Abstract
Interest in the production of l-(+)-lactic acid is presently growing in relation to its applications in the synthesis of biodegradable polymer materials. With the aim of obtaining efficient production and high productivity, we introduced the bovine l-lactate dehydrogenase gene (LDH) into a wild-type Kluyveromyces lactis yeast strain. The observed lactic acid production was not satisfactory due to the continued coproduction of ethanol. A further restructuring of the cellular metabolism was obtained by introducing the LDH gene into a K. lactis strain in which the unique pyruvate decarboxylase gene had been deleted. With this modified strain, in which lactic fermentation substituted completely for the pathway leading to the production of ethanol, we obtained concentrations, productivities, and yields of lactic acid as high as 109 g liter−1, 0.91 g liter−1 h−1, and 1.19 mol per mole of glucose consumed, respectively. The organic acid was also produced at pH levels lower than those usual for bacterial processes.
Over the past 50 years, plastics derived from petrochemicals have become indispensable materials in our daily life. Unfortunately, most of these materials are not biodegradable, and they cause significant disposal and pollution problems for both land and sea. Metabolic engineering (2) may well be useful to solve and/or reduce such problems by enabling the low-cost production of biological polymers and polymer precursors. In this regard, a group of compounds of potential interest is represented by the polyhydroxyalkanoate polyesters accumulated by different organisms as energy reserve materials (14, 15, 22). A different compound of potential interest is l-(+)-lactic acid. In fact, lactic acid can be used for the synthesis of biodegradable polymeric materials (for a detailed review, see reference 3), and it can be produced by microorganisms during fermentation. The most important industrial microorganisms belong to the genera Lactobacillus (3), Bacillus (3), and Rhizopus (3). During a typical lactic acid fermentation, the low pH (due to lactic acid production) has an inhibitory effect on the metabolic activities of the producing microbial cells (3, 6, 13). The addition of Ca(OH)2, CaCO3, NaOH, or NH4OH to neutralize the lactic acid is a conventional operation to minimize the negative effects of undissociated lactic acid accumulation in industrial processes (3, 6, 13). However, the neutralization of lactic acid during fermentation has major disadvantages. Additional operations are required to regenerate undissociated lactic acid from its salt and to dispose of or recycle the neutralizing cation (3, 6, 13). All the extra operations and expense could be reduced if undissociated lactic acid could be accumulated by microorganisms able to grow and metabolize at low pH levels (the pKa for lactic acid is 3.86) (3). Yeasts are well known to grow and survive at low pH levels, and the use of metabolically engineered strains of Saccharomyces cerevisiae expressing a lactate dehydrogenase gene (LDH) to shift the glycolytic flux toward the production of lactic acid has already been proposed (1, 10, 17). These heterolactic engineered strains exhibit both alcoholic and lactic fermentations. Because of the production of ethanol, lactic acid production from such processes is not competitive with that from lactic bacteria, even considering the coproduct value of ethanol. Moreover, S. cerevisiae possesses three pyruvate decarboxylase (PDC) genes, and the depletion of pyruvate decarboxylase (Pdc) activity comports a severe reduction of growth ability to this yeast (20). The Crabtree-negative yeast Kluyveromyces lactis possesses only one PDC gene, (5) and, thanks to well-established protocols for genetic manipulations, it could represent an alternative to S. cerevisiae and produce higher levels of lactic acid. In this study, we present the development of a homolactic K. lactis yeast strain which exhibits high concentration and high productivity of lactic acid at a pH level below 5 to 5.5, the typical minimum pH range for the production of lactate by conventional microbial cells (3, 6, 13).
MATERIALS AND METHODS
Strains and culture conditions.
The K. lactis strains (derived from CBS2359 strain) used were PM6-7A (MATa, adeT-600, uraA1-1, KlPDC1) (23) and PMI/C1 (MATa, adeT-600, uraA1-1, Klpdc1::ura3). The PMI/C1 strain was isolated by treatment with 5-fluoro-orotic acid (16) from a Klpdc1::URA3-deleted strain of PM6-7A (11). Engineered strains were tested in batch culture during growth on minimum synthetic medium (1.3% [wt/vol] yeast nitrogen base without amino acids (Difco, Detroit, Mich.), 200 mg of adenine liter−1, and 50 g of glucose liter−1). Media were buffered to pH 5.5 with 200 mM phosphate buffer.
