Abstract
Purpose:
This work aimed to assess the incidence of proliferative diabetic retinopathy (PDR) events and improvement to mild non-PDR (NPDR) or better after intravitreal aflibercept injection (IAI) or laser treatment (control) in diabetic macular edema (DME).
Methods:
PDR events in the VISTA (NCT01363440) and VIVID (NCT01331681) phase 3 clinical trials were evaluated in a combined IAI-treated group (IAI 2 mg every 4 weeks or 2 mg every 8 weeks after 5 initial monthly doses; n = 475) and a macular laser control group (n = 235) through week 100 in eyes without PDR at baseline (Diabetic Retinopathy Severity Scale [DRSS] score ≤ 53). Improvement in the DRSS score to 35 or better was evaluated in those with a baseline DRSS score of 43 or greater.
Results:
A lower proportion of eyes in the IAI group than in the laser group developed a PDR event through week 100 (4.4% vs 11.1%; adjusted difference, −6.7%; 97.5% CI, −11.7 to −1.6; nominal P = .0008). All PDR events occurred in eyes with a baseline DRSS score of 43, 47, or 53 and not in those with a score of 35 or less. A greater proportion of eyes in the IAI group than in the control group achieved a DRSS score of 35 or less (20.0% vs 3.8%; nominal P < .0001).
Conclusions:
Fewer eyes with NPDR and DME treated with IAI than eyes treated with a laser had a PDR event. More eyes treated with IAI improved to mild NPDR or better (DRSS score ≤ 35) through 100 weeks.
Keywords: anti-VEGF therapy, diabetic macular edema, diabetic retinopathy, intravitreal aflibercept, proliferative diabetic retinopathy, VISTA, VIVID
Introduction
Diabetic retinopathy (DR) is the most frequent microvascular complication of diabetes mellitus, 1 affecting up to one-third of all individuals with the disease. 2 DR can lead to severe vision loss, primarily through the development of retinal ischemia, pathologic retinal neovascularization, and diabetic macular edema (DME), the latter resulting from increased vascular permeability and/or capillary nonperfusion independent of retinopathy stage and severity. 3 At present, DR is the most common cause of vision loss among working-age adults worldwide. 4
In clinical trials, DR severity is often assessed using the Early Treatment Diabetic Retinopathy Study (ETDRS) Diabetic Retinopathy Severity Scale (DRSS). Overall, there are 13 distinct categories of DR severity on the DRSS, with nonproliferative DR (NPDR) ranging from mild to severe (score 35-53) followed by advanced sight-threatening proliferative DR (PDR) ranging from mild to advanced (score 61-85). 5
Antivascular endothelial growth factor (anti-VEGF) agents, such as intravitreal aflibercept injection (IAI), are widely used for the management of DME and DR. 6 In the VISTA (ClinicalTrials.gov identifier: NCT01363440) and VIVID (ClinicalTrials.gov identifier: NCT01331681) phase 3 trials of patients with DME, IAI significantly improved visual acuity (VA) and retinal thickness compared with laser photocoagulation through 100 weeks. 7 In addition, in the overall patient population, a significantly greater proportion of IAI-treated eyes than eyes in the laser control group had a 2-step or greater improvement in the DRSS score from baseline through week 100 7 and fewer eyes progressed to PDR. 8
This post hoc analysis of the VISTA and VIVID phase 3 clinical trials assessed the incidence of PDR events, defined as PDR (DRSS score ≥ 61), panretinal photocoagulation (PRP), or vitrectomy, and the rate of improvement to mild NPDR in eyes treated with IAI vs eyes in a laser photocoagulation control group by baseline DRSS severity through week 100.
