Abstract
Sarcopenia was recently identified as an entity in the ICD-10 classification of October 2016. According to the recommendation of the European Working Group on Sarcopenia in Older People (EWGSOP2), sarcopenia is defined as low muscle strength and low muscle mass, while physical performance is used to categorize the severity of sarcopenia. In recent years, sarcopenia has become increasingly common in younger patients with autoimmune diseases such as Rheumatoid arthritis (RA). Due to the chronic inflammation caused by RA, patients have reduced physical activity, immobility, stiffness, and joint destruction and all of that lead to the loss of muscle mass, muscle strength, disability and significantly lowering the patients’ quality of life. This article is a narrative review about sarcopenia in RA, with a special focus in its pathogenesis and management.
Keywords: Autoimmune disease, Inflammation, Rheumatoid arthritis, Sarcopenia
Introduction
Over time, the loss of muscle mass and strength is expected, especially after the seventh decade of life, where it decreases by 20-40%[1]. There is a difference in the loss of muscle mass between men and women that could be attributed to the action of hormones such as testosterone and growth hormone[2]. The decrease of muscle mass, strength or even physical performance associated with ageing are comprehensively named “sarcopenia”[3]. Sarcopenia predisposes to increased mortality, particularly in the elderly, due to falls, fractures, and prolonged immobilization[4,5]. Although the definition of sarcopenia was originally intended for the elderly, in recent years younger patients with autoimmune diseases have been recognized with sarcopenia. Age-related sarcopenia without any known cause is called “primary sarcopenia”, while “secondary sarcopenia” occurs when one or more causes, such as Rheumatoid arthritis (RA), have been identified (Table 1)[6,7].
Table 1.
Sarcopenia categories by cause (Adapted from Dionyssiotis Y et al.[7] with permission).
| Categories | Examples |
|---|---|
| Primary sarcopenia | |
| Age-related sarcopenia | Without any known cause |
| Secondary sarcopenia | |
| Activity-related sarcopenia | Can result from bed rest, sedentary lifestyle, deconditioning or zero-gravity conditions. |
| Disease-related sarcopenia | Associated with advanced organ failure (heart, lung, liver, kidney, and brain), inflammatory disease (such as Rheumatoid arthritis) malignancy or endocrine disease |
| Nutrition-related sarcopenia | Associated with inadequate caloric intake, malabsorption, gastrointestinal disorders, drug induced anorexia |
It has been observed that in autoimmune diseases such as RA, there is a predisposition to sarcopenia. In this narrative review of articles published between 2010 and 2022, we examined the existence of sarcopenia in RA patients. As it is analyzed further in this review, this is mainly attributed to inflammation and to the deregulated immune response, the latter being an important risk factor for sarcopenia.
Definition and Diagnosis of Sarcopenia
In1989, Irwin Rosenberg was the first to notice a decrease in muscle mass and attempted to state the first definition of sarcopenia. He used the Greek language to indicate the definition of this condition, with the words “sarx” or sarka meaning skin and “penia” meaning scarcity. In the following years (2010) the European Working Group on Sarcopenia in the Elderly (EWGSOP) defined sarcopenia as “a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as poor physical quality life and death.” In 2019 the EWGSOP released the updated EWGSOP2 sarcopenia definition. The main differences are: 1) diagnosis requires documentation of low muscle strength and low muscle mass, while physical performance is used to categorize the severity of sarcopenia; and 2) new cut-off points were recommended. EWGSOP2 uses low muscle strength as the primary parameter of sarcopenia; muscle strength is presently the most reliable measure of muscle function. Specifically, sarcopenia is probable when low muscle strength is detected. A sarcopenia diagnosis is confirmed by the presence of low muscle quantity or quality. When low muscle strength, low muscle quantity/quality and low physical performance are all detected, sarcopenia is considered severe[8].
Large organizations have tried to establish the definition of sarcopenia. The most representative ones are the already mentioned European Working Group on Sarcopenia in the Elderly (EWGSOP), the European Society for Clinical Nutrition and Metabolism (ESPEN-SIG) and the International Working Group on Sarcopenia (IWGSOP)[9].
The definitions they provided are:
Sarcopenia is probable when low muscle strength is detected, confirmed by the presence of low muscle quantity or quality and when low muscle strength, low muscle quantity/quality and low physical performance are all detected, sarcopenia is considered severe. (EWGSOP2).
The presence of low skeletal muscle mass and low muscle strength (ESPEN-SIG).
The presence of low skeletal muscle mass and physical performance (IWGS).
Τhe Asian Working Group on Sarcopenia (AWGS), used the same definition as the EWGSOP but used a different cut-off value for Asians, as the cut-off values for EWGSOP were set in reference to Caucasian populations[10].
The EWGSOP has established guidelines for diagnosing sarcopenia in clinical practice with the help of imaging modalities such as computed tomography (CT scan), magnetic resonance imaging (MRI), dual energy X-ray absorption (DXA) and bioimpedance analysis (BIA). Low muscle mass and strength and/or poor physical performance are two requirements for the diagnosis. The dual energy X-ray absorptiometry (DXA) method is the gold standard for measuring muscle mass since it is economical, simple to use, and accurate. The appendicular skeletal muscle mass (ASMM) can be estimated since it measures the skeletal muscle mass of the four limbs. The DXA’s measurement of ASMM is useful for sarcopenia diagnosis. To study ASMM, a number representing patient measurement is required, hence sarcopenia can be quantified by either ASMM/height2 or skeletal muscle mass index (SMI) in kilograms per square meter (kg/m2).