Cells were preinoculated in the synthetic medium. Exponentially growing cells were inoculated in 300-ml flasks containing 100 ml of fresh medium. The flasks were incubated at 30°C in a shaking bath (Dubnoff) at 150 rpm, and fermentation was monitored at regular intervals. Cell concentration was determined with an electronic counter (Coulter Counter ZBI; Coulter Electronics, Harpenden, United Kingdom) after sonication of the samples was carried out to avoid cellular aggregates (Fisher 300 sonicator, medium point, 35% power, 10 s) (18).
Inocula for the bioreactor were prepared by preculturing the transformed cells under the same conditions as above. Eighty milliliters of inoculum was transferred to a 14-liter stirred-tank bioreactor (BioFlo 3000 system; New Brunswick Scientific Co., Inc., Edison, N.J.) containing 8 liters of nutrient medium (30 g of dry solids liter−1 of light corn steep water [A. E. Staley Manufacturing Co., Decatur, Ill.], 10 g of yeast extract liter−1 [Difco], 200 mg of adenine liter−1, and 50 g of glucose liter−1). The bioreactor was kept at 30°C, stirred at 400 rpm, and aerated at 2 liters min−1 throughout the process. Antifoam (Antifoam 1520; Dow Corning Corp., Midland, Mich.) was added to control foaming. When controlled, the pH was maintained by automatic addition of 14.8 M ammonium hydroxide in water. Glucose (500 g liter−1) was discontinuously pulsed to the bioreactor in order to restore a glucose concentration of about 50 g liter−1.
Chemostat cultures were performed in a 0.8-liter bioreactor (Biostat-Q; B. Braun Biotech International, GmbH). The cultures were kept at 30°C and pH 4.5, were stirred at 800 rpm, and were aerated at 0.8 liters min−1 throughout the process. Medium used contained 30 g of glucose liter−1, 0.67% (wt/vol) yeast nitrogen base, and 200 mg of adenine liter−1.
Plasmid constructs and transformation procedures.
A standard site-directed mutagenesis (25) was performed in order to introduce an XbaI restriction before the starting ATG codon of the bovine LDH cDNA (17). The isolated DNA fragment was then inserted in the pALTER-1 vector (Promega, Madison, Wis.), yielding the pVC1 plasmid. The bovine LDH sequence, isolated as an XbaI-HindIII fragment of 1,675 bp from the pVC1 vector, was then cloned in the corresponding sites of the pBluescript II KS vector (Stratagene, La Jolla, Calif.). The KlPDC1 promoter was subcloned from vector pMD12 (11) into the SalI and XbaI sites of the pBluescript II KS vector with T4 DNA ligase by using standard procedures (21). Escherichia coli JM110 (obtained from the American Type Culture Collection) was transformed with the two new vectors, called pKSMD8/7 and pKSEXH/16, respectively. The KlPDC1 promoter and bovine LDH gene were thus isolated as SalI-XbaI fragments, and they were ligated in vitro with T4 DNA ligase at room temperature in the presence of SalI endonuclease to favor ligation at the XbaI ends. The ligation product was then cloned into the SalI cloning site of pE1 vector (4). The URA3 marker on the plasmid allows the complementation of the K. lactis uraA1-1 mutation (9). The vector obtained, called pEPL2, was stably maintained (>95%) during growth on both selective and complex media, and the plasmid copy number was approximately five copies per cells (data not shown).
E. coli and K. lactis strains were transformed as previously described (5).
LDH activity and metabolite determinations.
At different times, about 108 transformed cells were harvested, and the l-lactate dehydrogenase (LDH) activity was determined as previously described (17). Samples from the growth medium, obtained after removing cells by centrifugation, were analyzed for the presence of glucose, ethanol, acetic acid, and l-(+)- and d-(−)-lactic acid by using diagnostic kits from Boehringer GmbH, Mannheim, Germany (kits 716251, 176290, 148261, and 1112821, respectively), according to the manufacturer’s instructions.
RESULTS
Introduction of a bovine LDH into K. lactis.
