Abstract
Purpose:
This work assesses bilateral ganglion cell layer–inner plexiform layer (GCL-IPL) thickness changes in patients with unilateral neovascular age-related macular degeneration (nAMD) treated with antivascular endothelial growth factor (anti-VEGF).
Methods:
In this single-center, retrospective, cohort study, the medical records of patients with unilateral nAMD treated with anti-VEGF were reviewed. The treated group included eyes with newly diagnosed nAMD that subsequently underwent treatment with intravitreal anti-VEGF injections. The control group was the fellow eye with dry AMD. Eyes receiving at least 10 intravitreal injections were included. Measurement of GCL-IPL thickness was performed at different time points using spectral domain–optical coherence tomography.
Results:
A total of 216 eyes of 108 patients met the inclusion criteria. The mean age ± SD was 80.1 ± 10.7 years. Eyes in the treated group underwent a mean ± SD of 20.2 ± 7.2 injections in 21.3 ± 6.8 months. At baseline, average mean ± SD of GCL-IPL thickness was 73.71 ± 8.81 µm and 73.84 ± 8.26 µm in the treated and fellow eye, respectively (P = .795). After 10 injections the average thickness was 65.41 ± 14.08 µm and 68.77 ± 13.24 µm in the treated and fellow eye, respectively (P = .007). The absolute decrease in thickness was significantly greater in the treated eye than the fellow eye (mean ± SD, 8.31 ± 11.19 µm vs 5.07 ± 10.83 µm, respectively; P = .002).
Conclusions:
GCL-IPL thickness decreased significantly in the treated group more than in the control group after 10 anti-VEGF injections. The mechanism and clinical significance of this observation warrants further study.
Keywords: age-related macular degeneration, anti-VEGF, ganglion cell layer–inner plexiform layer, glaucoma, intravitreal injections
Introduction
Age-related macular degeneration (AMD) is the leading cause of central vision loss in patients older than 50 years in industrialized countries. 1 -3 In the past, the visual prognosis in eyes with neovascular AMD (nAMD) was guarded. However, the introduction of antivascular endothelial growth factor (anti-VEGF) injections revolutionized the treatment paradigm for this condition, and patients now usually maintain functional vision for many years following diagnosis. According to the randomized CATT (Comparison of the Age-Related Treatment Trial), eyes undergoing treatment with monthly bevacizumab gained a mean 8 letters of visual acuity (VA), whereas those receiving monthly ranibizumab injections gained a mean of 8.5 letters. 4 The VIEW (VEGF Investigation of Efficacy and Safety in Wet AMD) studies found that aflibercept produced noninferior safety and efficacy outcomes compared with monthly ranibizumab. 5 Recently, the HAWK and HARRIER studies reported that eyes treated with brolucizumab achieved greater anatomic response with less frequent injections than those treated with aflibercept. 6
One of the key facts frequently discussed with patients with nAMD is that their condition does not usually affect peripheral vision. However, recent studies have questioned this dogma and reported that regular intravitreal anti-VEGF injections used to treat the condition might affect the retinal nerve fiber layer (RNFL). 7 In turn, this would affect the patient’s peripheral vision. Martinez-de-la-Casa et al found that eyes treated with ranibizumab for nAMD sustained a statistically significant decrease in RNFL thickness compared with the control group after just 12 months. 8 Enders et al reported that intravitreal anti-VEGF injections cause moderate RNFL loss in nonglaucomatous eyes. 9 Other reports, however, including a meta-analysis, did not confirm these observations. 10 -12 Similarly, certain studies raised the concern that the ganglion cell layer–inner plexiform layer (GCL-IPL) is another aspect of the eye that might be affected in nAMD, 13 -17 although this remains unconfirmed 18,19 (Table 1). The goal of the present study was to assess GCL-IPL thickness changes in patients with unilateral nAMD treated with intravitreal anti-VEGF injections.
Table 1.
