- or delirium-free days, median (IQR) | 5 (2-5) | 3 (1-5) | 5(3-5) | 4 (2-5) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation, version 2; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GED, General Educational Development; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
Patients who were ineligible for or lost to follow-up included 4 who withdrew, 26 who declined follow-up assessment, 14 who did not consent to follow-up assessment, 1 who was incarcerated at the time of follow-up contact, and 45 who could not be contacted during their follow-up window despite multiple attempts.
In a previous report36 of primary outcome measures, 196 participants died at 180 days. An additional 2 participants died prior to the end of their 5- to 8-month window for completion of cognitive, psychological, and functional assessments.
Race and ethnicity were identified by self-report or researcher observation.
Other race and ethnicity included American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, mixed race, and other.
Educational level was reported by patient or surrogate during interview at enrollment and at follow-up. Unknown means the respondent did not know the highest educational level attained by the participant.
The APACHE II score ranges from 0 to 71, with higher scores indicating greater risk of hospital death. A score of 25 indicates a mortality probability of approximately 50%.
The SOFA score ranges from 0 to 24, with higher scores indicating greater severity of organ dysfunction. A score between 7 and 9 is associated with a 40% to 50% mortality risk.
Other included outside hospital, operating suite, inpatient rehabilitation unit, oncology unit, another ICU, emergency department observation unit, nursing home, and urgent care.
Table 2. Characteristics of Included Patients Stratified by Group.
| Characteristic | No. (%) | |
|---|---|---|
| Control group | Intervention group | |
| No. of participants | 105 | 108 |
| Age, median (IQR), y | 56 (46-66) | 59 (51-68) |
| Sex | ||
| Male | 53 (50.5) | 59 (54.6) |
| Female | 52 (49.5) | 49 (45.4) |
| Race and ethnicitya | ||
| Black | 37 (35.2) | 31 (28.7) |
| Hispanic or Latino | 7 (6.7) | 6 (5.6) |
| White | 57 (54.3) | 72 (66.7) |
| Otherb | 11 (10.5) | 5 (4.6) |
| Educational levelc | ||
| <High school | 18 (17.1) | 23 (21.3) |
| High school diploma or GED | 31 (29.5) | 24 (22.2) |
| Some college | 54 (51.4) | 58 (53.7) |
| Unknown | 2 (1.9) | 3 (2.8) |
| Medical history at enrollment | ||
| BMI, median (IQR) | 29 (23-34) | 28 (24-33) |
| Diabetes | 29 (27.6) | 35 (32.4) |
| Cardiovascular disease | 46 (43.8) | 52 (48.1) |
| Respiratory disease | 23 (21.9) | 26 (24.1) |
| Current cancer | 16 (15.2) | 12 (11.1) |
| Neurological disease | 22 (21.0) | 19 (17.6) |
| APACHE II score, median (IQR)d | 25 (16-30) | 25 (20-32) |
| SOFA scoree | 8 (5-10) | 8.5 (6-11) |
| Organ support at enrollment | ||
| Vasopressor | 29 (27.6) | 19 (17.6) |
| Ventilator | 46 (43.8) | 47 (43.5) |
| Both | 30 (28.6) | 42 (38.9) |
| Days on mechanical ventilation, median (IQR) | 0 (0-3) | 0 (0-3) |
| ICU admission source | ||
| Emergency | 74 (70.5) | 71 (65.7) |
| Hospital floor | 15 (14.3) | 19 (17.6) |
| Step-down unit | 7 (6.7) | 6 (5.6) |
| Intermediate care | 1 (0.5) | 0 |
| Otherf | 8 (7.6) | 12 (11.1) |
| Length of ICU stay, median (IQR), d | 3 (2-6) | 4 (2-6) |
| Admission reason | ||
| Sepsis | 73 (69.5) | 76 (70.4) |
| Other medical | 28 (26.7) | 25 (23.1) |
| Other surgical | 4 (3.8) | 7 (6.5) |
| Coma- or delirium-free days, median (IQR) | 5 (3-5) | 4 (2-5) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation, version 2; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GED, General Educational Development; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
Race and ethnicity were identified by self-report or researcher observation.
Other race and ethnicity included American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, mixed race, and other.
Educational level was reported by patient or surrogate during interview at enrollment and at follow-up. Unknown means the respondent did not know the highest educational level attained by the participant.