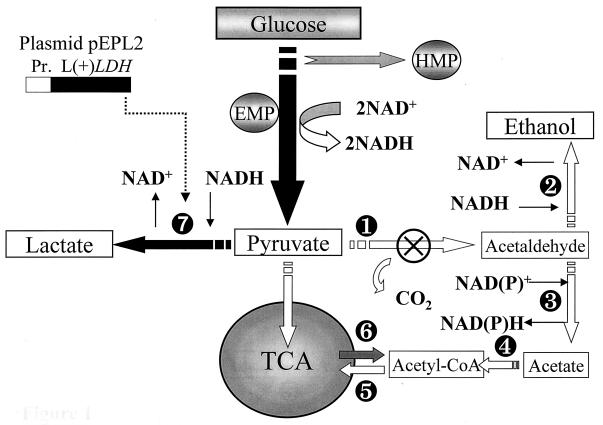
We constructed the replicative vector pEPL2 containing the gene encoding the bovine LDH (LDH-A) under the control of the promoter region of the pyruvate decarboxylase gene of K. lactis. The vector, a derivative of plasmid pKD1 from Kluyveromyces drosophilarum (12) which is stably maintained in K. lactis (4), was used to transform the K. lactis strain PM6-7A in order to drive its metabolism toward the production of l-lactic acid. Figure 1 shows the heterologous biosynthetic pathway introduced into yeast cells, along with the main pyruvate depletion pathways. The heterologous gene was cloned under the control of the KlPDC1 promoter. The choice of this promoter was based on the fact that the expression of KlPDC1 is strongly induced by glucose (5), and the size of the promoter region used was sufficient to include all the glucose responding elements (11). PM6-7A(pEPL2) transformants were tested for lactic acid production during growth on glucose-based media in flask cultures (Fig. 2). At the end of the fermentation, all the glucose supplied to the engineered cells was completely consumed and the final pH value was 3.0.
FIG. 1.
Schematic representation of the main pyruvate dissimilation pathways in K. lactis. EMP, Embden-Meyerhof pathway; HMP, hexose-monophosphate pathway; TCA, tricarboxylic acid cycle. Key enzymatic reactions at the pyruvate branch point are catalyzed by the following enzymes: ➊, pyruvate decarboxylase (EC 4.1.1.1; ⊗ indicates that this activity is absent in the K. lactic strain PMI/C1); ➋, alcohol dehydrogenase (EC 1.1.1.1); ➌, acetaldehyde dehydrogenase (EC 1.2.1.4 or EC 1.2.1.5); ➍, acetyl coenzyme A (CoA) synthetase (EC 6.2.1.1); ➎, acetyl CoA shuttle from the cytosol to mitochondria; ❻, acetyl CoA shuttle from mitochondria to the cytosol; and ❼, heterologous l-(+)-lactate dehydrogenase (EC 1.1.1.27). Enzymatic reactions involved in anaplerotic syntheses have been omitted. Black arrows indicate the metabolic pathway leading to the production of lactic acid from glucose in heterolactic and homolactic strains PM6-7A(pEPL2) and PMI/C1(pEPL2), respectively. A schematic representation of the expression cassette [KlPDC1 promoter and l-(+) LDH gene] on the plasmid pEPL2 is also shown.
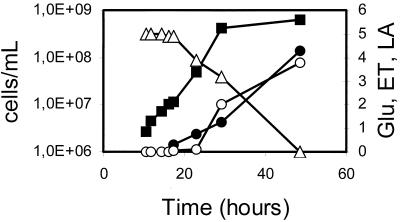
FIG. 2.
l-(+)-Lactic acid production from the heterolactic PM6-7A(pEPL2) transformed strain. Batch fermentation was carried out on yeast nitrogen base medium containing 50 g of glucose liter−1 buffered at T = 0 to pH 5.5, as described in Materials and Methods. Production of d-(−)-lactic acid was not detected. The LDH-specific activity was 4 to 5 U (mg of total cell proteins)−1 during the entire experiment. Final pH value was 3. ■, cells per milliliter; ○, ethanol (ET) production, grams per liter; ●, l-(+)-lactic acid (LA) production, grams per liter; and ▵, percent (wt/vol) residual glucose (Glu).
Strain improvement for higher productions.