Muscle strength is assessed using a handgrip dynamometer. Physical performance can be measured among other tests by gait speed, the Short Physical Performance Battery (SPPB), and the Timed-Up and Go test (TUG)[11]. The timed get-up-and-go test (TGUG) is a performance measurement that evaluates dynamic equilibrium by measuring the time required to complete a series of tasks (getting up from a chair, walk a short distance, turn around, go back and sit down again). The Short Physical Performance Battery (SPPB), one of the most reliable methods for assessing physical performance, is another exam. It comprises of a short series of tests intended to estimate how the lower limbs perform. There are three separate parts that make up this battery. The first test involves measuring balance in three different positions: walking with feet together for 10 seconds, walking in a semi-tandem position for 10 seconds (big toe side to heel bone), and walking in a tandem position once more for 10 seconds (toe behind the heel). The purpose of the second component is to evaluate a 4 meter linear gait speed. The final component examines the capability and time required to complete the sit-to-stand exercise five times in succession[12].
Rheumatoid arthritis (RA)
Musculoskeletal symptoms of RA, such as diffuse pain, articular swelling, and prolonged stiffness, discourage patients from physical exercise, leading to skeletal muscle atrophy. Most studies on the prevalence of sarcopenia in autoimmune diseases have included patients with RA and suggest that its prevalence is significantly higher in patients with RA[13].
A higher rate of sarcopenia was reported in the RA patients than in the control groups, estimated to be approximately 20-44%, depending on the diagnostic criteria[14]. In a prospective observational study involving 100 RA patients (the CHIKARA study), Masahiro Tada et al. (2018) estimated the rate of sarcopenia to be 28%[15], Mie Torii et al. 2019 in 388 women with RA in 37.1%[16] and Takeshi Mochizuki et al. 2019 in 240 RA patients aged over 65, in 29.6%[17]. Additionally, Yutaro Yamada et al. 2020 examined the presence of sarcopenia at the start of the study and one year later in 100 RA patients who were enrolled in the CHIKARA study. They discovered sarcopenia in 68 patients (68%) at disease onset and in 9 additional patients (13.4%) at the one-year later[18].
Rheumatoid arthritis and Sarcopenia
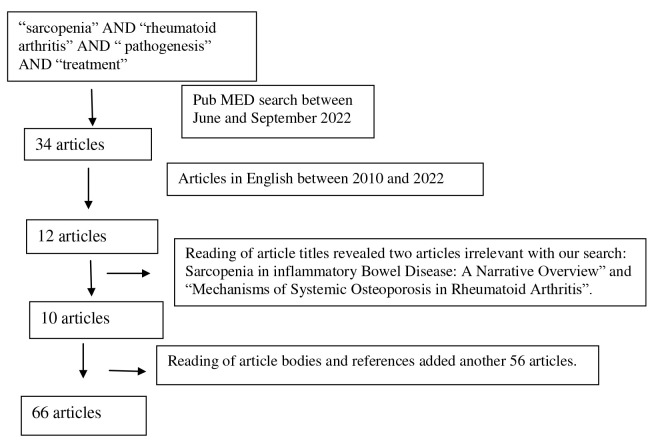
Between June and September 2022, we undertook a search in the PubMed database using the MeSH terms: “sarcopenia”, “rheumatoid arthritis”, “pathogenesis” and “treatment”. The search yielded 34 articles. The search was modified to include full text articles published between 2010 and 2022 and written in English. From the 12 articles that remained, two articles were excluded as their titles did not match our search criteria (“Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview” and “Mechanisms of Systemic Osteoporosis in Rheumatoid Arthritis”). After carefully reading the article bodies and their relevant references, another 56 articles came up to be included in our review. Finally, our review was based on 66 articles. The above are summarized in the following flow chart (Figure 1).
Figure 1.
Flow diagram of search results.
Rheumatoid arthritis is an autoimmune disease. It can manifest either as an oligoarticular syndrome with minimal joint damage or as a chronic progressive polyarthritis with reduced functional capacity. Patients with RA are at higher risk for sarcopenia with a reported prevalence ranging from17.1% to 60 %[19].
Delia Reina et al. 2019 in 89 women with RA with mean patient age 62±8 years, mean disease duration 14±9 months and mean disease activity score (DAS-28) 3.7±1.4 and mean Health Assessment Questionnaire 0.88±0.77 which assesses the functional capacity of the patients, who compared with 100 women with similar age without inflammatory rheumatic diseases, found a statistically lower lean body mass in all assessment sites, and a higher fat mass in RA patients compared to the control group. RA patients had a higher rate of sarcopenia (44% of patients and 19% of controls p<0.001)[20,21].
Compared to the general population, patients with RA are more likely to develop sarcopenia[22]. Giles et al. found that in women with RA and normal body mass index (BMI <25 kg/m2) the loss of lean muscle mass was three times higher than in healthy women. Their study also pointed that the loss of muscle mass was significantly associated with the presence of rheumatoid factor, joint deformities, increased Health Assessment Questionnaire Disability Index (HAQ DI) and increased C-reactive protein (CRP) as inflammation marker[23]. In addition to CRP, inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), which are involved in the pathogenesis of RA, are also thought to play an important role in the development of sarcopenia[24]. In addition other factors that can lead to sarcopenia are ageing, increased BMI, increased fat mass, longer disease duration, articular erosions, osteoporosis, malnutrition, low protein intake, serum rheumatoid factor (RF) and matrix metalloproteinase 3 (MMP3)[25-27].
