Abstract
Background and purpose:
There has not been a comprehensive study on the simultaneous effects of metformin, etoposide, and epirubicin on thyroid cancer cells. Hence, the current research proposed the in vitro study on the effect of metformin alone and in combination with etoposide and epirubicin on the rate of proliferation, apoptosis, necrosis, and migration against B-CPAP and SW-1736 cells as thyroid cancer cell lines.
Experimental approach:
MTT-based proliferation assay, combination index method, flow cytometry, and scratch wound healing assays were used to evaluate the simultaneous effects of the three approved drugs against thyroid cancer cells.
Findings/Results:
This study showed that the toxic concentration of metformin on normal Hu02 cells was more than 10 folds higher than B-CPAP and SW cancerous cells. Metformin in combination with epirubicin and etoposide could increase percentages of B-CPAP and SW cells in early and late apoptosis and necrosis phases in comparison with their single concentrations, significantly. Metformin in combination with epirubicin and etoposide could arrest the S phase in B-CPAP and SW cells, significantly. Metformin in combination with epirubicin and etoposide could reduce ~100% migration rate, whereas single concentrations of epirubicin and etoposide could reduce ~50% migration rate.
Conclusion and implication:
Combined treatment of metformin with anticancer drugs epirubicin and etoposide can increase the mortality in thyroid cancer cell lines and reduce the toxicity of these drugs on the normal cell line, which could be the starting point for proposing a new combination strategy in the therapy of thyroid cancer to induce more potency and reduce acute toxicity.
Keywords: Epirubicin, Etoposide, Metformin, Synergism, Thyroid cancer
INTRODUCTION
Thyroid cancer (TC) is the utmost common endocrine malignancy with more annual deaths than all other endocrine cancers combined. Medical centers in many parts of the world have a growing frequency of cases of thyroid cancer. TC derived from follicular cells includes differentiated TC (DTC) with papillary (PTC) or follicular (FTC) histology and undifferentiated (anaplastic-ATC) and poorly differentiated TC(1,2).
PTC is a common endocrine malignancy and the most highly differentiated cancer of the thyroid follicular epithelium. PTC tends to affect women more often than men, and thyroid cancer is the seventh most common cancer in women. 586,202 people globally have been diagnosed with thyroid cancer in 2020. Medullary TC (MTC) and PTC led to about 3% of all thyroid cancers(1,2).
Considering the incidence of thyroid cancer, so far different treatments have been carried out, such as surgery, radioiodine treatment, chemotherapy, and hormone treatment with thyroid-stimulating hormone suppression. For this purpose, new therapies and drugs are under development to treat cancer and reduce the risk of chemotherapy drugs(3).
The researchers have made many efforts to standardize chemotherapy drugs for thyroid malignancies such as other cancers. Among the common drugs, doxorubicin, epirubicin, etoposide, and bleomycin are used in relevant regimens for the treatment of thyroid carcinoma(3,4).
Epirubicin is an anticancer drug derived from doxorubicin that plays its anticancer role by interfering with the synthesis and function of DNA and is most effective during the S phase of the cell cycle(5).
Etoposide a topoisomerase II inhibitor is utilized as monotherapy in MTC and has had a response rate of 14%(6,7). Relevant neuroendocrine tumors, for instance, small-cell lung and peripheral neuroectodermal cancers, are highly responsive to etoposide in single and combination systems.
Due to the limited effectiveness of monotherapy, and the emergence of resistance, further research on combination therapy is considered to be important. Among the drugs that are used for combination therapy, epirubicin and etoposide can be mentioned(8).
Metformin, a famous insulin-sensitizer agent usually used for type 2 diabetes therapy, has lately emerged as a potentially attractive drug in oncology. It is low-cost and relatively safe and many reports have shown it to be effective in cancer prevention and therapy. Metformin applies antimitogenic action in several cancer histotypes through several molecular mechanisms(9,10,11).
It should be noted that in light of metformin’s effects on thyroid cancer; so far, a comprehensive study has not been carried out on the simultaneous effects of these drugs on thyroid cancer cells.
Metformin is an oral anti-hyperglycemic agent that has been widely purchased in the management of non-insulin-dependent diabetes mellitus based on multiple mechanisms of action, such as suppression of hepatic gluconeogenesis, increase in peripheral glucose uptake, and reduction in insulin resistance(11).
The intracellular effects of metformin are intervened by the activation of AMP-activated protein kinase (AMPK)(12). Investigations in breast, prostate, and ovarian cancer cells in vitro and in vivo, verified that metformin inhibits cancer cell growth by way of AMPK-dependent inhibition of the mTOR signaling pathway in addition to suppression of cyclin D1 expression(13,14,15). Furthermore, metformin can increase the cytotoxic effects of chemotherapeutic agents via AMPK and p53 signaling(16,17,18).
The previous findings have demonstrated that treatment with metformin is related to the sensitization of breast cancer cells to neoadjuvant therapy, and presented metformin as a part of a successful adjuvant treatment strategy against breast tumors in phase III clinical trials(19).
Previous studies investigating the mechanism of action of metformin have shown that activation of AMPK and inhibition of p70S6K/pS6 signaling are common incidents in all examined TC cells. Indeed, findings demonstrated that metformin can reduce and inhibit cell growth and suppress the expression of cyclin D1 in PTC-, MTC-, and FTC-derived cell lines in addition to inhibition of mTOR/p70S6K/pS6 signaling and suppression of p-extracellular signal-regulated kinase (ERK) in MTC-derived cell lines, TT and MZ-CRC-1 cells. Furthermore, TC cell treatment with metformin at concentrations that were adequate for the inhibition of cell growth did not reduce TC cell migration(20,21).
The retrospective analysis of clinicopathological characteristics in diabetic patients with TC demonstrated that tumor size was significantly smaller in metformin-treated compared to the groups not treated with metformin(22). Chemotherapy is a standard treatment regimen to treat a wide range of tumors; though, chemotherapy alone does not result in considerable improvement and regularly leads to drug resistance. On the contrary, combination treatment has shown to be an efficient strategy for TC treatment(23,24). Together, these data suggest the potential utility of metformin for the treatment of TCs.
Hence, the current research was designed to investigate the comprehensive in vitro effect of metformin alone and in combination with etoposide and epirubicin on the rate of proliferation, apoptosis, necrosis, and migration against B-CPAP and SW-1736 cells as TC cell lines.
