Abstract
Purpose: To assess the correlation between the change in central subfield thickness (CST) and change in best-corrected visual acuity (BCVA) in eyes with diabetic macular edema (DME) treated with fixed-dosing intravitreal aflibercept injection (IAI). Methods: This post hoc analysis of the VISTA and VIVID randomized controlled clinical trials, in which 862 eyes with central-involved DME were randomly assigned to IAI 2 mg every 4 weeks (2q4; 290 eyes), IAI 2 mg every 8 weeks after 5 initial monthly doses (2q8; 286 eyes), or macular laser (286 eyes) and followed through 100 weeks. Correlations between the change in CST and change in BCVA from baseline to weeks 12, 52, and 100 were assessed using the Pearson correlation. Results: The respective correlations (r [95% CI]) at weeks 12, 52, and 100 were −0.39 (−0.49 to −0.29), −0.27 (−0.38 to −0.15), and −0.30 (−0.41 to −0.17) in the 2q4 arm and −0.28 (−0.39 to −0.17), −0.29 (−0.41 to −0.17), and −0.33 (−0.44 to −0.20) in the 2q8 arm. Linear regression analysis of the correlation at week 100, adjusted for relevant baseline factors, showed CST changes accounted for 17% of the variance in BCVA changes; every 100-µm decrease in CST was associated with a 1.2-letter increase in BCVA (P = .001). Conclusions: Correlations between the change in CST and change in BCVA after 2q4 or 2q8 fixed-dosing IAI for DME were modest. Although a change in CST might be important in determining the need for antivascular endothelial growth factor for DME at follow-up, it was not a good surrogate for VA outcomes.
Keywords: aflibercept, anti-VEGF, central subfield thickness, correlation, diabetic macular edema, visual acuity
Introduction
Recent post hoc analyses from the Diabetic Retinopathy Clinical Research (DRCR) Retina Network found that eyes with diabetic macular edema (DME) treated with intravitreal antivascular endothelial growth factor (anti-VEGF) injections showed weak to moderate correlations between central subfield thickness (CST) changes on optical coherence tomography (OCT) and final visual acuity (VA) outcomes during 2 years of treatment across aflibercept, bevacizumab, and ranibizumab in Protocol T 1 and during 5 years of follow-up using ranibizumab in Protocol I. 2 Moreover, VA changes after 3 initial monthly injections had little predictive value for final VA at 2 years. 3 These results suggest that CST changes and early VA responses might not be good predictors of VA outcomes based on an as-needed treatment regimen, typically after an initial 6 monthly injections. To our knowledge, however, this correlation has not been evaluated for a fixed-dosing regimen of anti-VEGF injections every month or every other month after an initial set of monthly injections.
This study aimed to determine the correlation between changes in CST with changes in best-corrected VA (BCVA) in participants with DME in the VIVID or VISTA phase 3 clinical trials who were assigned to the fixed-dosing intravitreal aflibercept injection (IAI) arms through 100 weeks after the initial randomization visit.
Methods
VISTA and VIVID Study Design
VISTA and VIVID were 2 similarly designed, double-masked, randomized, active-controlled phase 3 clinical trials. VISTA (registered at www.clinicaltrials.gov; NCT01363440) was performed across 54 sites in the United States and randomly assigned 466 study participants from May 2011 to November 2014. VIVID (registered at www.clinicaltrials.gov; NCT01331681) was performed at 73 sites across Europe, Japan, and Australia and randomly assigned 406 study participants from May 2011 to March 2015. Each clinical site’s respective institutional review board (IRB)/ethics committee approved the study. All patients provided written informed consent before enrollment. Both studies were performed in compliance with the ethical guidelines of the Declaration of Helsinki and the US Health Insurance Portability and Accountability Act. The study design and eligibility criteria for the VISTA and VIVID trials have been described.4,5 This post hoc analysis started in August 2020 and was completed in October 2020 with IRB approval from Johns Hopkins University School of Medicine.