APACHE II score ranges from 0 to 71, with higher scores indicating greater risk of hospital death. A score of 25 indicates a mortality probability of approximately 50%.
SOFA score ranges from 0 to 24, with higher scores indicating greater severity of organ dysfunction. A score between 7 and 9 is associated with a 40% to 50% mortality risk.
Other included outside hospital, operating suite, inpatient rehabilitation unit, oncology unit, another ICU, and emergency department observation unit.
Cognitive Outcomes
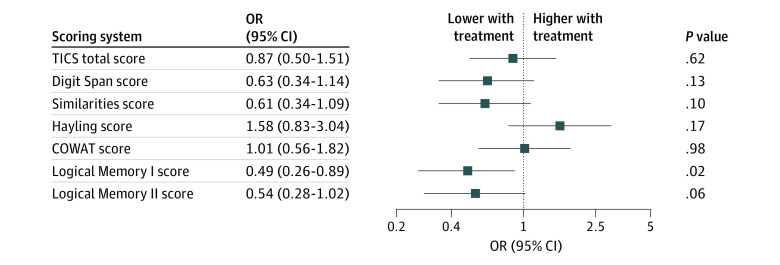
Cognitive outcomes at 6 months are provided in eTable 3 in Supplement 1, and treatment effects are summarized in Figure 2 and eAppendix 3 in Supplement 1. None of the participants who underwent cognitive assessment had delirium at the time of assessment. Treatment with vitamin C, thiamine, and hydrocortisone was generally associated with worse cognitive performance, with statistically significant worsening of immediate memory (Logical Memory I score; adjusted OR [aOR], 0.49; 95% CI, 0.26-0.89; P = .02) (Figure 2). The point estimate for the treatment effect was below 1 for all other cognitive assessments (range, 0.49-0.87) except Hayling Sentence Completion Test (aOR, 1.58; 95% CI, 0.83-3.04; P = .17) and Controlled Oral Word Association Test (aOR, 1.01; 95% CI, 0.56-1.82; P = .98) (Figure 2). Complete case analyses yielded similar effect sizes for all cognitive outcomes (eAppendix 4 and eTable 4 in Supplement 1).
Figure 2. Adjusted Odds Ratios (aORs) for Improvement in 6-Month Cognitive Outcomes With Treatment.
Participants in the intervention group had worse scores on the Weschler Adult Intelligence Scale IV Logical Memory I subtest (aOR, 0.49; 95% CI, 0.26-0.89), which measures immediate (short-term) memory. Effect sizes for scores on all other cognitive assessments except the Hayling Sentence Completion Test and the Controlled Oral Word Association Test (COWAT) were less than 1, suggesting a tendency toward worse cognitive outcomes with treatment. TICS indicates Telephone Interview for Cognitive Status.
Psychological and Functional Outcomes
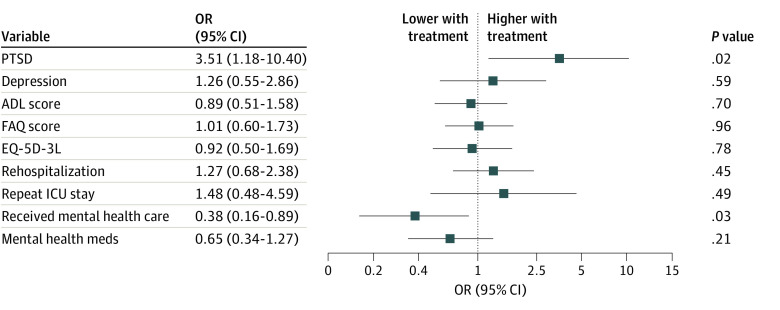
The ORs for adverse psychological and functional outcomes are depicted in Figure 3, with counts for each group provided in eTable 3 in Supplement 1 and probabilities plotted in eAppendix 3 in Supplement 1. We detected PTSD in 10 of 105 (9.5%) control participants and 18 of 108 (16.7%) participants in the intervention group (eTable 3 in Supplement 1). Treatment with vitamin C, thiamine, and hydrocortisone was associated with increased odds of PTSD (aOR, 3.51; 95% CI, 1.18-10.40; P = .02) (Figure 3). Depression was detected in 50 of 142 (35.2%) participants who completed the PROMIS-6. There was no statistical evidence of a treatment effect on depression (aOR, 1.26; 95% CI, 0.55-2.86; P = .59) (Figure 3).
