Summary
Hereditary angioedema (HAE) is a potentially life-threatening rare disease, which is mainly caused by the deficiency or dysfunction of C1-esterase inhibitor, and characterized by spontaneous, recurrent episodes of edema in various parts of the body including internal organs and the laryngeal area. Delayed diagnosis and treatment increase the burdens and risks of this condition. The current study aimed to understand the burden of illness for HAE patients in Japan before and after diagnosis through a patient reported outcome survey. A survey instrument was distributed to 121 adult patients with HAE by a patient organization via HAE treating physicians between July and November in 2016. Seventy patients (57.9%) returned the questionnaire. Patients reported high levels of medical resource utilization, including emergency procedures and services. Episodes of receiving laparotomy were somewhat less after diagnosis with HAE than before, but no apparent difference in episodes of tracheotomy between before and after the diagnosis. The economic burden, including direct and indirect medical costs, was highest before diagnosis, but still perceived as substantial after diagnosis. Patients reported disruption of work and school life, with 40% reporting that they miss 10 or more days from work or education per year. Sixty percent of patients reported that HAE affected their daily activities. We concluded that HAE is associated with considerable physical, social, economic and psycho-social burdens even after diagnosis, and that higher attack frequency is associated with a heavy disease burden for patients in Japan.
Keywords: hereditary angioedema, burden of illness, Japan, patient reported outcomes, quality of life
1. Introduction
Hereditary angioedema (HAE) is a potentially life-threatening rare disease that is characterized by spontaneous and recurrent episodes of edema in various parts of the body, including internal organs and the laryngeal area (1,2). The frequency and severity of these episodes varies not only between patients, but also across a single patient's life course (3). Attacks are unpredictable, frequently painful and disfiguring, and may cause significant temporary disability (4,5). Symptoms are self-limiting, generally lasting between 1-4 days, but family history may include asphyxiation as a result of an untreated laryngeal attack (3-6).
HAE symptoms are the result of increased activity of plasma kallikrein which triggers a cascade leading to excessive bradykinin production. The background to this for the majority of patients is mainly the deficiency (Type I) or dysfunction (Type II) of C1-esterase inhibitor (C1- INH) (1,2). However, since the early 2000s a third type of HAE has been described in the literature, HAE with normal C1-INH. Research into diagnostic biomarkers is ongoing, but this rare form of HAE is poorly understood. C1-INH function is normal but swelling is considered to be the result of excessive bradykinin production and/or function (7-9).
The prevalence of HAE has been estimated at 1 case per 50,000 people, with no reported bias among different ethnic groups (3,4). Based on population size, there would be an estimated 2,300-3,000 Japanese HAE patients, but unofficial data collated from case reports in Japan and from several pharmaceutical company databases suggests that the number of diagnosed HAE cases in Japan is between 500-550, meaning around 20% of the expected population with HAE.
In 2020, we reported on the results of the first patient reported outcome (PRO) questionnaire survey of Japanese patients covering a wide number of clinical topics, including the pathway to diagnosis, number of attacks and treatment, time from an attack to the treatment and then resolution, and satisfaction with treatment (10). This is the companion paper of reference 10, reporting the burden of illness for HAE patients in Japan both before and after diagnosis in the treatment environment at the time of the data collected, and patient perceptions of what they would like to see to increase their quality of life. Major categories of concern are medical resource utilization, physical, mental and socio-economic burdens on patients, as well as work, education and leisure impairments and restrictions to daily living. At the time that we conducted this PRO study, only one human plasma derived C1-INH concentrate was available as an acute attack treatment for HAE and this had to be administered in a hospital. Some patients were using tranexamic acid and attenuated androgens aiming for prophylactic treatment, but no medications for this designated by an international guideline for HAE had been approved in Japan (10). Our data-set offers an important baseline against which future studies can investigate the impact of a diversification of treatment options. At the same time, it offers a historic picture of the disease burden before self-administrated acute attack treatment and/or prophylactic treatment options were available.
2. Materials and Methods
In this study, we collected data between July and November 2016 from adult patients who had been diagnosed with HAE, as reported by Iwamoto, et al. in 2020 (10). Through discussion with physicians and patients involved in HAE practice, a questionnaire was developed with the goal of of understanding the burden and unmet needs of patients, and obtaining knowledge that would be useful in clinical practice. A total of 48 questions were generated through discussion to collect the necessary information while minimizing the burden on patients.
The survey was distributed to 121 patients between July and November in 2016 by a Japanese HAE patient organization (https://haej.org) via HAE treating physicians and analyzed in 2017. Patients without a confirmed diagnosis of HAE or patients under 20 years of age were excluded from the study. Patients completed the questionnaire and returned it to Anterio Co., Ltd., an organization specializing in pharmaceutical market research (https://www.intage-healthcare.co.jp). The data from the returned surveys were compiled by Anterio Co., Ltd.
After removing invalid responses from each questionnaire, the data were statistically analyzed using GraphPad Prism8 (GraphPad Software, San Diego, CA). Statistical significance between the period before and after diagnosis was calculated by the Wilcoxon matched-pairs signed rank test.
The protocol for this study was approved by the Ethics Committee of Hiroshima University (No. E_339) and was conducted on the basis of the principles of the Declaration of Helsinki. Informed consent was obtained from all participants.
3. Results
3.1. Demographics of respondents by gender
Of the 70 respondents, 78.6% of respondents were female (15 males, 55 females). The average age was 44.9 ± 18.8 years (mean ± SD, min-max, 8-84 years).
3.2. Segmentation by frequency of attacks
As attack frequency has been shown to be linked to the burden of disease and impact on health-related quality of life (HRQoL) (11-14), we divided patients into segmented subgroups based on reported attack frequency over the previous year. Patients reporting 20 or more attacks were categorized as a high frequency group (n = 19/70, 27.1%), those reporting 1-19 attacks were described as a low frequency group (n = 29/70, 41.4%), patients reporting a history of attacks, but none over the previous year were categorized as a no attack group (n = 7/70, 10.0%), and those reporting no attack onset were classified as an asymptomatic group (n = 7/70, 10.0%). An "unclassified" group was created to label those who did not indicate the number of attacks on average that they experienced in a year (n = 8/70, 11.4%).
