Abstract
Objectives:
To evaluate the prevalence of sarcopenia in patients referred to a Multidisciplinary Chronic Pancreatitis (CP) Clinic at the University Hospitals of Leicester.
Methods:
All patients who had undergone CT scans were identified. Controls were identified from CT colonograms with no features of malignancy or pancreatic pathology. The psoas muscle index (PMI) was calculated using the formula: total psoas muscle cross-sectional area at the third lumbar vertebral level (cm2)/ the patient’s height squared (m2). PMI cut-offs were <6.31cm2/m2 and <3.91cm2/m2 for males and females, respectively.
Results:
58 CP CT scans were available for analysis along with 62 control scans. 71.9% of CP patients had a PMI below the cut-off for their gender, compared to 45.2% of the controls. The mean PMI (±SD) for male CP patients and male controls were 5.54cm2/m2 (±1.60) and 6.73 cm2/m2 (±1.54), (P=0.0023). The mean PMI (±SD) for female CP patients and female controls were 3.82 cm2/m2 (+/-1.46) and 4.98 cm2/m2 (+/-1.43), (P=0.0021).
Conclusions:
CP patients had a mean PMI below the cut-off value, suggesting that CP patients are largely sarcopenic. As malnutrition is a significant feature of CP, optimisation of nutrition may help to ameliorate sarcopenia in CP patients.
Keywords: Chronic Pancreatitis, Frailty, Nutrition, Sarcopenia
Introduction
Sarcopenia is a syndrome characterised by a progressive reduction in skeletal muscle and strength. These characteristics are associated with adverse complications including impaired quality of life (QOL) and disability[1]. Loss of skeletal muscle mass and strength secondary to ageing alone can be described as ‘primary sarcopenia’; if it is due to another underlying disease process it can be described as ‘secondary sarcopenia’[2]. Predisposing factors include malnutrition, alcohol excess, limited physical mobilisation and cigarette smoking have been described. Sarcopenia must be differentiated from other syndromes such frailty and cachexia which is hampered by the absence of a widely accepted formal definition. This has prompted various publications from independent groups such as; the European Working Group on Sarcopenia in Older People (EWGSOP), the International Working Group on Sarcopenia (IWGS) and the European Society for Clinical Nutrition and Metabolism Special Interest Groups (ESPEN-SIG). The definitions published all demonstrate a consensus requiring low skeletal muscle mass and loss of muscle strength/function in the diagnosis. Utilisation of CT has been described in the literature for evaluating sarcopenia, however use of modalities such as whole-body dual-energy X-ray absorptiometry, bioelectrical impedance and functional tests such as grip dynamometry have also been described[3].
Chronic Pancreatitis is an inflammatory disease associated with a variety of characteristics including malnutrition secondary to pancreatic exocrine insufficiency. Sarcopenia is thought to be a frequent association with CP[4]. The syndrome is also frequently associated with the geriatric population with a prevalence estimated to be as high as 50% in individuals over the age of 80[5]. There has been little in the way of studies exploring the prevalence of sarcopenia in these patients so far. However, this topic is the subject of increasing research interest in the last few years, with recent reviews estimating the prevalence of sarcopenia amongst chronic pancreatitis patients to be between 17-62%[3,6]. In this patient group, sarcopenia was significantly associated with reduced quality of life, reduced survival, increased hospitalisation and worse surgical outcomes in patients undergoing total pancreatectomy with islet autotransplantation[4,7,8].
In June 2017, a dedicated, outpatient multidisciplinary CP clinic was set up at University Hospitals of Leicester NHS Trust. The aim of this clinic is to improve disease control and reduce emergency surgical admissions in this complex and often-multimorbid patient group. This study examined the prevalence of sarcopenia in patients referred to this clinic, with the aim of contributing to the emerging literature in this topic of study.