Previous Studies Investigating Ganglion Cell–Inner Plexiform Layer Thickness in the Setting of Anti-VEGF Injections for Treatment of Neovascular Age-Related Macular Degeneration.
| Authors | Publication y | Sample size (treated eyes) | Mean No. of anti-VEGF injections | Conclusions |
|---|---|---|---|---|
| Kim et al 15 | 2020 | 48 | 6.29 | GCL-IPL thickness decreased significantly after 3 consecutive injections |
| Lee et al 17 | 2020 | 52 | 5.1 | GCL-IPL thickness significantly decreased in aflibercept and total groups, but not ranibizumab treatment group |
| Abdolrahimzadeh et al 13 | 2019 | 24 | 10.4 | GCL-IPL thickness significantly decreased at 1 and 2 y |
| Zucchiatti et al 18 | 2017 | 24 | 5.3 | Nonsignificant reduction in thickness of GCL-IPL after 1 y |
| Lee et al 16 | 2017 | 16 | 10.6 | Rate of GCL-IPL thinning significantly greater in eyes receiving injections vs fellow eyes |
| Beck et al 14 | 2016 | 34 | 31.5 | Significant decrease in GCL-IPL thickness vs fellow eye; RNFL decrease not significant |
| Perdicchi et al 19 | 2015 | 32 | 3 | Slight reduction in GCL-IPL thickness that was not statistically significant |
Abbreviations: anti-VEGF, antivascular endothelial growth factor; GCL-IPL, ganglion cell–inner plexiform layer; RNFL, retinal nerve fiber layer.
Methods
Study Design
A single-center, retrospective, cohort study was designed. Data were collected via review of electronic medical records (MDIntelleChart, Nextech Systems LLC).
Inclusion and Exclusion Criteria
The medical records of adult patients who underwent treatment with intravitreal injections between 2011 and 2017 were screened. Patients with newly diagnosed nAMD in one eye and dry AMD in the fellow eye were evaluated. The eye with nAMD was treatment-naive at baseline and subsequently underwent intravitreal anti-VEGF injections per the treat-and-extend protocol. The fellow eye served as the control and did not develop nAMD during the study period. The study participants underwent a minimum of 10 injections in the treated eye, and their baseline VA was anywhere between 20/20 and light perception.
Exclusion criteria were any of the following: (1) history of glaucoma, (2) history of ocular hypertension, (3) cup-to-disc asymmetry on fundoscopic examination, (4) cup-to-disc ratio of 0.6 or greater, and (5) RNFL asymmetry or (6) GCL-IPL asymmetry based on spectral-domain optical coherence tomography (SD-OCT). Eyes that underwent surgical interventions (eg, vitrectomy or cataract extraction) during the study period were excluded. 20
Intravitreal Injections
The study eyes underwent intravitreal anti-VEGF injections with a 30-gauge needle through the pars plana while under topical or subconjunctival anesthesia. A strict treat-and-extend protocol was followed. One of the following medications was administered: bevacizumab 1.25 mg (Avastin, Genentech Inc), aflibercept 2 mg (Eylea, Regeneron), or ranibizumab 0.5 mg (Lucentis, Novartis). Eyes that underwent additional treatment (eg, intravitreal steroids, off-label higher or lower dose of the anti-VEGF medication) were excluded.
Treat-and-Extend Protocol
The treat-and-extend protocol 21 included a loading phase with 3 monthly injections on initial diagnosis. Lack of detectable fluid on SD-OCT at any subsequent visit would prompt an extension of the treatment interval by 1 to 2 weeks with a maximum of 12 weeks possible. However, eyes with signs of exudation on SD-OCT at any subsequent visit had their treatment interval shortened by 1 week up to a minimum of every 4-week dosing.