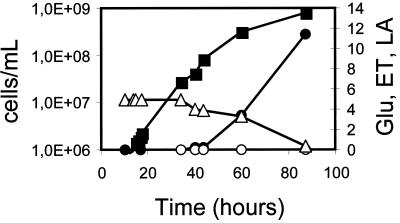
Due to the coproduction of ethanol, the overall concentration, productivity, and yield of lactic acid obtained by the heterolactic strain described above were not satisfactory. Similar conclusions have been reached with metabolically engineered S. cerevisiae cells (17). Further restructuring of the cellular metabolism could potentially divert a larger fraction of the glycolytic flux to lactic acid. In fact, it can be anticipated that improved lactic acid production might be obtained by engineering yeast strains to partially or completely replace the conversion of pyruvate to ethanol. These strains can be obtained by several methods; for instance, they can be obtained by inactivating or suppressing enzymatic activities involved in the production of ethanol (e.g., pyruvate decarboxylase activity). An example of a K. lactis strain harboring a deleted PDC gene has been reported by Bianchi et al. (5). The strain had no Pdc activity and did not produce ethanol, but it showed a wild-type ability to grow on synthetic-glucose-based media. The ΔKlpdc1 K. lactis strain PMI/C1 (see Materials and Methods) was thus transformed with the plasmid pEPL2. Expression of the LDH gene in this yeast strain gave higher concentrations of lactic acid than the heterolactic analogue without the formation of ethanol (Fig. 3). In this case the final pH value was 2.95.
FIG. 3.
l-(+)-Lactic acid production from the homolactic PMI/C1(pEPL2) transformed strain. Batch fermentation was carried out as described in Fig. 2. The LDH-specific activity was 55 to 60 U (mg of total cell proteins)−1 during the entire experiment. Final pH value was 2.95. ■, cells per milliliter; ○, ethanol (ET) production, grams per liter; ●, l-(+)-lactic acid (LA) production, grams per liter; and ▵, percent (wt/vol) residual glucose (Glu).
The performances of the hetero- and homolactic engineered strains are summarized and compared in Table 1. With the heterolactic engineered strain, about 1 mmol of lactic acid was produced per 2 mmol of ethanol, while in the homolactic ΔKlpdc1 strain, otherwise isogenic to the PM6-7A strain, the overall production of ethanol was completely replaced by lactic acid production. The lactate yield was increased from 0.17 to 0.46 mol per mole of glucose, a number still far below 2.0, the maximum theoretical value. Although an accurate carbon balance is unobtainable in shake-flask experiments, and taking into consideration the ethanol evaporation for the heterolactic strain, it is clear that a large part of the carbon source is used for the syntheses of other products. However, K. lactis strains are well-known producers of many organic compounds, such as organic acid esters (24).
TABLE 1.
Lactate, ethanol, and acetate productions from wild-type, heterolactic, and homolactic K. lactis strains during batch growth on 5% (wt/vol) glucose-yeast nitrogen base medium
| Strain | Concentration of (mM)a:
|
Yieldb | U of LDHc | ||
|---|---|---|---|---|---|
| Lactate | EtOH | Acetate | |||
| PM6-7A | 0.0 | 161.0 | 0.5 | 0.00 | 0.0 |
| PM6-7A(pEPL2) | 47.7 | 84.8 | 0.3 | 0.17 | 4–5 |
| PMI/C1 | 0.0 | 0.0 | 1.7 | 0.00 | 0.0 |
| PMI/C1(pEPL2) | 126.5 | 0.0 | 0.3 | 0.46 | 55–60 |
Highest values measured. Numbers did not vary by more than 10% between independent experiments.
Yield is expressed as millimoles of lactic acid produced liter−1 divided by millimoles of glucose consumed liter−1.
Specific activity of the heterologous LDH enzyme. Units are given per milligram of total cell protein.
Moreover, we must note that the heterologous LDH activity is about 10 times higher in the PMI/C1(pEPL2) strain than in the PM6-7A(pEPL2) strain (Table 1). This data has been validated by Northern analyses of the heterologous mRNA transcripts in the two transformed strains (data not shown) and confirms that the transcriptional activity of the KlPDC1 promoter is negatively modulated by the presence of the Pdc enzyme in a wild-type background (11).
We then tested lactic acid production during classical chemostat cultivation. Continuous and stable production (1.5 g liter−1 h−1) of lactic acid has been obtained for at least 3 weeks (dilution rate, 0.1 h−1) by using the transformed K. lactis PMI/C1(pEPL2) cells.
