Abstract
Purpose:
This report aims to describe a case of bilateral, multifocal neurosensory retinal detachments that developed during erdafitinib therapy for metastatic urothelial carcinoma.
Methods:
A case report with color fundus imaging and spectral-domain optical coherence tomography imaging is presented.
Results:
A 50-year-old man with metastatic urothelial carcinoma had an unremarkable baseline ophthalmic examination prior to starting erdafitinib. At 3-month follow up, an examination revealed bilateral, multifocal retinal detachments. Because the patient was asymptomatic and erdafitinib was the only drug to which his tumor had responded, he was kept on the medication with close ophthalmic monitoring.
Conclusions:
Erdafitinib, a fibroblast growth factor receptor inhibitor, can cause bilateral, multifocal retinal detachments. Continuation of erdafitinib may be considered in patients without significant visual impairment when the overall benefit of the medication appears to outweigh the risks.
Keywords: drug reactions, erdafitinib, fibroblast growth factor receptor inhibitor, serous retinal detachments, urothelial carcinoma
Introduction
Erdafitinib (Balversa) is a small-molecule fibroblast growth factor receptor inhibitor (FGFRi) that was recently approved for the treatment of locally advanced or metastatic urothelial carcinoma with genetic alterations of FGFR2 or FGFR3. Inhibition of the FGFR pathway affects a host of downstream signal transduction pathways, leading to the inhibition of cell proliferation and cell death in FGFR-overexpressing tumor cells. In patients with these tumors, there was significant clinical response that compared favorably to immune checkpoint inhibitors, leading to accelerated approval of erdafitinib by the US Food and Drug Administration in 2019. 1
In an open-label, phase 2 study that led to erdafitinib’s approval, significant ocular adverse effects were noted. The most serious ocular manifestation was described as central serous retinopathy (CSR) and retinal pigment epithelial detachment, which occurred in 21% of total patients with a median time to first onset of 50 days. Of the patients with ocular effects, 86% required a dosage interruption or reduction and 14% required discontinuation; no patients were maintained on their current dosage. This prompted a recommendation that patients given erdafitinib be routinely monitored by an ophthalmologist during treatment and that temporary cessation occur and decreased dosage of medication given upon resumption for those affected by retinopathy. 2,3
Despite these recommendations and the high rate of adverse events reported, minimal data exist on the ocular manifestations of erdafitinib. Two cases of serous retinal detachments (RDs) associated with erdafitinib use were described prior to the medication’s approval and argued for medication discontinuation at the onset and discovery of serous RDs. 4 As such, the current package label recommends discontinuing the medication until ophthalmic adverse effects resolve, then restarting the medication at a decreased dosage. However, Parikh et al has recently described a case of a patient treated with erdafitinib who developed a single serous RD in the central macula in each eye that was safely observed on erdafitinib. 5 Given the significant survival benefit of erdafitinib for patients in whom there is no alternative, the risks of medication discontinuation may outweigh the unclear risks of ocular adverse effects. We describe the clinical course of a patient who received erdafitinib over 5 months and developed bilateral, multifocal serous RDs that were morphologically distinct from what has been previously described.
Methods
This case report was documented with color fundus and spectral-domain optical coherence tomography (SD-OCT) imaging.
Results
A 50-year-old man with a history of rapidly progressive stage IV urothelial carcinoma with metastases to the liver, lungs, spine, and hip was referred by his oncologist to the ophthalmology department for a baseline eye examination prior to starting erdafitinib. He had a complicated course of pharmacotherapy for metastatic urothelial carcinoma that failed to resolve with paclitaxel, carboplatin, and pembrolizumab. He had completed 3 weeks of dexamethasone taper following palliative radiation several weeks prior to the initial examination.
Prior to being given erdafitinib, he had no symptoms. His visual acuity (VA) was 20/30 in the right eye and 20/40 in the left eye. Visually significant cataracts and enlarged cup-to-disc ratios of 0.7 in both eyes were noted. Findings from the remainder of his examination were unremarkable including normal macula, vessels, and periphery. SD-OCT of the macula also confirmed normal findings.