Doğan SC et al. 2015 studied 30 women with RA and 30 control women. They assessed body composition with DXA, skeletal mass index (SMI), body mass index, levels of the laboratory inflammatory markers CRP and TKE, and disease activity with the Disease Activity Score- 28 joints (DAS28). Sarcopenia was more frequent in the RA group (p=0.004), both in women with normal body weight and in women who were overweight but not obese, while a correlation was found with CRP levels (p=0.230) but not with disease activity as assessed byDAS-28 of joints with TCE (p=0.530)[28].
In Meltem Alkan Melikoğlu’s 2017 study of 40 women with RA aged 31-66 compared to 40 healthy female controls of similar body mass index, aged 31-58, RA patients found a significant negative correlation between skeletal mass index (SMI) and functional capacity [with Health Activity Quality (HAQ) score] (p<0.05)[29]. In the study by Masahiro Tada et al. 2018 in 100 patients with RA, sarcopenia was positively related to radiographic joint, CRP, body mass index, as well as body fat mass. Of particular interest in this study, sarcopenia was associated with levels of matrix metalloproteinase 3 (MMP3, 0.11 mg/dL versus 70.3 ng/mL), which has it does with its insult and thus the insult of the anatomical structure and function of the joints[30].
Another study used whole-body DXA in a series of women with a mean age of 47.7 years. Almost one in two RA patients (43.3%) had muscle mass loss, whereas this condition was prevalent in only 10% of the healthy controls. Women with RA and low BMI were almost twice as likely (61.5% vs. 38.5%) to develop sarcopenia[31].
Researching sarcopenia in RA is important because it is causative to falls, fractures, osteoporosis due to immobilization and an increased cardiometabolic risk, all of which lead to increased cumulative mortality[32].
Pathogenesis of sarcopenia in RA
The sarcopenia observed in RA is due to inflammatory cytokines (IL-6, TNF-α and IL-1β), oxidative stress and reduced physical activity, leading to an unbalanced anabolism and catabolism of proteins[33]. The inflammatory cytokine IL-6 has a protein-catabolic effect on skeletal muscle and inhibits IGF-1-induced anabolic activity. In addition, high levels of IL-6 and low levels of IGF-1 reduce mobility and are associated with loss of muscle strength. The combination of low IGF-I and high IL-6 levels confers a high risk for progressive disability and sarcopenia in patients with RA, suggesting a global effect of dysregulation on the endocrine and immune systems. The joint effects of IGF-I and IL-6 may be important targets for therapies to prevent or minimize the disability associated with sarcopenia[34].
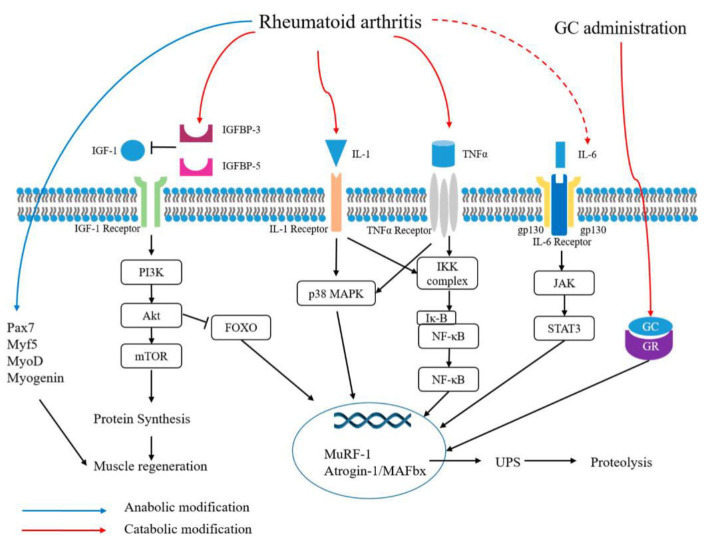
TNF-α is involved in protein catabolism in myofibers. In a study, activation of IκB kinase (IKK)/NF-κB by TNF-α increased MuRF1 expression, leading to muscle atrophy. IL-1β induces increased expression of 8 MAPK which leads to increased activation of MAFbx/Atrogen-1 and MuRF1 and causes muscle protein degradation. TNF-α works synergistically with IL-1β and over expression of IL-1β has been associated with anorexia and an accelerated muscle loss (Figure 2). Therefore, not controlling the disease can lead to prolonged exposure to inflammation, resulting in loss of muscle mass and muscle strength. For this reason, treatment under bDMARDs such as anti-TNF and anti-IL6 directly block TNF-α and IL-6, inhibiting protein catabolism caused by inflammatory cytokines[35,36].
Figure 2.
Mechanisms of sarcopenia and metabolic modifications in rheumatoid arthritis (by An HJ et al.[36] published under CC BY 4.0 license) IGF-1, insulin-like growth factor-1; IGFBP, insulin-like growth factor binding protein; IL-1, interleukin-1; TNF-α, tumor necrosis factor-α; GC, glucocorticoid; GR, glucocorticoid receptor; gp 130, glycoprotein 130; Pax7, paired box 7; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; MAPK, mitogen-activated protein kinase; IKK, IκB kinase; IκB, inhibitor of nuclear factor kappa B; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; JAK, Janus kinase; STAT3, signal transducer and activator of transcription 3; MuRF-1, muscle RING-finger 1; UPS, ubiquitin proteasome system.
Furthermore, the use of glucocorticoids as a pharmacological treatment for RA symptoms may cause sarcopenia. More specifically, chronic administration of glucocorticoids has been associated with muscle atrophy. More specifically, glucocorticoids have been shown to induce muscle atrophy (steroid myopathy) through activation of the transcription factor FOXO or repression of mTOR signaling, leading to protein catabolism. Glucocorticoid-induced skeletal muscle atrophy easily causes fatigue in daily physical activities, such as climbing stairs and walking at a fast pace, and reduces body movements to cause reduced ability to perform physical activity leading to the appearance of sarcopenia[37].