Data Collection and Analysis
At each visit, VA and intraocular pressure (IOP) were measured. OCT of the macula was obtained with special attention to GCL-IPL thickness. Baseline information including demographics, VA, IOP, and baseline GCL-IPL thickness was collected. At each visit, SD-OCT (Cirrus HD-OCT, Carl Zeiss Meditec Inc) was obtained and dilated fundoscopic examination was performed. The average GCL-IPL thickness was calculated using the “macular cube analysis” (macular cube 514 × 128 protocol) of the automated Cirrus SD-OCT software.
As part of the OCT analysis, scans were divided into 6 sectors: superior, superonasal, inferonasal, inferior, inferotemporal, and superotemporal. The mean thickness value was then calculated based on these 6 values and subsequently included in the analysis.
Results
Baseline demographic and clinical characteristics are shown in Table 2. The mean age was 80.1 ± 10.71 years. A total of 216 eyes from 108 patients met the inclusion criteria and were analyzed. Of these, 5.5% (6 of 108) were treated with bevacizumab, 57.4% (62 of 108) with ranibizumab, 14.8% (16 of 108) with aflibercept, and 22.2% (24 of 108) with a combination of the aforementioned drugs. All participants received a total of 10 injections (Figure 1).
Table 2.
Baseline Patient Demographics and Clinical Characteristics.
| Age, y, mean ± SD | 80.10 ± 10.71 | Paired-sample t test | |
| No. of injections | 10 | ||
| Follow-up period, mo, mean ± SD | 21.3 ± 6.8 | ||
| Treated eye | Fellow eye | ||
| Baseline average thickness, µm, mean ± SD | 73.71 ± 8.81 | 73.84 ± 8.26 | P = .795 |
| Baseline visual acuity, logMAR (Snellen)± SD | 0.47 (20/59) ± 0.44 | 0.29 (20/38) ± 0.42 | P < .001 |
| Baseline IOP, mm Hg, mean ± SD | 15.50 ± 2.89 | 15.83 ± 2.88 | P = .067 |
Abbreviation: IOP, intraocular pressure.
Figure 1.
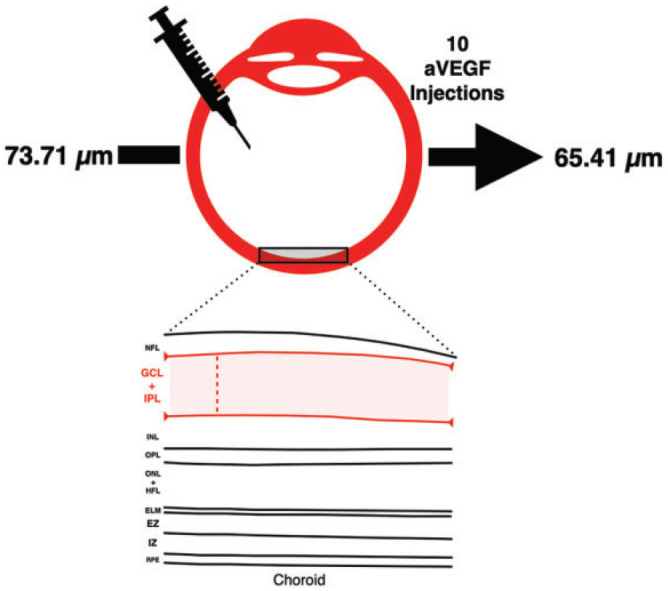
Graphical overview demonstrating the mean number of injections and decrease in ganglion cell–inner plexiform layer thickness of the treated eye. Retinal layers are outlined, including the nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), Henle fiber layer (HFL), external limiting membrane (ELM), ellipsoid zone (EZ), interdigitation zone (IZ), and retinal pigment epithelium (RPE). anti-VEGF indicates antivascular endothelial growth factor.
Clinical characteristics, including IOP, best-corrected VA (BCVA), and GCL-IPL thickness, are listed in Table 3. A significant difference was noted in the average thickness at final follow-up between the treated and fellow eye (P = .007). In addition, the difference in GCL-IPL thickness from baseline to final follow-up visit was significantly greater in the treated eye compared with the fellow eye (P = .002). VA remained significantly worse in the treated eye compared with the fellow eye despite treatment (P = .041), although the difference decreased as a result of treatment with anti-VEGF intravitreal injections.