Transferring the process from laboratory scale to a productive perspective.
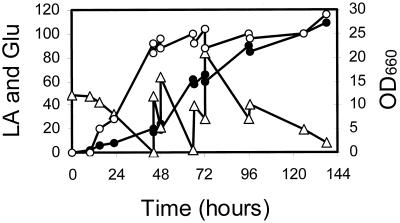
Cloning a gene and restructuring cellular metabolism are only two of the steps required for metabolic engineering applications. Lactic acid production by PMI/C1(pEPL2) was further tested by cultivation in a 14-liter, stirred-tank bioreactor. We used a nutrient medium based on corn steep water, which is more economical from an industrial standpoint than the synthetic yeast nitrogen base. Initial glucose concentration was 50 g liter−1. After 48 h, glucose (500 g liter−1) was intermittently fed into the medium (Fig. 4). Aeration rate was 2 liters min−1, and pH was maintained at 4.5 throughout the process. The maximum lactate concentration obtained was 109 g liter−1 (1.2 M) with a productivity of 0.795 g liter−1 h−1. Under the same conditions, the transformed PM6-7A(pEPL2) strain accumulated ethanol and lactate with a shape similar to that observed in shake-flask experiments (31 and 30 g liter−1, respectively).
FIG. 4.
l-(+)-Lactic acid production from the homolactic PMI/C1(pEPL2) transformed strain in a bioreactor. The fermentation pH was controlled at 4.5 throughout the fermentation. Production of lactic acid (LA) (●, grams per liter), residual glucose (Glu) (▵, grams per liter), and optical density of the biomass at 660 nm (OD660) (○) are shown.
Since the accumulation of fermentation products should be favored by low oxygen availability, some fermentations were run at lower aeration rates (0.5 and 0.05 liters min−1); however, in these conditions the lactic acid production is dramatically reduced or arrested, respectively (data not shown).
We further tested different strategies of pH control. The production of lactate appears to be dependent on the pH of the fermentation mixture. The results are summarized in Table 2. It is important to emphasize that the total amounts of free lactic acid are higher in tests 2 and 3 than in test 1.
TABLE 2.
Lactic acid production by transformed PMI/C1(pEPL2) cells in a bioreactor
| Test no.a | Elapsed time (h) | Total production (g liter−1) | Yield of lactic acidb | Final pH | Undissociated lactic acid
|
|
|---|---|---|---|---|---|---|
| % | g liter−1 | |||||
| 1 | 137 | 109 | 1.19 | 4.5 | 19 | 20.7 |
| 2 | 97 | 35 | 0.88 | 3.0 | 88 | 30.8 |
| 3 | 72 | 29 | 0.70 | 2.8 | 92 | 26.7 |
Lactic acid production was tested as follows: test 1, the fermentation pH was controlled at 4.5 throughout the fermentation (Fig. 4); test 2, the initial fermentation pH was maintained at 4.0 until 1 to 1.2 mol of ammonium hydroxide was added, then pH control was discontinued; and test 3, the initial fermentation pH was 5.0 and no neutralizing agent was added during the fermentation. The elapsed time was measured from the time of inoculation. Values are the highest measured, corresponding to the end point.
Yield is expressed as millimoles of lactic acid produced liter−1 divided by millimoles of glucose consumed liter−1.