The GH axis and its final active metabolite, IGF-1, might also contribute to the development of sarcopenia but has not been fully elucidated.IGF-1, named for its high homology to insulin, is a small structurally related peptide of 70 amino acids. It is secreted by many tissues including the liver and skeletal muscle. At rest, circulating IGF-1 is relatively constant, and young adults have significantly higher concentrations compared to older individuals. Pathological conditions such as the use of high therapeutic doses of glucocorticoids and high disease activity in RA patients are significantly associated with low muscle IGF-1 levels. The decrease in IGF-1 causes a decrease in the levels of protein anabolism in skeletal muscle cells, which ultimately leads to changes in the structure and function of skeletal muscle cells. Therefore, the impairment of GH/IGF-1 plays a key role in the loss of skeletal muscle mass[38]. In muscle, IGF-1 is stimulated by mechanical loading and contraction in which the IGF receptor (IGFR) is activated. IGF-1 is secreted from muscle fibers into the extracellular matrix (ECM) in which it binds to IGF-binding proteins (IGFBPs-3, IGFBPs-5). IGF-1 enters the cell via IGFR, activates phosphoinositol 3-kinase (P13-K) to generate phosphatidylinositol (4, 5)-diphosphate (PIP2), leading to the production of phosphatidylinositol 3, 4, 5-triphosphate (PIP3). PIP3 is then free to bind to phosphoinositide-dependent kinase-1 (PDK1), which binds to the pleskstrin homology (PH) domain of Akt, allowing translocation to the cell membrane that precedes phosphorylation at Akt308[39].
Studies in mice with induced arthritis by Ibanez de Caceres et al. found that there was an increase in cyclooxygenase (COX)-2 gene and a decrease in IGF-1. Therapeutic intervention with non-steroidal anti-inflammatory drugs (NSAIDs) seemed to reverse the negative feedback of COX activation on inflammation and to increase serum IGF-1. The authors concluded that COX2 expression was responsible for the development of sarcopenia associated with inflammatory arthritis by intervening in the GH-IGF axis and the function of proteasome-ubiquitin pathways[40].
Treatment
Exercise, diet and pharmaceutical treatment of RA are basic elements in the treatment of sarcopenia.
• Exercise
Exercise, such as endurance exercises, increases muscle strength and mass, while aerobic exercise increases muscle fibers and improves insulinsensitivity. However, exercise should be properly supervised by qualified individuals and carried out continuously[41]. Resistance exercise (RE) is recommended as the first-line therapy to address the deleterious effects of sarcopenia in patients with RA. A RE program consisting of two exercise sessions per week and including a combination of upper and lower body exercises performed at relatively high effort for 1-3 sets of 6-12 repetitions is recommended[42]. Reports have shown improvement in muscle strength and physical function after 6 months of relatively simple daily exercises such as squats, one leg stands, heel raises and 20-30 minutes of walking[43]. Studies such as those of Kent-Braun et al. and Freiberger et al. argue that physical activity has a positive effect on sarcopenia, muscle loss or endurance, while low levels of physical activity are associated with an accelerated reduction in muscle mass and strength[44].
In particular, aerobic exercise induces an increase in muscle mass, while progressive resistance exercise programs or training with weight leads to a positive effect in sarcopenia. The existing literature suggests that patients who train with low-weight-bearing exercise have improved their performance in simple activities such as walking and sitting up. What’s more, resistance exercise improved the performance of the elderly in daily activities such as swimming and preparing a meal. Slow-velocity exercise has been shown to increase muscle mass through activation of protein synthesis, proliferation of satellite cells, increase in anabolic proteins, and reduction in catabolic cytokine production. Three very important limitations are placed by Malafarina et al regarding the improvement of muscle mass and strength: a) resistance exercises should not be interrupted, otherwise their benefits are lost quickly, b) there are certain consequences when the exercise is performed regularly, especially for the elderly, c) resistance exercises may not be sufficient to prevent muscle loss, especially in the elderly[45].
When an exercise was sought to better treat sarcopenia and improve daily activities, the exercise with resistance at increased speed was developed. This exercise has been linked to better scores in sarcopenia parameters, because it increases type II muscle fibers[46].
• Diet
Adequate nutrition is one of the main interventions for the prevention and management of sarcopenia. Yannis Dionyssiotis states through research that one of the interventions for the prevention and treatment of sarcopenia is nutrition. It has been proven that older adults probably need 1.0–1.2 gr/kg protein intake per day. Creatine supplements, high vitamin D levels and other nutrients under investigation may provide further help[46].
Nutritional treatment for sarcopenia including 20 gr whey protein and 800 IU vitamin D twice daily improves lower limb strength. Exercise therapy for sarcopenia, such as resistance training and 6 months of home exercise, improves muscle strength and physical function. Combination therapy including both nutritional and exercise therapy improves gait speed and knee extension strength more than either exercise alone or nutritional therapy alone[47]. In a randomized, double-blind, placebo-controlled trial, 62 elderly subjects participated twice weekly in a progressive resistance exercise program. They were supplemented twice daily with either milk protein (2 ´ 15 g) or placebo. The supplemented group increased their total daily protein intake from 1.0 to 1.3 g/kg/day. As a result, lean body mass increased by 1.3 kg in the protein-supplemented group, while no change was observed in the placebo group. A meta-analysis also corroborates this finding showing significant, albeit modest, higher lean mass gains[48]. Nutritional deficiencies need to be corrected in order to both prevent and combat sarcopenia. Calorie intake should be increased due to the increased demands placed on exercise. Dietary protein requirements increase in patients with sarcopenia. It should reach to more than 1.2 g per kilogram of body weight per day, except in patients with severe renal impairment. Leucine, β-hydroxy-β-methyl ester (HMB) and creatine can have beneficial effects on skeletal muscle protein balance. Additionally, the correction of vitamin D deficiencies is essential for muscle function[49,50].
