Abstract
Purpose:
This report describes a case of bilateral macular holes (MHs) in adult vitelliform macular dystrophy (AVMD).
Methods:
A retrospective case report of a patient with AVMD and sequential onset of bilateral MHs is presented.
Results:
Bilateral MHs were observed after vitreomacular traction was identified on optical coherence tomography. Holes in both eyes were repaired with pars plana vitrectomy (PPV) with C3F8 (perfluoropropane) gas tamponade; only the right eye underwent internal limiting membrane peeling. In the right eye, 2 PPVs were required for hole closure. In both eyes, long-term atrophy of the retina and retinal pigment epithelium was observed.
Conclusions:
MHs in AVMD may be preceded by vitreomacular traction. Surgical repair with PPV and gas tamponade was successful. Retinal and retinal pigment epithelium atrophy developed postoperatively, but the patient’s vision still improved.
Keywords: adult vitelliform macular dystrophy, internal limiting membrane peel, macular hole, vitreomacular traction
Introduction
First described by Gass in 1974, 1 adult vitelliform macular dystrophy (AVMD) presents with a yellow elevated lesion with central or surrounding pigment in both eyes. Age at presentation is 30 to 50 years, and vision loss is slowly progressive and only mild to moderate, typically with preservation of reading ability. 1 The yellow material likely represents defective phagocytosis, which leads to subretinal accumulation of lipofuscin, photoreceptor outer segments, and retinal pigment epithelial (RPE) cells. 2 The yellow lesion often disappears over time and may be associated with RPE and retinal atrophy. 2 Histologic examination shows focal loss of photoreceptor segments and outer nuclear layer that may predispose the retina to macular hole (MH) formation. 2 MHs have been previously reported in AVMD and other diseases with abnormal subretinal deposits. 3 -8 Reported here is a case of bilateral MHs in AVMD repaired surgically but with chronic development of foveal retinal and RPE atrophy.
Case Report
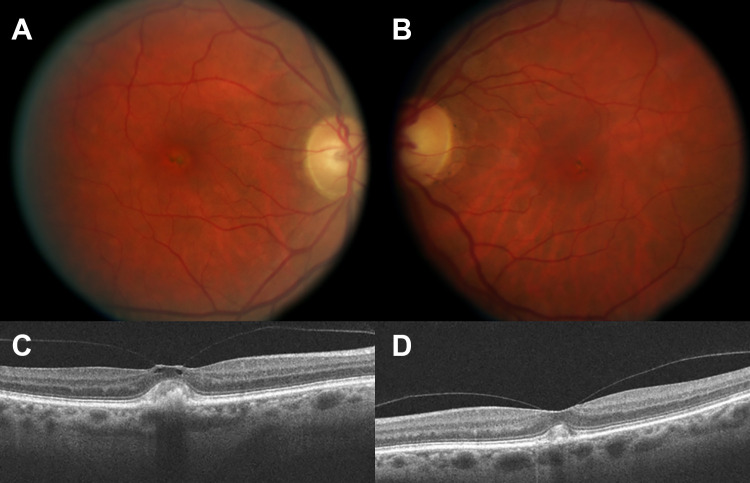
A 67-year-old woman with a history of AVMD presented with metamorphopsia in the right eye. Three years prior, the patient was diagnosed with AVMD based on her examination, which found symmetrical, bilateral, yellow subretinal deposits, and her electrophysiology testing, which had a focal foveal depression on multifocal electroretinogram and normal findings on an electrooculogram, ruling out Best disease. On examination, her vision was 20/40 in the right eye and 20/20 in the left. Fundus examination was remarkable for vitelliform lesions in both eyes (Figure 1, A and B). Optical coherence tomography (OCT) also showed vitelliform lesions in both eyes, with vitreomacular traction (VMT) in the right eye (Figure 1, C and D).
Figure 1.
Color fundus photograph of the (A) right and (B) left eyes at presentation, with vitelliform lesions and pigmentary changes in both maculae. Optical coherence tomography of the (C) right eye shows vitreomacular traction, whereas in (D) the left eye, only adhesion is observed. Subretinal deposits corresponding to the vitelliform lesions are present in both eyes.