Figure 3. Adjusted Odds Ratios (aORs) for 6-Month Psychological and Functional Outcomes With Treatment.
Participants in the intervention group had higher odds of having a positive screening result for PTSD (aOR, 3.51; 95% CI, 1.18-10.40) and lower odds of receiving mental health care (aOR, 0.38; 95% CI, 0.16-0.89). Symptom burden (ie, the number of related symptoms reported) and functional capabilities were roughly equivalent between the groups. ADL indicates activities of daily living; EQ-5D-3L, EuroQoL 5-Dimensions 3-Level; FAQ, Functional Activities Questionnaire; ICU, intensive care unit; and PTSD, posttraumatic stress disorder.
Functional status did not differ between the intervention and control groups. The aOR was 0.89 (95% CI, 0.51-1.58; P = .70) for the Katz Activities of Daily Living Scale, 1.01 (95% CI, 0.60-1.73; P = .96) for the Functional Activities Questionnaire, and 0.92 (95% CI, 0.50-1.69; P = .78) for overall health status on the EQ-5D-3L. The complete case analysis for psychological and functional outcomes yielded similar results (eAppendix 4 and eTable 4 in Supplement 1).
Health Care Use
The odds of rehospitalization were similar between the intervention and control groups (aOR, 1.09; 95% CI, 0.56-2.12; P = .79) (eTable 4 in Supplement 1). Participants in the intervention group had lower odds than those in the control group of receiving formal psychiatric, psychological, or mental health care during the 6 months after discharge (aOR, 0.38; 95% CI, 0.16-0.89; P = .03) (Figure 3). The intervention group also had lower odds than the control group of reporting medication use for depression or anxiety, although this difference was not statistically significant (aOR, 0.65; 95% CI, 0.34-1.27; P = .21) (Figure 3).
Discussion
In this prespecified secondary analysis of sepsis survivors in the VICTAS trial, we found no evidence of the benefit of early treatment with vitamin C, thiamine, and hydrocortisone on 6-month cognitive, psychological, and functional outcomes. On the contrary, participants in the intervention group had lower immediate memory scores and higher odds of PTSD compared with participants in the control group. These findings refute the hypothesis that antioxidant and anti-inflammatory therapy during critical illness might mitigate the development of long-term, PICS-related disabilities in survivors of sepsis. The absence of benefit is consistent with results of prior randomized clinical trials that found no improvement in mortality and other hospital-based outcomes with vitamin C therapy.23,31,32,33,34 Yet the observation of possible long-term concerns among participants in the intervention group is a signal that warrants caution. Fewer participants in the intervention group than in the control group received mental health care despite these participants more frequently demonstrating possible PTSD. Future studies evaluating the association between acute treatment and longer-term outcomes should consider whether intercurrent therapy is a confounding factor or an outcome in and of itself.
The cognitive scores of both the intervention and control groups were similar in magnitude to those reported in other cohorts of ICU survivors.47,48 The median differences in scores for the cognitive assessments were 1 to 2 points on scales ranging from 0 to 10 or 20 (range of 0-100 in Controlled Oral Word Association Test), reflecting a relatively modest potential effect of the intervention on 6-month cognitive outcome. The rates of depression, PTSD, and functional disability were also commensurate with those previously reported49,50 and consistent with the outcomes typically observed in PICS.51,52,53
Could antioxidant-based therapy truly play a role in increased risk of adverse cognitive, psychological, and functional sequelae among sepsis survivors? The increased odds of PTSD that was observed in the intervention group may have been mediated less by the role of vitamin C as an antioxidant and more by its modulation of neurotransmitter metabolism in the brain. Vitamin C facilitates the conversion of dopamine to norepinephrine (also known as noradrenaline).54,55 The latter neurotransmitter is the primary messenger of the locus coeruleus, which mediates physical and emotional responses to stress56,57 and facilitates the consolidation of aversive memories.58,59 Blockade of norepinephrine receptors enables fear extinction in animals that are subjected to stressful stimuli,60 reinforcing the hypothesis that norepinephrine transmission plays an important role in the pathogenesis of PTSD.61 If the prevention of vitamin C deficiency during sepsis preserved norepinephrine metabolism, it is possible that this process affected the strength of aversive memory formation during the stressful ICU experience, leading to higher odds of PTSD. Coadministration of hydrocortisone with norepinephrine potentiates fear memory responses62 and may have acted synergistically to increase risk of PTSD in the VICTAS trial participants. Alternatively, exogenous norepinephrine and/or corticosteroids may have played a role. A post hoc review of the clinical data revealed that 73.7% of