Following are some sensible suggestions for patients’ ideal protein intake and muscular contraction, given the volume of research that is now available and some contradicting data:
Daily protein consumption is approximately 1.6–1.8 g/kg/d.
Three main meals with 0.6 g/kg of sources of high-quality protein.
A minimum of 5 g of leucine per serving.
When protein supplements are required, give preference to high-quality, quickly digestible proteins.
More long-term data are need to effectively prescribe supplemental leucine, despite acute and short-term evidence demonstrating benefits of isolated leucine supplementation to mixed meals.
Make sure you have enough energy because a negative energy balance makes it harder to consume protein post-meal and worsens anabolic resistance.
At least twice a week, do some resistance training.
Lessen your sedentary time50.
• Pharmaceutical treatment
In the last decade, studies have been conducted to develop medications that could be applied in the treatment of sarcopenia. The most studied drugs so far include: Testosterone, Growth Hormone, Dehydroepiandrosterone, Vitamin D, Myostatin, Ursolic Acid and Omega-3 Acids[51].
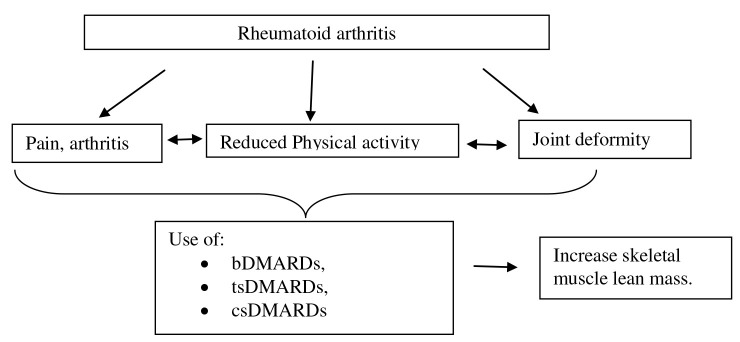
By blocking inflammatory mediators and their signaling or by activating anti-inflammatory and regulatory pathways, the available treatments for RA seek to cause disease remission[52]. Medications that treat rheumatic diseases and prevent joint deterioration are known as disease-modifying antirheumatic drugs (DMARDs)[53]. Medication comprises of biologic DMARDs (bDMARDs), targeted synthetic DMARDs (tsDMARDs), conventional synthetic DMARDs (csDMARDs), which include methotrexate, leflunomide, sulfasalazine, and hydroxychloroquine[54] (Figure3).
Figure 3.
Mechanisms of treatment in RA patients. Biologic DMARDs (bDMARDs), targeted synthetic DMARDs (tsDMARDs), conventional synthetic DMARDs (csDMARDs).
Infliximab, adalimumab, certolizumab, golimumab, and tocilizumab are monoclonal antibodies that target TNF and IL-6, respectively. Etanercept is a soluble TNF receptor, abatacept is a T-cell costimulation inhibitor, and rituximab is a monoclon CD20 B-cell-destroying antibody[55]. The intracellular signaling of type I and II cytokines is the target of tsDMARDs, which are inhibitors of the Janus tyrosine kinase (JAK) family (tofacitinib, baricitinib, upadacitinib). In order to prevent joint damage in RA patients, tsDMARD reduces T cells and leukocyte recruitment to the joint. This reduces joint inflammation and T cell numbers[56,57].
Data from multiple research suggest that targeting inflammatory cytokines can prevent muscle atrophy. In various animal models, TNF-blockade can partially cure muscle atrophy by inhibiting the NF-B pathway, and it can stop old mice from surviving[58]. Treatments with anti-IL-6 and anti-TNF are more likely than other DMARDs to result in an increase in lean mass. According to a recent review[59], tocilizumab can increase appendiceal and total lean mass in RA patients, which can increase muscle mass. Studies have applied the biological agent tocilizumab, a monoclonal antibody against the IL-6 receptor, whose use for a one-year period showed an increase in skeletal muscle lean mass[60].
Additionally, RA patients’ skeletal muscles are known to be negatively affected by glucocorticoids (CG), which can limit the disease activity in RA[61]. CG use has been reported to be positively associated with low lean muscle mass and sarcopenia in patients with RA[62]. The advantage with cortisone administration is the quick reduction of inflammation and bone loss, but a major disadvantage lies in the decrease of muscle mass. In the study by Yutaro Yamada et al. 2020 in 100 RA patients, univariate analysis found that sarcopenia was associated with older age (r=0.28 with p=0.022), higher mean corticosteroid dosage in year of the study reflecting greater disease activity (r=0.25 with p=0.043), as well as lower body mass index (r=-0.28 with p=0.019). In the multivariate analysis, a dosage of ≥3.25 mg prednisolone per day was significantly associated with the occurrence of sarcopenia (odds ratio=8.81 with 95% confidence interval 1.14-67.9 and p=0.037). Thus, it is arguable that corticosteroid administration in RA should be of small dosage and of short duration[63,64].
The correlations between RA treatment and the prevalence of sarcopenia were examined by Dao et al. (2021). The authors demonstrated that, in comparison to RA patients treated with bDMARDs, those receiving csDMARDs exhibited a decreased prevalence of sarcopenia. Sarcopenia was not associated with the use of tsDMARDs[65].