DISCUSSION
To the best of our knowledge, the present data represent the first example of a total replacement of a fermentation product (ethanol versus lactic acid) mediated by metabolic engineering techniques in yeast genera. The resulting recombinant yeast strain PMI/C1(pEPL2) does not produce ethanol, thus allowing lactic acid production with a higher yield than that of the yeast strain having a wild-type ability to produce ethanol (PM6-7A[pEPL2]). In this regard, K. lactis shows an interesting potential when compared with S. cerevisiae. In fact, lactic acid production from S. cerevisiae is affected by its strong inclination toward ethanol fermentation. Using a wild-type S. cerevisiae strain transformed with the bovine LDH gene, we obtained, in aerobic flask and bioreactor cultures, lactate yields of about 0.2 mol per mole of glucose consumed (17). When heterolactic S. cerevisiae cells, transformed with the same LDH gene, are cultured in anaerobic batch cultures, the yield increases to about 0.3 mol per mole of glucose consumed (1), and the deletion of PDC1 (one of the three PDC genes coding for pyruvate decarboxylase activity in S. cerevisiae) results in a further increase of the yields of anaerobic batch cultures, to about 0.4 mol per mole of glucose consumed (1). All of these values are lower than those obtained with the homolactic K. lactis strain. Furthermore, it is important to remember that S. cerevisiae cells bearing deletions in all the structural PDC genes are no longer able to grow on glucose-based synthetic media (20). This is not the case for K. lactis (reference 5 and this work), in which the pyruvate flux towards ethanol formation can be fully replaced by lactic acid production (Table 1). However, yields obtained are still distant from the highest theoretical value (1.19 versus 2 mol of lactate per mole of glucose). This is probably due to the strong respiratory metabolism exhibited by K. lactis. Unfortunately, besides the very high LDH activity (50 to 60 U mg−1), low aeration rates strongly reduce or completely arrest lactate production (data not shown). One possible explanation could be related to a limitation in the secretion of lactic acid under such conditions. In fact, lactic acid can freely diffuse through the membranes only in its undissociated form (8). Since the cytoplasmic pH value in yeast cells is much higher than the lactic acid pKa value (i.e., 3.86), almost all of the lactic acid produced is in the dissociated form and has to be actively transported outside the cells. A simple reduction of lactic acid transport will inevitably lead to an increase of the cytoplasmic lactate concentration, inhibiting LDH activity and leading to the reduction or arrest of lactate production.
The same phenomenon could be responsible for the reduction of lactate production observed when the pH value of the medium is much lower than the lactic acid pKa value (Table 2). In fact, below this pH value, the undissociated form becomes predominant in the medium and can freely diffuse back into the cell, increasing the intracellular lactate concentration. Indeed, we proved in vitro that a lactate concentration of as low as 333 mM (30 g liter−1) reduces overall LDH activity by about 70%.
To further test the relevance of the lactate secretion on the overall production, we overexpressed JEN1 in heterolatic S. cerevisiae cells (19). Jen1 is considered to be the lactate transporter in budding yeast (7). Preliminary data showed that the overexpression of JEN1 was associated with the doubling of both lactate production and yield from heterolactic S. cerevisiae cells. Unfortunately, the lactate transporter(s) in K. lactis has not yet been identified, and further studies of lactate secretion from K. lactis cells are required.
Finally, the demonstrated concentration and production of lactic acid begins to approach those of established lactic acid microbes (19). However, production can be obtained at pH values lower than those used by bacteria.
ACKNOWLEDGMENTS
We thank BIOPOLO s.c.r.l., The Consortium for Biotechnology Development and Research (Milan, Italy), for supporting the project.
REFERENCES
- 1.Adachi E, Torigoe M, Sugiyama M, Nikawa J J, Shimizu K. Modification of metabolic pathways of Saccharomyces cerevisiae by the expression of lactate dehydrogenase and deletion of pyruvate decarboxylase genes for the lactic acid fermentation at low pH value. J Ferment Bioeng. 1998;86:284–289. [Google Scholar]
- 2.Bailey J E. Toward a science of metabolic engineering. Science. 1991;252:1668–1675. doi: 10.1126/science.2047876. [DOI] [PubMed] [Google Scholar]
