Abstract
Purpose:
This work aims to evaluate the clinical utility and feasibility of a novel scanning laser ophthalmoscope-based navigated ultra-widefield swept-source optical coherence tomography (UWF SS-OCT) imaging system.
Methods:
A retrospective, single-center, consecutive case series evaluated patients between September 2019 and October 2020 with UWF SS-OCT (modified Optos P200TxE, Optos PLC) as part of routine retinal care. The logistics of image acquisition, interpretability of images captured, nature of the peripheral abnormality, and clinical utility in management decisions were recorded.
Results:
Eighty-two eyes from 72 patients were included. Patients were aged 59.4 ± 17.1 years (range, 8-87 years). During imaging, 4.4 series of images were obtained in 4.1 minutes, with 86.4% of the image series deemed to be diagnostic of the peripheral pathology on blinded image review. The most common pathologic findings were chorioretinal scars (18 eyes). In 31 (38%) eyes, these images were meaningful in supporting clinical decision-making with definitive findings. Diagnoses imaged included retinal detachment combined with retinoschisis, retinal hole with overlying vitreous traction and subretinal fluid, vitreous inflammation overlying a peripheral scar, Coats disease, and peripheral retinal traction in sickle cell retinopathy.
Conclusions:
Navigated UWF SS-OCT imaging was clinically practical and provided high-quality characterization of peripheral retinal lesions for all eyes. Images directly contributed to management plans, including laser, injection or surgical treatment, for a clinically meaningful set of patients (38%). Future studies are needed to further assess the value of this imaging modality and its role in diagnosing, monitoring, and treating peripheral lesions.
Keywords: imaging, ultra-widefield, optical coherence tomography, Optos, retinal holes, retinoschisis, retinal detachment
Introduction
Optical coherence tomography (OCT) was introduced in 1996 and has become the standard of care for various macular conditions, such as preretinal membranes, macular edema, choroidal neovascularization, and many others. 1 OCT has also served a critical research role to better characterize retinal disease pathophysiology, advancing the understanding and management of these entities. 2 Since its development, macular OCT scans have become an indispensable component of routine retinal practice.
Conventional OCT devices, however, are limited to imaging a relatively narrow posterior field. Many important vitreoretinal abnormalities encountered in routine vitreoretinal care are located in the retinal periphery, identifiable only on skilled peripheral retinal examination, or more recently with the use of 2-dimensional ultra-widefield (UWF) imaging. 3 Widefield imaging is used to image the retina up to a 50° field of view, whereas UWF-OCT is used to image up to 200° of retina out to the periphery. 4,5 There has been significant research and interest in the use of UWF devices to describe pathologic findings and guide therapy for diabetic retinopathy, sickle retinopathy, retinoschisis, and retinal detachment, among many other diseases. 6 -9
A recurring theme in retina practice, as illustrated by the examples of macular OCT and UWF, has been the implementation of new imaging technologies leading to novel discovery, classification, and thus paradigm shifts in practice patterns. With novel imaging one gains the ability to visualize new anatomic details in both normal and abnormal eyes, spurring discoveries and clinical progress. Recently, there has been increased emphasis on the application of OCT to the retinal periphery through the use of novel lenses, angling devices, or combinations of viewing systems. Although these approaches have increased peripheral views, they have not necessarily captured far peripheral images classified as UWF. 3,4,10,11
One of these combinations has included merging a scanning laser ophthalmoscope (SLO) fundus photograph using the Optos P200TxE (Optos PLC) imaging system with spectral domain (SD)–OCT (Heidelberg Engineering Co). 3 Other studies have used modifications or preexisting SD-OCT devices to allow further peripheral access (Cirrus, Carl Zeiss, and Heidelberg Engineering Co) 5 or have used time-domain OCT (Stratus 3, Carl Zeiss) with color fundus photographs of the periphery. 4 All these combinations produced good peripheral OCT images, but the clinical feasibility of routinely obtaining these images is questionable. The techniques require adequate dilation, clear media, high patient cooperation, sometimes imaging on 2 different machines, and a skilled photographic technician to angle the devices to obtain these peripheral images.