The overall management of sarcopenia in RA is complex. It should combine an adequate intake of protein and fatty acids, physical exercise and immunosuppressive medication to reduce inflammation. The administration of corticosteroids was accompanied by a decrease in skeletal muscle mass and strength, while the biological disease-modifying drugs such as monoclonal antibodies against TNF-α or IL-6, their increase combined with a reduction in the risk of developing sarcopenia. Although there is no targeted medication for sarcopenia in RA, modification of the disease itself seems to be helpful in managing it[66].
Conclusions
Sarcopenia occurs in about 66% of patients with RA. Consequences of sarcopenia include disability, disturbed quality of life, falls, osteoporosis, dyslipidemia, increased cardiovascular risk, metabolic syndrome and immunosuppression. There is well-established knowledge that DMARDS control disease activity, reduce inflammatory cytokines and thus prevent the onset of sarcopenia. Additionally, a specialised protein diet accompanied by systematic exercise successfully prevents sarcopenia. Treatment regimens with high doses of GC are a definite risk factor and should be administered with caution. Therefore, further research is needed to investigate the pathogenesis of sarcopenia in RA, so that potent targeted treatments are developed.
Footnotes
Edited by: Yannis Dionyssiotis
References
- 1.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia:Aging-Related Loss of Muscle Mass and Function. Physiol Rev. 2019;99(1):427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.West DW, Phillips SM. Anabolic processes in human skeletal muscle:restoring the identities of growth hormone and testosterone. Phys Sportsmed. 2010;38(3):97–104. doi: 10.3810/psm.2010.10.1814. [DOI] [PubMed] [Google Scholar]
- 3.Ni HJ, Hsu TF, Chen LK, Chou HL, Tung HH, Chow LH, et al. Effects of Exercise Programs in older adults with Muscle Wasting:A Systematic Review and Meta-analysis:Effects of Exercise Programs in Muscle Wasting. Arch Gerontol Geriatr. 2022;99:104605. doi: 10.1016/j.archger.2021.104605. [DOI] [PubMed] [Google Scholar]
- 4.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults:results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68(9):1001–7. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 5.Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia:facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5(4):253–9. doi: 10.1007/s13539-014-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An HJ, Tizaoui K, Terrazzino S, Cargnin S, Lee KH, Nam SW, et al. Sarcopenia in Autoimmune and Rheumatic Diseases:A Comprehensive Review. Int J Mol Sci. 2020;21(16):5678. doi: 10.3390/ijms21165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dionyssiotis Y, Kapsokoulou A, Samlidi E, Angoules AG, Papathanasiou J, Chronopoulos E, et al. Sarcopenia:From definition to treatment. Hormones (Athens) 2017;16(4):429–439. doi: 10.14310/horm.2002.1764. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia:revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia:consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia:revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dionyssiotis Y. Sarcopenia in the Elderly. Eur Endocrinol. 2019;15(1):13–14. doi: 10.17925/EE.2019.15.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietzel R, Wiegmann S, Borucki D, Detzer C, Zeiner KN, Schaumburg D, et al. Prevalence of sarcopenia in patients with rheumatoid arthritis using the revised EWGSOP2 and the FNIH definition. RMD Open. 2022;8(2):e002600. doi: 10.1136/rmdopen-2022-002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tekgoz E, Colak S, Ozalp Ates FS, Sonaeren I, Yilmaz S, Cinar M. Sarcopenia in rheumatoid arthritis:Is it a common manifestation?Int J Rheum Dis. 2020;23(12):1685–1691. doi: 10.1111/1756-185X.13976. [DOI] [PubMed] [Google Scholar]
- 15.Masahiro T, Yutaro Y, Koji M, Noriaki H. Matrix Metalloprotease 3 Is Associated With Sarcopenia in Rheumatoid Arthritis - Results From the CHIKARA Study. Int J Rheum Dis. 2018;21(11):1962–1969. doi: 10.1111/1756-185X.13335. [DOI] [PubMed] [Google Scholar]
- 16.Torii M, Hashimoto M, Hanai A, Fujii T, Furu M, Ito H, et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol. 2019;29(4):589–595. doi: 10.1080/14397595.2018.1510565. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki T, Yano K, Ikari K, Okazaki K. Sarcopenia-associated factors in Japanese patients with rheumatoid arthritis:A cross-sectional study. Geriatr Gerontol Int. 2019;19(9):907–912. doi: 10.1111/ggi.13747. [DOI] [PubMed] [Google Scholar]
- 18.Yamada Y, Tada M, Mandai K, Hidaka N, Inui K, Nakamura H. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis:from the CHIKARA study. Clin Rheumatol. 2020;39(6):1757–1764. doi: 10.1007/s10067-020-04929-4. [DOI] [PubMed] [Google Scholar]
- 19.Letarouilly J-G, et al. Body composition in patients with rheumatoid arthritis:a narrative literature review. Ther Adv Musculoskelet Dis. 2021;13:1759720X211015006. doi: 10.1177/1759720X211015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reina D, Gómez-Vaquero C, Díaz-Torné C, Solé JMN. Rheumatology Service. Hospital Moisès Broggi. Assessment of nutritional status by dual X-Ray absorptiometry in women with rheumatoid arthritis:A case-control study. Medicine (Baltimore) 2019;98(6):e14361. doi: 10.1097/MD.0000000000014361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galanopoulos NG, Dionyssiotis Y. Ορόλοςτηςσαρκοπενίας, τηςκαχεξίαςκαιτηςαδυναµίας/ευπάθειαςστουςασθενείςµερευµατικέςπαθήσεις[The role of sarcopenia, cachexia and weakness/frailty in patients with rheumatic diseases. Skeletal health. 2021;20(1):4–17. [Google Scholar]