While 78.6% of the sample were female, women made up 94.7% of those in the high attack group (n = 18/19). Male respondents were slightly over-represented in the low and asymptomatic groups, and more substantially over-represented in the no attack group (n = 7/29, 24.1%; n = 2/7, 28.6%; n = 3/7, 42.9%, respectively) (Figure 1).
Figure 1.
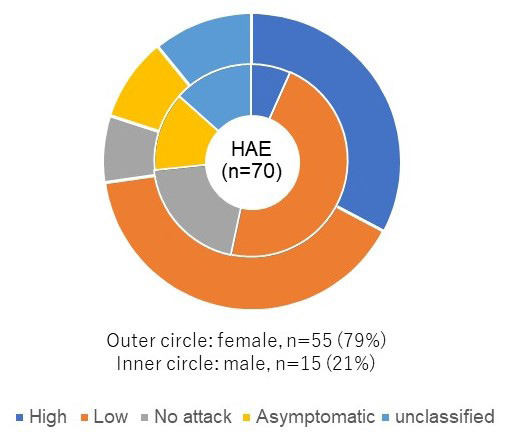
Demographics of respondents by gender and frequency of attacks. Of the 70 respondents, 55 (78.6%) respondents were female, and 15 (21.4%) were male. Those patients reporting on average 20 or more attacks were categorized as a high frequency group (n =19/70, 27.1%), those reporting 1-19 attacks were described as a low frequency group (n = 29/70, 41.4%), patients reporting a history of attacks, but none over the previous year were categorized as a no attack group (n = 7/70, 10.0%), and those reporting no attack onset were classified as an asymptomatic group (n = 7/70, 10.0%). An "unclassified" group was created to label those who did not indicate the number of attacks on average that they experience in a year (n = 8/70, 11.4%). Women made up 94.7% of those in the high attack group (n = 18/19). Male respondents were slightly over-represented in the low and asymptomatic groups, and more substantially over-represented in the no attack group (n = 7/29, 24.1%; n = 2/7, 28.6%; n = 3/7, 42.9%, respectively).
3.3. Medical resource utilization
While the companion paper published elsewhere introduced a part of the data on medical resource utilization to compare the pre- and post-diagnosis periods, we expanded the analysis from the viewpoint of the burden of the disease for patients. In this report, we added attack frequency as an additional point of analysis, and we framed medical utilization as an indicator of the physical and psychological burden of HAE.
Patients were asked how often they visit the hospital or clinic where they receive regular care for HAE, whether for consultations or treatment. Answers to this question showed that there is a high utilization of medical resources for routine care of HAE. Eighteen out of 70 (25.7%) respondents reported visiting a medical facility every month, including 5 (7.1%) respondents who reported weekly visits. An additional 33 (47.1%) respondents reported that they went to their regular treating hospital or clinic once every 2-3 months. Patients in the high attack group reported higher usage of routine care with 8 out of 19 (42.1%) respondents reporting that they go weekly or twice monthly (Figure 2).
Figure 2.
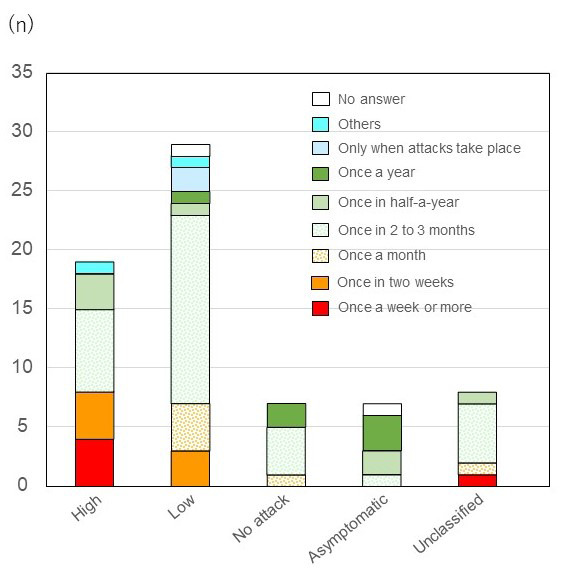
Frequency of visiting hospital or clinic where the patient receives routine care for HAE. Eighteen out of 70 (25.7%) respondents reported visiting a medical facility every month, including 5 (7.1%) respondents who reported weekly visits. An additional 33 (47.1%) respondents reported that they went to their regular treating hospital or clinic once every 2-3 months. Patients in the high attack group reported higher usage of routine care with 8 out of 19 (42.1%) respondents reporting that they go weekly or twice monthly. Abbreviation: HAE, Hereditary angioedema.
Utilization of emergency services was also commonly reported by patients, especially those in the high attack group. Patients were asked the approximate number of times they had used an ambulance for HAE symptoms, both before and after diagnosis (Figure 3A). Twenty-five out of 63 (39.7%) respondents reported having used an ambulance at least once in the pre-diagnosis period and 22 (34.9%) respondents in the post-diagnosis period. The mean number of uses by patients in the high attack group after diagnosis was 8.2 times compared to 2.2 times by patients in the low attack group. In the high and low attack groups, there were slightly higher utilization of emergency ambulance services after compared to prior to diagnosis (8.2 to 7.9, and 2.2 to 2.0, respectively). It is noteworthy that 2 out of 7 (28.6%) respondents in the "no attack" group reported having used an ambulance for HAE attacks both in the pre-diagnosed and post-diagnosed period.
Figure 3.