Table 3.
GCL-IPL Thickness and Clinical Values for Treated and Fellow Eye After 10 Total Injections.
| Treated eye | Fellow eye | Paired-sample t test | |
|---|---|---|---|
| Average thickness, µm, mean ± SDa | 65.41 ± 14.08 | 68.77 ± 13.24 | P = .007 |
| Δ Average thickness, µm, mean ± SDb | 8.31 ± 11.19 | 5.07 ± 10.83 | P = .002 |
| Best-corrected visual acuity, logMAR (Snellen) ± SD | 0.43 (20/53) ± 0.42 | 0.38 (20/47) ± 0.53 | P = .041 |
| IOP, mm Hg, mean ± SD | 14.76 ± 3.18 | 14.57 ± 3.51 | P = .335 |
Abbreviations: GCL-IPL, ganglion cell–inner plexiform layer; IOP, intraocular pressure.
a Thickness of GCL-IPL after 10 antivascular endothelial growth factor injections.
b Difference in GCL-IPL thickness from baseline to after 10 injections.
A case example denoting decreased GCL-IPL thickness is demonstrated in Figure 2. The right eye underwent intravitreal anti-VEGF injections at regular intervals with a decrease in GCL-IPL thickness from an average thickness of 74 µm to 37 µm over a 4-year span.
Figure 2.
Case example of a 90-year-old patient with age-related macular degeneration who underwent antivascular endothelial growth factor injections to the right eye at regular intervals. This patient’s baseline average ganglion cell–inner plexiform layer thickness was (A) 74 µm, which decreased to (B) 57 µm after 2 years and then to (C) 37 µm after 4 years.
Conclusions
Intravitreal anti-VEGF injections remain the mainstay treatment of nAMD with proven safety and efficacy. 22,23 However, some long-term implications remain debatable. These include, but are not limited to, cerebrovascular accidents, 24 myocardial ischemic events, 25 intraocular inflammation, 26 and geographic atrophy. 27 -29 Prior studies also investigated the treatment’s impact on optic nerve health. 8,11 -16,30 A meta-analysis of 46 related studies by de Vries et al showed a significant decrease in RNFL thickness. 29 A dose-response relationship between RNFL thinning and the number of anti-VEGF injections for nAMD has also been shown, thereby suggesting a cumulative effect. 7 However, other studies have shown conflicting evidence that demonstrates no change in RNFL thickness, even after a follow-up period of 2 years. 10,11
GCL-IPL thinning following intravitreal anti-VEGF injections also remains controversial. Several studies, with a sample size anywhere between 16 to 52 eyes, have demonstrated a significant decrease in GCL-IPL thickness. 13,15 -17 However, in the largest cohort of these, Lee et al showed that this outcome might also depend on the pharmacologic agent. 17 Although ranibizumab was not found to significantly decrease the GCL-IPL thickness, this was not the case with aflibercept. 17 On the other hand, certain findings showed that the GCL-IPL decrease was not significant. 18,19,31 The objective of the present study was to elucidate the relationship between anti-VEGF injections and GCL-IPL thinning using longer follow-up and a larger sample size.