- 22.Kasher M, Gabdulina G, Beissebayeva A, Mussabaeva D, et al. Rheumatoid arthritis is associated with exacerbated body composition deterioration in Kazakh females. Nutrition. 2019;66:219–226. doi: 10.1016/j.nut.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Son KM, Kang SH, Seo YI, Kim HA. Association of body composition with disease activity and disability in rheumatoid arthritis. Korean J Intern Med. 2021;36(1):214–222. doi: 10.3904/kjim.2019.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Targowski T. Sarcopaenia and rheumatoid arthritis. Reumatologia. 2017;55(2):84–87. doi: 10.5114/reum.2017.67603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torii M, Hashimoto M, Hanai A, Fujii T, et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol. 2019;29(4):589–595. doi: 10.1080/14397595.2018.1510565. [DOI] [PubMed] [Google Scholar]
- 26.Müller R, Kull M, Põlluste K, Valner A, et al. Factors Associated With Low Lean Mass in Early Rheumatoid Arthritis:A Cross- Sectional Study. Medicina (Kaunas) 2019;55(11):730. doi: 10.3390/medicina55110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngeuleu A, Allali F, Medrare L, Madhi A, et al. Sarcopenia in rheumatoid arthritis:prevalence, influence of disease activity and associated factors. Rheumatol Int. 2017;37(6):1015–1020. doi: 10.1007/s00296-017-3665-x. [DOI] [PubMed] [Google Scholar]
- 28.Doğan SC, Hizmetli S, Hayta E, Kaptanoğlu E, Erselcan T, Güler E. Sarcopenia in women with rheumathoid arthritis. Eur J Rheumatol. 2015;2:57–61. doi: 10.5152/eurjrheum.2015.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alkan Melikoğlu M. Presarcopenia and its Impact on Disability in Female Patients With Rheumatoid Arthritis. Arch Rheumatol. 2017;32(1):53–59. doi: 10.5606/ArchRheumatol.2017.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tada M, Yamada Y, Mandai K, Hidaka N. Matrix metalloprotease 3 is associated with sarcopenia in rheumatoid arthritis - results from the CHIKARA study. Int J Rheum Dis. 2018;21(11):1962–1969. doi: 10.1111/1756-185X.13335. [DOI] [PubMed] [Google Scholar]
- 31.Doğan SC, Hizmetli S, Hayta E, Kaptanoğlu E, Erselcan T, Güler E. Sarcopenia in women with rheumatoid arthritis. Eur J Rheumatol. 2015;2(2):57–61. doi: 10.5152/eurjrheum.2015.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngeuleu A, Allali F, Medrare L, Madhi A, et al. Sarcopenia in rheumatoid arthritis:prevalence, influence of disease activity and associated factors. Rheumatol Int. 2017 Jun;37(6):1015–1020. doi: 10.1007/s00296-017-3665-x. [DOI] [PubMed] [Google Scholar]
- 33.El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int J Mol Sci. 2022;23(15):8713. doi: 10.3390/ijms23158713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahmani J, Montesanto A, Giovannucci E, Zand H, Barati M, Kopchick JJ, et al. Association between IGF-1 levels ranges and all-cause mortality:A meta-analysis. Aging Cell. 2022;21(2):e13540. doi: 10.1111/acel.13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little RD, Prieto-Potin I, Pérez-Baos S, Villalvilla A, Gratal P, Cicuttini F, et al. Compensatory anabolic signaling in the sarcopenia of experimental chronic arthritis. Sci Rep. 2017;7(1):6311. doi: 10.1038/s41598-017-06581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An HJ, Tizaoui K, Terrazzino S, Cargnin S, Lee KH, Nam SW, et al. Sarcopenia in Autoimmune and Rheumatic Diseases:A Comprehensive Review. Int J Mol Sci. 2020;21(16):5678. doi: 10.3390/ijms21165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MK, Jeong HH, Kim MJ, Ryu H, Baek J, Lee B. Nutrients against Glucocorticoid-Induced Muscle Atrophy. Foods. 2022;11(5):687. doi: 10.3390/foods11050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chew J, Tay L, Lim JP, Leung BP, Yeo A, Yew S, et al. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J Nutr Health Aging. 2019;23(10):979–986. doi: 10.1007/s12603-019-1255-1. [DOI] [PubMed] [Google Scholar]
- 39.Barclay RD, Burd NA, Tyler C, Tillin NA, Mackenzie RW. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front Nutr. 2019;6:146. doi: 10.3389/fnut.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martín AI, Priego T, Moreno-Ruperez Á, González-Hedström D, Granado M, López-Calderón A. IGF-1 and IGFBP-3 in Inflammatory Cachexia. Int J Mol Sci. 2021;22(17):9469. doi: 10.3390/ijms22179469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee WJ, Liu LK, Peng LN, Lin MH, Chen LK. ILAS Research Group. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people:results from the I-Lan longitudinal aging study. J Am Med Dir Assoc. 2013;14(7):528.e1–7. doi: 10.1016/j.jamda.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Hurst C, Sayer AA. Improving muscle strength and physical function in older people living with sarcopenia and physical frailty:Not all exercise is created equal. J R Coll Physicians Edinb. 2022;52(2):166–171. doi: 10.1177/14782715221104859. [DOI] [PubMed] [Google Scholar]