Recently, another device that combines UWF-SLO with a navigable swept-source (SS) OCT (Optos Silverstone, Optos PLC) has become commercially available. This device produces an initial high-resolution SLO fundus image up to 200° and then uses navigated SS-OCT line or volume scans of the far retinal periphery as mapped by UWF-SLO images. As with prior imaging modalities, over the coming years the implications of this new device for defining and monitoring peripheral retinal disease will become clearer. We sought to characterize the early implementation of UWF SS-OCT in routine clinical practice. The goals of the present study were to (1) describe the cohort of patients who underwent UWF SS-OCT imaging as part of routine clinical practice (ie, what anatomic features providers were seeking to image); (2) assess the logistics of image acquisition and image interpretability in these patients; and (3) determine which indications for imaging offered insightful clinical information about the specific abnormality at hand, either by changing follow-up duration or helping to guide clinical or surgical decision-making.
Methods
This was a retrospective, consecutive case series of patients who visited the retina service at Weill Cornell Department of Ophthalmology (New York, New York) between September 2019 and October 2020.
Patient Cohort and Data Collection
All patients who underwent UWF SS-OCT imaging in our department were included in the study. Eighty-two eyes from 72 patients underwent UWF-navigated SS-OCT of peripheral retinal findings at Weill Cornell Medicine Department of Ophthalmology. The device used was a modified Optos P200TxE (Optos PLC) with navigated SS-OCT capabilities (similar to the commercially available Optos Silverstone). Discretion to perform testing was made by the physician based on findings from either primary UWF-SLO imaging (performed with either the Optos California P200DTx or modified Optos P200TxE, Optos PLC) or indirect dilated fundus examination. Demographic information was recorded for all patients, as well as ocular history, diagnoses, treatment, and interpretation of UWF SS-OCT imaging. A note was made in the patient's record whether imaging findings helped guide management decisions for that visit.
Image Acquisition and Processing
All patients were dilated prior to images being obtained as part of routine practice of the vitreoretinal specialists at our center. On installation of the imaging device, photographers received instructions from the manufacturer as to how to navigate and capture images, but no distinct skill acquisition was needed beyond the initial device training and familiarity with using the Optos UWF imaging systems. A single provider (attending, fellow, or resident) and photographer interaction took place in which the relevant lesion was indicated to the photographer on a prior UWF-SLO image. The provider was not necessarily present for the duration of image acquisition (but sometimes was out of interest in the device).
The modified Optos P200TxE captured SLO fundus images up to 200° of the retina, and the ophthalmic photographer then used this image to navigate a 2-dimensional line marked on top of the required peripheral abnormality that was captured with SS-OCT. Both steps were performed while the patient was seated on the same device. Targets for SS-OCT could be refined and further identified and illustrated to the photographer by the practitioner on the UWF-SLO images if there were concerns about the correct lesion being captured. Both individual line raster scans and volume series were obtained in all patients. Images were evaluated for their interpretability. Just as with routine UWF images, the UWF SS-OCT images were digitally transferred to the imaging server accessible for review with patients.
In a blinded fashion, reviewers (K.D.K. and M.A.M.) evaluated all images from a UWF SS-OCT session for (1) number of imaging series (both series of line rasters and volume series) acquired for each patient (“image series obtained”); (2) number of series that captured interpretable retinal tissue with adequate signal strength for evaluation and no significant mirror inversions (“interpretable series”); and (3) number of series that captured the lesion in question and were diagnostic of the peripheral retinal lesion (“diagnostic series”). The duration of UWF SS-OCT image acquisition in minutes was also recorded based on the time between the first and last images’ time stamps. Given the nature of this retrospective, descriptive case series, statistical analysis was not performed.