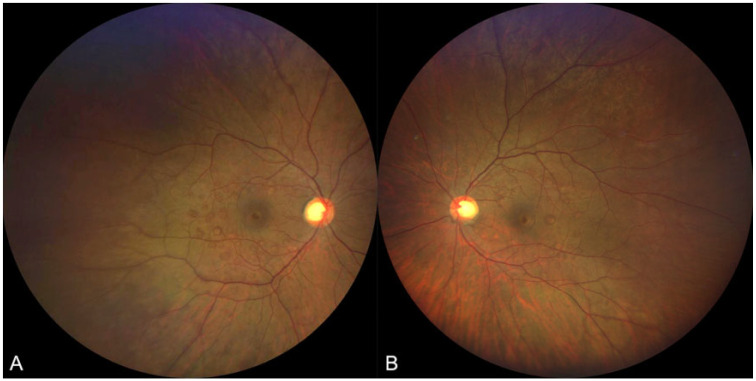
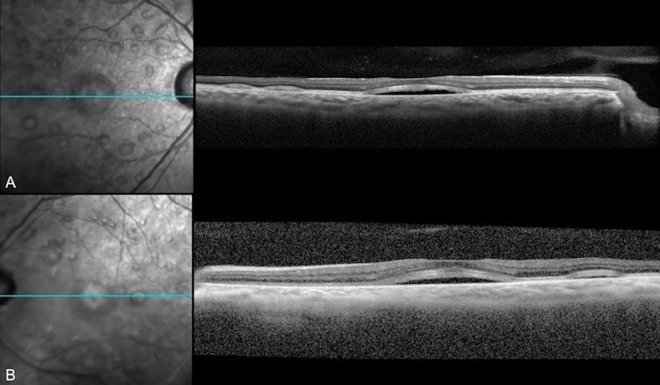
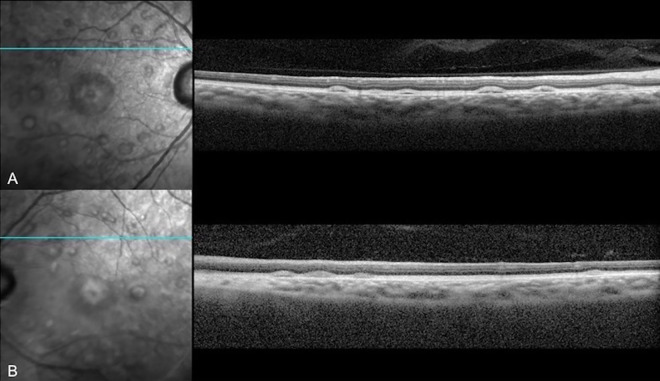
The patient was prescribed 8-mg erdafitinib once daily, and computed tomography and magnetic resonance imaging showed significant tumor response in the nodes, liver, lungs, and bones. At his 3-month follow-up with the ophthalmology department, he denied any ocular complaints. VA and anterior segment examination results were stable from his previous visit without any evidence of intraocular inflammation, but his fundus examination demonstrated new multiple, elevated lesions in the posterior pole of both eyes (Figure 1). Fundus autofluorescence demonstrated hyperautofluorescent areas surrounding the macula that corresponded to the lesions seen on fundus examination. SD-OCT revealed bilateral serous neurosensory RDs with irregular hyperreflective outer retinal thickening and associated subretinal fluid (Figures 2 and 3).
Figure 1.
Color fundus photograph of (A) the right eye and (B) left eye after 3 months of erdafitinib use showing multifocal lesions in the macula that correspond to serous retinal detachments seen on spectral-domain optical coherence tomography.
Figure 2.
Spectral-domain optical coherence tomography of (A) the right eye and (B) left eye after 3 months of erdafitinib use demonstrating serous retinal detachments in the central and temporal macula.