Methods
VISTA and VIVID Study Design
Details of the study designs and methods of VISTA and VIVID have been reported. 9 In brief, VISTA and VIVID were 2 similarly designed, double-masked, multicenter, randomized, 148-week phase 3 clinical trials that compared the efficacy and safety of 2 dosing regimens (IAI vs macular laser photocoagulation [laser control]) in 872 patients with clinically significant DME. VISTA was performed across 54 sites in the United States, and VIVID was conducted at 73 sites across Australia, Europe, and Japan. The study protocols were approved by each participating clinical site’s respective institutional review board or ethics committee before study commencement, and all patients provided written informed consent. Both studies were performed in compliance with the ethical guidelines of the Declaration of Helsinki and the U.S. Health Insurance Portability and Accountability Act.
Adult patients with type 1 or type 2 diabetes mellitus who presented with central DME involvement (defined as retinal thickening involving the 1.0 mm central [optical coherence tomography] subfield thickness) were eligible for enrollment if the best-corrected VA was between 73 and 24 letters (20/40 to 20/320 Snellen equivalent) in the study eye. Only 1 eye per patient was enrolled in the study.
Eyes were randomly assigned in a 1:1:1 ratio to receive either (1) treatment with IAI 2 mg every 4 weeks (2q4 subgroup), (2) 2 mg every 8 weeks (2q8 subgroup) after 5 initial monthly doses with sham injections on nontreatment visits or (3) treatment with laser photocoagulation at baseline and sham injections at every visit (laser control group). Eyes were treated through week 96. All treatment groups were eligible for rescue treatment from week 24 onward as described previously. 9
Post Hoc Analysis
This post hoc integrated analysis of VISTA and VIVID assessed the effect of IAI (in the combined IAI 2q4 and 2q8 subgroups) compared with the effect of laser treatment on the development of PDR events in eyes with DME and NPDR at baseline (DRSS score ≤ 53) and the proportion of eyes with a baseline DRSS score of 43 or greater who achieved mild NPDR or better (DRSS score ≤ 35) through 100 weeks. The development of PDR events was also assessed in eyes with a baseline DRSS score of 47 or 53, reflecting the eligibility criteria for the PANORAMA study (ClinicalTrials.gov identifier: NCT02718326). PDR events were defined as PDR (DRSS score ≥ 61), PRP, or vitrectomy through week 100.
All eyes in the full analysis set of VISTA and VIVID (all randomized eyes that received any study medication and had at least 1 baseline and 1 postbaseline assessment) were included in this analysis if they had a gradable DRSS score at baseline. Eyes with PDR at baseline were excluded from analyses of the development of PDR events. Eyes with PDR at baseline were included in the analysis of eyes with baseline a DRSS score of 43 or greater that achieved mild NPDR or better.
Fundus photographs were used to assess DRSS scores by independent central reading centers (Digital Angiography Reading Center, Great Neck, New York [VISTA]; Vienna Reading Center, Vienna, Austria [VIVID]) at baseline and at weeks 24, 52, and 100. DRSS assessment was based on the ETDRS study scale and included the following scores with increasing severity: 10 = DR absent; 20 = microaneurysm only; 35, 43, 47, and 53 = mild, moderate, moderately severe, and severe NPDR, respectively; and 61, 65, and 71/75 = mild, moderate, and high-risk PDR, respectively. 5 For eyes that received rescue treatment, data were censored from the time rescue treatment was given.