MATERIALS AND METHODS
Cell culture
B-CPAP (poorly differentiated thyroid gland carcinoma) ATCC CRL-1803, SW-1736 (thyroid gland undifferentiated (anaplastic) carcinoma) IBRC C10311, and Hu02 (human foreskin fibroblast) IBRC C10309, were provided from Iranian Biological Resource Center (Tehran, Iran). They were plated in 25 and 75 cm2 cell culture flasks in RPMI 1640 plus 10% fetal bovine serum (Biosera, France) and 2 g/L of (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (HEPES; Biosera, France), under a 5% CO2 atmosphere with pH 7.4. Cells with 70-80% of confluence were removed by trypsin-ethylenediaminetetraacetic acid (EDTA) solution 1X (Biosera, France). For each test, all cells with a density of 104 cells/cm2 in 96-multiwell plates were seeded for MTT and 105 cells/cm2 in 24-multiwell plates for cell cycle and cell proliferation tests (three wells for each sample and treatment), in that order.
MTT cell proliferation assay
The toxicity properties of metformin (0.48, 2.4, 12.8, 64, and 320 µL/mL), etoposide (8, 40, 200, and 1000 µL/mL), and epirubicin (0.32, 1.6, 8, 40, and 200 µL/mL) were determined by RPMI 1640 and assessed by of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; thiazolyl blue) test (Sigma-Aldrich, Germany). The cells with RPMI 1640 were adjusted to 1.0 × 104 cells/cm2 to plate in 96-well plates (200 µL/well) and incubated for 24 h under a 5% CO2 atmosphere. Following treatment with pointed compounds, each well obtained 20 µL of MTT (5 g/L) and incubated for 4 h. After removing the medium and adding 100 µL/well DMSO, the plates were analyzed by multiwell scanning spectrophotometer (ELISA reader, Organon Tekninka, Netherlands) at a wavelength of 545 nm. The percentage of cell toxicity was obtained by the following equations(25):

In addition, based on the MTT assay, the effects of etoposide and epirubicin in single concentrations and in combination with metformin on two cell lines were evaluated after 24 h of treatment, as above-mentioned.
Annexin V-propidium iodide staining-based assay to assess the induction of apoptosis and necrosis
B-CPAP and SW cells were incubated in 24-well plates under various treatments for 48 h (1.0 × 105 cells/cm2) with entire, the non-adherent cells were collected, and adherent cells were quickly washed with phosphate-buffered saline (PBS) buffer and were taken away with trypsin-EDTA solution 1X at room temperature. Subsequently, the procedure was performed based on the manufacturer staining instruction for annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) solutions (IQ Products BV, Netherlands). Then the results were analyzed by a flow cytometer as CyFlow (Partec, Germany)(26).
Analysis of cell cycle
The cancerous cells (1.0 × 105 cells/cm2) were treated with entire compounds for 24 h. After that, the treated B-CPAP and SW cells were washed in cold PBS (Biosera, France), fixed in cold 70% ethanol (Sigma-Aldrich, Germany), and kept at -20 °C for 6 h. Before analysis, cells were washed twice in PBS, resuspended in staining solution (final concentration 0.1% Triton™ X-100, 0.5 mg/mL ribonuclease A (Sinaclon, Iran), and 0.025 mg/mL propidium iodide (Sigma-Aldrich, Germany)) to incubate in the dark place at room temperature for 30 min and analyze by CyFlow (Partec, Germany)(27).
Scratch wound healing assay to evaluate cell migration
B-CPAP and SW cells were seeded in 24-well plates at a confluence density, incubated for 48 h at 37 °C, and scratched using a yellow pipette tip when the cells covered the well. The cells were washed with PBS twice to clear the floating cells and various concentrations of compounds were added. Images were captured at 48 h(28). The migration rate is calculated by the equation below:

Statistical analysis
The presented data were mean ± SEM or mean ± SD of three triplicates (n = 3) and IC50s were examined by Graph Pad Prism 5.0 program (GraphPad, La Jolla, CA, USA) with a 95% confidence interval. Statistical comparisons were carried out by ANOVA followed by Tukey's post hoc test. The differences were considered significant when P < 0.05.
To analyze the interaction between metformin and the anticancer drug, the combination index (CI) method was applied. CI method calculated by ComboSyn (ComboSyn Inc., NY, USA) is based on the CI theorem of Chou and Talalay and presented a quantitative measure based on the mass-action law of the degree of drug interaction in terms of synergism (CI < 1), additive (CI = 1), and antagonism (CI > 1) for a particular endpoint of the effect measurement. CI has resulted from n(CI)x = Σn j = 1(D)j/(D50)j where j, D, and D50 are defined as each of n chemicals that exerts x% inhibition in combination, dose, and median-effect dose, the dose produces a 50% effect as IC50 respectively.
RESULTS
Effects of single and combined concentrations of drugs on cell proliferation
The MTT assay was utilized for the measurement of cell toxicity caused by the metformin and two drugs in single (Table 1) and combination (Table 2) manners on B-CPAP, SW, and Hu02 cell lines. Findings of the MTT assay demonstrated that metformin is toxic on B-CPAP and SW cell lines in its single concentrations.
Table 1.
IC50s (mean ± SEM) of the epirubicin, etoposide, and metformin on B-CPAP, SW, and Hu02 cell lines after 24 h incubation.
| Sample | IC50 (μg/mL) |
|---|---|
| Epirubicin on B-CPAP cells | 7.21 ± 0.558 |
| Epirubicin on SW cells | 92.9 ± 0.704 |
| Epirubicin on Hu02 cells | 914.96 ± 0.998 |
| Etoposide on B-CPAP cells | 77.51 ± 0.798 |
| Etoposide on SW cells | 43.62 ± 0.801 |
| Etoposide on Hu02 cells | 408.31 ± 1.00 |
| Metformin on B-CPAP cells | 16.62 ± 0.625 |
| Metformin on SW cells | 7.34 ± 0.916 |
| Metformin on Hu02 cells | ~1487.25 ± 0.68 |
Table 2.