Figure 3.
Spectral-domain optical coherence tomography of (A) the right eye and (B) left eye after 3 months of erdafitinib use demonstrating serous retinal detachments in the superior macula.
The patient’s new ocular findings and options of discontinuation, dosage reduction, or continuation of the current dosage of erdafitinib were discussed with his oncologist. A decision to continue the medication at its therapeutic dosage under close monitoring was made based on the asymptomatic nature of the ocular findings and because erdafitinib was the only drug to which the patient's metastatic lesions had responded. At month 4 of erdafitinib, he demonstrated unchanged VA with stable bilateral, multifocal serous RDs. Again, because there was no change in symptoms or clinical examination findings, a decision to keep him on erdafitinib with close ophthalmologic supervision was made in conjunction with the oncology team.
At month 5 of erdafitinib, the patient remained without any subjective vision decline but visual assessment revealed decreased VA of 20/50 in the right eye and stable VA of 20/40 in the left eye. While the serous RDs in the macula were stable on examination, fundus photography, and SD-OCT, there was a progression in the number of the peripheral serous RDs. Computed tomography and magnetic resonance imaging also showed progression of his tumor despite erdafitinib therapy, and the medication was stopped. The patient opted for palliative care, did not return for ophthalmic follow-up, and died 4 months later.
Conclusions
We describe an early case of retinopathy associated with erdafitinib, in which erdafitinib was continued despite the onset of bilateral neurosensory RDs. It is also the first case to our knowledge to demonstrate multifocality of bilateral neurosensory RDs on erdafitinib use, distinct from what has been previously described by Parikh et al. 5 The medication was continued because there was no decline in subjective vision or VA and, after discussion with the patient's oncologist, the benefits of tumor reduction outweighed the visual risks.
Although this reaction to erdafitinib has previously been thought to be CSR, 3 the retinopathy from erdafitinib we observed was both clinically and morphologically distinct. CSR is typically associated with the pachychoroid, which was not seen in this case. Further, CSR rarely has as numerous subretinal fluid pockets.
The mechanism through which erdafitinib may cause neurosensory RDs is likely through its indirect effect on the FGFR pathway. FGFR signaling is known to occur upstream of the mitogen-activated protein kinase (MAPK) or MEK pathways. 6,7 Perturbing the MAPK or MEK pathways with inhibitors such as pimasertib and dabrafenib has been shown to cause bilateral central serous RDs and more rarely bilateral cystoid macular edema. 8,9 It thus follows that perturbing the MAPK pathway upstream through FGFR could have a similar effect. While the mechanism of bilateral neurosensory RD in MAPK inhibition is not clearly understood, there is evidence that the MAPK pathway regulates tight junctions between retinal pigment epithelial cells and that inhibition interferes with fluid transport and causes fluid accumulation below the fovea. 10 Similarly, this may be the underlying mechanism of serous RD seen with erdafitinib. We support using the term FGFR inhibitor–associated retinopathy over central serous retinopathy to describe the multifocal neurosensory RDs that occur in the setting of FGFR inhibitor use.
As previously noted in BCL2001 clinical trial, the CSR/pigment epithelial detachment–like phenomenon led to an interruption or reduction of dosage or discontinuation of erdafitinib in 86% and 14% of patients with the reaction, respectively. 11 The package insert recommends withholding erdafitinib until resolution of these lesions and resuming it at 1 or 2 lower dosage levels if resolution is achieved within 4 weeks. 3 In our particular case, retinopathy occurred in the absence of change in VA and subjective vision, allowing the patient to remain on and benefit from erdafitinib systemically. It was not until 5 months of therapy that the patient was noted to have a 2-line decrease in VA in 1 eye, which may have been due to a greater number and size of macular lesions in the affected eye and resultant gradual degenerative changes of photoreceptors. The patient also had progression of peripheral lesions in both eyes and, despite continued treatment, eventual failure of tumor response to therapy. Since we were unable to observe this patient when medication was stopped, it is unknown if the VA would have rebounded to baseline level in a manner similar to quiescent CSR or if the changes would have been long-lasting.