Results
UWF-navigated SS-OCT images were obtained on 82 eyes of 72 patients. The average age was 59.4 ± 17.1 years with a range from 8 to 87 years. All UWF SS-OCT images to be included in this series were captured with sufficient quality and resolution, as deemed by the evaluating physician. The most common abnormalities imaged were chorioretinal scars. Other abnormalities that were imaged included retinoschisis, peripheral drusen, retinal detachments, retinal holes, lattice degeneration, and choroidal nevi (Table 1). Some eyes had more than 1 peripheral finding imaged. For the purposes of analysis, the results demonstrate the primary abnormality imaged for a given eye.
Table 1.
Peripheral Retinal Abnormalities Imaged With Ultra-Widefield Swept-Source Optical Coherence Tomography in Routine Clinical Practice at a Tertiary Center.
| Peripheral abnormality | Eyes | Guided management (%)a | Received treatment (%)b |
|---|---|---|---|
| Chorioretinal scar | 18 | 0 | 0 |
| Retinoschisis | 13 | 8 (62) | 1 (8) |
| Drusen | 13 | 0 | 0 |
| Retinal hole/tear | 9 | 5 (56) | 3 (30) |
| Retinal detachment | 3 | 3 (100) | 2 (66) |
| Sickle cell retinopathy with focal TRD | 1 | 1 (100) | 1 (100) |
| Coats disease | 1 | 0 | 0 |
| Hemorrhage | 4 | 2 (50) | 2 (50) |
| Retinoschisis with retinal detachment | 4 | 4 (100) | 3 (75) |
| Lattice | 4 | 2 (50) | 0 |
| Nevus | 3 | 3 (100) | 0 |
| White without pressure | 3 | 0 | 0 |
| Reticular degeneration | 2 | 0 | 0 |
| CMV retinitis | 2 | 2 (100) | 1 (50) |
| Torpedo maculopathy | 1 | 0 | 0 |
| RP, bone spicules | 1 | 0 | 0 |
| Total | 82 | 31 (38) | 13 (16) |
Abbreviations: CMV, cytomegalovirus; RP, retinitis pigmentosa; TRD, traction retinal detachment.
aEyes for which the imaging helped guide or support a diagnosis, planned follow-up period, or a decision for treatment. bPatients who ultimately received intervention (including laser, injection, or surgery) in part based on or supported by imaging findings.
Patients had on average 4.4 OCT series obtained per imaging session, including both individual rasters and volume cubes (Table 2). Of this total, an average of 4.0 series (90.9%) demonstrated images that had substantial signal strength to be interpretable and were without substantial artifact. On average 3.8 series (86.4%) captured the peripheral retinal lesion of interest in sufficient quality to inform the diagnosis by a masked reviewer. Based on the elapsed time between the first and last images captured, image acquisition took approximately 4.1 minutes. Four patients had serial examinations with UWF SS-OCT; on average 229.5 days elapsed between examinations.
Table 2.
Mean Values of Image Acquisition and Interpretability Parameters.
| Image series obtained per session | 4.4 (range, 1-12) |
| Interpretable series per session | 4.0 (90.9%) |
| Diagnostic series per session | 3.8 (86.4%) |
| Image acquisition time, min | 4.1 (range, 1-12) |
| Patients with serial examinations | 4 |
| Average time between examinations, d | 229.5 (range, 28-434) |
A review of the medical records and acquired images showed that in 31 of the 82 eyes (38%), the UWF SS-OCT images were deemed clinically significant and supported decision-making regarding follow-up time, became the standard to monitor the peripheral findings over time, or helped confirm the decision for intervention (laser, injection, or surgery). One condition monitored in this fashion was retinoschisis with associated retinal detachment, laser barricade, chronic subretinal fluid, or demarcation lines. In these cases, UWF-OCT images helped to establish a baseline of the fluid level to compare on subsequent visits. Other cases included nevi with overlying drusen, minimal elevation, and absence of subretinal fluid, which could also be measured accurately and serially imaged to ensure stability. In the cases of these nevi, review of prior ultrasound measurements noted the choroidal lesions to be “flat” without significant discernable thickness, whereas slight thickening was measurable on UWF-OCT.