Our study found that after 10 injections, the GCL-IPL thickness decreased a mean of 8.3 µm in the treated eye, whereas the fellow eye decreased 5.1 µm on average (P = .002). This finding has 4 potential mechanisms. First, the neurodegenerative process in eyes with AMD might be affecting the thickness of the entire retina. Previous studies found that GCL-IPL thickness is significantly reduced in eyes with advanced nAMD compared with eyes with earlier stages of the disease, 32 and this effect might be attributed to the loss of bipolar cells and the reduced excitatory signals in eyes with advanced nAMD. 33 Second, IOP has been shown to transiently increase following an intravitreal injection of 0.05 mL. 34 De Vries et al found that the mean weighted difference in IOP before and after intravitreal injection was 25.95 mm Hg immediately after injection, but 2.61 mm Hg 30 minutes after the injection. 29 These IOP spikes, although short-lived, may result in glaucomatous damage in eyes undergoing regular treatment and in GCL-IPL thickness loss without resulting in a detectable change of the IOP. 35
Third, the anti-VEGF antibodies themselves may contribute to GCL-IPL thickness loss. As previously shown in various animal models, VEGF has been proven to have neuroprotective properties. 36 -38 Therefore, anti-VEGF intravitreal injections may neutralize these neuroprotective cascades and result in oxidative stress leading to detrimental cytotoxic effects. In human eyes, this cytotoxic effect may translate into neuronal damage and GCL-IPL thickness loss. Last, this decrease in GCL-IPL thickness may result from an overall “drying” effect of the anti-VEGF injections.
Furthermore, our findings align with previous studies demonstrating that, despite the improvement of BCVA with anti-VEGF injections, final BCVA in the treated group was inferior to that in the control group. 14,15 This result is noteworthy because it affects patient counseling and expectation management, given that visual improvement with anti-VEGF treatment has certain limitations.
The strengths of the present study include its long follow-up period and large sample size. Its main limitation is its retrospective nature, as well as the fact that it has anatomic data without additional outcomes about the patients’ visual function, such as perimetry. Additionally, the study evaluates patients who received a variety of anti-VEGF injections, therefore, there may be unaccounted drug-specific effects at play. In conclusion, long-term anti-VEGF injections might lead to GCL-IPL thickness loss when compared with the fellow eye. This loss may be a contributor to the statistically significant difference in VA noted between eyes despite treatment. The mechanism and significance of this process, however, warrants further investigation.
Footnotes
Ethical Approval: The protocol was approved by the Rutgers Institutional Review Board (protocol No. RCV-DR-007). This project was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner and complied with the federal regulations of the United States.
Statement of Informed Consent: Informed consent was obtained prior to performing the procedure, including permission for publication of all photographs and images including herein.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration—emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–471. doi:10.1080/07853890600946724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Congdon N, O’Colmain B, Klaver CCW; et al. Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi:10.1001/archopht.122.4.477 [DOI] [PubMed] [Google Scholar]
- 3. Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. doi:/S0042-96862004001100009 [PMC free article] [PubMed] [Google Scholar]