Combination effects of metformin with epirubicin and etoposide on B-CPAP, SW, and Hu02 cell lines after 24 h incubation
| Treatments | Concentration (μg/mL) | Combination index | Fraction affected | |
|---|---|---|---|---|
| Epirubicin plus metformin on B-CPAP | Epirubicin | Metformin | ||
| 40.0 | 12.8 | 0.46928 | 0.60 | |
| 8.0 | 12.8 | 0.56380 | 0.47 | |
| 1.6 | 12.8 | 0.82040 | 0.20 | |
| 40.0 | 64.0 | 0.10368 | 0.90 | |
| 8.0 | 64.0 | 0.23452 | 0.79 | |
| 1.6 | 64.0 | 0.38884 | 0.69 | |
| Epirubicin plus metformin on SW | Epirubicin | Metformin | ||
| 40.0 | 12.8 | 0.50044 | 0.45 | |
| 8.0 | 12.8 | 0.71628 | 0.39 | |
| 1.6 | 12.8 | 0.99153 | 0.34 | |
| 40.0 | 64.0 | 0.49497 | 0.58 | |
| 8.0 | 64.0 | 0.64338 | 0.55 | |
| 1.6 | 64.0 | 0.83846 | 0.52 | |
| Epirubicin plus metformin on Hu02 | Epirubicin | Metformin | ||
| 200.0 | 12.8 | 1.66940 | 0.24 | |
| 40.0 | 12.8 | 1.76751 | 0.18 | |
| 8.0 | 12.8 | 18.9641 | 0.11 | |
| 200.0 | 64.0 | 1.23445 | 0.31 | |
| 40.0 | 64.0 | 1.56363 | 0.24 | |
| 8.0 | 64.0 | 6.85770 | 0.18 | |
| Etoposide plus metformin on B- CPAP | Etoposide | Metformin | ||
| 40.0 | 12.8 | 0.42649 | 0.53 | |
| 8.0 | 12.8 | 0.34288 | 0.42 | |
| 1.6 | 12.8 | 0.29298 | 0.39 | |
| 40.0 | 64.0 | 0.27690 | 0.58 | |
| 8.0 | 64.0 | 0.16057 | 0.53 | |
| 1.6 | 64.0 | 0.03371 | 0.59 | |
| Etoposide plus metformin on SW | Etoposide | Metformin | ||
| 40.0 | 12.8 | 0.42368 | 0.44 | |
| 8.0 | 12.8 | 0.75130 | 0.36 | |
| 1.6 | 12.8 | 0.80864 | 0.31 | |
| 40.0 | 64.0 | 0.33246 | 0.57 | |
| 8.0 | 64.0 | 0.60507 | 0.48 | |
| 1.6 | 64.0 | 0.63103 | 0.47 | |
| Etoposide plus metformin on Hu02 | Etoposide | Metformin | ||
| 200.0 | 12.8 | 1.83292 | 0.29 | |
| 40.0 | 12.8 | 1.41884 | 0.16 | |
| 8.0 | 12.8 | 3.84215 | 0.12 | |
| 200.0 | 64.0 | 1.23736 | 0.39 | |
| 40.0 | 64.0 | 1.41884 | 0.25 | |
| 8.0 | 64.0 | 3.84215 | 0.20 |
Indeed, analyzing the combined effects of metformin with etoposide and epirubicin showed that two tested concentrations of metformin can increase the toxicity effects of etoposide and epirubicin in their IC50 and less than IC50 concentrations synergistically (CI < 1). The mentioned data were the same for both cancer cell lines.
Effects of single and combined concentrations of drugs on inducing apoptosis and necrosis
The annexin V-FITC vs PI quantitation-based assay was employed to assess the cell toxicity caused by the compounds and two drugs in single and combination manners on B-CPAP and SW cell lines (Figs. 1-4).
Fig. 1.
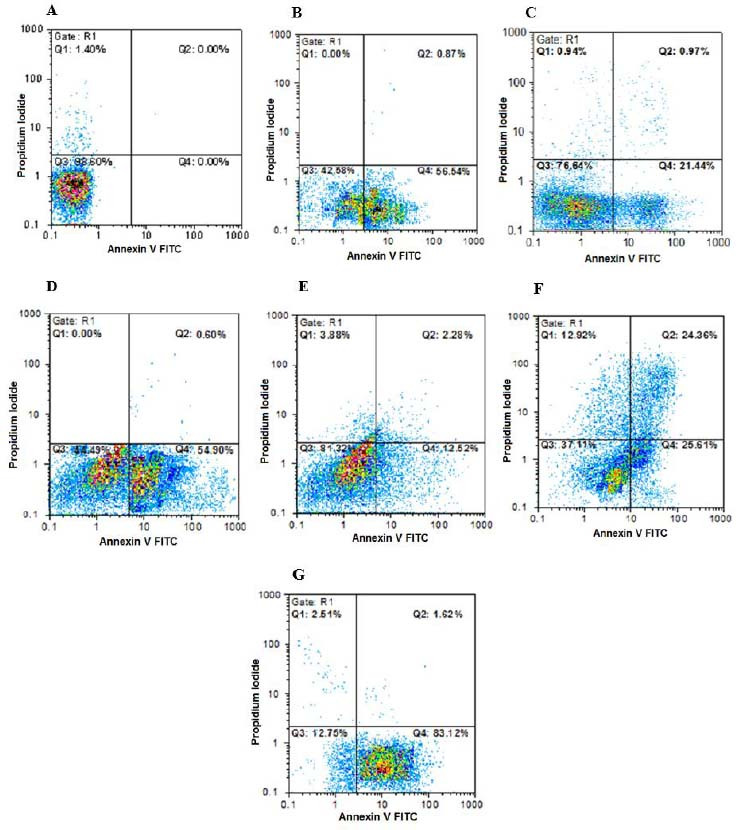
Findings for annexin V-FITC vs propidium iodide quantitation of B-CPAP cells in (A) non-treated group (RPMI as negative control) in addition to (B) B-CPAP cells treated by epirubicin (10 µg/mL), (C) epirubicin (2 µg/mL), (D) etoposide (80 µg/mL), (E) etoposide (16 µg/mL), (F) metformin (20 µg/mL) in combination with epirubicin (2 µg/mL), and (G) metformin (20 µg/mL) in combination with etoposide (16 µg/mL). FITC, Fluorescein isothiocyanate.
Fig. 4.