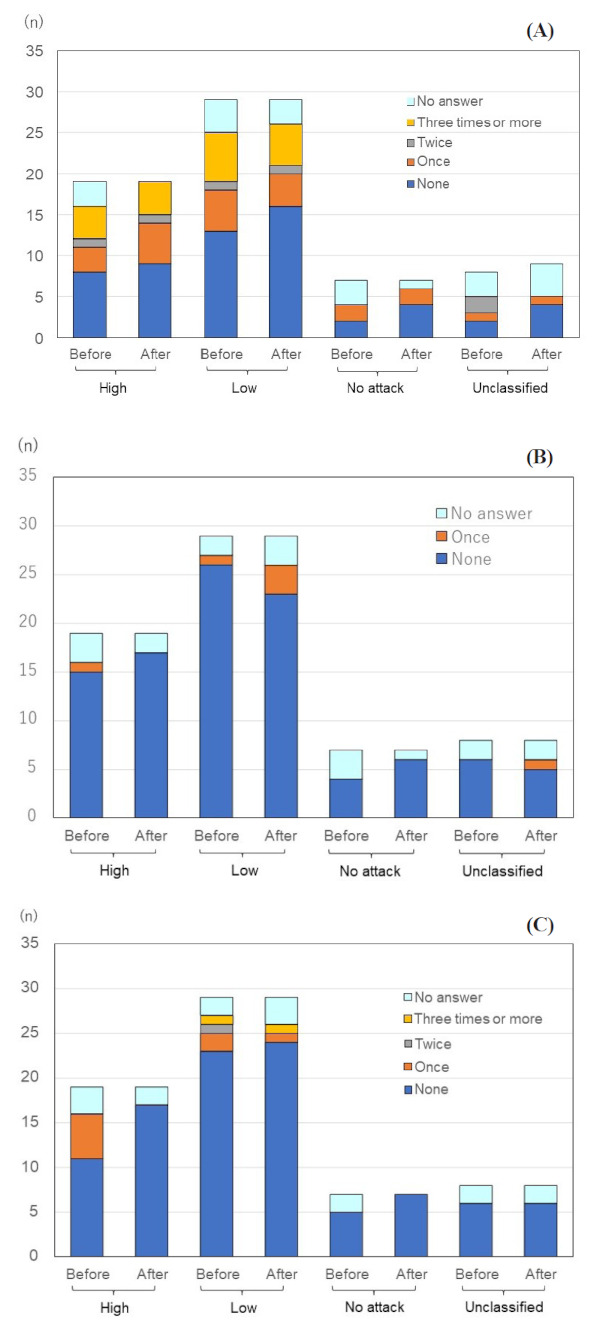
Utilization of emergency services and procedures. (A) Total episodes of being ambulanced to hospitals. Twenty-five out of 63 (39.7%) respondents reported having used an ambulance at least once in the pre-diagnosis period and 22 (34.9%) respondents in the post diagnosis period. It is noteworthy that 2 out of 7 (28.6%) respondents in the "no attack" group reported having used an ambulance for HAE attacks both in the pre-diagnosed and diagnosed period. (B) Total episodes of receiving a tracheotomy. Two out of 63 (3.2%) respondents reported experience of a tracheotomy in the pre-diagnosed period, and this increased to 4 (6.3%) in the post-diagnosed period, with this concentrated in the low attack group (n = 3/29, 10.3%). (C) Total frequency of ever receiving a laparotomy. Seven out of 63 (11.1%) respondents reported "one experience" of a laparotomy, one (1.6%) respondent reported "twice" and one (1.6%) respondent reported "3 times or more" in the pre-diagnosed period. The incidence of this surgery was higher in the high attack group, with 5 out of 19 (26.3%) respondents reporting at least once incidence. After diagnosis, only 2 out of 63 (3.2%) respondents report experience of a laparotomy. Abbreviation: HAE, Hereditary angioedema.
Additional indicators of emergency medical resource utilization in this study were experience of tracheotomy or laparotomy. There were reports of both procedures in the pre-diagnosed and post-diagnosed periods. Two out of 63 (3.2%) respondents reported experience of a tracheotomy in the pre-diagnosed period, and this increased to 4 (6.3%) in the post-diagnosed period, with this concentrated in the low attack group (n = 3/29, 10.3%) (Figure 3B).
The opposite trend can be seen with reported incidence of laparotomy, with fewer incidences reported in the post-diagnosed period. Seven out of 63 (11.1%) respondents reported "one experience" of a laparotomy, one (1.6%) respondent reported "twice" and one (1.6%) respondent reported "3 times or more" in the pre-diagnosed period. The incidence of this surgery was higher in the high attack group, with 5 out of 19 (26.3%) respondents reporting at least one incidence. After diagnosis, only 2 out of 63 (3.2%) respondents report experience of a laparotomy (Figure 3C).
3.4. Physical burden
Frequent hospital visits and the pattern of emergency medical resource utilization already point to the heavy physical burden of the disease. In our previous report by Iwamoto, et al., we covered the time needed between onset of attack and treatment, but here we report on the time to get to hospital for regular check-ups for an outpatient appointment (10). For patients who regularly meet their doctor for a consultation or treatment, the commute time to the hospital creates both a physical and economic burden. The economic burden will be discussed below. Thirty-six out of 70 (51.4%) respondents are attending a medical institution regularly for HAE that takes more than 30 minutes to get to from where they live. Twelve (17.1%) respondents report that the journey time is more than one hour. Three out of 19 (15.8%) respondents in the high attack group reported time between 1 hour to 1.5 hours for them to reach their regular treating hospital. Some patients reported the physical burden not only of traveling to hospital, but also the long waits on arrival.
The coding of open-ended responses created two categories that related to the physical burden of the condition linked to frequent hospital visits. Six out of 70 (8.6%) respondents indicated in their response that they felt that the waiting time for hospital visits was too long. For the high attack group, 3 out of 19 (15.8%) respondents referred to the long waiting time as something that troubled them. Nine out of 70 (12.9%) respondents responded that their life would be improved if they could receive treatment at a local hospital. For the high attack group, 5 out of 19 (26.3%) respondents indicated that being able to get treatment at a local hospital would improve their lives.