Statistical Analysis
All analyses were performed on the full analysis set using observed data. For comparisons of binary outcomes between 2 groups, differences with CIs were calculated using the Mantel-Haenszel weighting method adjusted by study (VISTA vs VIVID). Associated P values were calculated using the stratified Cochran-Mantel-Haenszel test. The time to an event and cumulative incidence were evaluated by the Kaplan-Meier method. The log-rank test was used to assess the difference between 2 cumulative incidence curves. The hazard ratio (HR) between the combined IAI treatment group and laser control group was estimated using Cox proportional hazards analysis adjusted by study. The time at risk for each eye was defined as the minimum of time from randomization to the first of any of the following: (1) the date a patient discontinued the study, (2) the date of the first episode of the evaluated event, and (3) the end of the study. The time at risk was expressed as person-years at risk (PYR), with the rate expressed as the number of events/PYR. All analyses described were post hoc and performed in an exploratory manner. Analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Of the 872 eyes randomly assigned in VISTA and VIVID, 862 were included in the full analysis set. 9 Among the full analysis set, 748 eyes had a gradable fundus image at baseline and were included in this post hoc analysis (Supplemental Figure 1). Of these, 710 eyes did not have PDR at baseline and were included in the analyses examining the development of PDR events. DRSS scores ranged from 10 to 75, with 688 eyes (92.1%) having NPDR (DRSS score 35-53) and 38 eyes (5.1%) having PDR (DRSS score 61-75) at baseline (Table 1). Patients with higher DRSS scores tended to be younger (aged < 60 years) and were more often male, with higher hemoglobin A1c levels, poorer vision, and a greater central subfield thickness than patients with lower DRSS scores (Table 1). In general, baseline characteristics were comparable between the IAI combined group and laser control group within each baseline DRSS score subgroup (Supplemental Table 1).
Table 1.
Baseline Characteristics by Baseline DRSS Score. a
| Characteristic | Baseline DRSS score (N = 748) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 (n = 9) | 20 (n = 12) | 35 (n = 24) | 43 (n = 256) | 47 (n = 153) | 53 (n = 256) | 61 (n = 9) | 65 (n = 22) | 71 (n = 6) | 75 (n = 1) | |
| Age, mean ± SE, y | 65.7 ± 4.5 | 64.4 ± 2.2 | 63.8 ± 1.8 | 65.0 ± 0.5 | 63.8 ± 0.7 | 60.9 ± 0.6 | 55.7 ± 3.2 | 52.4 ± 1.8 | 47.8 ± 4.3 | 64 ± N/A |
| Female, n (%) | 5 (55.6) | 7 (58.3) | 10 (41.7) | 111 (43.4) | 69 (45.1) | 102 (39.8) | 3 (33.3) | 7 (31.8) | 0 (0.0) | 0 (0.0) |
| HbA1c | ||||||||||
| Mean ± SE, % | 7.1 ± 0.2 | 7.0 ± 0.3 | 8.0 ± 0.3 | 7.7 ± 0.1 | 7.7 ± 0.1 | 7.8 ± 0.1 | 7.9 ± 0.4 | 8.0 ± 0.4 | 8.1 ± 0.7 | 8.5 ± N/A |
| ≤ 8%, n (%) | 8 (88.9) | 10 (83.3) | 15 (62.5) | 173 (67.6) | 91 (59.5) | 165 (64.5) | 6 (66.7) | 14 (63.6) | 3 (50.0) | 0 (0.0) |
| Diabetes mellitus, n | 9 | 12 | 24 | 255 | 151 | 255 | 8 | 22 | 6 | 1 |
| Duration, mean ± SE, y | 17.6 ± 3.5 | 24.3 ± 3.6 | 18.5 ± 2.2 | 18.1 ± 0.7 | 16.3 ± 0.8 | 13.9 ± 0.6 | 13.0 ± 3.2 | 11.4 ± 1.8 | 16.5 ± 3.2 | 17.0 ± N/A |
| Study eye | ||||||||||
| BCVA, mean ± SE, ETDRS | 61.8 ± 2.2 | 63.3 ± 2.7 | 60.2 ± 2.4 | 60.4 ± 0.6 | 62.4 ± 0.8 | 58.5 ± 0.7 | 45.1 ± 5.5 | 55.0 ± 3.2 | 51.3 ± 6.5 | 39.0 ± N/A |
| CST, mean ± SE, µm | 490.4 ± 40.0 | 497.9 ± 34.7 | 475.8 ± 30.5 | 476.3 ± 8.7 | 475.6 ± 10.6 | 530.1 ± 10.3 | 611.9 ± 83.1 | 567.1 ± 44.5 | 560.3 ± 122.3 | 851.0 ± N/A |
Abbreviations: BCVA, best-corrected visual acuity; CST, central subfield thickness; DR, diabetic retinopathy; DRSS, Diabetic Retinopathy Severity Scale; ETDRS, Early Treatment Diabetic Retinopathy Study letters; HbA1c hemoglobin A1c; N/A, not applicable; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
DRSS score: 10, DR absent; 20, DR questionable, microaneurysms only; 35, mild NPDR; 43, moderate NPDR; 47, moderately severe NPDR; 53, severe NPDR; 61, mild PDR; 65, moderate PDR; 71, high-risk PDR; 75, high-risk PDR.