This case demonstrates that erdafitinib use can lead to bilateral multifocal serous RDs similar to those observed with MAPK/MEK inhibition. Further, this case demonstrates that in the absence of alternative cancer therapy and significant visual change, it may be reasonable to continue erdafitinib, a potentially life-saving therapy, in the setting of close ophthalmic monitoring. Discussion with a patient’s oncologists is essential to further understanding the benefits and alternatives of erdafitinib, and an individualized approach with close monitoring should be taken when retinopathy occurs.
Future studies should aim at better characterizing FGFRi-associated retinopathy, including its frequency, time to resolution, the extent of visual impairment, its relationship to both dosage and tumor response, and long-term effects. Our case highlights that FGFRi-associated retinopathy can be multifocal and distributed throughout the retina rather than limited to the central macula as previously described. When retinopathy is present, the benefits and alternatives of life-prolonging treatment should be discussed with the patient’s oncologist and an individualized approach should be taken.
Footnotes
Ethical Approval: This case report was conducted in accordance with Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner.
Statement of Informed Consent: Informed consent including permission for publication of all photographs and images was obtained.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. FDA Oncology Update. Am Health Drug Benefits. 2019;12(4):198–200. [PMC free article] [PubMed] [Google Scholar]
- 2. Loriot Y, Necchi A, Park SH, et al. BLC2001 Study Group. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–348. doi:10.1056/NEJMoa1817323 [DOI] [PubMed] [Google Scholar]
- 3. BALVERSA (erdafitinib) for Healthcare Professionals. Prescribing information. Janssen Biotech Inc; Accessed December 5, 2020. [Google Scholar]
- 4. Prensky C, Marlow E, Gupta M, Sales C, Kiss S, D’Amico DJ. Reversible macular lesions in the setting of oral pan-fibroblast growth factor inhibitor for the treatment of bladder cancer. J Vitreoretin Dis. 2018;2(2):111–114. doi:10.1177/2474126417751724 [Google Scholar]
- 5. Parikh D, Eliott D, Kim LA. Fibroblast growth factor receptor inhibitor-associated retinopathy. JAMA Ophthalmol. 2020;138(10):1101–1103. doi:10.1001/jamaophthalmol.2020.2778 [DOI] [PubMed] [Google Scholar]
- 6. Stjepanovic N, Velazquez-Martin JP, Bedard PL. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann Oncol. 2016;27(6):998–1005. doi:10.1093/annonc/mdw100 [DOI] [PubMed] [Google Scholar]
- 7. Purbrick RMJ, Osunkunle OA, Talbot DC, Downes SM. Ocular toxicity of mitogen-activated protein kinase inhibitors. JAMA Oncol. 2017;3(2):275–277. doi:10.1001/jamaoncol.2016.4213 [DOI] [PubMed] [Google Scholar]
- 8. Schoenberger SD, Kim SJ. Bilateral multifocal central serous-like chorioretinopathy due to MEK inhibition for metastatic cutaneous melanoma. Case Rep Ophthalmol Med. 2013;2013:673796. doi:10.1155/2013/673796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. AlAli A, Bushehri A, Park JC, Krema H, Lam WC. Pimasertib and serous retinal detachments. Retin Cases Brief Rep. 2016;10(2):191–196. doi:10.1097/ICB.0000000000000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang Q, Cao C, Lu S, et al. MEK/ERK pathway mediates UVB-induced AQP1 downregulation and water permeability impairment in human retinal pigment epithelial cells. Int J Mol Med. 2009;23(6):771–777. doi:10.3892/ijmm_00000191 [DOI] [PubMed] [Google Scholar]
- 11. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–348. doi:10.1056/nejmoa1817323 [DOI] [PubMed] [Google Scholar]