- 4. CATT Research Group; Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi:10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heier JS, Brown DM, Chong V; et al. VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 6. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89–99. doi:10.1016/j.ophtha.2020.06.028 [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Swaminathan SS, Yang J, et al. Dose-response relationship between recurrent intravitreal injections and retinal nerve fiber layer thinning in exudative age-related macular degeneration. Ophthalmol Retina. 2020;5(7):648–654. doi:10.1016/j.oret.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 8. Martinez-de-la-Casa JM, Ruiz-Calvo A, Saenz-Frances F, et al. Retinal nerve fiber layer thickness changes in patients with age-related macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 2012;53(10):6214–6218. doi:10.1167/iovs.12-9875 [DOI] [PubMed] [Google Scholar]
- 9. Enders P, Sitnilska V, Altay L, Schaub F, Muether PS, Fauser S. Retinal nerve fiber loss in anti-VEGF therapy for age-related macular degeneration can be decreased by anterior chamber paracentesis. Ophthalmologica. 2017;237(2):111–118. doi:10.1159/000457907 [DOI] [PubMed] [Google Scholar]
- 10. Shin HJ, Kim SN, Chung H, Kim TE, Kim HC. Intravitreal anti-vascular endothelial growth factor therapy and retinal nerve fiber layer loss in eyes with age-related macular degeneration: a meta-analysis. Invest Ophthalmol Vis Sci. 2016;57(4):1798–1806. doi:10.1167/iovs.15-18404 [DOI] [PubMed] [Google Scholar]
- 11. Horsley MB, Mandava N, Maycotte MA, Kahook MY. Retinal nerve fiber layer thickness in patients receiving chronic anti-vascular endothelial growth factor therapy. Am J Ophthalmol. 2010;150(4):558–561.e1. doi:10.1016/j.ajo.2010.04.029 [DOI] [PubMed] [Google Scholar]
- 12. El-Ashry MF, Lascaratos G, Dhillon B. Evaluation of the effect of intravitreal ranibizumab injections in patients with neovascular age related macular degeneration on retinal nerve fiber layer thickness using optical coherence tomography. Clin Ophthalmol. 2015;9:1269–1274. doi:10.2147/OPTH.S80704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdolrahimzadeh S, Gharbiya M, Formisano M, Bertini F, Cerini A, Pacella E. Anti-vascular endothelial growth factor intravitreal therapy and macular ganglion cell layer thickness in patients with neovascular age-related macular degeneration. Curr Eye Res. 2019;44(9):1000–1005. doi:10.1080/02713683.2019.1610179 [DOI] [PubMed] [Google Scholar]
- 14. Beck M, Munk MR, Ebneter A, Wolf S, Zinkernagel MS. Retinal ganglion cell layer change in patients treated with anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Am J Ophthalmol. 2016;167:10–17. doi:10.1016/j.ajo.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 15. Kim SY, Yoon MH, Chin HS. Changes in the ganglion cell-inner plexiform layer after consecutive intravitreal injections of anti-vascular endothelial growth factor in age-related macular degeneration patients. Korean J Ophthalmol. 2020;34(1):11–18. doi:10.3341/kjo.2019.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee WJ, Kim YK, Kim YW, et al. Rate of macular ganglion cell-inner plexiform layer thinning in glaucomatous eyes with vascular endothelial growth factor inhibition. J Glaucoma. 2017;26(11):980–986. doi:10.1097/IJG.0000000000000776 [DOI] [PubMed] [Google Scholar]
- 17. Lee SW, Sim HE, Park JY, et al. Changes in inner retinal layer thickness in patients with exudative age-related macular degeneration during treatment with anti-vascular endothelial growth factor. Medicine (Baltimore). 2020;99(17):e19955. doi:10.1097/MD.0000000000019955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zucchiatti I, Cicinelli MV, Parodi MB, et al. Effect of intravitreal ranibizumab on ganglion cell complex and peripapillary retinal nerve fiber layer in neovascular age-related macular degeneration using spectral domain optical coherence tomography. Retina. 2017;37(7):1314–1319. doi:10.1097/IAE.0000000000001360 [DOI] [PubMed] [Google Scholar]
- 19. Perdicchi A, Peluso G, Iacovello D, et al. Ganglion cell complex evaluation in exudative age-related macular degeneration after repeated intravitreal injections of ranibizumab. Biomed Res Int. 2015;2015:268796. doi:10.1155/2015/268796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shin IH, Lee WH, Lee JJ, Jo YJ, Kim JY. Thickness of the macula, retinal nerve fiber layer, and ganglion cell-inner plexiform layer in the age-related macular degeneration: the Repeatability Study of Spectral Domain Optical Coherence Tomography. Retina. 2018;38(2):253–262. doi:10.1097/IAE.0000000000001535 [DOI] [PubMed] [Google Scholar]