Materials and Methods
Patient selection
Patients were identified from attendances to a chronic pancreatitis specialist clinic, and were all newly enrolled. Sample size was determined by sequential enrolment into the clinic over a two-year period. Anthropometric data, in the form of body mass index (BMI), was collected at clinic appointments and cross checked with electronic hospital records. Functional assessments (such as grip dynamometry and timed up and go test) were not within the scope of our study. These patients were then subsequently included in the study if they fulfilled the criteria of available cross-sectional imaging undertaken for diagnostic or follow-up purposes. Clinic letters were reviewed for basic patient data, including smoking status.
Fifty eight patients with known chronic pancreatitis were retrospectively identified. Sixty two appropriate patients were identified for the control group. The studies were collated from patients referred to the colorectal cancer two week-wait pathway via primary care, where their eligibility for selection was determined by no identifiable features of malignancy and pancreatic pathology on their respective CT scans. BMI was classified according to the National Institute for Health and Care Excellence (NICE) guidelines[9].
Outcome variable
The scans were then assessed by two radiologists, one of whom is a board certified sub-specialist gastrointestinal radiologist. Regions of interest were drawn around the psoas muscles bilaterally at the L3 vertebral body as per accepted validated methods. The sum of the psoas muscle area was subsequently divided by the square of the corresponding individual’s recorded height in metres in order to determine the psoas muscle index. Psoas muscle index cut-off values were sourced from a retrospective study which calculated optimal cut-off values by receiver operating characteristic analysis for survival[10].
Statistical analysis
Continuous variables were described with mean and standard deviation or median and interquartile range (IQR). Categorical variables were frequency and percentages. When appropriate, parametric comparisons of continuous data were conducted with the Student’s t test while non-parametric comparisons were conducted with the Mann Whitney U test. When appropriate, comparisons of categorical data were conducted with the χ2 test. All data was analysed using GraphPad Prism software version 7.04 (GraphPad Software, San Diego, United States).
Results
Of the 60 patients enrolled in the clinic, the total number of chronic pancreatitis patients with suitable scans was 58, (40 males and 18 females). Three patients without appropriate recent CT imaging were excluded. The median (IQR) age of CP patients was 53.5 (44.5-60.5). The total number of controls was 62, 36 males and 26 females. The median age of controls was 62 (IQR 12.75).
Fifty scans were performed prior to clinic review, and median time between initial scan and subsequent clinic date was 125 (61-258.3) days. Of the scans performed following clinic enrolment, the median time between initial clinic and subsequent scan was 61.5 (28-120.8) days.
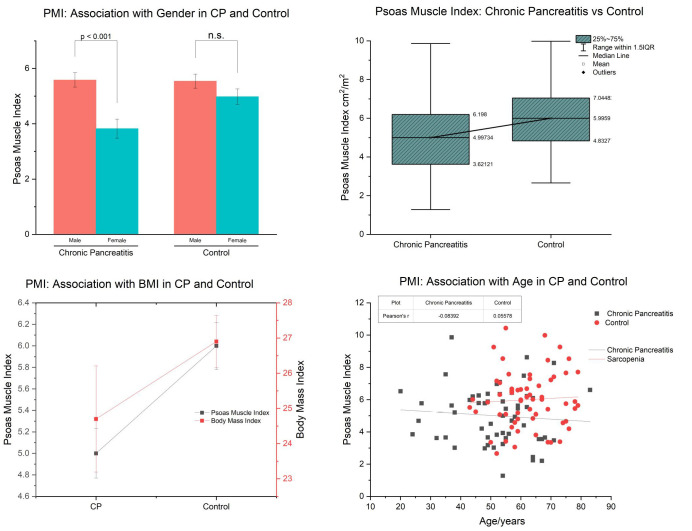
Basic demographic and anthropometric data are represented in Table 1. Nutritional parameters and management were described in Table 2. Using cut-off values of 6.31cm2/m2 for males and 3.91cm2/m2 for females, 70.7% of CP patients had a PMI (psoas muscle index) below the cut-off value for their respective gender, compared to 40.3% of the control group (p=0.0008) (Figure 1). The mean PMI (±SD) for male chronic pancreatitis patients and male controls were 5.54cm2/m2 (±1.60) and 6.73 cm2/m2 (±1.54), respectively (P=0.0023) (Table 3). The mean PMI (±SD) for female chronic pancreatitis patients and female controls were 3.82 cm2/m2 (+/-1.46) and 4.98 cm2/m2 (+/-1.43), respectively (P=0.0211) (Table 3). Of the CP patients with PMI below the gender-specific cut-off value, 17.5% were classified as underweight, 60% healthy weight and 22.5% classified as overweight, according to the NICE BMI classification.)