- 3.Benninga H A. A history of lactic acid making. Dordrecht, The Netherlands: Kluyver Academic Publishers; 1990. [Google Scholar]
- 4.Bianchi M M, Falcone C, Chen X J, Wésolowski-Louvel M, Frontali L, Fukuhara H. Transformation of the yeast Kluyveromyces lactis by new vectors derived from the 1.6 μm circular plasmid pKD1. Curr Genet. 1987;12:185–192. [Google Scholar]
- 5.Bianchi M M, Tizzani L, Destruelle M, Frontali L, Wésolowski-Louvel M. The ‘petite-negative’ yeast Kluyveromyces lactis has a single gene expressing pyruvate decarboxylase activity. Mol Microbiol. 1996;19:27–36. doi: 10.1046/j.1365-2958.1996.346875.x. [DOI] [PubMed] [Google Scholar]
- 6.Buchta K. Lactic acid. In: Dellweg H, editor. Biotechnology. Vol. 3. Weinheim, Federal Republic of Germany: Verlag Chemie; 1983. pp. 409–417. [Google Scholar]
- 7.Casal M, Paiva S, Andrade R P, Gancedo C, Leao C. The lactate-proton symport of Saccharomyces cerevisiae is encoded by JEN1. J Bacteriol. 1999;181:2620–2623. doi: 10.1128/jb.181.8.2620-2623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassio F, Leao C, van Uden N. Transport of lactate and other short-chain monocarboxylic acids in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol. 1987;53:509–513. doi: 10.1128/aem.53.3.509-513.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Louvencourt L, Fukuhara H, Heslot H, Wésolowski M. Transformation of Kluyveromyces lactis by killer plasmid DNA. J Bacteriol. 1983;154:737–742. doi: 10.1128/jb.154.2.737-742.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dequin S, Barre P. Mixed lactic acid-alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus caseil(+)-LDH. Bio/Technology. 1994;12:173–177. doi: 10.1038/nbt0294-173. [DOI] [PubMed] [Google Scholar]
- 11.Destruelle M, Menghini R, Frontali L, Bianchi M M. Regulation of the expression of the Kluyveromyces lactis PDC1 gene: carbon source responsive elements and autoregulation. Yeast. 1999;15:361–370. doi: 10.1002/(SICI)1097-0061(19990330)15:5<361::AID-YEA378>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Falcone C, Saliola M, Chen X J, Frontali L, Fukuhara H. Analysis of a 1.6 μm circular plasmid from the yeast Kluyveromyces drosophilarum: structure and molecular dimorphism. Plasmid. 1986;15:248–252. doi: 10.1016/0147-619x(86)90044-2. [DOI] [PubMed] [Google Scholar]
- 13.Hongo M, Nomura Y, Iwahara M. Novel methods of lactic production by electrodialysis fermentation. Appl Environ Microbiol. 1986;32:227–234. doi: 10.1128/aem.52.2.314-319.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S Y. Bacterial polyhydroxyalkanoates. Biotechnol Bioeng. 1996;49:1–14. doi: 10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Marchessault R H. Tender morsels for bacteria: recent developments in microbial polyesters. Trends Polymer Sci. 1996;4:163–168. [Google Scholar]
- 16.McCusker J H, Davis R W. The use of proline as a nitrogen source causes hypersensitivity to, and allows more economical use of, 5FOA in Saccharomyces cerevisiae. Yeast. 1991;7:607–608. doi: 10.1002/yea.320070608. [DOI] [PubMed] [Google Scholar]
- 17.Porro D, Brambilla L, Ranzi B M, Martegani E, Alberghina L. Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnol Prog. 1995;11:294–298. doi: 10.1021/bp00033a009. [DOI] [PubMed] [Google Scholar]
- 18.Porro D, Martegani E, Ranzi B M, Alberghina L. Developments of high cell density culture of engineered Saccharomyces cerevisiae able to grow on lactose. Biotechnol Lett. 1992;14:1085–1088. [Google Scholar]
- 19.Porro, D., M. M. Bianchi, B. M. Ranzi, L. Frontali, M. Vai, A. A. Winkler, and L. Alberghina. March 1999. Yeast strains for the production of lactic acid. PCT WO 99/14335.
- 20.Pronk J T, Steensma Y H, van Dijken J P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast. 1996;12:1607–1633. doi: 10.1002/(sici)1097-0061(199612)12:16<1607::aid-yea70>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 22.Sim S J, Snell K, Hogan S, Stubble J, Rha C, Sinskey A. PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat Biotechnol. 1997;15:63–67. doi: 10.1038/nbt0197-63. [DOI] [PubMed] [Google Scholar]
- 23.Wésolowski-Louvel M, Prior C, Bornecque D, Fukuhara H. Rag− mutations involved in glucose metabolism in yeast: isolation and genetic characterization. Yeast. 1992;8:711–719. [Google Scholar]
- 24.Wésolowski-Louvel M, Breunig K D, Fukuhara H. Kluyveromyces lactis. In: Wolf K, editor. Nonconventional yeasts in biotechnology. Berlin, Germany: Springer-Verlag; 1996. pp. 139–202. [Google Scholar]
- 25.Zoller M J, Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. Methods Enzymol. 1987;154:329–350. doi: 10.1016/0076-6879(87)54083-6. [DOI] [PubMed] [Google Scholar]