Patients were eligible for enrollment in VISTA and VIVID if they were adults with type 1 or type 2 diabetes mellitus who presented with central-involved DME (defined as retinal thickening involving the central 1.0 mm as measured by spectral-domain [SD]-OCT CST) and had a BCVA in the study eye between 73 and 24 Early Treatment Diabetic Retinopathy Study letters score (20/40–20/320 approximate Snellen equivalent). Only 1 eye per patient was eligible for enrollment.
Eyes were randomly assigned in a 1:1:1 ratio to receive IAI 2 mg every 4 weeks (2q4), IAI 2 mg every 8 weeks after 5 initial monthly doses (2q8), or macular laser photocoagulation (laser control) at baseline. Study eyes in all treatment groups were assessed for laser treatment if criteria were met beginning at week 12. Study eyes in the 2q4 and 2q8 groups received sham laser treatment, and those in the laser control group received active laser treatment if treatment criteria were met, but not more frequently than every 12 weeks. Beginning at week 24, when the prespecified additional (rescue) treatment criteria were met, study eyes in the 2q4 and 2q8 groups received active laser treatment while those in the laser control group received 5 doses of 2q4 followed by dosing every 8 weeks. Eyes were treated through week 96.4,5
Post Hoc Analysis
Study Population
All participants who were in the full analysis set (defined as patients who were randomly assigned and had baseline and at least 1 post-baseline BCVA assessment) were included.
Outcome Measures
Randomized clinical trial data were used to evaluate the association between the change in CST as measured by SD-OCT and the change in BCVA in patients in each treatment arm. Only participants with observed data were used; there was no imputation for missing data. Data were censored after rescue medication was given.
Statistical Analyses
Data from the VISTA and VIVID studies were integrated because the outcomes across the 2 studies (not shown) were consistent. The Pearson correlation coefficient (r) was used to evaluate the association between CST and BCVA at baseline and at weeks 12, 52, and 100 as well as between the changes in CST and changes in BCVA from baseline to week 12, 52, and 100.
The Fisher z transformation was used to estimate the 95% CIs of the Pearson correlation coefficients. The relationship between 2 variables was considered negligible for an r value from 0 to less than 0.1, weak for an r value from 0.1 to less than 0.40, moderate for an r value from 0.4 to less than 0.7, strong for an r value from 0.7 to less than 0.9, and very strong for an r value from 0.9 to 1.6,7 Generalized linear regression models were used to calculate the slope of the regression line for the change in CST and the coefficient of determination (R2) for each visit.
At 100 weeks, baseline factors of interest (age, glycated hemoglobin A1c level, Diabetic Retinopathy Severity Scale [DRSS] score [(≤ 35, 43-53, > 60)], BCVA) as well as treatment and treatment interaction with BCVA were included in the model for adjustment to assess the effect of change in CST on change in BCVA. All patients with available values for these factors were included in the analysis.
All P values reported were 2-sided; P values less than 0.05 were considered nominally significant because there were no adjustments for multiplicity and the investigations were post hoc analyses. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Of 862 eyes in the full analysis set, the number of eyes with BCVA and CST measurements available for analysis at weeks 12, 52, and 100 was 275 (95%), 249 (86%), and 216 (74%), respectively, in the 2q4 arm and 277 (97%), 242 (85%), and 203 (71%), respectively, in the 2q8 arm.
Correlation Between BCVA and CST
At baseline, the r values (95% CIs) between CST and BCVA were −0.45 (−0.53 to −0.35) in the 2q4 arm and −0.47 (−0.55 to −0.37) in the 2q8 arm. At weeks 12, 52, and 100, the respective values were −0.28 (−0.38 to −0.17), −0.08 (−0.20 to −0.04), and −0.06 (−0.19 to 0.08) in the 2q4 arm and −0.14 (−0.26 to −0.03), −0.11 (−0.23 to −0.02), and 0.02 (−0.11 to 0.16) in the 2q8 arm (Table 1). Supplemental Figures S1 to S3 show the linear regression between absolute CST and absolute VA at baseline and weeks 12, 52, and 100 across all treatment arms.