As we reported in the previous analysis, approximately 55% of all attacks in the previous year had been treated (10). Here we further report that only 16 out of 47 (34.0%) respondents reported that they sought treatment every time they had an attack. Among the high attack group, one out of 19 (5.3%) respondents reported that they did not seek treatment for any attacks, and 3 (15.8%) respondents for less than 20% of attacks. The main reason given for not receiving treatment (multiple responses allowed), was that the symptoms were judged by the patient to be mild (n = 34/37, 91.9%). Other reasons given for not seeking treatment include the distance to the hospital (n = 7/37, 18.9%), the treating hospital not offering treatment at night (n = 4/37, 10.8%), going to the hospital to be treated would involve missing work or school (n = 8/37, 21.6%) and the treatment was too expensive (n = 6/37, 16.2%) (Figure 4).
Figure 4.
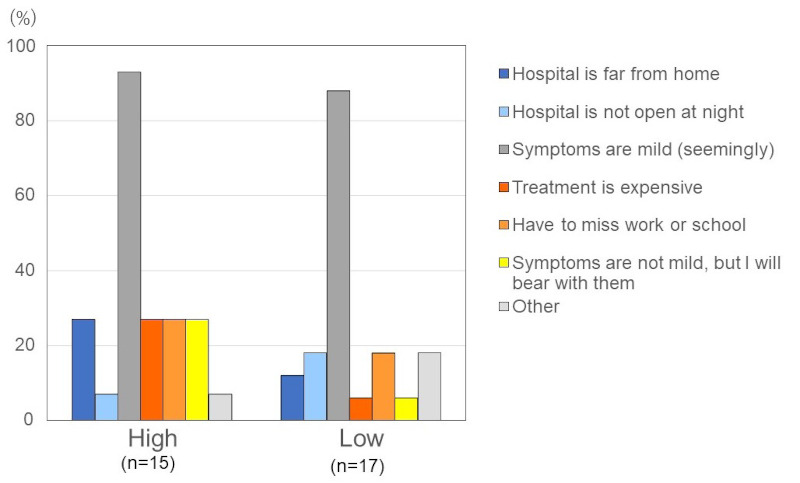
Reason for not seeking treatment for attacks. The main reason given for not receiving treatment (multiple responses allowed), was that the symptoms were judged by the patient to be mild (n = 34/37, 91.9%). Other reasons given for not seeking treatment include the distance to the hospital (n = 7/37, 18.9%), the treating hospital not offering treatment at night (n = 4/37, 10.8%), going to the hospital to be treated would involve missing work or school (n = 8/37, 21.6%) and the treatment was too expensive (n = 6/37, 16.2%).
3.5. Economic burdens and associated impairments
Once a patient has been diagnosed with HAE, they can apply through their local health center for public remuneration under the Specified Disease Law. Patients were asked if they were currently receiving public reimbursement for out-of-pocket expenses. Fifty-five out of 70 (78.6%) respondents answered yes, but 14 (20.0%) respondents said that they were not receiving any public renumeration. All respondents of the high attack group (n = 19/19, 100%) and 22 out of 29 (75.9%) respondents in the low attack group were receiving public renumeration. Those in the no attack and asymptomatic groups were more likely to report that they do not receive public renumeration (n = 2/7, 28.6% and n = 4/7, 57.1%, respectively) (Figure 5). By age, those in their 50s and 30s were most likely to be receiving public renumeration (n = 12/13, 92.3% and n = 16/18, 88.9%, respectively), and those in their 60s the least likely (n = 5/8, 62.5%).
Figure 5.
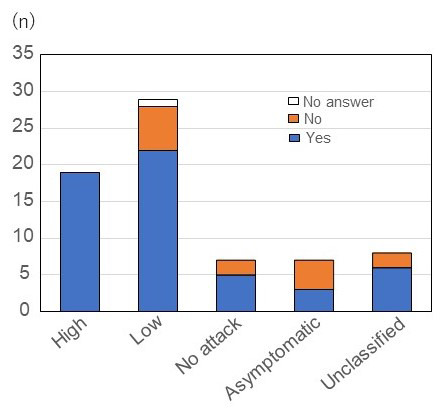
Patients receiving or qualified to receive public financial support for HAE treatment according to attack frequency. We found that all respondents of the high attack group (n = 19/19, 100%) and 22 out of 29 respondents (75.9%) in the low attack group were receiving public renumeration. Those in the no attack and asymptomatic groups were more likely to report that they do not receive public renumeration (n = 2/7, 28.6% and n = 4/7, 57.1%, respectively). Abbreviation: HAE, Hereditary angioedema.
Patients who had experienced at least one attack (n = 62) were asked about HAE related out-of-pocket costs for treatment and tests per year, both in the pre-diagnosed and post-diagnosed periods. The mean out-of-pocket expenses per year were estimated to be 123,615 Japanese yen (JPY) (936.86 United States dollar - USD; conversion rate as of 6th February 2023, 1 JPY = 0.00758349 USD) prior to diagnosis, falling to 92,392 JPY (700.18 USD) after diagnosis in large part due to the public renumeration system. Those currently receiving financial renumeration report on average higher out-of-pocket expenses per year in the pre-diagnosed period (n = 52, 83.9%, a mean of 138,269 JPY; 1,048.22 USD) compared to those not currently receiving financial support (n = 10, 16.1%, 64,050 JPY; 485.80 USD), but also in the post-diagnosed period (100,172 JPY; 759.78 USD compared to 59,070 JPY; 448.01 USD) (Figure 6).
Figure 6.
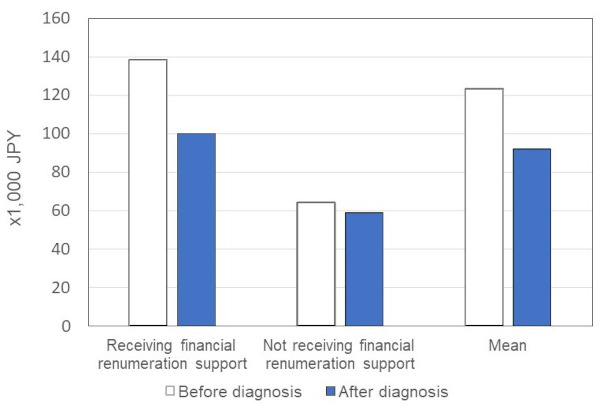
Out-of-pocket money for drug therapies/tests in a typical year. The mean out-of-pocket expenses per year was estimated to be 123, 615 JPY (938.08 USD) prior to diagnosis, falling to 92,392 JPY (701.14 USD) after diagnosis in large part due to the public renumeration system. Those currently receiving financial renumeration reported on average higher out-of-pocket expenses per year in the pre-diagnosed period (n = 52, 83.9%, a mean of 138,269 JPY; 1,049.16 USD) compared to those not currently receiving financial support (n = 10, 16.1%, 64,050 JPY; 485.98 USD), but also in the post-diagnosed period (100,172 JPY; 759.87 USD compared to 59,070 JPY; 448.19 USD). Abbreviation: JPY, Japanese yen; USD, United States dollar.