- 21. Skelly A, Bezlyak V, Liew G, Kap E, Sagkriotis A. Treat and extend treatment interval patterns with anti-VEGF therapy in nAMD patients. Vision (Basel). 2019;3(3):41. doi:10.3390/vision3030041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi:10.1056/NEJMoa042760 [DOI] [PubMed] [Google Scholar]
- 23. Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112(6):1035–1047. doi:10.1016/j.ophtha.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 24. Starr MR, Dalvin LA, AbouChehade JE, et al. Classification of strokes in patients receiving intravitreal anti-vascular endothelial growth factor. Ophthalmic Surg Lasers Imaging Retina. 2019;50(5):e140–e157. doi:10.3928/23258160-20190503-14 [DOI] [PubMed] [Google Scholar]
- 25. Mikačić I, Bosnar D. Intravitreal bevacizumab and cardiovascular risk in patients with age-related macular degeneration: systematic review and meta-analysis of randomized controlled trials and observational studies. Drug Saf. 2016;39(6):517–541. doi:10.1007/s40264-016-0408-y [DOI] [PubMed] [Google Scholar]
- 26. Greenberg JP, Belin P, Butler J; et al. Aflibercept Sterile Inflammation Research Group. Aflibercept-related sterile intraocular inflammation outcomes. Ophthalmol Retina. 2019;3(9):753–759. doi:10.1016/j.oret.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 27. Zinkernagel MS, Schorno P, Ebneter A, Wolf S. Scleral thinning after repeated intravitreal injections of antivascular endothelial growth factor agents in the same quadrant. Invest Ophthalmol Vis Sci. 2015;56(3):1894–1900. doi:10.1167/iovs.14-16204 [DOI] [PubMed] [Google Scholar]
- 28. Sadda SR, Guymer R, Monés JM, Tufail A, Jaffe GJ. Anti-vascular endothelial growth factor use and atrophy in neovascular age-related macular degeneration: systematic literature review and expert opinion. Ophthalmology. 2020;127(5):648–659. doi:10.1016/j.ophtha.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 29. de Vries VA, Bassil FL, Ramdas WD. The effects of intravitreal injections on intraocular pressure and retinal nerve fiber layer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):13248. doi:10.1038/s41598-020-70269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin HY, Park HYL, Jung KI, Park CK. Comparative study of macular ganglion cell-inner plexiform layer and peripapillary retinal nerve fiber layer measurement: structure-function analysis. Invest Ophthalmol Vis Sci. 2013;54(12):7344–7353. doi:10.1167/iovs.13-12667 [DOI] [PubMed] [Google Scholar]
- 31. Zhang A, Kumar N, Desai U. Effect of intravitreal bevacizumab on the retina ganglion cell layer. Abstract presented at: ARVO Annual Meeting; April 28-May 2, 2019; Vancouver, British Columbia, Canada. [Google Scholar]
- 32. Zucchiatti I, Parodi MB, Pierro L, et al. Macular ganglion cell complex and retinal nerve fiber layer comparison in different stages of age-related macular degeneration. Am J Ophthalmol. 2015;160(3):602–607.e1. doi:10.1016/j.ajo.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 33. Saha S, Greferath U, Vessey KA, Grayden DB, Burkitt AN, Fletcher EL. Changes in ganglion cells during retinal degeneration. Neuroscience. 2016;329:1–11. doi:10.1016/j.neuroscience.2016.04.032 [DOI] [PubMed] [Google Scholar]
- 34. Sharei V, Höhn F, Köhler T, Hattenbach LO, Mirshahi A. Course of intraocular pressure after intravitreal injection of 0.05 mL ranibizumab (Lucentis). Eur J Ophthalmol. 2010;20(1):174–179. doi:10.1177/112067211002000124 [DOI] [PubMed] [Google Scholar]
- 35. Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011;95(8):1111–1114. doi:10.1136/bjo.2010.180729 [DOI] [PubMed] [Google Scholar]
- 36. Foxton RH, Finkelstein A, Vijay S, et al. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 2013;182(4):1379–1390. doi:10.1016/j.ajpath.2012.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. doi:10.2353/ajpath.2007.061237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brar VS, Sharma RK, Murthy RK, Chalam KV. Bevacizumab neutralizes the protective effect of vascular endothelial growth factor on retinal ganglion cells. Mol Vis. 2010;16:1848–1853. [PMC free article] [PubMed] [Google Scholar]