participants in the follow-up cohort (68.6% in the control group, and 78.7% in the intervention group) received parenteral norepinephrine; 31.0% received a concomitant open-label corticosteroid. The differential roles of exogenous vs endogenous neurotransmitters in PTSD pathogenesis have yet to be elucidated. The lower rate of mental health care and higher rate of rehospitalization in the intervention group may also have influenced the risk of PTSD. Regardless of whether the difference in outcomes between groups is directly associated with treatment, the observed rate of PTSD (13.1% [28 of 213 participants in the follow-up cohort) was higher than the past-year prevalence of 3.5% to 6.1% in the general population,63,64 lending further support to the Society of Critical Care Medicine recommendations for post-ICU screening of PTSD and other PICS-related symptoms.65
Putative mechanisms of postsepsis cognitive decline include intracerebral oxidative stress, inflammation, and endothelial dysfunction.66 Vitamin C, hydrocortisone, and thiamine protect neurons from oxidative damage and stem the systemic inflammatory response and thus were expected to decrease the burden of long-term neurological compromise.16 Yet we found no clinical evidence of such an effect in this trial. It is possible that the dose, timing, or duration of therapy was insufficient to provide adequate cellular protection. The intervention was limited to the ICU stay (median of 3 days among the follow-up cohort). Mouse models of sepsis suggest that, although vitamin C depletion occurs during the acute illness period,19 there are distinct phases of the neuroinflammatory response after injury.67 Long-term effects are observed in the central nervous system after continuous activation of microglial cells.68,69 Individuals may depend on prolonged vitamin C repletion throughout the recovery period to counteract the oxidative burden and protect against postinjury cognitive decline. Future studies with long-term vitamin C supplementation after sepsis may test this hypothesis.
Sedation and paralysis practices—in particular, number of ICU days with opiates or neuromuscular blockade—may contribute to psychiatric symptoms after critical illness.70 Benzodiazepines are factors in increased risk of ICU delirium,71 which is also associated with long-term cognitive impairment.47 Daily sedation interruption and similar modern critical care bundles and practices limit this risk.72 Future studies evaluating the effect of acute therapeutic measures on long-term cognitive and psychiatric outcomes should track adherence to Society of Critical Care Medicine guidelines on the management of pain, agitation, and delirium.73
Limitations
This study has several limitations. First, interpretation was restricted to those who survived sepsis. How survivorship influenced the interpretation of results was unclear, although understanding the differences in patient-reported outcomes based on survivorship remains valuable. Second, a common problem among studies of cognitive and psychological outcomes after critical illness is the unplanned nature of inclusion and the resultant difficulty in adequately assessing for premorbid cognitive and psychological disorders. Despite randomization, there could be baseline differences in cognitive measures among participants in the follow-up cohort that cannot be quantified or controlled in the analysis. We did not collect baseline cognitive or psychological data, which warrants the cautious interpretation of the results.
Third, 40 of 303 eligible participants (13.2%) did not consent to follow-up assessment. The fact that trial participants were not automatically included in this secondary analysis and had to express willingness to participate may have increased attrition. Furthermore, the VICTAS trial was administratively terminated due to withdrawal of funding, leading to a truncated follow-up window for a small proportion (4.6%) of eligible participants. Nonetheless, we were able to obtain at least partial follow-up assessments for approximately 85% of eligible participants, and these sepsis survivors did not differ in characteristics from those who withdrew, refused assessment, or were ultimately lost to follow-up. Some participants were unable or unwilling to complete a few of the assessments, and the rates of missing data reached nearly 25% for several assessments. Among participants who were included in the follow-up cohort, the most common reason for failure to complete an assessment was patient refusal (eTable 2 in Supplement 1). The rates of missing data were similar between the intervention and control groups, and a separate analysis using a complete case approach yielded similar findings. These observations suggest that completion rates did not significantly affect the overall comparisons. Fourth, for pragmatic purposes, we selected brief screening tools as psychological outcome measures. Although these tools are well accepted and have high sensitivity and specificity for detection of PTSD and depression, formal diagnosis requires in-depth evaluation by a trained mental health professional.