In 13 of 82 eyes (16%) imaging helped definitively confirm the decision to proceed with treatment, including laser, injections, or surgery. These most commonly included retinal breaks with vitreous traction, with or without subretinal fluid, which were treated with laser barricade. One eye included sickle cell retinopathy with a small, localized traction retinal detachment that underwent laser barricade, as well as panretinal photocoagulation. Two of these cases included retinoschisis with adjacent retinal detachment that, after confirmation with UWF SS-OCT, were taken to the operating room and treated with pars plana vitrectomy and a scleral buckle.
Conclusions
The present study demonstrated the clinical utility and feasibility of UWF SS-OCT in routine clinical practice at an academic retinal practice. The UWF SS-OCT images were readily interpretable and often held bearing on medical decision-making, often supporting or confirming clinical assessments. UWF SS-OCT has the potential to fundamentally change retinal practice by redefining commonly encountered peripheral retinal abnormalities with definitive assessments, adding a new means of disease-monitoring that can ultimately minimize the subjectivity of indirect ophthalmoscopy. However, it is a new imaging modality that has not been previously assessed regarding specific diseases in clinical practice. All images obtained by the ophthalmic photographers were interpretable by the treating physician. Most images obtained during the examinations (86.4%) were interpretable and diagnostic of the peripheral retinal lesions. Furthermore, 38% of the images helped guide follow-up plans or confirm management decisions with definitive objective evidence. To our knowledge, this is one of the first studies to delineate these clinical features of this novel imaging device.
Our study demonstrated specific areas where UWF SS-OCT imaging provided insight that directly affected management decisions. One obvious area was in differentiating retinoschisis from retinal detachment, or monitoring retinal detachment associated with retinoschisis. Prior UWF studies have used tools like autofluorescence to aid in differentiating retinal detachment from retinoschisis. 6,12 -14 However, UWF SS-OCT appears to provide the most definitive evidence of splitting vs detachment by more directly observing intrinsic retinal layers and subsequent retinoschisis cavities on cross-section (Figure 1). Likewise, sometimes on clinical examination degenerative intraretinal cystic changes overlying retinal detachment can mimic the appearance of retinoschisis, which can also readily be assessed with cross-sectional visualization from OCT (Figure 2). Previously, these have been described as macrocysts when they become apparent enough to be visualized on ophthalmoscopy or ultrasonography; however, there is likely a spectrum of degenerative cystic changes yet to be elucidated with OCT that exists in overlying chronic retinal detachments. 15 -17
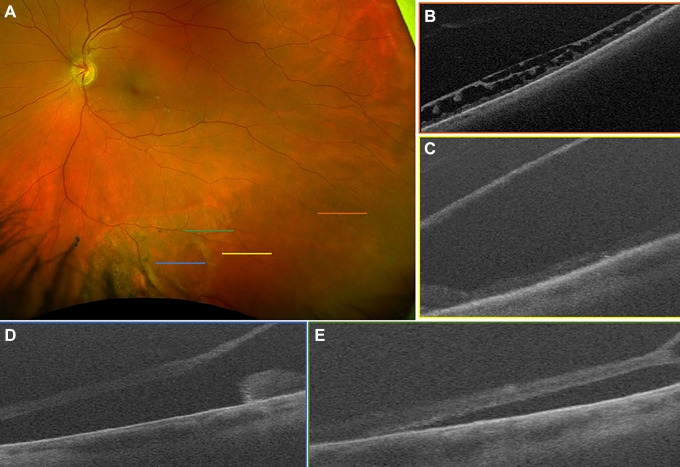
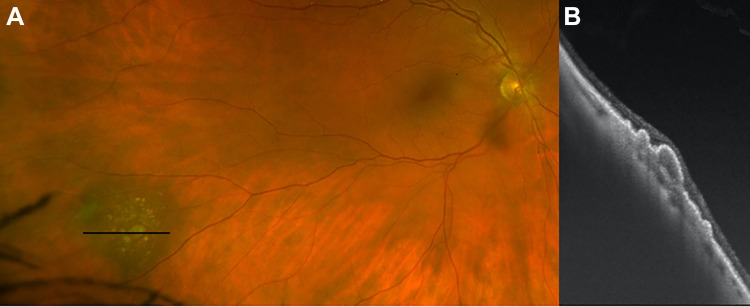
Figure 1.