Table 1.
Correlation Between Absolute Best-Corrected Visual Acuity and Absolute Central Subfield Thickness by Treatment Group.
| Timepoint | Laser Control | IAI 2q8 | IAI 2q4 | |||
|---|---|---|---|---|---|---|
| n | r (95% CI) | n | r (95% CI) | n | r (95% CI) | |
| Baseline | 286 | −0.36 (−0.46 to −0.26) | 286 | −0.47 (−0.55 to −0.37) | 290 | −0.45 (−0.53 to −0.35) |
| Week 12 | 267 | −0.45 (−0.54 to −0.34) | 277 | −0.14 (−0.26 to −0.03) | 275 | −0.28 (−0.38 to −0.17) |
| Week 52 | 179 | −0.31 (−0.43 to −0.17) | 242 | −0.11 (−0.23 to 0.02) | 249 | −0.08 (−0.21 to 0.04) |
| Week 100 | 139 | −0.27 (−0.41 to −0.10) | 203 | 0.02 (−0.11 to 0.16) | 216 | −0.06 (−0.19 to 0.08) |
Abbreviations: 2q4, 2 mg every 4 weeks; 2q8, 2 mg every 8 weeks after 5 initial monthly doses; IAI, intravitreal aflibercept injection; r, correlation.
Correlation Between Change in CST and Change in BCVA
At weeks 12, 52, and 100, the r values (95% CIs) were −0.39 (−0.49 to −0.29), −0.27 (−0.38 to −0.15), and −0.30 (−0.41 to −0.17), respectively, in the 2q4 arm and −0.28 (−0.39 to −0.17), −0.29 (95% CI −0.41 to −0.17), and −0.33 (−0.44 to −0.20), respectively, in the 2q8 arm (Table 2). Figures 1 and 2 show the linear regression between the change in VA by the change in CST at 12, 52, and 100 weeks in the 2q4 and 2q8 treatment arms. The corresponding linear regression in the laser control group is shown in Supplemental Figure S4.
Table 2.
Correlation Between Changes in Best-Corrected Visual Acuity and Changes in Central Subfield Thickness From Baseline by Treatment Group.
| Timepoint | Laser Control | IAI 2q8 | IAI 2q4 | |||
|---|---|---|---|---|---|---|
| n | r (95% CI) | n | r (95% CI) | n | r (95% CI) | |
| Week 12 | 267 | −0.31 (−0.41 to −0.20) | 277 | −0.28 (−0.39 to −0.17) | 275 | −0.39 (−0.49 to −0.29) |
| Week 52 | 179 | −0.21 (−0.34 to −0.06) | 242 | −0.29 (−0.41 to −0.17) | 249 | −0.27 (−0.38 to −0.15) |
| Week 100 | 139 | −0.17 (−0.32 to 0.00) | 203 | −0.33 (−0.44 to −0.20) | 216 | −0.30 (−0.41 to −0.17) |
Abbreviations: 2q4, 2 mg every 4 weeks; 2q8, 2 mg every 8 weeks after 5 initial monthly doses; IAI, intravitreal aflibercept injection; r, correlation.
Figure 1.