Conclusions
In the VICTAS trial, for survivors of sepsis who were treated early with vitamin C, thiamine, and hydrocortisone in the ICU, the cognitive, psychological, and functional outcomes at the 6-month follow-up either were unaffected by the regimen or were worse than for patients who received placebo. These results do not support antioxidant and anti-inflammatory therapy and suggest that antioxidant and anti-inflammatory therapy does not mitigate the development of long-term cognitive, psychological, and functional impairment in patients with sepsis who require cardiovascular or respiratory support in the ICU.
eAppendix 1. Descriptions and Scoring Methods of Outcome Assessment Tools
eTable 1. Demographic and Clinical Characteristics of the Full Cohort of Included Participants, Stratified by Treatment Group
eAppendix 2. Missingness and Reasons for Assessment Non-Completion
eTable 2. Reasons for Assessment Non-Completion
eTable 3. Outcomes at 6 Months in Sepsis Survivors With and Without Vitamin C, Thiamine & Hydrocortisone Therapy
eAppendix 3. Evaluation of the Proportional Odds Assumption and Probability Plots
eAppendix 4. Adjusted Models (Complete Case Analysis)
eTable 4. Effect of Vitamin C, Thiamine & Hydrocortisone on 6-Month Outcomes After Sepsis
eReferences
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchman TG, Simpson SQ, Sciarretta KL, et al. Sepsis among Medicare beneficiaries: 1. the burdens of sepsis, 2012-2018. Crit Care Med. 2020;48(3):276-288. doi: 10.1097/CCM.0000000000004224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee C, Dantes R, Epstein L, et al. ; CDC Prevention Epicenter Program . Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241-1249. doi: 10.1001/jama.2017.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-1794. doi: 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yende S, Austin S, Rhodes A, et al. Long-term quality of life among survivors of severe sepsis: analyses of two international trials. Crit Care Med. 2016;44(8):1461-1467. doi: 10.1097/CCM.0000000000001658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276-1283. doi: 10.1097/CCM.0b013e3181d8cc1d [DOI] [PubMed] [Google Scholar]
- 7.Semmler A, Widmann CN, Okulla T, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84(1):62-69. doi: 10.1136/jnnp-2012-302883 [DOI] [PubMed] [Google Scholar]
- 8.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486-552. doi: 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 9.Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063-e1143. doi: 10.1097/CCM.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 10.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502-509. doi: 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 11.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195-203. doi: 10.1016/j.molmed.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 12.Hernandes MS, D’Avila JC, Trevelin SC, et al. The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J Neuroinflammation. 2014;11:36. doi: 10.1186/1742-2094-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36(10):605-613. doi: 10.1016/j.it.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lochhead JJ, McCaffrey G, Quigley CE, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30(9):1625-1636. doi: 10.1038/jcbfm.2010.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galea I. The blood-brain barrier in systemic infection and inflammation. Cell Mol Immunol. 2021;18(11):2489-2501. doi: 10.1038/s41423-021-00757-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison FE, Bowman GL, Polidori MC. Ascorbic acid and the brain: rationale for the use against cognitive decline. Nutrients. 2014;6(4):1752-1781. doi: 10.3390/nu6041752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrada L, Barahona MJ, Salazar K, Vandenabeele P, Nualart F. Vitamin C controls neuronal necroptosis under oxidative stress. Redox Biol. 2020;29:101408. doi: 10.1016/j.redox.2019.101408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns JJ. Missing step in man, monkey and guinea pig required for the biosynthesis of L-ascorbic acid. Nature. 1957;180(4585):553. doi: 10.1038/180553a0 [DOI] [PubMed] [Google Scholar]
- 19.Consoli DC, Jesse JJ, Klimo KR, et al. A cecal slurry mouse model of sepsis leads to acute consumption of vitamin C in the brain. Nutrients. 2020;12(4):911. doi: 10.3390/nu12040911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armour J, Tyml K, Lidington D, Wilson JX. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol (1985). 2001;90(3):795-803. doi: 10.1152/jappl.2001.90.3.795 [DOI] [PubMed] [Google Scholar]
- 21.Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21(1):300. doi: 10.1186/s13054-017-1891-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann I, Briegel J, Schliephake F, et al. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization-dependent neutrophil functions. Intensive Care Med. 2008;34(2):344-349. doi: 10.1007/s00134-007-0868-8 [DOI] [PubMed] [Google Scholar]