Proliferative Diabetic Retinopathy Events
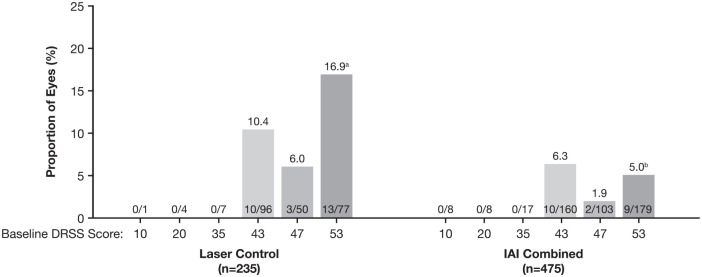
The proportion of eyes that developed a PDR event (PDR, PRP, or vitrectomy) from baseline to week 100 was 4.4% and 11.1% in the combined IAI group and laser control group, respectively (adjusted difference, −6.7%; 97.5% CI, −11.7 to −1.6; P = .0008). Within each treatment group, all PDR events occurred in eyes with a baseline DRSS score of 43, 47, or 53, with rates being statistically higher only for those with a baseline DRSS score of 53 vs 47 in the laser control group (% difference [95% CI], 10.9 [0.2-21.5]; P = .0451) (Figure 1).
Figure 1.
Distribution of incidences of PDR events by baseline DRSS score through week 100. Full analysis set.
Observed case, with data censored from the time of rescue therapy. PDR event defined as PDR (DRSS score ≥ 61), PRP, or vitrectomy.
Abbreviations: DRSS, Diabetic Retinopathy Severity Scale; IAI, intravitreal aflibercept injection; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation.
aP = .0451 compared with eyes with baseline DRSS score 47 in the laser control group (difference: 10.9% [95% CI, 0.2 to 21.5]).
bP = .0095 compared with eyes with baseline DRSS score 53 in the laser control group (difference: −11.9% [95% CI, −20.8 to −2.9]).
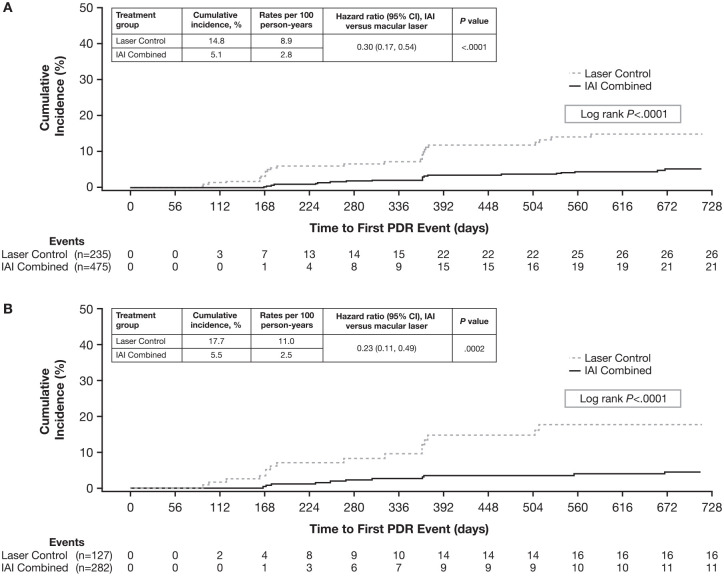
Comparing across treatment groups, the rates of PDR events were relatively lower in the combined IAI group than in the laser control group in the subgroups of eyes with baseline DRSS scores of 43, 47, or 53; the difference between the combined IAI group and laser control group was significant only for eyes with a baseline DRSS score of 53 (% difference vs laser [95% CI], −11.9 [−20.8 to −2.9]; P = .0095) (Figure 1). PDR events occurred earlier in the laser control group than in the combined IAI treatment group (log-rank P < .0001) (Figure 2A). The cumulative incidence of PDR events through 100 weeks was 5.1% (combined IAI) vs 14.8% (laser control), with an HR for progressing to PDR of 0.30 (95% CI, 0.17-0.54; P < .0001).