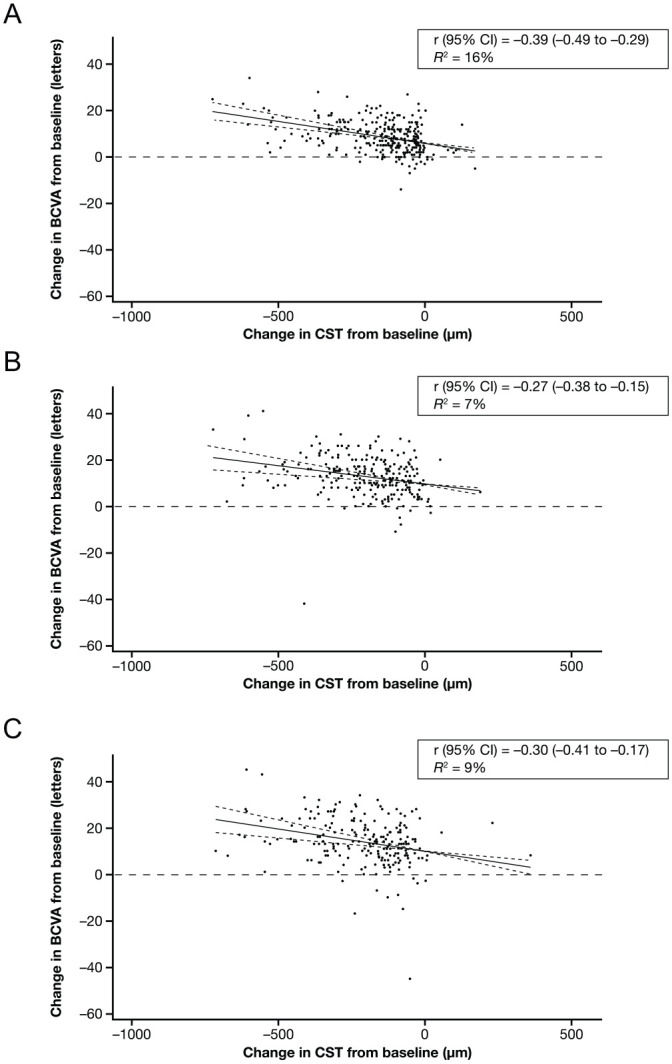
Correlations between changes in BCVA and changes in CST from baseline in patients treated with IAI 2q4 at (A) week 12, (B) week 52, and (C) week 100. Solid lines indicate the correlation line, and dashed lines indicate the 95% CI. Abbreviations: 2q4, 2 mg every 4 weeks; BCVA, best-corrected visual acuity; CST, central subfield thickness; IAI, intravitreal aflibercept injection; r, correlation; R2, coefficient of determination.
Figure 2.
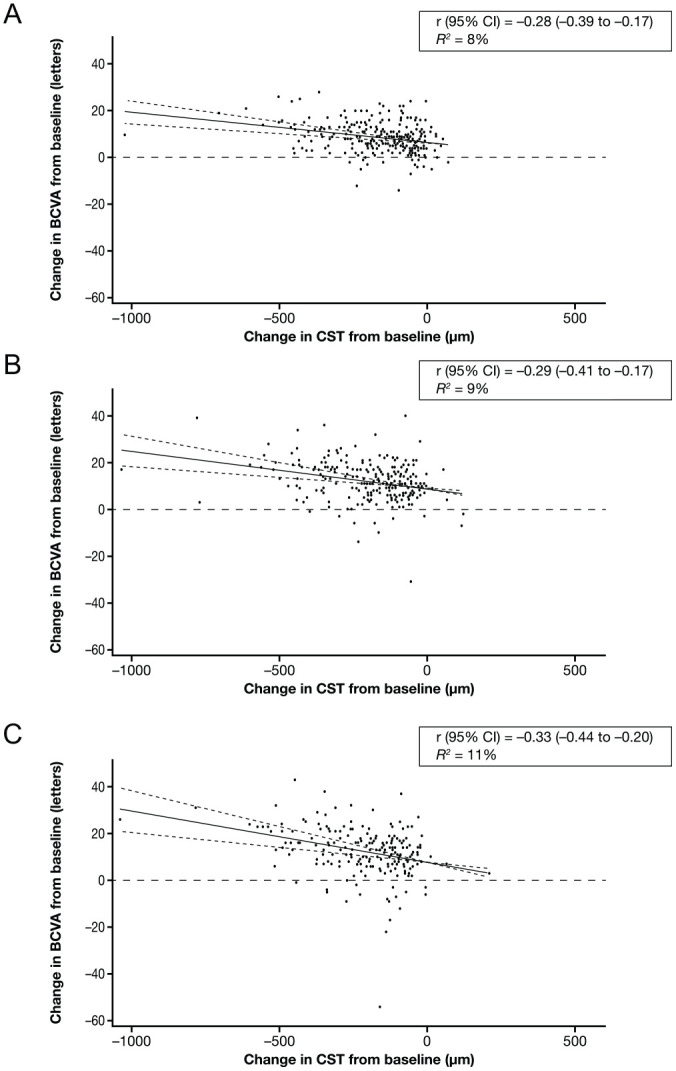
Correlations between changes in BCVA and changes in CST from baseline in patients treated with IAI 2q8 at (A) week 12, (B) week 52, and (C) week 100. Solid lines indicate the correlation line, and dashed lines indicate the 95% CI. Abbreviations: 2q8, 2 mg every 8 weeks after 5 initial monthly doses; BCVA, best-corrected visual acuity; CST, central subfield thickness; IAI, intravitreal aflibercept injection; r, correlation; R2, coefficient of determination.