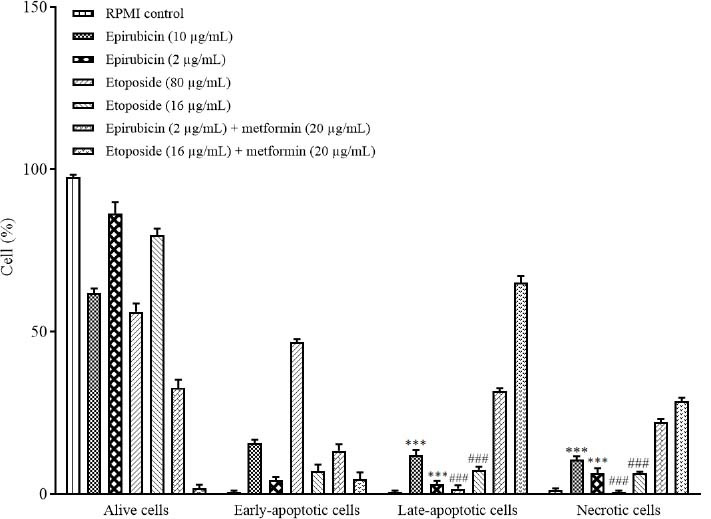
Comparison of the cytotoxic effects of epirubicin and etoposide in single concentrations and in combination with metformin on SW cells based on annexin V-fluorescein isothiocyanate vs propidium iodide quantitation method after 48 h incubation. Data are presented as mean ± SD. The percentage of SW cells inhibited in the late-apoptotic and necrotic stages has increased significantly in groups treated by metformin in combination with epirubicin in comparison with the groups treated by both doses of epirubicin alone. In addition, it has increased significantly in the late-apoptotic and necrotic stages, in groups treated with metformin in combination with etoposide in comparison with the groups treated with both doses of etoposide alone. ***P < 0.001 Indicates significant differences compared to the group treated with metformin in combination with epirubicin and ###P < 0.001 versus the group treated with metformin in combination with etoposide.
Fig. 2.
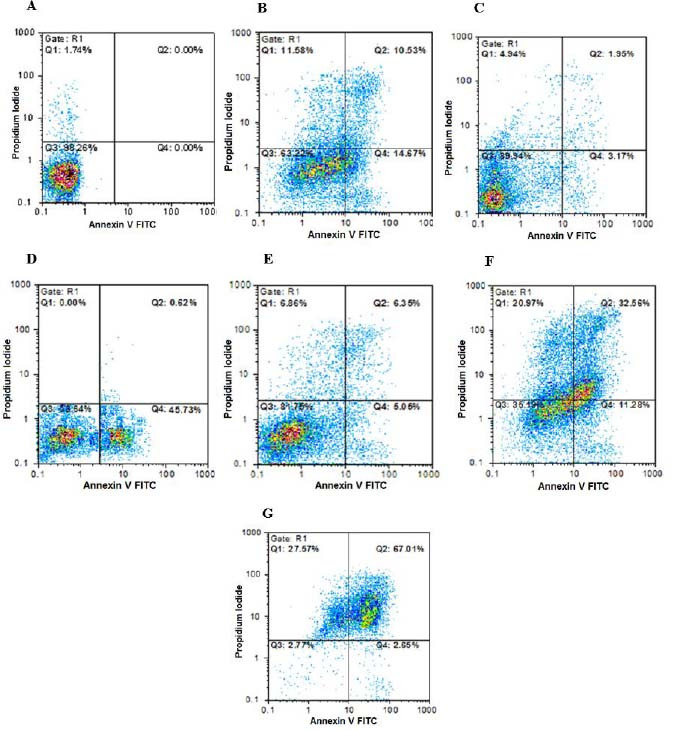
Findings for annexin V-FITC vs propidium iodide quantitation of SW cells in (A) non-treated group (RPMI as negative control) in addition to (B) SW cells treated by epirubicin (100 µg/mL), (C) epirubicin (20 µg/mL), (D) etoposide (40 µg/mL), (E) etoposide (5 µg/mL), (F) metformin (10 µg/mL) in combination with epirubicin (20 µg/mL), and (G) metformin (10 µg/mL) in combination with etoposide (5 µg/mL). FITC, Fluorescein isothiocyanate.
Fig. 3.
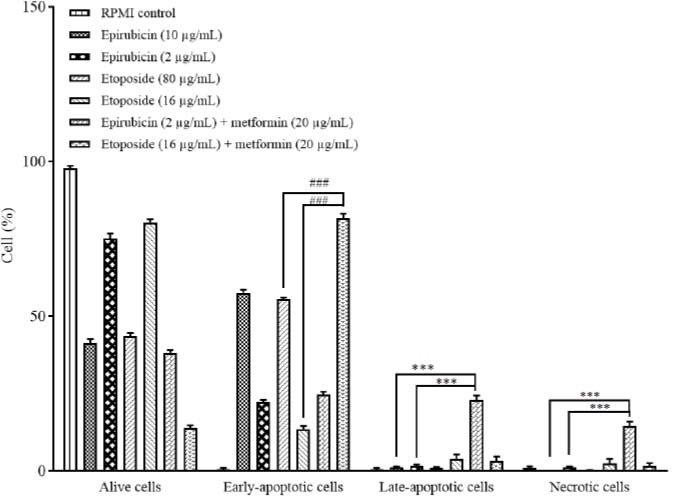
Comparison of the cytotoxic effects of epirubicin and etoposide in single doses and in combination with metformin on B-CPAP cells based on annexin V-fluorescein isothiocyanate vs propidium iodide quantitation method after 48 h incubation. Data are presented as mean ± SD. The percentage of B-CPAP cells inhibited in the early-apoptotic stage has increased significantly (###P < 0.001) in groups treated with metformin in combination with etoposide in comparison with the groups treated with both doses of etoposide alone. In addition, it has increased significantly (***P < 0.001) in the late-apoptotic and necrotic stages, in the groups treated with metformin in combination with epirubicin in comparison with groups treated with both doses of epirubicin alone.
Data showed that metformin (20 µg/mL) in combination with etoposide (16 µg/mL) could significantly increase the early apoptosis percentage of B-CPAP cell count in comparison with single doses of etoposide (80 and 16 µg/mL). Metformin (20 µg/mL) in combination with epirubicin (2 µg/mL) could remarkably increase late apoptosis and necrotic percentages of B-CPAP cell count in comparison with single doses of epirubicin (10 and 2 IC50 doses). Metformin (10 µg/mL) in combination with epirubicin (20 µg/mL) and etoposide (8 µg/mL) could significantly increase late apoptosis and necrotic percentages of SW cell count in comparison with single doses of epirubicin (100 and 20 µg/mL) and etoposide (40 and 8 µg/mL).
Effects of single and combined concentrations of drugs on TC cell cycle
The DNA-binding dye including PI was used to analyze occurring changes in cell cycle phases based on the quantitation of DNA by the compounds and two drugs in single and combination manners on B-CPAP and SW cell lines (Figs. 5-8).
Fig. 5.