Figure 2.
Cumulative incidence of PDR events through week 100 by baseline DRSS score of: (A) ≤ 53 and (B) 47 or 53.
PDR event defined as PDR (DRSS score ≥ 61), PRP, or vitrectomy.
Abbreviations: DRSS, Diabetic Retinopathy Severity Scale; IAI, intravitreal aflibercept injection; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation.
PDR Events in Eyes With Baseline DRSS Scores of 47 or 53
The proportion of eyes with a baseline DRSS score of 47 (moderately severe NPDR) or 53 (severe NPDR) that developed a PDR event through week 100 was lower in the combined IAI group than in the laser control group (3.9% vs 12.6%; adjusted difference, −8.7%; 97.5% CI, −15.8 to −1.6; P = .0011). The cumulative incidence of PDR events through week 100 for eyes with a baseline DRSS score of 47 or 53 was 5.5% in the combined IAI group and 17.7% in the laser control group, with an HR for progressing to PDR of 0.23 (95% CI, 0.11-0.49; P = .0002) (Figure 2B).
Eyes With Baseline DRSS Score of 43 or Greater That Achieved DRSS Score of 35 or Less
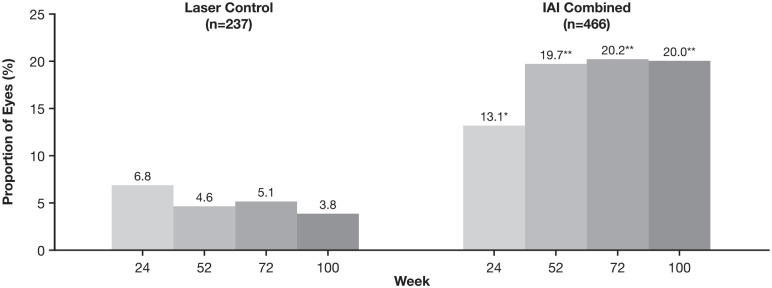
Figure 3 shows the proportions of eyes with a baseline DRSS score of 43 or greater (moderate NPDR or worse) who achieved a DRSS score of 35 or less (mild NPDR or better) through week 100. In the laser control group, the proportion achieving a DRSS score of 35 or less tended to decrease numerically from week 24 to week 100; in the combined IAI group, the proportion achieving a DRSS score of 35 or less increased from week 24 to week 52 and was maintained to week 100. The proportion of eyes that achieved a DRSS score of 35 or less was higher in the combined IAI group than in the laser control group at each timepoint; at week 100, 20.0% in the combined IAI group and 3.8% in the laser control group achieved a DRSS score of 35 or less (P < .0001) (Figure 3).
Figure 3.
Proportion of eyes with a baseline DRSS score of 43 or greater who achieved a DRSS score of 35 or less through week 100.
Abbreviations: DRSS, Diabetic Retinopathy Severity Scale; IAI, intravitreal aflibercept injection.
*P = .0023 compared with laser control. **P < .0001 compared with laser control.