Linear Regression Analysis Adjusted for Baseline Factors
The linear regression model, after adjusting for baseline factors (age, BCVA, glycated hemoglobin A1c, DRSS score, treatment, treatment by baseline BCVA interaction), showed that CST changes in the combined IAI groups (n = 360) accounted for only 17% of the variance in BCVA changes at week 100, with every 100 µm decrease in CST associated with a 1.2 letter increase in BCVA (95% CI, 0.5-1.9; P = .001).
Conclusions
This post hoc analysis evaluating eyes that received fixed 2q4 or 2q8 dosing regimens of IAI showed weak to moderate correlations between changes in CST and the changes in BCVA across 12 weeks, 52 weeks, and 100 weeks of treatment without relation to the sequential timepoint. Even with more frequent injection in the 2q4 arm, no substantial difference was found compared with the 2q8 arm. For any given change in CST from baseline, there was a broad range of changes in BCVA from baseline at weeks 12, 52, and 100. These findings are consistent with previous reports from the DRCR Retina Network Protocol T, in which eyes with central-involved DME and VA of 20/32 to 20/320 were treated with an as-needed regimen after 6 monthly injections (r value, 0.33; 95% CI, 0.26-0.41 at week 104 in combined treatment group). 1 Similarly, Ou et al 8 reported moderate correlations in pooled DME eyes that received ranibizumab in as-needed and treat-and-extend regimens after 4 monthly injections (r value, −0.45; 95% CI, −0.56 to −0.32 at month 12). Regardless of the treatment regimen, changes in CST were not well correlated with changes in VA in these clinical trials.
In the current analysis, using a linear regression model adjusting for baseline factors, the magnitude of VA improvement (1.2 letters) in response to a relatively large reduction in CST (100 µm) was very small and unlikely to be clinically relevant in predicting outcomes, similar to ranibizumab-treated eyes (2.7 letters) in the study by Ou et al. 8 Moreover, in the IAI arm of Protocol T, changes in CST at 12 weeks were not associated with changes in BCVA; a 1% CST relative change accounted for an estimated 0.06 letter change in BCVA, 3 similar to the small magnitude of the findings using fixed-dosing IAI regimens in VISTA and VIVID.
Although the design of this investigation cannot determine why there are weak-to-moderate correlations between retinal thickness and VA outcomes, several hypotheses seem plausible. Studies have noted that as CST gets thinner, there might be little visual gain. Presumably this can occur if the thickness or edema were associated with ischemia and subsequent cell death in the macula in some of the eyes. As the edema resolves, thinning would be noted but there would be no improvement in VA.9,10
The strengths of this post hoc investigation include that VISTA and VIVID were relatively large randomized controlled clinical trials that were performed across several countries. Eyes were included in the study only if they had a verified BCVA and CST and adhered to the fixed-dosing regimen in VISTA and VIVID. 4 To our knowledge, this is one of the first DME studies analyzing the relationship between change in VA and change in CST in eyes receiving fixed-dosing regimens.