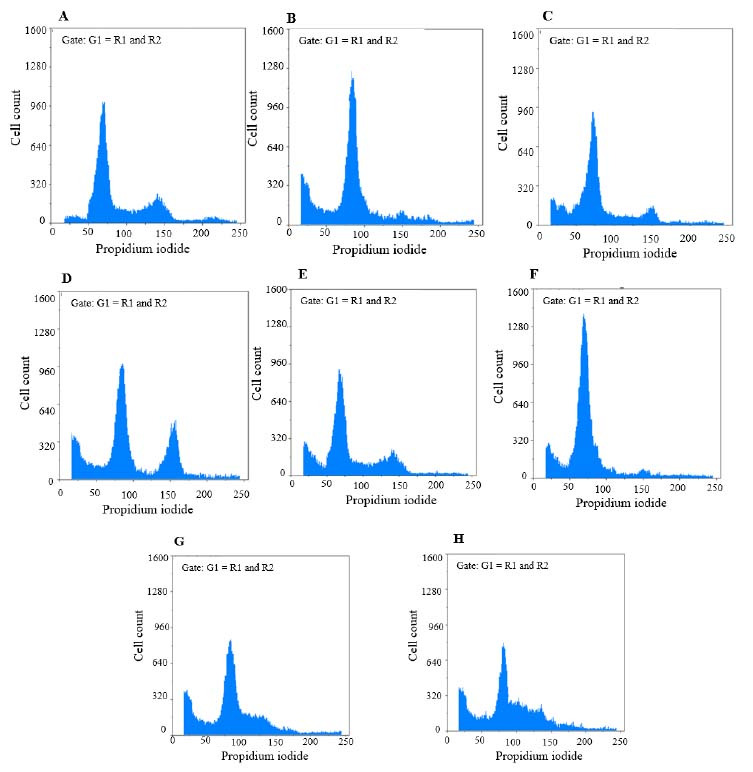
Findings for annexin V-fluorescein isothiocyanate vs propidium iodide quantitation of B-CPAP cells in (A) non- treated group (RPMI as negative control) in addition to (B) B-CPAP cells treated by epirubicin (10 µg/mL), (C) epirubicin (2 µg/mL), (D) etoposide (80 µg/mL), (E) etoposide (16 µg/mL), (F) metformin (20 µg/mL), (G) metformin (20 µg/mL) in combination with epirubicin (2 µg/mL), and (H) metformin (20 µg/mL) in combination with etoposide (16 µg/mL).
Fig. 8.
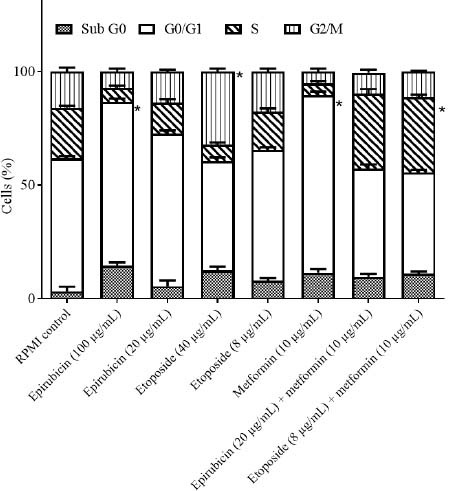
The effects of epirubicin and etoposide in single concentration (values are the real concentrations which are closest to calculated IC50 and one fifth of IC50) and in combination with metformin on cell cycle distribution of SW cell line after 48 h incubation. Data are presented as mean ± SD. *P < 0.05 Indicate significant differences compared to RPMI as the control group.
Fig. 6.
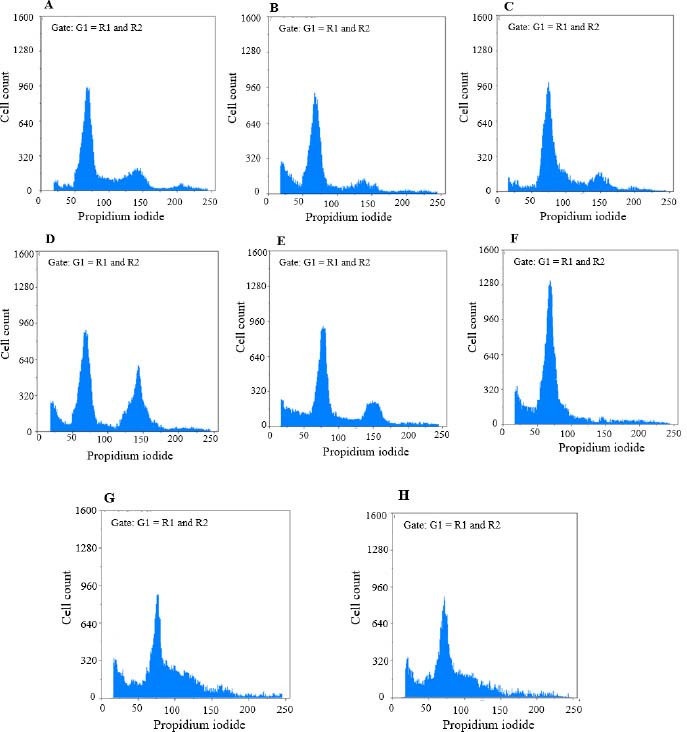
Findings for annexin V-fluorescein isothiocyanate vs propidium iodide quantitation of SW cells in (A) non-treated group (RPMI as negative control) in addition to (B) SW cells treated by epirubicin (100 μg/mL), (C) epirubicin (20 μg/mL), (D) etoposide (40 μg/mL), (E) etoposide (5 μg/mL), (F) metformin (10 μg/mL), (G) metformin (10 μg/mL) in combination with epirubicin (20 μg/mL), and (H) metformin (10 μg/mL) in combination with etoposide (5 μg/mL).
Fig. 7.
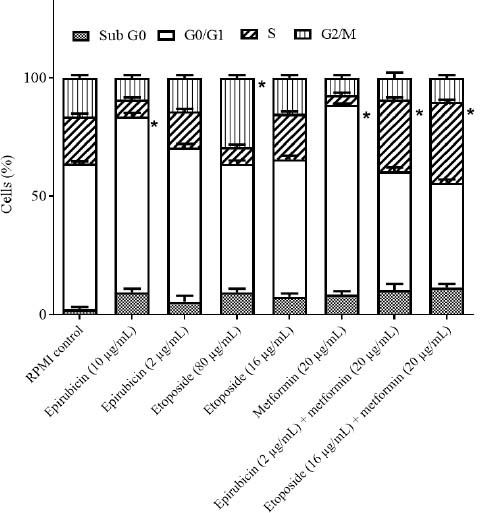
The effects of epirubicin and etoposide in single concentration (values are the real concentrations which are closest to calculated IC50 and one-fifth of IC50;) and in combination with metformin on cell cycle distribution of B-CPAP cell line after 48 h incubation. Data are presented as mean ± SD. *P < 0.05 Indicate significant differences compared to RPMI as the control group.