Table 1.
Patient demographics and Characteristics.
| Patient Demographics | CP (n=58) | Control (n=62) | P value |
|---|---|---|---|
| Gender | |||
| Male (%) | 40 (69) | 36 (58.1) | 0.216 |
| Age/years (IQR) | 53.5 (44.5-60.5) | 62 (55.75-69.25) | <0.0001 |
| BMI /kg/m2 (SEM) | 24.7 +/- 1.51 | 26.9 +/- 0.753 | 0.0011 |
| Co-morbidities (%) | |||
| Liver cirrhosis | 11 (19) | 2 (3.23)** | 0.0056 |
| DM* | 29 (50) | 9 (14.5) | <0.0001 |
| History of alcohol excess | 27 (46.6) | 2 (0.03) | <0.0001 |
| Smoking (%) | |||
| Current smoking | 28 (48.3) | *** | NA |
| Number of cigarettes per day (IQR) | 15 (15-20) | *** | NA |
CP Chronic pancreatitis, BMI body mass index, DM diabetes mellitus, NA not applicable.
Includes type 1 DM, type 2 DM and type 3c DM.
Other potentially relevant co-morbidities included: 1x Crohn’s disease and 1x oesophagitis.
Unable to acquire accurate data corresponding to parameter.
Table 2.
Nutritional Parameters in Chronic Pancreatitis group.
| CP (n=58) | |
|---|---|
| Pancreatic enzyme replacement therapy (PERT) | |
| PERT commenced prior to CT scan (%) | 22 (37.9) |
| PERT total daily dose/units* | 75000 (75,000 – 125,000) |
| PERT duration/months | 21 (6.75-37.5) |
| Nutritional supplements prior to CT scan (%) | 20 (34.5) |
| Serum vitamin and micronutrients | |
| Serum zinc/ umol/L (prevalence of deficiency/ %) | 9.45 +/- 0.33 (46.9) |
| Serum selenium/ umol/L (prevalence of deficiency/ %) | 0.646 +/- 0.0394 (78) |
| Serum vitamin D/ nmol/L (prevalence of deficiency/ %) | 37.3 +/- 2.81 (70.4) |
| Serum iron / µmol/L (prevalence of deficiency) | 13.1 +/- 1.78 (71.4) |
| Serum vitamin B12/ ng/L (prevalence of deficiency/ %) | 446 +/- 33.3 (5.77) |
| Serum folate/ ug/L (prevalence of deficiency/ %) | 8.06 +/- 0.865 (3.33) |
| Serum albumin g/L | 42.3 +/- 0.594 |
| Serum HbA1c IFCC (mmol/mol) | 49.6 +/- 2.97 |
| Serum vitamin A/ µmol/L (prevalence of deficiency/ %) | 1.65 +/- 0.0995 (11.1) |
| Serum vitamin E/ µmol/L (prevalence of deficiency/ %) | 22 +/- 1.16 (2.27) |
Derived from PERT dosages utilised for regular meals
Figure 1.
a) PMI in CP and control stratified by gender b) PMI in CP vs. Control c) PMI in CP vs. Control correlated with age d) PMI in CP vs. Control correlated with BMI.
Table 3.