Limitations of this study include the post hoc design; results should be considered exploratory to aid in building hypotheses for future studies and should not necessarily be used for treatment considerations. The study analyzed the correlations at 3 timepoints and appeared to have gross results. Analyses with multiple timepoints might yield more sophisticated results but were not pursued because they were unlikely to show a difference. Furthermore, the analysis included only the quantitative features of CST as determined by the SD-OCT device; it included neither structural nor qualitative features noted on OCT, such as cystoid abnormalities, loss of ellipsoid layer, subretinal fluid, or disorganization of retinal inner layers (DRIL) nor features noted on OCT angiography. Some of these features have been reported to be associated with visual outcomes; for example, loss of ellipsoid layer and DRIL were associated with negative visual outcomes, although not necessarily as predictive factors at baseline for VA outcomes when controlling for both baseline VA and CST.11,12 Other quantitative measurements (eg, loss of outer retinal layer thickness defined as loss of the ellipsoid layer or DRIL) was reported to have a better correlation with VA than the total retinal thickness at baseline, 13 although, again, not necessarily as a predictive factor at baseline when controlling for both baseline VA and CST. 1 Thinning of the outer retinal layer was also reported to have a negative visual outcome in eyes with DME receiving ranibizumab in an as-needed regimen. 10 The analysis and interpretation across these other studies must be compared with caution because the eligible criteria and methods of each study are different.
To our knowledge, none of these other features has been shown to independently predict BCVA outcomes with any greater magnitude of correlation when analyzed using methods similar to those in this investigation. Even in another post hoc analysis of VIVID and VISTA data, the baseline subretinal fluid showed no meaningful impact on visual outcomes in eyes treated with IAI. 14 Further studies that include analyses of structural changes or other features on OCT, or combined with angiographic or pathologic studies, might provide further insight into the correlations between these changes and the treatment response in patients with DME.
In eyes with central-involved DME and VA loss, whether treated with fixed-dosing intravitreal anti-VEGF as with IAI in the VISTA and VIVID trials or with as-needed treatment regimens with aflibercept, bevacizumab, or ranibizumab as in the DRCR Retina Network Protocol T, the correlations between the change in CST and change in BCVA were modest. Although the change in CST might be an important factor in determining whether anti-VEGF treatment should be withheld, continued, or resumed at a specific visit, CST changes alone do not appear to be a good surrogate for predicting VA outcomes.
Supplemental Material
Supplemental material, sj-docx-1-vrd-10.1177_24741264221099429 for Correlation of Change in Macular Thickness With Change in Visual Acuity in Diabetic Macular Edema: Post Hoc Analysis of VISTA and VIVID Trials by Onnisa Nanegrungsunk, Sophie Z. Gu, Susan B. Bressler, Weiming Du, Fouad Amer, Hadi Moini and Neil M. Bressler in Journal of VitreoRetinal Diseases
Footnotes
Authors’ Note: Portions of this investigation were presented at Macula Society 2021 (virtually February 6–7, 2021) and the Association for Research in Vision and Ophthalmology 2021 (virtually May 1–7, 2021).
Ethical Approval: VISTA (registered at www.clinicaltrials.gov: NCT01363440) was conducted across 54 sites in the United States, and VIVID (registered at www.clinicaltrials.gov: NCT01331681) was conducted at 73 sites across Australia, Europe, and Japan. The study protocols were approved by each participating clinical site’s respective institutional review board or ethics committee prior to study commencement, and all patients provided written informed consent. Both studies were carried out in compliance with ethical guidelines of the Declaration of Helsinki and the US Health Insurance Portability and Accountability Act. This post hoc analysis was designed starting in August 2020 and completed in October 2020 with IRB approval from Johns Hopkins University School of Medicine.