Looking only at those who are currently receiving financial renumeration, the high attack group report higher out-of-pocket medical expenses on average than the low and no attack group both in the pre-diagnosed (mean of 209,444 JPY; 1,588.52 USD compared to 130,769 JPY; 991.58 USD and 5,000 JPY; 37.91 USD, respectively) and post-diagnosed periods (143,081 JPY; 1,084.91 USD compared to 92,328 JPY; 700.08 USD and 13,333 JPY; 101.09 USD, respectively).
Patients were also asked the estimated yearly out-of-pocket costs associated with receiving surgery or for ambulance use for HAE-like or HAE symptoms in the pre- and post-diagnosed period. This showed a dramatically higher average expenditure in the pre-diagnosed (n = 63, 102,543 JPY; 777.50 USD) compared to the post-diagnosed period (n = 63, 30,291 JPY; 229.68 USD).
Those currently receiving pubic renumeration (n = 52, 83.9%) reported a dramatic decrease in out-of-pocket expenses due to surgery and ambulance use in the post diagnosed period; before diagnosis an average of 111,034 JPY (841.92 USD) decreasing to 30,203 JPY (229.03 USD) after diagnosis. For those currently not receiving public renumeration (n = 10, 16.1%) the decrease was less dramatic with the average out-of-pocket costs in the pre-diagnosed period 50,800 JPY (385.20 USD) declining to 37,000 JPY (280.56 USD) in the post-diagnosed period.
3.6. Psycho-social burdens and areas of unmet need
Due to the lack of self-administered options for acute attack treatment at the time of the survey, seeking treatment resulted in work, education and leisure impairments due to the time involved in a hospital visit. As reported in the previous section, patients make frequent visits to their treating hospital and 27 out of 55 (49.1%) respondents reported that they had been hospitalized on average for "one or more days" per year. In addition, 18 out of 47 (38.3%) respondents reported missing "10 or more days" from work/school because of HAE attacks.
We were able to gain insights retrospectively on the longer-term impacts of HAE on work and school by asking about three periods: the time between onset of symptoms and first seeking medical advice; the period between first seeing a physician or other health care practitioner and being diagnosed; the period after diagnosis. This showed that the highest mean time for days missed from work or school per year was in the period after seeking medical advice and before diagnosis (n = 45, mean days lost 17.7 days). After diagnosis this was reduced to a mean of 11.9 days lost (n = 47) per year.
The coding of open-ended question data offered some insight into the mental health burden of HAE for Japanese patients at the time of the survey. Eleven out of 70 (15.7%) respondents reported issues that we coded as 'mental anxiety' due to HAE. This was highest in the high attack group (n = 6/19, 31.6%%), but was also notable in the low attack and non-attacks groups (n = 4/29, 13.8% and n = 1/7, 14.3%, respectively).
Another category retrieved from the data were statements that we coded "being troubled by attacks" (n = 11/70, 15.7%), which we also interpreted as a mental health burden. Statements suggesting patients were troubled by attacks were seen more frequently in the high attack group (n = 7/19, 36.8%), but were also present in the low attack (n = 3/29, 10.3%) and no attack (n = 1/7, 14.3%) groups.
Anxiety around HAE attacks impacts whether a patient feels able to work or not, and if they do seek employment it impacts the kind of work they feel they can do. One patient reported that she/he cannot take a night shift or any shift where there are few workers in case she/he gets sick. The responses also show that patients may alter their treatment-seeking behavior when an attack occurs, e.g. holding out until the evening to avoid losing pay or bothering co-workers. This behavior could result in delayed treatment, which is associated with a higher level of attack morbidity. While in school, an attack can involve missing school or spending time in the infirmary which leads to missed classes (Table 1).
Table 1. Impacts of HAE on work and school, open-responses.
| About work and school: |
| • I want to work, but I can't when thinking about what I might do if an attack takes place. I would trouble others around me. |
| • HAE affects the type of work I can do, how often I can go out, the amount I can eat, and what I can do before needing to rest. |
| • I suddenly become unable to perform any work. |
| • I can't do a night shift or work where there are not many other workers [in case I am ill] |
| • I can't afford to keep missing days from work. So I go to the hospital at night more often, which burdens my children. |
| • When attacks occur, I have stomach pain and vomit and can't do any house work. I need help from family. I'm scared about attacks. |
| • I miss school, or sometimes have to rest at the school infirmary. |
| • I can get a swelling just from holding or running around with my child. |
| • I can't look after my children, I can't do the housework. [when I have an attack] |
Abbreviation: HAE, Hereditary angioedema.
Patients were asked whether HAE impacted their ability to make plans, or restricted travel and daily life activities. Roughly equal numbers of patients responded that 'they try not to travel' and 'daily life activities are restricted'. The percentage reporting that they were unable to make plans, restricted travel and daily life activities was considerably higher for the high attack group. Seventeen out of 19 (89.5%) respondents reported that they restricted their daily activities, compared to group (n = 8/29, 27.6%) for the low attack group (Figure 7).
Figure 7.