The calculated psoas muscle index and prevalence of sarcopenia in the chronic pancreatitis and control group.
| Psoas muscle index (cm2/m2) Mean +/-SD | P value | Prevalence of sarcopenia (%) | P value | ||
|---|---|---|---|---|---|
| Total | CP | 5 +/- 1.74 | 0.0029 | 41 (70.7) | 0.0008 |
| Control | 6 +/- 1.72 | 25 (40.3) | |||
| Male | CP | 5.54 +/- 1.60 | 0.0023 | 29 (72.5) | 0.0244 |
| Control | 6.73 +/- 1.54 | 12 (66.7) | |||
| Female | CP | 3.82 +/- 1.46 | 0.0021 | 17 (47.2) | 0.0187 |
| Control | 4.98 +/- 1.43 | 8 (30.8) | |||
| CP | PERT | 5.07 +/- 1.91 | 0.658 | 16 (72.7) | 0.967 |
| No PERT | 4.89 +/- 1.47 | 26 (72.2) | |||
CP Chronic Pancreatitis, PERT Pancreatic Enzyme Replacement Therapy.
Discussion
Sarcopenia is an under-appreciated and under-recognised pathological process despite the wide-ranging negative outcomes associated with it. It has been shown to correlate with mortality, functional impairment, disability, reduced QOL and an appreciable increase in healthcare costs[11-14]. A 2004 United States study by Janssen et al., estimated the healthcare costs attributable to sarcopenia to be $18.5 billion in the year 2000 – a number that perhaps underestimates the cost today due to the aging population[15]. Identification of sarcopenia in CP and the risk factors that contribute to its development in this patient group will potentially identify therapies that positively impact outcomes.
In this study, we compared the psoas muscle index, derived from computerised tomography, in chronic pancreatitis patients and control with gender-specific PMI cut-off values to identify a potential association between chronic pancreatitis and sarcopenia. This, to our knowledge, is the first study in the available literature evaluating for sarcopenia in CP patients as compared to controls. We identified a significant association in males and females between chronic pancreatitis and low psoas muscle index which may suggest chronic pancreatitis is a risk factor for the development of sarcopenia. Sarcopenia is also observed as part of the normal aging process. Muscle mass decreases of 1-2% per year after the age of 50 have been reported[16]. Muscle strength also declines with age. The aetiology of age-related sarcopenia is thought to be multifactorial – it is suggested that immobility, malnutrition, and systemic inflammation (amongst other factors), contribute to sarcopenia development[17]. Chronic diseases such as chronic kidney disease, in part due to the release of proinflammatory cytokines, may also predispose to the development of sarcopenia[18]. By the same mechanism, it is reasonable to postulate a pro-inflammatory state (along with malnutrition and relative immobility due to chronic pain), which is often found in chronic pancreatitis, contributes to the development of sarcopenia[19].
The predominant imaging modalities used for assessing sarcopenia are currently dual-energy X-ray absorptiometry (DXA), computed tomography (CT), magnetic resonance imaging (MRI) and ultrasonography (US). Non-imaging methods have also been described, including bioelectrical impedance (BIA), which estimates total muscle mass by evaluating current conduction[20]. A known limitation of this modality is lack of standardised, consensus cut-off values at least in part due to appreciable variability with device and population[21].
Abdominal CT is a considered imaging examination due to being more frequently performed for diagnostic and follow-up purposes than other areas, such as thigh and neck, and therefore provides the largest dataset for retrospective analysis. The majority of evidence in literature for using CT to evaluate for sarcopenia comes from measurements of total abdominal wall musculature, with thigh musculature, psoas muscle and other areas including the neck and upper limb being less common[22].
Various methods of assessing muscle mass have been described, including skeletal muscle index (SMI)[23]. SMI is the most widely described measure in use for evaluating sarcopenia on CT, and is calculated by cross-sectional muscle area divided by height in metres^2. Typically, a single slice is taken at a reference site, for abdominal CT usually at L3 vertebral level, and the SMI is calculated. Studies have explored and demonstrated that measurement of SMI from a single image correlates with total body skeletal muscle volume[24].
Currently, there is a lack of established sarcopenia cut-off values on CT, which renders assessment challenging. The preferred cut-off values used in this study were referenced from a 2017 Yuri et al paper, in which receiver operator characteristics analysis was utilised to find the optimal psoas muscle index that correlated with a significant increase in mortality. A predictable weakness is the difference in demographics between this cohort and their cohort - however given the absence of contemporary values that correspond to the group of interest, a decision was made to use these values.