Conclusions
In this post hoc analysis of integrated data from the phase 3 VISTA and VIVID studies in eyes with DME, PDR events occurred only in those with moderate to severe NPDR (baseline DRSS scores 43-53). In these eyes, the incidence of PDR events was significantly lower in the IAI treatment group than in the laser control group in eyes with a DRSS score of 53. PDR events occurred earlier in the laser control group than in the IAI treatment group. In addition, a greater proportion of eyes treated with IAI than with eyes treated with a laser improved to mild NPDR or better (DRSS score ≤ 35) through week 100. Taken altogether, these findings are consistent with those in previous studies reporting that the rate of DR progression was associated with DR severity10,11 and further suggest that IAI reduces DR progression in eyes with DME.
The results in this study should be interpreted with caution because this was a post hoc analysis and all P values are considered nominal. In addition, PDR events were not prespecified endpoints. Vitreous hemorrhage and anterior segment neovascularization were not included as PDR events because the information was insufficient to confirm a relationship with PDR. Approximately 14% of fundus images from the full analysis set were nongradable, which might have disproportionately reflected on the number of eyes in the subgroups evaluated in this study. Furthermore, the role of other factors associated with DR progression, including poor glycemic control, duration of diabetes, retinal nonperfusion, systemic hypertension, and dyslipidemia,11,12 were not evaluated in our study because of the small sample and inadequate collection of systemic disease characteristics.
Nevertheless, our findings showing a lower incidence of PDR events after treatment with IAI of eyes with DME in the VISTA and VIVID trials are consistent with findings reported in the RISE, RIDE, and Protocol I studies of ranibizumab in a similar patient population and the PANORAMA and Protocol W studies that evaluated IAI in eyes with moderate to severe NPDR without DME.13–17 In both PANORAMA and Protocol W, through year 2, a greater proportion of eyes in the IAI-treated groups experienced a 2-step or greater improvement in the DRSS score and a reduced rate of proliferative disease than eyes in the sham-treated groups. 15 In addition, both studies reported that the development of center-involved DME was significantly lower in IAI-treated eyes than in sham-treated eyes.15,16 Collectively, these findings show that anti-VEGF agents can reduce or prevent DR progression regardless of the presence or absence of DME.
Supplemental Material
Supplemental material, sj-docx-1-vrd-10.1177_24741264221093914 for Proliferative Diabetic Retinopathy Events in Patients With Diabetic Macular Edema: Post Hoc Analysis of VISTA and VIVID Trials by Diana V. Do, Carmelina Gordon, Ivan J. Suñer, Kimberly Reed, Hadi Moini, Andrea Gibson, Weiming Du and Chirag P. Shah in Journal of VitreoRetinal Diseases
Acknowledgments
Medical writing support was provided by Nila Bhana, MSc, Rhutika Dessai, MSc, and Keng Jin Lee, PhD, of Prime Global (Knutsford, UK) according to Good Publication Practice Guidelines (Link), and funded by Regeneron Pharmaceuticals, Inc.
Footnotes
Authors’ Note: Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the indication has been approved by major health authorities if there is legal authority to share the data and there is not a reasonable likelihood of participant reidentification. Submit requests to https://vivli.org/.