In the B-CPAP cell line; epirubicin (10 μg/mL) could markedly induce the accumulation of cells in the G0/G1 phase in comparison with the RPMI group. Etoposide (80 μg/mL) could notably induce the accumulation of cells in the G2/M phase in comparison with the RPMI group. Metformin in (20 μg/mL) could significantly induce the accumulation of cells in the G0/G1 phase in comparison with the RPMI group. Metformin (20 μg/mL) in combination with epirubicin (2 μg/mL) could notably induce the accumulation of cells in the S phase compared to the RPMI group. Metformin (20 μg/mL) in combination with etoposide (16 μg/mL) could significantly induce the accumulation of cells in the S phase in contrast with the RPMI group. RPMI was used as negative control (95% confidence interval). In the SW cell line; etoposide in (40 μg/mL) could significantly induce the accumulation of cells in the G2/M phase in comparison with the RPMI group. Metformin (10 μg/mL) could markedly induce the accumulation of cells in the G0/G1 phase contrasted with the RPMI group. Metformin (10 μg/mL) in combination with epirubicin (20 μg/mL) could induce a notable accumulation of cells in the S phase compared to the RPMI group. Metformin (10 μg/mL) in combination with etoposide (16 μg/mL) could induce a significant accumulation of cells in the S phase in comparison with the RPMI group. RPMI was used as negative control (95% confidence interval).
Effects of single and combined concentration of drugs on migration rate
The scratch wound healing assay was used to analyze occurring changes by the compounds and two drugs in single and combination manners on B-CPAP (Fig. 9) and SW (Fig. 10) cell lines. Data showed that single doses of epirubicin (5 µg/mL on B-CPAP cells and 50 µg/mL on SW cells) and etoposide (40 µg/mL on B-CPAP cells and 20 µg/mL on SW cells) could reduce ~50% migration rate.
Fig. 9.
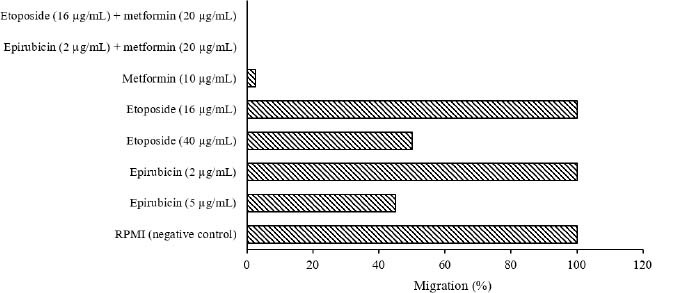
Migration percent of B-CPAP cells treated with epirubicin, etoposide, and metformin in single and combined concentrations based on scratch wound healing assay.
Fig. 10.
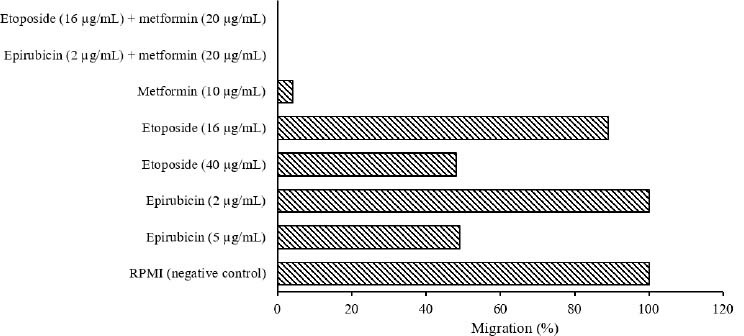
Migration percent of SW cells treated with epirubicin, etoposide, and metformin in single and combined concentrations based on scratch wound healing assay
A single dose of metformin (10 µg/mL on B-CPAP cells and 5 µg/mL on SW cells) could reduce ~98% and 96% migration rates, respectively. Single doses of epirubicin (2 µg/mL on B-CPAP cells and 20 µg/mL on SW cells) and etoposide (16 µg/mL on B-CPAP cells and 8 µg/mL on SW cells) could not reduce the migration rate. Furthermore, metformin (10 µg/mL on B-CPAP cells and 5 µg/mL on SW cells) in combination with epirubicin (2 µg/mL on B-CPAP cells and 20 µg/mL on SW cells) and etoposide (16 µg/mL on B-CPAP cells and 8 µg/mL on SW cells) could reduce the migration rate ~100%, respectively.
DISCUSSION
Symptoms of cancer are a set of functional characteristics that a cell acquires during malignant development. Relevant studies present that metformin regulates approximately the entire cancer symptoms. Metformin is one of the therapeutic agents with the potential for synergy with other chemotherapeutic agents, with low cost, low side effects, and high positive outcomes(24).
The current research was performed to indicate the comprehensive in vitro anti-thyroid cancer effect of metformin in single and combined doses with etoposide and epirubicin as two anticancer drugs with reported toxic effects against several TC cell lines. The impact of these adjuvant treatment strategies on the rate of proliferation, apoptosis, necrosis, and migration in B-CPAP and SW cell lines was evaluated
Data showed that the toxic concentration of metformin on normal Hu02 cells was more than 10 folds higher than B-CPAP and SW cancerous cells. The pointed findings during the current research have confirmed the anticancer effect of metformin alone on TC cells reported in previous studies. Several investigations have proved that metformin showed antimitogenic activity in human ATC and DTC cells, as well as in human thyroid primary cell cultures and rat follicular thyroid cells that it was correlated with induction of apoptosis and inhibition of cell growth and migration by metformin(24).
Metformin increased p-AMPK, decreased mTOR phosphorylation, decreased S6K1 / S6 signaling, and inhibited cyclin D1 and c-myelocytomatosis oncogene product via the mTOR pathway(29). In addition, metformin modulated the expression of epithelial-mesenchymal transition markers E-cadherin, N-cadherin, and SNAIL(29). Destruction of the tuberous sclerosis complex 2 mTOR inhibitor, or rapamycin therapy (mTOR blockade), confirmed metformin suppression of TC cell proliferation, migration, and epithelial-mesenchymal transition(29).
Anti-proliferative activity of metformin was also observed in doxorubicin-resistant ATC cell lines, and silencing AMPK in ATC cells partially improved mTOR phosphorylation and metformin inhibition of cell growth(20).
Metformin can target the growth of TC through cellular metabolism. Cancer cells, despite having oxygen, often change from mitochondrial oxidative phosphorylation to glycolysis to produce ATP, a metabolic reprogramming called the Warburg effect(31,32).