The results also suggest that low BMI in chronic pancreatitis patients does not necessarily correlate with low PMI. Almost two thirds of CP patients with a low PMI had a normal BMI, and indeed 22.5% were overweight according to the NICE classification. This has important ramifications for appropriate identification of sarcopenic CP patients, as the majority of these patients may appear to have a normal body composition on basic anthropometric measurement.
There is emerging evidence that optimisation of nutrition and exercise has the potential to treat sarcopenia. A 2017 review by Yoshimura et al. demonstrated there is evidence to suggest that effective exercise and nutrition may improve muscle strength and function[25]. Protein supplementation has been shown to improve muscle function and strength in sarcopenic patients[26]. The chronic pancreatitis multidisciplinary team clinic aims to address, amongst a number of other issues, the nutritional deficits that CP patients often have secondary to pancreatic exocrine insufficiency. Specialist dietetic input to the clinic facilitates the tailoring of pancreatic enzyme replacement regimes and nutrition plans to the individual CP patient. Further randomised controlled trials are needed to investigate the effect of diet and exercise on sarcopenia.
The cohorts were not matched by age and gender, which are two potentially significant confounders. The CP group were significantly younger than the control group, which perhaps renders the result more notable, as sarcopenia is known to develop with increasing age. Another limitation of this study is the absence of functional assessment or assessment of physical activity alongside measurement of skeletal muscle mass. Our study focussed on retrospectively investigating the relationship between CP and radiological evidence of sarcopenia, with a diagnostic cut-off value that predicts morbidity and mortality – as such functional outcomes were not assessed. Measures such as handgrip strength, the Short Physical Performance Battery, and gait speed have been described as methods of doing so. Our work highlights the need for widely-accepted CT cut-off values for sarcopenia in CP patients (which correspond with significantly reduced skeletal muscle function), in order to potentially utilise CT as an independent screening tool for sarcopenia. This will allow us to target individual CP patient with bespoke treatment regimens, which may include nutrition optimisation and focussed physical conditioning. Although a significant association between chronic pancreatitis and low PMI has been demonstrated here, a number of potential confounders such as disease aetiology, co-morbidity, psychosocial health and smoking exist, and would benefit from multivariate, prospective analysis in future with larger cohort sizes so as to limit bias, which is another drawback of our small retrospective study.
Conclusion
Sarcopenia is associated with several adverse outcomes in the literature. In the study, both male and female chronic pancreatitis patients had a mean PMI below the cut-off value, suggesting the patients in the CP clinic are largely sarcopenic. This is despite many patients having a normal BMI. Currently, the evidence base for the diagnosis and management of sarcopenia is limited. However, preliminary evidence indicates that optimising exercise and nutrition may be effective in treating sarcopenia. As malnutrition is a significant element of chronic pancreatitis, it is our hope that the optimisation of nutrition, facilitated by various members of the multidisciplinary team, will aid in the treatment of sarcopenia. Further work is needed to determine if rehabilitation and nutrition optimisation can reverse CP-associated sarcopenia.
Authors’ contributions
Study conception and design: NB, GG. Acquisition of data: TO, AM, JS, RB. Analysis and interpretation of data: TO, AM, JS, RB. Drafting of manuscript: TO, AM. Critical revision of manuscript: TO, AM, NB, GG, JS, RB.