Statement of Informed Consent: All patients provided written informed consent before enrollment.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs Nanegrungsunk and Gu declare no conflict of interest. Dr S. Bressler has received grants to Johns Hopkins University School of Medicine from Bayer, Biocon, Biogen, Boehringer-Ingleheim Pharma GmbH & Co, EyePoint, Genentech (Roche), Mylan Inc, Notal Vision, Novartis, and Regeneron Pharmaceuticals Inc, and has a contract with Amgen as chair of the Data and Safety Monitoring Committee; Drs Du, Amer, and Moini are salaried employees with and have stock ownership in Regeneron Pharmaceuticals, Inc. Dr N. Bressler has received grants to Johns Hopkins University from Bayer, Biogen, Genentech (Roche), Novartis, Regeneron Pharmaceuticals, Inc, and Samsung Bioepis, and has contract with the American Medical Association as editor-in-chief of JAMA Ophthalmology.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Regeneron Pharmaceuticals, Inc and by philanthropic grants awarded to the Johns Hopkins University School of Medicine (principal investigator Neil M. Bressler, MD). Regeneron Pharmaceuticals, Inc participated in the design of the study, data analysis, and preparation of the manuscript.
Supplemental Material: Supplemental material is available online with this article.
References
- 1. Bressler NM, Odia I, Maguire M, et al. ; DRCR Retina Network. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a post hoc analysis of the Protocol T randomized clinical trial. JAMA Ophthalmol. 2019;137(9):977-985. doi: 10.1001/jamaophthalmol.2019.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dugel PU, Campbell JH, Kiss S, et al. Association between early anatomic response to anti-vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of Protocol I study data. Retina. 2019;39(1):88-97. doi: 10.1097/IAE.0000000000002110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bressler NM, Beaulieu WT, Maguire MG, et al. ; Diabetic Retinopathy Clinical Research Network. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in Protocol T. Am J Ophthalmol. 2018;195:93-100. doi: 10.1016/j.ajo.2018.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122(10):2044-2052. doi: 10.1016/j.ophtha.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 5. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254. doi: 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 6. Fisher RA. On the “probable error” of a coefficient of correlation deduced from a small sample. Metron. 1921;1:1-32. [Google Scholar]
- 7. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763-1768. doi: 10.1213/ANE.0000000000002864 [DOI] [PubMed] [Google Scholar]
- 8. Ou WC, Brown DM, Payne JF, Wykoff CC. Relationship between visual acuity and retinal thickness during anti-vascular endothelial growth factor therapy for retinal diseases. Am J Ophthalmol. 2017;180:8-17. doi: 10.1016/j.ajo.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 9. Markan A, Agarwal A, Arora A, Bazgain K, Rana V, Gupta V. Novel imaging biomarkers in diabetic retinopathy and diabetic macular edema. Ther Adv Ophthalmol. 2020;12:2515841420950513. doi: 10.1177/2515841420950513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ebneter A, Wolf S, Abhishek J, Zinkernagel MS. Retinal layer response to ranibizumab during treatment of diabetic macular edema: thinner is not always better. Retina. 2016;36(7):1314-1323. doi: 10.1097/IAE.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 11. Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309-1316. doi: 10.1001/jamaophthalmol.2014.2350 [DOI] [PubMed] [Google Scholar]
- 12. Muftuoglu IK, Mendoza N, Gaber R, Alam M, You Q, Freeman WR. Integrity of outer retinal layers after resolution of central involved diabetic macular edema. Retina. 2017;37(11):2015-2024. doi: 10.1097/IAE.0000000000001459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong RL, Lee JW, Yau GS, Wong IY. Relationship between outer retinal layers thickness and visual acuity in diabetic macular edema. Biomed Res Int. 2015;2015:981471. doi: 10.1155/2015/981471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korobelnik JF, Lu C, Katz TA, et al. Effect of baseline subretinal fluid on treatment outcomes in VIVID-DME and VISTA-DME studies. Ophthalmol Retina. 2019;3(8):663-669. doi: 10.1016/j.oret.2019.03.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-vrd-10.1177_24741264221099429 for Correlation of Change in Macular Thickness With Change in Visual Acuity in Diabetic Macular Edema: Post Hoc Analysis of VISTA and VIVID Trials by Onnisa Nanegrungsunk, Sophie Z. Gu, Susan B. Bressler, Weiming Du, Fouad Amer, Hadi Moini and Neil M. Bressler in Journal of VitreoRetinal Diseases