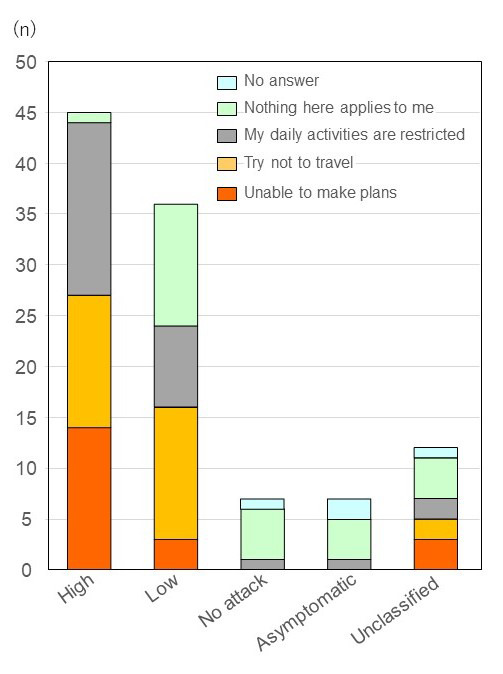
Daily activity impairments. Patients were asked whether HAE impacted their ability to make plans, or restricted travel and daily life activities. Roughly equal numbers of patients responded that "they try not to travel" and "daily life activities are restricted". The percentage reporting that they were unable to make plans, restricted travel and daily life activities was considerably higher for the high attack group. Seventeen out of 19 respondents (89.5%) reported that they restricted their daily activities, compared to group (n = 8/29, 27.6%) for the low attack group. Abbreviation: HAE, Hereditary angioedema.
Patients were asked what changes would improve their lives. Our coding of these open-ended responses reveals several areas where patients would like to see change. Ten out of 70 (14.3%) respondents reported that being able to self-administer medications for their acute attack would improve their lives. In the high attack group, 7 out of 19 (36.8%) respondents described that self-administration would improve their lives. Access to C1-INH concentrate for prophylactic treatment options was also indicated as something that would bring improvements to their lives by 7 out of 70 (10.0%) respondents, and 4 out of 19 (21.1%) respondents in the high attack group. Ten out of 70 (14.3%) respondents also wished for greater awareness of the condition among physicians, this figure rising to 4 out of 19 (21.1%) respondents in the high attack group (Table 2).
Table 2. Mental health burden of HAE.
| Items | Total (n = 70) |
High (n = 19) |
Low (n = 29) |
No attack (n = 7) |
Asymptomatic (n = 7) |
Unclassified (n = 8) |
|---|---|---|---|---|---|---|
| Troubled with attacks | 11 | 7 | 3 | 1 | 0 | 0 |
| Mentally worried | 11 | 6 | 4 | 1 | 0 | 0 |
| Want access to self-injection, treatment at home | 10 | 7 | 3 | 0 | 0 | 0 |
| Raise awareness among doctors | 10 | 4 | 4 | 1 | 0 | 1 |
| Want access to a hospital that would offer treatment nearby | 9 | 5 | 3 | 1 | 0 | 0 |
| Want new products and formulations | 9 | 5 | 3 | 0 | 0 | 1 |
| Prophylactic use of Berinert or a drug that has preventive effect | 7 | 4 | 1 | 0 | 1 | 1 |
| Not enough information | 4 | 0 | 4 | 0 | 0 | 0 |
| Waiting time is too long | 6 | 3 | 2 | 0 | 0 | 1 |
| Supporting costs for genetic tests for family | 4 | 2 | 2 | 0 | 0 | 0 |
| Scared of going on trips | 5 | 1 | 4 | 0 | 0 | 0 |
| Concerned about the side effects of drugs | 4 | 1 | 2 | 1 | 0 | 0 |
| Wish to have no more attacks; Want a cure | 3 | 1 | 0 | 0 | 1 | 1 |
| Financial burden | 4 | 2 | 1 | 1 | 0 | 0 |
| Impact of treatment is unknown; Have unexplained symptoms | 3 | 0 | 3 | 0 | 0 | 0 |
| Cannot get my medication at a pharmacy | 1 | 0 | 1 | 0 | 0 | 0 |
| Burden on family | 1 | 0 | 1 | 0 | 0 | 0 |
| How to deal with throat swelling | 1 | 0 | 0 | 0 | 0 | 1 |
| A third-party counselor specializing in HAE | 1 | 0 | 0 | 0 | 1 | 0 |
| Nothing at this moment | 4 | 0 | 2 | 1 | 1 | 0 |
| No answer | 18 | 1 | 9 | 2 | 3 | 3 |
Abbreviation: HAE, Hereditary angioedema.
Patients were also asked hypothetically whether they would want to self-inject, if plasma derived C1- INH concentrate was authorized for home use for acute attack therapy and prophylactic use. Thirty-seven out of 51 (72.5%) respondents patients answered that they would want to self-inject for acute attack therapy, and 30 out of 51 (58.8%) respondents did so if it were available for prophylactic therapy. In the high attack group, this intension was even higher; 16 out of 18 (88.9%) respondents said that they would want to self-inject for acute attack treatment and 13 (72.2%) respondents for prophylactic therapy.
4. Discussion
Through a PRO survey instrument designed in consultation with patients, we were able to clarify the burden of HAE in key domains, including medical resource utilization, physical, psycho-social and economic burdens, and associated work and school impairments. We also elicited responses about what changes might reduce these burdens. Through this instrument we were able to clarify not only the current burden of illness for patients at the time of the survey, but also the impact in the pre-diagnosed period, which is something that most HAE burden of illness research has not attempted to do.
In keeping with other HAE burden of illness studies, we found that the heaviest burden in all domains was experienced by those with a higher attack frequency. This burden was evident both in the pre- and post-diagnosed periods. As comparable survey instruments have not been used in the various HAE burden of illness studies that have been carried out to-date, it is hard to precisely compare Japanese patients with their counterparts overseas (11-20). However, our study confirms that there is a very high level of work, school and activity impairment for HAE patients in Japan at the time of this survey, which is consistent with studies carried out in the United States and Europe (11-20).