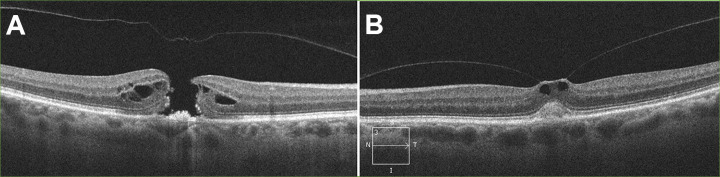
On follow-up 1 year later, the patient reported a new central scotoma in the right eye over the past month. Vision in the right eye was 20/250; the left eye was 20/25. Fundus examination and OCT were remarkable for a full-thickness MH in the right eye (Figure 2A). The left eye also had evidence of VMT (Figure 2B).
Figure 2.
(A) Optical coherence tomography of the right eye with full-thickness macular hole and release of vitreomacular traction. (B) Optical coherence tomography of the left eye with vitreomacular traction.
Pars plana vitrectomy (PPV) with indocyanine green (ICG) staining, internal limiting membrane (ILM) peeling, and 10% C3F8 (perfluoropropane) tamponade was performed 6 weeks later, yet the hole remained persistently open. A second PPV was performed 4 months later, and ICG staining confirmed complete ILM removal; 10% C3F8 was again used as tamponade. Postoperatively, the hole closed, yet the vision was 20/600, limited by cataract and macular atrophy (Figure 3).
Figure 3.
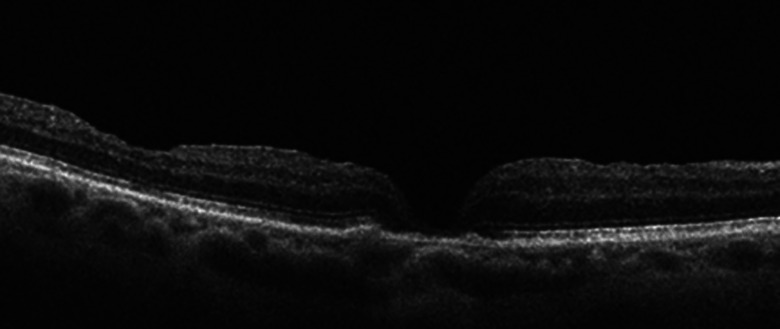
Postoperative optical coherence tomography of the right eye with a closed hole but retinal atrophy.
Two years following treatment in the right eye, the patient developed a MH in the left eye (Figure 4A), with declined vision of 20/60 and increased metamorphopsia.
Figure 4.
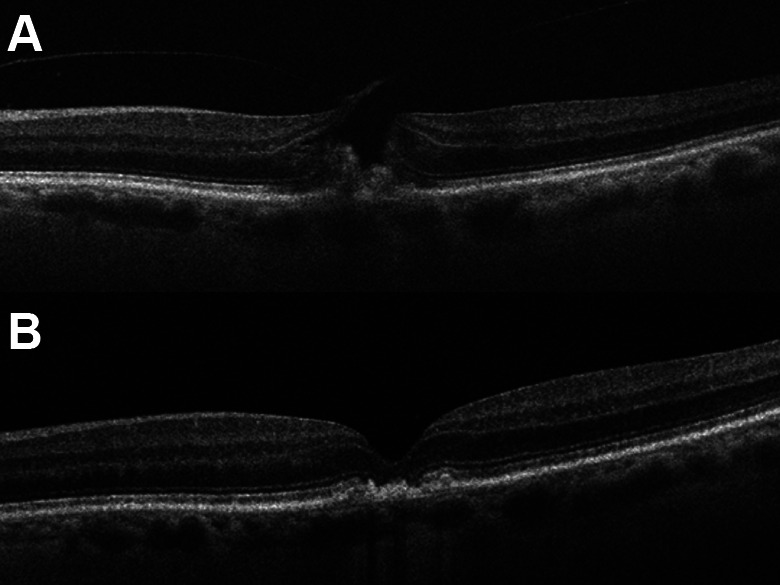
(A) Optical coherence tomography of the left eye with a full-thickness macular hole and persistent vitreomacular traction on the nasal side of the hole. A residual subretinal vitelliform lesion remains. (B) Postoperative optical coherence tomography with hole closure.