- 23.Fowler AA III, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261-1270. doi: 10.1001/jama.2019.11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest. 2017;152(5):954-962. doi: 10.1016/j.chest.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker WH, Rhea EM, Qu ZC, Hecker MR, May JM. Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am J Physiol Cell Physiol. 2016;311(4):C652-C662. doi: 10.1152/ajpcell.00076.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuck JL, Bastarache JA, Shaver CM, et al. Ascorbic acid attenuates endothelial permeability triggered by cell-free hemoglobin. Biochem Biophys Res Commun. 2018;495(1):433-437. doi: 10.1016/j.bbrc.2017.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plevin D, Galletly C. The neuropsychiatric effects of vitamin C deficiency: a systematic review. BMC Psychiatry. 2020;20(1):315. doi: 10.1186/s12888-020-02730-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229-1238. doi: 10.1016/j.chest.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 29.de Andrade JAA, Gayer CRM, Nogueira NPA, et al. The effect of thiamine deficiency on inflammation, oxidative stress and cellular migration in an experimental model of sepsis. J Inflamm (Lond). 2014;11:11. doi: 10.1186/1476-9255-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson GE, Hirsch JA, Fonzetti P, Jordan BD, Cirio RT, Elder J. Vitamin B1 (thiamine) and dementia. Ann N Y Acad Sci. 2016;1367(1):21-30. doi: 10.1111/nyas.13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii T, Luethi N, Young PJ, et al. ; VITAMINS Trial Investigators . Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. 2020;323(5):423-431. doi: 10.1001/jama.2019.22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskowitz A, Huang DT, Hou PC, et al. ; ACTS Clinical Trial Investigators . Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial. JAMA. 2020;324(7):642-650. doi: 10.1001/jama.2020.11946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang SY, Ryoo SM, Park JE, et al. ; Korean Shock Society (KoSS) . Combination therapy of vitamin C and thiamine for septic shock: a multi-centre, double-blinded randomized, controlled study. Intensive Care Med. 2020;46(11):2015-2025. doi: 10.1007/s00134-020-06191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamontagne F, Masse MH, Menard J, et al. ; LOVIT Investigators and the Canadian Critical Care Trials Group . Intravenous vitamin C in adults with sepsis in the intensive care unit. N Engl J Med. 2022;386(25):2387-2398. doi: 10.1056/NEJMoa2200644 [DOI] [PubMed] [Google Scholar]
- 35.Hager DN, Hooper MH, Bernard GR, et al. The Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) protocol: a prospective, multi-center, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials. 2019;20(1):197. doi: 10.1186/s13063-019-3254-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sevransky JE, Rothman RE, Hager DN, et al. ; VICTAS Investigators . Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. JAMA. 2021;325(8):742-750. doi: 10.1001/jama.2020.24505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsell CJ, McGlothlin A, Nwosu S, et al. Update to the Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) protocol: statistical analysis plan for a prospective, multicenter, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials. 2019;20(1):670. doi: 10.1186/s13063-019-3775-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christie JD, Biester RC, Taichman DB, et al. Formation and validation of a telephone battery to assess cognitive function in acute respiratory distress syndrome survivors. J Crit Care. 2006;21(2):125-132. doi: 10.1016/j.jcrc.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 39.Marcantonio ER, Michaels M, Resnick NM. Diagnosing delirium by telephone. J Gen Intern Med. 1998;13(9):621-623. doi: 10.1046/j.1525-1497.1998.00185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandt J, Spencer M, Folstein MF. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111-117. [Google Scholar]
- 41.Hansen M, Andersen TE, Armour C, Elklit A, Palic S, Mackrill T. PTSD-8: a short PTSD inventory. Clin Pract Epidemiol Ment Health. 2010;6:101-108. doi: 10.2174/1745017901006010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group . The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol Assess. 2014;26(2):513-527. doi: 10.1037/a0035768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged—the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 45.Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323-329. doi: 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- 46.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343. doi: 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 47.Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators . Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316. doi: 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sukantarat KT, Burgess PW, Williamson RC, Brett SJ. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia. 2005;60(9):847-853. doi: 10.1111/j.1365-2044.2005.04148.x [DOI] [PubMed] [Google Scholar]