We can also confirm that patients are frequent users of medical resources, both routine care and emergency care, which creates physical, mental and economic burdens for the patients and their caregivers. Our results show that for many patients, especially those with frequent attacks who make regular trips to the hospital, these visits often require a long travel time and then a long waiting time for treatment. It is notable that in overseas studies on the burden of illness of HAE patient, the issue of travel time to receive treatment is not raised, possibly due to the high prevalence of self-administration. Nevertheless, a systematic review of studies looking at the association between travel times to receive treatment for a variety of conditions, but not HAE, and health outcomes of countries in the global north found that 83 out of 108 (76.9%) studies revealed a negative relationship. The longer the travel time, the worse the health outcome and the higher the level of non-attendance for required treatments (21). While none of these studies were concerned with HAE, the study conclusions alert us to the possible HRQoL-related implications of longer travel times.
The survey instrument was able to clarify, to some extent, the psycho-social and economic burdens of HAE and the associated impairments on work, education and leisure activities, as well as the direct financial costs associated with treatment and the extent to which they were ameliorated by the public remuneration system.
The system of public renumeration for rare disease patients greatly reduces the direct costs associated with medical resource utilization. Yet, it is noteworthy that 14 out of 70 (20%) respondents of patients overall had not applied for public numeration. While 6 out of 29 (20.7%) respondents in the low attack group reported they were not receiving public renumeration, all patients in the high attack group were receiving public renumeration.
Patients in the high attack group were shown to have shouldered the heavier direct medical costs during the pre-diagnosed period. These results would suggest that those with heavier medical resource utilization history have a higher motivation to file for public renumeration to offset the economic burden. Nevertheless, we need greater insights into why some patients do not apply for public renumeration and the impact of this on how they access effective acute attack treatments.
While public renumeration reduces the financial burden of direct medical costs, we saw that some patients perceive the costs of receiving treatment for each attack as too high and this influences treatment seeking behavior. When we consider the economic burden of HAE, we also need to factor in lifetime costs, including those in the pre-diagnosed period, in which patients experience multiple misdiagnoses with high out-of-pocket expenses. This result confirms that an early diagnosis is not only important to reduce the risks of mortality and morbidity associated with undiagnosed HAE but also reduce the economic burden (13). It goes without saying that until a definitive diagnosis has been reached, an HAE patient cannot apply for public remuneration.
Due to the lack of self-administered options for disease specific treatments at the time of the survey, seeking treatment for HAE attacks resulted in work, education and leisure impairments due to the time involved in a hospital visit. Patients make frequent visits to their treating hospital and this is not only time consuming, but results in time lost from work and educational activities.
Patients in the study pointed to three areas of possible improvement to reduce the burden of the disease. First, they called for self-administered HAE treatments, both for acute attack and to prevent attacks. Second, they called for the authorization of C1-INH concentrate, the only authorized disease-specific available treatment at the time of the survey, for prophylactic use. Finally, they called for greater awareness of the disease by physicians. The first two requests point to patients' desire to reduce the burden of being treated and, in the case of prophylactic care, reducing the frequency of attacks. Self-administration of acute attack and prophylactic medications has shown to reduce the burden of HAE in studies conducted outside of Japan (16).
The limitations of this study lie in the use of a non-validated survey instrument. Nevertheless, conversely, the strengths of this study are that the survey instrument was designed in consultation with patients and physicians, and that the content reflects key concerns that were generated in the consultation process. In addition, the survey was carried out in a treatment environment where only one disease-specific treatment was authorized, human plasma derived C1-INH concentrate, solely for an acute attack. Treatment required the patient to visit a designated hospital to receive intravenous administration from a physician or a nurse. Other studies on burden of illness have been carried out in environments where patients had access to a wider variety of treatments and to self-administration, largely in the United States and Europe (11-20). Second, patients were asked to recall attack history, medical resource utilization and direct economic costs in the pre-diagnosed period that varies by patient in length of time and distance from the present day. The reliance on retrospective reporting is another limitation. Nevertheless, it did allow us to clarify the cumulative impact of the disease in broad brush strokes. Even if in future, the burden is reduced by the introduction of new treatments and/or widening the access to the current treatment by the authorization of self-administration, the historical accumulation of impairments and costs will not be eliminated. A third limitation is that the patient population included in this study might be biased to those attending a specialist clinic on a regular basis or at least on an irregular basis.
It is important to note that HAE treatment environment in Japan has undergone rapid change over the last five years. Human plasma derived C1-INH concentrate has been available for acute attack treatment since 1990, but it became available for short-term prophylaxis as well in the first quarter of 2017, after our survey was administered. For both indications, treatment must be carried out in a hospital setting under the supervision of a physician. Additionally, subcutaneous administration of a bradykinin-B2-receptor antagonist (icatibant) and its self-administration was approved for acute attack in the final quarter of 2018. In the first quarter of 2021, an oral plasma kallikrein inhibitor (berotralstat) was approved for prophylactic indications, followed by the approval of monoclonal antibody against plasma kallikrein (lanadelumab) in Japan as treatment options for long-term prophylaxis to prevent attacks. In the context of this improving treatment environment, our data-set offers an important baseline against which future studies can investigate the impact of these improvements in HAE treatment options on the burden or illness experiences by patients. It also offers a historic picture of the disease burden before self-administrated acute attack treatment and/or prophylactic treatment options were available.
In conclusion, based on a PRO survey this paper revealed that HAE patients in Japan experience heavy burdens of illness in the physical, social, economic, and psycho-social domains, causing a decrease in HRQoL. As the burden of disease in these domains was shown to be even higher before than after diagnosis, we can conclude that early diagnosis is a necessary condition to reduce the lifetime burden of this disease. Patients felt that access to self-administered treatments would reduce the physical and social burdens associated with frequent hospital visits. We identify this as an area of unmet need at the time of the survey. Results of this study should be a basis for comparison in coming studies after the introduction of self-administered modalities for acute attack and prophylactic treatments.
Acknowledgements
We thank members of the steering committee of the patient organization HAEJ for their help in the design and distribution of the questionnaire, especially Kazuna and Naohiro Takagishi.
Funding:
This work was supported by a research grant from the Shire (now a part of Takeda) Japan.