Footnotes
Edited by: Yannis Dionyssiotis
References
- 1.Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177–80. [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia:European consensus on definition and diagnosis:Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bundred J, Thakkar RG, Pandanaboyana S. Systematic review of sarcopenia in chronic pancreatitis:prevalence, impact on surgical outcomes, and survival. Expert Rev Gastroenterol Hepatol. 2022;16(7):665–72. doi: 10.1080/17474124.2022.2091544. [DOI] [PubMed] [Google Scholar]
- 4.Olesen SS, Büyükuslu A, Køhler M, Rasmussen HH, Drewes AM. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology. 2019;19(2):245–51. doi: 10.1016/j.pan.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24(6):623–7. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuan LL, Dennison AR, Garcea G. Prevalence and Impact of Sarcopenia in Chronic Pancreatitis:A Review of the Literature. World J Surg. 2021;45(2):590–7. doi: 10.1007/s00268-020-05828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieliuniene E, Brøndum Frøkjær J, Pockevicius A, Kemesiene J, Lukosevičius S, Basevicius A, et al. CT- and MRI-Based Assessment of Body Composition and Pancreatic Fibrosis Reveals High Incidence of Clinically Significant Metabolic Changes That Affect the Quality of Life and Treatment Outcomes of Patients with Chronic Pancreatitis and Pancreatic Cancer. Medicina (Kaunas) 2019;55(10) doi: 10.3390/medicina55100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trikudanathan G, Feussom G, Teigen L, Munigala S, Price K, Dirweesh A, et al. Pre-operative Sarcopenia Predicts Low Islet Cell Yield Following Total Pancreatectomy with Islet Autotransplantation for Chronic Pancreatitis. J Gastrointest Surg. 2020;24(10):2423–30. doi: 10.1007/s11605-020-04687-3. [DOI] [PubMed] [Google Scholar]
- 9.Excellence NNIfHaC. Obesity:identification, assessment and management. 2014 [Google Scholar]
- 10.Yuri Y, Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, et al. Implication of Psoas Muscle Index on Survival for Hepatocellular Carcinoma Undergoing Radiofrequency Ablation Therapy. J Cancer. 2017;8(9):1507–16. doi: 10.7150/jca.19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7(3):290–8. doi: 10.1002/jcsm.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 13.Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13(1):3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 14.Beaudart C, Locquet M, Reginster JY, Delandsheere L, Petermans J, Bruyère O. Quality of life in sarcopenia measured with the SarQoL®:impact of the use of different diagnosis definitions. Aging Clin Exp Res. 2018;30(4):307–13. doi: 10.1007/s40520-017-0866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–5. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 16.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia:facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129–33. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty TJ. Invited review:Aging and sarcopenia. J Appl Physiol (1985) 2003;95(4):1717–27. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa S, Yakabe M, Akishita M. Age-related sarcopenia and its pathophysiological bases. Inflammation and Regeneration. 2016;36(1):17. doi: 10.1186/s41232-016-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domański M, Ciechanowski K. Sarcopenia:A Major Challenge in Elderly Patients with End-Stage Renal Disease. Journal of Aging Research. 2012;2012:754739. doi: 10.1155/2012/754739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis--part I:review of principles and methods. Clin Nutr. 2004;23(5):1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez MC, Barbosa-Silva TG, Heymsfield SB. Bioelectrical impedance analysis in the assessment of sarcopenia. Current Opinion in Clinical Nutrition &Metabolic Care. 2018;21(5) doi: 10.1097/MCO.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 22.Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography:A Systematic Review. J Gerontol A Biol Sci Med Sci. 2019;74(10):1671–8. doi: 10.1093/gerona/glz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenchik L, Boutin RD. Sarcopenia:Beyond Muscle Atrophy and into the New Frontiers of Opportunistic Imaging, Precision Medicine, and Machine Learning. Semin Musculoskelet Radiol. 2018;22(3):307–22. doi: 10.1055/s-0038-1641573. [DOI] [PubMed] [Google Scholar]
- 24.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes:estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97(6):2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for Treating Sarcopenia:A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J Am Med Dir Assoc. 2017;18(6):553.e1–e16. doi: 10.1016/j.jamda.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Anton SD, Hida A, Mankowski R, Layne A, Solberg LM, Mainous AG, et al. Nutrition and Exercise in Sarcopenia. Curr Protein Pept Sci. 2018;19(7):649–67. doi: 10.2174/1389203717666161227144349. [DOI] [PubMed] [Google Scholar]