- 49.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50-56. doi: 10.1164/ajrccm.160.1.9708059 [DOI] [PubMed] [Google Scholar]
- 50.Jackson JC, Pandharipande PP, Girard TD, et al. ; Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) Study Investigators . Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369-379. doi: 10.1016/S2213-2600(14)70051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5(2):90-92. doi: 10.1515/jtim-2016-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marra A, Pandharipande PP, Girard TD, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018;46(9):1393-1401. doi: 10.1097/CCM.0000000000003218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jutte JE, Erb CT, Jackson JC. Physical, cognitive, and psychological disability following critical illness: what is the risk? Semin Respir Crit Care Med. 2015;36(6):943-958. doi: 10.1055/s-0035-1566002 [DOI] [PubMed] [Google Scholar]
- 54.Diliberto EJ Jr, Allen PL. Mechanism of dopamine-beta-hydroxylation: semidehydroascorbate as the enzyme oxidation product of ascorbate. J Biol Chem. 1981;256(7):3385-3393. doi: 10.1016/S0021-9258(19)69620-1 [DOI] [PubMed] [Google Scholar]
- 55.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009;46(6):719-730. doi: 10.1016/j.freeradbiomed.2008.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCall JG, Al-Hasani R, Siuda ER, et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605-620. doi: 10.1016/j.neuron.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daviu N, Bruchas MR, Moghaddam B, Sandi C, Beyeler A. Neurobiological links between stress and anxiety. Neurobiol Stress. 2019;11:100191. doi: 10.1016/j.ynstr.2019.100191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90(3):576-579. doi: 10.1016/j.nlm.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76(1):130-141. doi: 10.1016/j.neuron.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald PJ, Giustino TF, Seemann JR, Maren S. Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proc Natl Acad Sci U S A. 2015;112(28):E3729-E3737. doi: 10.1073/pnas.1500682112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giustino TF, Maren S. Noradrenergic modulation of fear conditioning and extinction. Front Behav Neurosci. 2018;12:43. doi: 10.3389/fnbeh.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gazarini L, Stern CA, Takahashi RN, Bertoglio LJ. Interactions of noradrenergic, glucocorticoid and endocannabinoid systems intensify and generalize fear memory traces. Neuroscience. 2022;497:118-133. doi: 10.1016/j.neuroscience.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 63.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein RB, Smith SM, Chou SP, et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. 2016;51(8):1137-1148. doi: 10.1007/s00127-016-1208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mikkelsen ME, Still M, Anderson BJ, et al. Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness. Crit Care Med. 2020;48(11):1670-1679. doi: 10.1097/CCM.0000000000004586 [DOI] [PubMed] [Google Scholar]
- 66.Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13(6):630-636. doi: 10.1016/S1474-4422(14)70017-1 [DOI] [PubMed] [Google Scholar]
- 67.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witcher KG, Eiferman DS, Godbout JP. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci. 2015;38(10):609-620. doi: 10.1016/j.tins.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muscat SM, Barrientos RM. The perfect cytokine storm: how peripheral immune challenges impact brain plasticity & memory function in aging. Brain Plast. 2021;7(1):47-60. doi: 10.3233/BPL-210127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang M, Parker AM, Bienvenu OJ, et al. ; National Institutes of Health, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network . Psychiatric symptoms in acute respiratory distress syndrome survivors: a 1-year national multicenter study. Crit Care Med. 2016;44(5):954-965. doi: 10.1097/CCM.0000000000001621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21-26. doi: 10.1097/00000542-200601000-00005 [DOI] [PubMed] [Google Scholar]
- 72.Bourenne J, Hraiech S, Roch A, Gainnier M, Papazian L, Forel JM. Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Ann Transl Med. 2017;5(14):291. doi: 10.21037/atm.2017.07.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873. doi: 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Descriptions and Scoring Methods of Outcome Assessment Tools
eTable 1. Demographic and Clinical Characteristics of the Full Cohort of Included Participants, Stratified by Treatment Group
eAppendix 2. Missingness and Reasons for Assessment Non-Completion
eTable 2. Reasons for Assessment Non-Completion
eTable 3. Outcomes at 6 Months in Sepsis Survivors With and Without Vitamin C, Thiamine & Hydrocortisone Therapy
eAppendix 3. Evaluation of the Proportional Odds Assumption and Probability Plots
eAppendix 4. Adjusted Models (Complete Case Analysis)
eTable 4. Effect of Vitamin C, Thiamine & Hydrocortisone on 6-Month Outcomes After Sepsis
eReferences
Nonauthor Collaborators
Data Sharing Statement