Surgical repair was performed 1 week later. After the outcome of RPE atrophy of the right eye, ICG toxicity was a concern, and therefore PPV was performed without dye staining or ILM peeling. The Tano Diamond Dusted Membrane Scraper (Synergetics, Inc) was gently applied to the edges of the hole to ensure absence of epiretinal membrane. Gas tamponade with 10% C3F8 was used. The hole was successfully closed after this surgery (Figure 4B), and vision improved to 20/30.
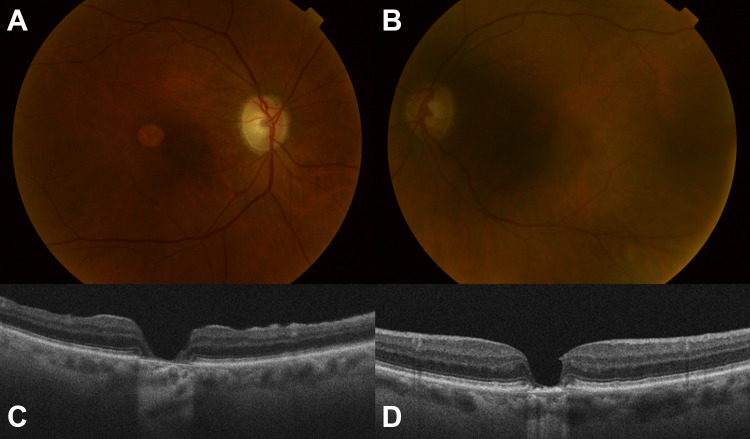
The patient was subsequently treated with cataract extraction of both eyes. At the most recent follow-up, 43 months following PPV of the right eye and 23 months following PPV of the left eye, vision in the right eye was 20/80 and the left was 20/40. Examination and OCT revealed closed holes in both eyes, with resolution of the vitelliform lesions but progressive RPE and outer retinal atrophy in both eyes (Figure 5).
Figure 5.
Color fundus photograph of the (A) right and (B) left eyes with atrophy of the retinal pigment epithelium subfoveally. Optical coherence tomography of the (C) right and (D) left eyes with closed holes but retinal and retinal pigment epithelium atrophy.
Conclusions
MH formation has been reported in AVMD by prior authors. Noble and Chang 3 reported a case of bilateral MHs; in 1 eye, hole formation was preceded by disappearance of the yellow material and associated with a posterior vitreous detachment. Goldberg and Freund 5 reported hole formation secondary to progressive retinal thinning with retinal and RPE atrophy. This observed progression contrasted with the preceding VMT seen in idiopathic MHs. These authors did not describe surgical repair or the clinical course of these holes, and the natural history without surgical repair is unknown.
AVMD-associated MH with surgical closure was reported by de Souza et al 4 , and it required 2 PPVs, the second using heavy silicone oil. Preoperative OCT in that case documented VMT to the nasal aspect of the hole. In both eyes of the present case, hole formation was preceded by VMT on OCT, suggesting that pathophysiology of these holes does have some overlap with idiopathic MHs rather than being a purely atrophic phenomenon. Galvin et al 8 also reported surgical repair of AVMD-associated MHs, with the successful use of a gas tamponade. In the present case, the right eye was followed for 43 months after surgical repair and to our knowledge is the longest reported follow-up of any AVMD-related MH after surgical repair. The duration of the previously longest-reported cases were 25 months by de Souza and colleagues 4 and Galvin et al. 8 Neither reported the RPE and outer retinal atrophy observed in our case.