Conflict of Interest
B.Y. has received honoraria, and/or served as a consultant and/or participated in advisory boards for CSL Behring, Takeda/Shire, Torii Pharmaceutical Company Ltd, BioCryst and Phavaris. D.H. has received honoraria as a speaker/advisor, and/ or participated in advisory boards for CSL Behring, Takeda/Shire, and Torii Pharmaceutical Company Ltd. I.O. has received honoraria as a speaker/advisor, and/or participated in advisory boards for CSL Behring, Takeda/ Shire, Torii Pharmaceutical Company Ltd, and BioCryst. K.I. have no conflicts of interest to declare. T.H. has received speaker/advisor honoraria from CSL Behring, Takeda and Torii Pharmaceutical Company Ltd. A.F. has received speaker/advisor honoraria and/or participated in advisory boards for CSL Behring and Takeda/Shire. J.M. has received speaker/advisor honoraria and/or participated in advisory boards for CSL Behring and Takeda/Shire. K.Y. has received speaker/advisor honoraria from CSL Behring and Takeda Pharmaceutical Company Ltd. M.H. has received honoraria from CSL Behring, Takeda and Torii Pharmaceutical Company Ltd, consulting fee from BioCryst, KalVista and Pharvaris.
References
- 1. Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006; 119:267-274. [DOI] [PubMed] [Google Scholar]
- 2. Agostoni A, Cicardi M. Hereditary and acquired C1- inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine. 1992; 71:206-215. [DOI] [PubMed] [Google Scholar]
- 3. Cicardi M, Agostoni A. Hereditary angioedema. N Engl J Med. 1996; 334:1666-1667. [DOI] [PubMed] [Google Scholar]
- 4. Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008; 359:1027-1036. [DOI] [PubMed] [Google Scholar]
- 5. Bygum A, Aygören-Pürsün E, Beusterien K, Hautamaki E, Sisic Z, Wait S, Boysen HB, Caballero T. Burden of illness in hereditary angioedema: a conceptual model. Acta Derm Venereol. 2015; 95:706-710. [DOI] [PubMed] [Google Scholar]
- 6. Bork K, Hardt J, Schicketanz KH, Ressel N. Clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Arch Intern Med. 2003; 163:1229-1235. [DOI] [PubMed] [Google Scholar]
- 7. Bork K, Machnig T, Wulff K, Witzke G, Prusty S, Hardt J. Clinical features of genetically characterized types of hereditary angioedema with normal C1 inhibitor: a systematic review of qualitative evidence. Orphanet J Rare Dis. 2020; 15:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKibbin L, Barber C, Kalicinsky C, Warrington R. Review of the Manitoba cohort of patients with hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol. 2019; 15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaplan AP, Maas C. The Search for Biomarkers in Hereditary Angioedema. Front Med (Lausanne). 2017; 4:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iwamoto K, Yamamoto B, Ohsawa I, Honda D, Horiuchi T, Tanaka A, Fukunaga A, Maehara J, Yamashita K, Akita T, Hide M. The diagnosis and treatment of hereditary angioedema patients in Japan: A patient reported outcome survey. Allergol Int. 2021; 70:235-243. [DOI] [PubMed] [Google Scholar]
- 11. Lumry WR, Castaldo AJ, Vernon MK, Blaustein MB, Wilson DA, Horn PT. The humanistic burden of hereditary angioedema: impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc. 2010; 31:407-414. [DOI] [PubMed] [Google Scholar]
- 12. Aygören-Pürsün E, Bygum A, Beusterien K, Hautamaki E, Sisic Z, Wait S, Boysen HB, Caballero T. Socioeconomic burden of hereditary angioedema: results from the hereditary angioedema burden of illness study in Europe. Orphanet J Rare Dis. 2014; 9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caballero T, Prior N. Burden of illness and quality-of-life measures in angioedema conditions. Immunol Allergy Clin North Am. 2017; 37:597-616. [DOI] [PubMed] [Google Scholar]
- 14. Longhurst H, Bygum A. The humanistic, societal, and pharmaco-economic burden of angioedema. Clin Rev Allergy Immunol. 2016; 51:230-239. [DOI] [PubMed] [Google Scholar]
- 15. Lin XJ, Lin IM, Fan SY. Methodological issues in measuring health-related quality of life. Tzu Chi Medical Journal. 2013; 25:8-12. [Google Scholar]
- 16. Lumry WR, Settipane RA. Hereditary angioedema: epidemiology and burden of disease. Allergy Asthma Proc. 2020; 41:S08-S13. [DOI] [PubMed] [Google Scholar]
- 17. Lumry WR, Craig T, Zuraw B, et al. Health-related quality of life with subcutaneous C1-inhibitor for prevention of attacks of hereditary angioedema. J Allergy Clin Immunol Pract. 2018; 6:1733-1741.e3. [DOI] [PubMed] [Google Scholar]
- 18. Banerji A, Davis KH, Brown TM, Hollis K, Hunter SM, Long J, Jain G, Devercelli G. Patient-reported burden of hereditary angioedema: findings from a patient survey in the United States. Ann Allergy Asthma Immunol. 2020; 124:600-607. [DOI] [PubMed] [Google Scholar]
- 19. Mendivil J, Murphy R, de la Cruz M, Janssen E, Boysen HB, Jain G, Aygören-Pürsün E, Hirji I, Devercelli G. Clinical characteristics and burden of illness in patients with hereditary angioedema: findings from a multinational patient survey. Orphanet J Rare Dis. 2021; 16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bouillet L, Launay D, Fain O, Boccon-Gibod I, Laurent J, Martin L, Montauban V, Finck K, Bouée S, Gompel A, Kanny G; French National Reference Center for Hereditary Angioedema (CREAK). Hereditary angioedema with C1 inhibitor deficiency: clinical presentation and quality of life of 193 French patients. Ann Allergy Asthma Immunol. 2013; 111:290-294. [DOI] [PubMed] [Google Scholar]
- 21. Kelly C, Hulme C, Farragher T, Clarke G. Are differences in travel time or distance to healthcare for adults in global north countries associated with an impact on health outcomes? A systematic review. BMJ Open. 2016; 6:e013059. [DOI] [PMC free article] [PubMed] [Google Scholar]