Combining drugs is a very motivating approach to treat cancer. Sorafenib is an approved multinokase inhibitor for TC therapy. One of the important issues is that it often causes severe side effects and requires dose reduction. Surafenib in combination with metformin demonstrated a synergistic suppression of cell growth and inhibition of sphere formation in ATC cells. In addition, metformin caused a 25% diminution in a dose of sorafenib for a similar inhibitory property.
Consecutively, combination therapy with vemurafenib (a selective BRAFV600E mutant protein inhibitor that essentially activates MAPK signaling) with metformin and rapamycin, significantly inhibited cell growth in 8505 (ATC) and B-CPAP-vemurafenib resistant cells (PTC-BRAFV600E)(33). In B-CPAP, a similar decrease in cell growth was reported after combination therapy. In recent times, synergistic effects leading to a decrease in p-ERK and an increase in p-AMPK in the combination of metformin and morafenib were obtained in T-238, BCPAP, and HTH7 models(34).
Synergistic activity on cytotoxicity was observed in PTC cells after combination therapy of metformin with hemigliptin (dipeptidyl peptidase-IV inhibitor) by activation of AMPK and AKT. Hemigliptin amplified inhibition of metformin-mediated proliferation and migration by declining MMP9, VCAM-1, and p-ERK and rising p53 and p21(35). In ATC cells, the combination of metformin with pioglitazone decreased the expression of AKT3, DEPTOR, EIF4E, ILK, MTOR, PIK3C, and PRKCA and amplified the expression of some tumor suppressor genes (e.g. EIF4EBP1, EIF4EBP2, PTEN)(36).
The results obtained in the present study concerning the effect of metformin combination treatment with two anticancer drugs on two TC cell lines confirmed the results of the aforementioned studies, which indicates the synergistic effect of metformin with other thyroid anticancer compounds.
The obtained data showed that metformin in combination with the entire tested concentrations of etoposide and epirubicin has antagonistically toxic effects on Hu02 cells, whereas metformin can induce the toxicity effects of etoposide and epirubicin on both B-CPAP and SW cell lines, synergistically.
The results of quantitative assays of annexin V-FITC vs PI show that the percentage of B-CPAP cells inhibited in the early apoptotic stage by the combined dose of metformin with etoposide and in the late stage of apoptosis and necrosis by the combined treatment of metformin with epirubicin increased significantly, compared with the single-dose treatment of etoposide and epirubicin.
Furthermore, data demonstrated that the percentage of SW cells was increased in the late apoptosis and necrosis phases by metformin in combination with etoposide and epirubicin in comparison with single doses of etoposide and epirubicin, remarkably.
Analysis of the cell cycle based on quantitation of DNA content confirmed that G0/G1 phase was arrested by a single dose of epirubicin and G2/M by etoposide on B-CPAP and SW cells, significantly. Indeed, metformin could arrest the G0/G1 phase on B-CPAP and SW cells in a single dose. Following that, metformin in combination with etoposide as well as epirubicin could significantly induce an accumulation of cells in the S phase on both B-CPAP and SW cells in comparison with RPMI as a control group. The accumulation of cells in the S phase is probably because forced entry into the S phase causes cells to start DNA replication before everything is ready. Consequently, the amplifier fork experiences propagation stress and stops your cells in phase S due to the activation of the checkpoint inside phase S. Metformin alone arrests the TC cell cycle in the G0/G1 phase with trigging cell death and apoptosis and modulates cell cycle distribution in CD133 high / CD44 high cells, but in combination with etoposide and epirubicin, it causes cells to accumulate in the S phase. Given that during phase S, the etoposide gradually affects the distribution of replication proteins, dispersing replication plants and creating foci of large nuclei containing the replication protein A, binding single-stranded DNA and subnuclear reorganization of replication structures(37), it can be concluded that the synergistic effect of metformin with etoposide in the simultaneous treatment of these two drugs may increase the inhibitory effect of etoposide on the TC cell replication phase and accumulation of the cells in S phase of the cell cycle.
Moreover, in the treatment of TC cells with a combination dose of metformin and epirubicin, since epirubicin exerts its antitumor effects by interfering with DNA synthesis and function and is most active during the S phase of the cell cycle(38), it can be said that the combination of metformin with epirubicin in the simultaneous treatment of TC cells can synergistically lead to these cells accumulation in the S phase.
Data for scratch wound healing test on B-CPAP and SW cells showed that metformin in combination with epirubicin and etoposide could reduce ~100% migration rate, whereas single doses of epirubicin and etoposide could reduce ~50% migration rate.
CONCLUSION
The findings from the entire performed assays during the current research demonstrated that metformin may be defined as a possible innovative combination chemotherapy strategy for the treatment of human thyroid tumors with the potential for synergy with other chemotherapeutic agents, with low cost, low side effects, and high positive outcomes. However, additional in vitro, in vivo, and clinical studies are necessary for further development of the findings.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors' contributions
S. Sardari as the supervisor contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Gh. Ghavami, R. Ebrahimi Kiasari, and F. Pakzad. The first draft of the manuscript was written by Gh. Ghavami. All authors read and approved the finalized article.
Acknowledgments
This study was funded by the National Institute for Medical Research Development (NIMAD), Deputy of Research and Technology, Ministry of Health and Medical Education of Iran through Grant No. 963566).