MHs have also been described with other diseases with subretinal abnormalities, such as Best disease 9 and age-related macular degeneration. 6,7 Postoperative disappearance of drusen has been reported with surgical hole closure, postulated to occur secondary to activation of phagocytosis by macrophages. 6
In AVMD, the yellow deposits may regress with time, leaving a corresponding focal area of geographic atrophy indicative of underlying RPE damage. 2 Although ICG toxicity was a concern after the surgical experience of the first eye, the symmetrical subfoveal atrophy in the left eye indicates that the observed RPE changes may be a progression of the underlying AVMD. Alternatively, RPE changes may have been iatrogenic and have been reported after routine idiopathic MH repair, attributed to light toxicity or physical debridement during membrane peel. 7
Surgical repair of MHs in AVMD likely has a lower success rate compared with idiopathic MHs. The right eye presented in this report and in the case presented by de Souza et al 4 required 2 PPVs for hole closure. Likely the abnormal and atrophic RPE contributes to difficulty in hole closure. The recommendation of de Souza and colleagues 4 is thus to use longer-acting tamponade with silicone oil; however, the holes of both eyes in our case closed with C3F8 gas.
To our knowledge from a literature search, this is the first report of surgical repair of bilateral MHs from AVMD. This case is also unique in the degree of retinal and RPE atrophy observed postoperatively. Whereas the more significant RPE atrophy in the right eye after surgical repair may be due in part to ICG toxicity, the symmetrical outer retinal atrophy cannot be attributed to the ICG, which was not used in the left eye repair. Both eyes, however, benefited from surgery, and vitrectomy is still recommended despite possible progression of underlying disease.
Footnotes
Ethical Approval: This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner. Review by the Institutional Review Board of the University of Wisconsin is not required for case reports.
Statement of Informed Consent: Informed consent is not required because protected health information is not disclosed.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by an unrestricted grant from Research to Prevent Blindness.
ORCID iD: Kathleen A. Regan, MD  https://orcid.org/0000-0003-0504-3053
https://orcid.org/0000-0003-0504-3053
References
- 1. Gass JD. A clinicopathologic study of a peculiar foveomacular dystrophy. Trans Am Ophthalmol Soc. 1974;72:139–156. [PMC free article] [PubMed] [Google Scholar]
- 2. Arnold JJ, Sarks JP, Killingsworth MC, Kettle EK, Sarks SH. Adult vitelliform macular degeneration: a clinicopathological study. Eye (Lond). 2003;17(6):717–726. doi:10.1038/sj.eye.6700460 [DOI] [PubMed] [Google Scholar]
- 3. Noble KG, Chang S. Adult vitelliform macular degeneration progressing to full-thickness macular hole. Arch Ophthalmol. 1991;109(3):325. doi:10.1001/archopht.1991.01080030027024 [DOI] [PubMed] [Google Scholar]
- 4. de Souza CF, Polkinghorne PJ, Riley AF. Heavy silicone oil effective in macular hole surgery associated with adult vitelliform macular dystrophy. Clin Exp Ophthalmol. 2012;40(1):e111–e112. doi:10.1111/j.1442-9071.2011.02585.x [DOI] [PubMed] [Google Scholar]
- 5. Goldberg N, Freund KB. Progression of an acquired vitelliform lesion to a full-thickness macular hole documented by eye-tracked spectral-domain optical coherence tomography. Arch Ophthalmol. 2012;130(9):1221–1223. doi:10.1001/archophthalmol.2012.407 [DOI] [PubMed] [Google Scholar]
- 6. Dithmar S, Pollithy S, Ach T. Disappearance of central confluent soft drusen following vitrectomy and ILM peeling. Eye (Lond). 2013;27(6):779–781. doi:10.1038/eye.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaudhry NA, Flynn HW, Jr, Smiddy WE, Thompson JT. Macular hole surgery in the presence of prominent macular drusen. Arch Ophthalmol. 2000;118(1):131–132. [PubMed] [Google Scholar]
- 8. Galvin JC, Chua BE, Fung AT. Successful closure of full-thickness macular holes secondary to macular vitelliform lesions. Retin Cases Brief Rep. 2019;13(3):199–201. doi:10.1097/ICB.0000000000000566 [DOI] [PubMed] [Google Scholar]
- 9. Nourinia R, Roshandel D, Lima BS, Sayanjali S. Best disease associated with macular hole. Retin Cases Brief Rep. 2015;9(1):7–12. doi:10.1097/ICB.0000000000000068 [DOI] [PubMed] [Google Scholar]