Ethical Approval: VISTA (ClinicalTrials.gov identifier: NCT01363440) was conducted across 54 sites in the United States, and VIVID (ClinicalTrials.gov identifier: NCT01331681) was conducted at 73 sites across Australia, Europe, and Japan. The study protocols were approved by each participating clinical site’s respective institutional review board or ethics committee before study commencement, and all patients provided written informed consent. Both studies were carried out in compliance with the ethical guidelines of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
Statement of Informed Consent: All patients provided written informed consent before study commencement.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs Do, Gordon, and Suñer have no proprietary interest in the study drug. Dr Do is a consultant to Allergan, Asclepix, Boehringer Ingelheim, Clearside, Genentech, Kodiak Sciences, and Regeneron Pharmaceuticals, Inc, and has received research funding from Asclepix, Boehringer Ingelheim, Genentech, and Regeneron Pharmaceuticals, Inc. Dr Gordon has received research funding from Alcon, Alimera, Allergan, Genentech, the National Institutes of Health (NIH), Novartis, Regeneron Pharmaceuticals, Inc, Santen Pharmaceutical Co, Ltd, and Topco. Dr Suñer is a consultant to Alimera, Allergan, Genentech, Novartis, and Regeneron Pharmaceuticals, Inc; a speaker for Alimera, Genentech, and Regeneron Pharmaceuticals, Inc; and an investigator for Alimera, Allergan, Genentech, Kodiak Pharmaceuticals, Inc, and Regeneron Pharmaceuticals, Inc. Drs Reed, Moini, and Du are salaried employees and shareholders of Regeneron Pharmaceuticals, Inc. Dr Gibson was a salaried employee of Regeneron Pharmaceuticals, Inc, at the time these analyses were performed. Dr Shah is a consultant to and investigator for Regeneron Pharmaceuticals, Inc, and an investigator for Alcon, Allergan, Apellis, Ellex, Genentech, NIH, and Novartis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The VISTA and VIVID studies were funded by Regeneron Pharmaceuticals, Inc, Tarrytown, New York, and Bayer HealthCare, Berlin, Germany. This post hoc analysis was funded by Regeneron Pharmaceuticals, Inc.
ORCID iD: Ivan J. Suñer  https://orcid.org/0000-0003-0123-846X
https://orcid.org/0000-0003-0123-846X
Supplemental Material: Supplemental material is available online with this article.
References
- 1. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227-1239. doi: 10.1056/NEJMra1005073 [DOI] [PubMed] [Google Scholar]
- 2. Yau JW, Rogers SL, Kawasaki R, et al. ; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14):e93751. doi: 10.1172/jci.insight.93751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee CS, Lee AY, Baughman D, et al. ; UK DR EMR Users Group. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group: report 3: baseline retinopathy and clinical features predict progression of diabetic retinopathy. Am J Ophthalmol. 2017;180:64-71. doi: 10.1016/j.ajo.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. Early Treatment Diabetic Retinopathy Study report number 12. Ophthalmology. 1991;98(5 suppl):823-833. doi: 10.1016/S0161-6420(13)38014-2 [DOI] [PubMed] [Google Scholar]
- 6. Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: the International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608-1622. doi: 10.1016/j.ophtha.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 7. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044-2052. doi: 10.1016/j.ophtha.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 8. Mitchell P, McAllister I, Larsen M, et al. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmol Retina. 2018;2(10):988-996. doi: 10.1016/j.oret.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 9. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254. doi: 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 10. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796-1806. [PubMed] [Google Scholar]
- 11. Ghamdi AHA. Clinical predictors of diabetic retinopathy progression; a systematic review. Curr Diabetes Rev. 2020;16(3):242-247. doi: 10.2174/1573399815666190215120435 [DOI] [PubMed] [Google Scholar]
- 12. Nicholson L, Ramu J, Chan EW, et al. Retinal nonperfusion characteristics on ultra-widefield angiography in eyes with severe nonproliferative diabetic retinopathy and proliferative diabetic retinopathy. JAMA Ophthalmol. 2019;137(6):626-631. doi: 10.1001/jamaophthalmol.2019.0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nguyen QD, Brown DM, Marcus DM, et al. ; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 14. Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina. 2018;2(10):997-1009. doi: 10.1016/j.oret.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 15. Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139(9):946-955. doi: 10.1001/jamaophthalmol.2021.2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maturi RK, Glassman AR, Josic K, et al. ; DRCR Retina Network. Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139(7):701-712. doi: 10.1001/jamaophthalmol.2021.0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e35. doi: 10.1016/j.ophtha.2010.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-vrd-10.1177_24741264221093914 for Proliferative Diabetic Retinopathy Events in Patients With Diabetic Macular Edema: Post Hoc Analysis of VISTA and VIVID Trials by Diana V. Do, Carmelina Gordon, Ivan J. Suñer, Kimberly Reed, Hadi Moini, Andrea Gibson, Weiming Du and Chirag P. Shah in Journal of VitreoRetinal Diseases