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Radu TG, Ciurea ME, Mogoanta SS, Busuioc CJ, Grosu F, Tenovici M, et al. Papillary thyroid cancer stroma - histological and immunohistochemical study. Rom J Morphol Embryol. 2016;57:801–809. PMID:27833974. [PubMed] [Google Scholar]
- 3.Hoskin PJ, Harmer C. Chemotherapy for thyroid cancer. Radiother Oncol. 1987;10(3):187–194. doi: 10.1016/s0167-8140(87)80004-x. [DOI] [PubMed] [Google Scholar]
- 4.Massart C, Barbet R, Genetet N, Gibassier J. Doxorubicin induces Fas-mediated apoptosis in human thyroid carcinoma cells. Thyroid. 2004;14(4):263–270. doi: 10.1089/105072504323030915. [DOI] [PubMed] [Google Scholar]
- 5.Santini F, Bottici V, Elisei R, Montanelli L, Mazzeo S, Basolo F, et al. Cytotoxic effects of carboplatinum and epirubicin in the setting of an elevated serum thyrotropin for advanced poorly differentiated thyroid cancer. J Clin Endocrinol Metab. 2002;87(9):4160–4165. doi: 10.1210/jc.2001-011151. [DOI] [PubMed] [Google Scholar]
- 6.Karpinich NO, Tafani M, Rothman RJ, Russo MA, Farber JL. The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c. J Biol Chem. 2002;277(19):16547–16552. doi: 10.1074/jbc.M110629200. [DOI] [PubMed] [Google Scholar]
- 7.Panomuppakarn N, Witoonpanich P, Waisayarat J, Jiarpinitnun C, Ativitavas T, Ngamphaiboon N. Uncommon response of cisplatin and etoposide for treatment of advanced medullary thyroid carcinoma. Clin Case Rep. 2017;5(10):1628–1633. doi: 10.1002/ccr3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayat Mokhtari, R Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. 2017;8:38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng X, Xu S, Chen G, Derwahl M, Liu Ch. Metformin and thyroid disease. J Endocrinol. 2017;233:R43–R51. doi: 10.1530/JOE-16-0450. [DOI] [PubMed] [Google Scholar]
- 10.Courtois S, Durán R, Giraud J, Sifré E, Izotte J, Mégraud F, et al. Metformin targets gastric cancer stem cells. Eur J Cancer. 2017;84:193–201. doi: 10.1016/j.ejca.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Rena G, Hardie G, Pearson E. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 13.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol. 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahra IB, Marchand-Brustel YL, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9(5):1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer: doses mechanisms and the dandelion and hormetic phenomena. Cell Cycle. 2010;9(6):1057–1064. doi: 10.4161/cc.9.6.10994. [DOI] [PubMed] [Google Scholar]
- 16.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71(9):3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha GZ, Dias MM, Ropelle ER, Osorio-Costa F, Rossato FA, Vercesi AE, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res. 2011;17(12):3993–4005. doi: 10.1158/1078-0432.CCR-10-2243. [DOI] [PubMed] [Google Scholar]
- 18.Petrushev B, Tomuleasa C, Soritau O, Aldea M, Pop T, Susman S, et al. Metformin plus PIAF combination chemotherapy for hepatocellular carcinoma. Exp Oncol. 2012;34:17–24. PMID:22453143. [PubMed] [Google Scholar]
- 19.Goodwin PJ, Stambolic V, Lemieux J, Chen BE, Parulekar WR, Gelmon KA, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Tret. 2011;126:215–220. doi: 10.1007/s10549-010-1224-1. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells suppresses self-renewal of derived cancer stem cells and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012;97(4):510–520. doi: 10.1210/jc.2011-1754. [DOI] [PubMed] [Google Scholar]
- 21.Klubo-Gwiezdzinska J, Jensen K, Costello J, Patel A, Hoperia V, Bauer A, et al. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocr Relat Cancer. 2012;19(3):447–456. doi: 10.1530/ERC-12-0046. [DOI] [PubMed] [Google Scholar]
- 22.Klubo-Gwiezdzinska J, Costello J, Patel A, Bauer A, Jensen K, Mete M, et al. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab. 2013;98(8):3269–3279. doi: 10.1210/jc.2012-3799. [DOI] [PubMed] [Google Scholar]
- 23.Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. Clin. Endocr. 1998;48(3):265–273. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 24.Morale MG, Tamura RE, Rubio IGS. Metformin and cancer hallmarks: molecular mechanisms in thyroid, prostate and head and neck cancer models. Biomolecules. 2022;12(3):1–18. doi: 10.3390/biom12030357. 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Meth. 1989;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 26.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82(2):331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 27.Takac P, Kello M, Pilatova MB, Kudlickova Z, Vilkova M, Slepcikova P, et al. New chalcone derivative exhibits antiproliferative potential by inducing G2/M cell cycle arrest mitochondrial-mediated apoptosis and modulation of MAPK signalling pathway. Chem Biol Interact. 2018;292:37–49. doi: 10.1016/j.cbi.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Liang CC, Park A, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 29.Han B, Cui H, Kang L, Zhang X, Jin Z, Lu L, et al. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the MTOR pathway. Tumor Biol. 2015;36(8):6295–6304. doi: 10.1007/s13277-015-3315-4. [DOI] [PubMed] [Google Scholar]
- 30.Bikas A, Jensen K, Patel A, Costello J, McDaniel D, Klubo-Gwiezdzinska J, et al. Glucose-deprivation increases thyroid cancer cells sensitivity to metformin. Endocr Relat Cancer. 2015;22(6):919–932. doi: 10.1530/ERC-15-0402. [DOI] [PubMed] [Google Scholar]
- 31.Coelho RG, Fortunato RS, Carvalho DP. Metabolic reprogramming in thyroid carcinoma. Front Oncol. 2018;8:1–15. doi: 10.3389/fonc.2018.00082. 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Cao L, Wang L, Liu L, Huang Y, Gong X. Metformin inhibits proliferation of human thyroid cancer TPC-1 cells by decreasing LRP2 to suppress the JNK pathway. Onco Targets Ther. 2020;13:45–50. doi: 10.2147/OTT.S227915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin HS, Sun HJ, Whang YM, Park YJ, Park DJ, Cho SW. Metformin reduces thyroid cancer tumor growth in the metastatic niche of bone by inhibiting osteoblastic RANKL productions. Thyroid. 2021;31(15):760–771. doi: 10.1089/thy.2019.0851. [DOI] [PubMed] [Google Scholar]
- 34.Durai L, Ravindran S, Arvind K, Karunagaran D, Vijayalakshmi R. Synergistic effect of metformin and vemurufenib (PLX4032) as a molecular targeted therapy in anaplastic thyroid cancer: an in vitro study. Mol Biol Rep. 2021;48(11):7443–7456. doi: 10.1007/s11033-021-06762-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG, Yoo HJ, et al. Synergistic cytotoxicity of the dipeptidyl peptidase-IV inhibitor gemigliptin with metformin in thyroid carcinoma cells. Endocrine. 2018;59(2):383–394. doi: 10.1007/s12020-017-1503-2. [DOI] [PubMed] [Google Scholar]
- 36.Kutbay NO, Avci CB, Yurekli BS, Kurt CC, Shademan B, Gunduz C, et al. Effects of metformin and pioglitazone combination on apoptosis and AMPK/mTOR signaling pathway in human anaplastic thyroid cancer cells. J Biochem Mol Toxicol. 2020;34(10):1–11. doi: 10.1002/jbt.22547. e22547. [DOI] [PubMed] [Google Scholar]
- 37.Montecucco A, Zanetta F, Biamonti G. Molecular mechanisms of etoposide. EXCLI J. 2015;14:95–108. doi: 10.17179/excli2015-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cersosimo RJ, Hong WK. Epirubicin: a review of the pharmacology, clinical activity, and adverse effects of an adriamycin analogue. J Clin Oncol. 1986;4(3):425–439. doi: 10.1200/JCO.1986.4.3.425. [DOI] [PubMed] [Google Scholar]