- 43.Kakehi S, Wakabayashi H, Inuma H, Inose T, Shioya M, Aoyama Y, et al. Rehabilitation Nutrition and Exercise Therapy for Sarcopenia. World J Mens Health. 2022;40(1):1–10. doi: 10.5534/wjmh.200190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freiberger E, Sieber C, Pfeifer K. Physical activity, exercise, and sarcopenia - future challenges. Wien Med Wochenschr. 2011;161(17-18):416–25. doi: 10.1007/s10354-011-0001-z. [DOI] [PubMed] [Google Scholar]
- 45.Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Sarcopenia in the elderly:diagnosis, physiopathology and treatment. Maturitas. 2012;71(2):109–14. doi: 10.1016/j.maturitas.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Dionyssiotis Y. Sarcopenia in the ElderlyEur Endocrinol. 2019;15(1):13–14. doi: 10.17925/EE.2019.15.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kakehi S, Wakabayashi H, Inuma H, Inose T, Shioya M, Aoyama Y, et al. Rehabilitation Nutrition and Exercise Therapy for Sarcopenia. World J Mens Health. 2022;40(1):1–10. doi: 10.5534/wjmh.200190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogeri PS, Zanella R, Jr, Martins GL, Garcia MDA, Leite G, Lugaresi R, et al. Strategies to Prevent Sarcopenia in the Aging Process:Role of Protein Intake and Exercise. Nutrients. 2021;14(1):52. doi: 10.3390/nu14010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marques DL, Neiva HP, Marinho DA, Marques MC. Manipulating the Resistance Training Volume in Middle-Aged and Older Adults:A Systematic Review with Meta-Analysis of the Effects on Muscle Strength and Size, Muscle Quality, and Functional Capacity [published online ahead of print, 2022 Oct 28] Sports Med. 2022;10 doi: 10.1007/s40279-022-01769-x. 1007/s40279-022-01769-x. [DOI] [PubMed] [Google Scholar]
- 50.Morley JE. Frailty and sarcopenia in elderly. Wien Klin Wochenschr. 2016;128(Suppl 7):439–445. doi: 10.1007/s00508-016-1087-5. [DOI] [PubMed] [Google Scholar]
- 51.Rooks D, Roubenoff R. Development of Pharmacotherapies for the Treatment of Sarcopenia. J Frailty Aging. 2019;8(3):120–130. doi: 10.14283/jfa.2019.11. [DOI] [PubMed] [Google Scholar]
- 52.Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs:2016 update. Ann Rheum Dis. 2017;76:960–77. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 53.Buttgereit F. Views on glucocorticoid therapy in rheumatology:the age of convergence. Nat Rev Rheumatol. 2020;16:239–46. doi: 10.1038/s41584-020-0370-z. [DOI] [PubMed] [Google Scholar]
- 54.Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs:2019 update. Ann Rheum Dis. 2020;79:S685–99. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 55.Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis:A Review. JAMA. 2018;320(13):1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 56.Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–81. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 57.Lundquist LM, Cole SW, Sikes ML. Efficacy and safety of tofacitinib for treatment of rheumatoid arthritis. World J Orthop. 2014;5:504–11. doi: 10.5312/wjo.v5.i4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sciorati C, Gamberale R, Monno A, Citterio L, Lanzani C, De Lorenzo R, et al. Pharmacological blockade of TNFαprevents sarcopenia and prolongs survival in aging mice. Aging (Albany NY) 2020;12:23497–508. doi: 10.18632/aging.202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavalheiro R, Hein T, Xavier RM. The effect of pharmacological treatment on rheumatoid arthritis related sarcopenia:an integrative review. Curr Rheumatol Res. 2021;2(1):5–11. [Google Scholar]
- 60.Hein TR, Peterson L, Bartikoski BJ, Portes J, Espírito Santo RC, Xavier RM. The effect of disease-modifying anti-rheumatic drugs on skeletal muscle mass in rheumatoid arthritis patients:a systematic review with meta-analysis. Arthritis Res Ther. 2022;24(1):171. doi: 10.1186/s13075-022-02858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada Y, Tada M, Mandai K, Hidaka N, Inui K, Nakamura H. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis:from the CHIKARA study. Clin Rheumatol. Germany. 2020;39:1757–64. doi: 10.1007/s10067-020-04929-4. [DOI] [PubMed] [Google Scholar]
- 62.Yun HW, Kim CJ, Kim JW, Kim HA, et al. The Assessment of Muscle Mass and Function in Patients with Long-Standing Rheumatoid Arthritis. J Clin Med. 2021;10(16):3458. doi: 10.3390/jcm10163458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fenton CG, Webster JM, Martin CS, Fareed S, et al. Therapeutic glucocorticoids prevent bone loss but drive muscle wasting when administered in chronic polyarthritis. Arthritis Res Ther. 2019;21(1):182. doi: 10.1186/s13075-019-1962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada Y, Tada M, Mandai K, Hidaka N, et al. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis:from the CHIKARA study. Clin Rheumatol. 2020;39(6):1757–1764. doi: 10.1007/s10067-020-04929-4. [DOI] [PubMed] [Google Scholar]
- 65.Dao T, Kirk B, Phu S, Vogrin S, Duque G. Prevalence of sarcopenia and its association with antirheumatic drugs in middle-aged and older adults with rheumatoid arthritis:a systematic review and meta-analysis. Calcif Tissue Int. 2021;109:475–89. doi: 10.1007/s00223-021-00873-w. [DOI] [PubMed] [Google Scholar]
- 66.Tournadre A, Vial G, Capel F, Soubrier M, et al. Sarcopenia. Joint Bone Spine. 2019;86(3):309–314. doi: 10.1016/j.jbspin.2018.08.001. [DOI] [PubMed] [Google Scholar]