(A) Scanning laser ophthalmoscope image demonstrating inferior retinoschisis with large cavity, outer retinal break, and associated retinal detachment. No inner retinal break was identified on examination. (B) Shallow retinoschisis, located temporally and correlating with the orange raster marker in (A), was noted on ultra-widefield swept-source optical coherence tomography. (C) A more bullous retinoschisis cavity was clearly demonstrated to correlate with the yellow raster marker in (A). (D) An outer retinal break with rolled margins was captured with the blue raster marker in (A), with (E) subretinal fluid nasal and just posterior (green raster marker in [A]) to the retinoschisis cavity. The patient has been observed without progression for 6 months.
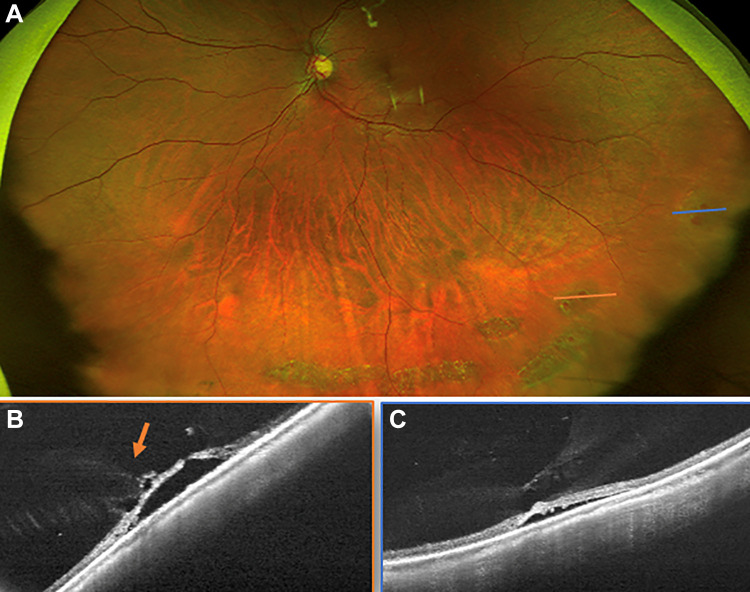
Figure 2.
(A) Ultra-widefield swept-source optical coherence tomography (UWF SS-OCT) images used to confirm the presence of asymptomatic retinoschisis. (B) Occasionally, subretinal fluid cuffs adjacent to an area of retinoschisis can be diagnosed and monitored with serial UWF SS-OCT images. Patients in both (A) and (B) were asymptomatic. (C) The appearance was distinct from subretinal fluid (arrow) in combined retinoschisis and retinal detachment in a symptomatic patient with acute peripheral vision loss. (D) Degenerative retinal cyst in an area of chronically detached retina can sometimes clinically be mistaken for retinoschisis; however, it can definitively be identified with UWF SS-OCT.
Another area where UWF SS-OCT proved to be clinically useful was in assessing the presence of subretinal fluid and vitreous traction on retinal breaks, particularly in the setting of lattice degeneration (Figures 3 -5). While the overall incidence of atrophic hole and lattice-associated retinal detachment is low, these lesions are present in a disproportionate number of patients with rhegmatogenous retinal detachment. 18 -20 As such, one avenue for future research would be to determine which patients with atrophic holes and lattice are at higher risk for developing rhegmatogenous detachments. UWF SS-OCT appeared to clearly demonstrate which lesions exhibited active traction as opposed to scarred margins of the break, in the present study informing physicians' decisions in the present study as to whether to apply prophylactic laser barrier retinopexy.
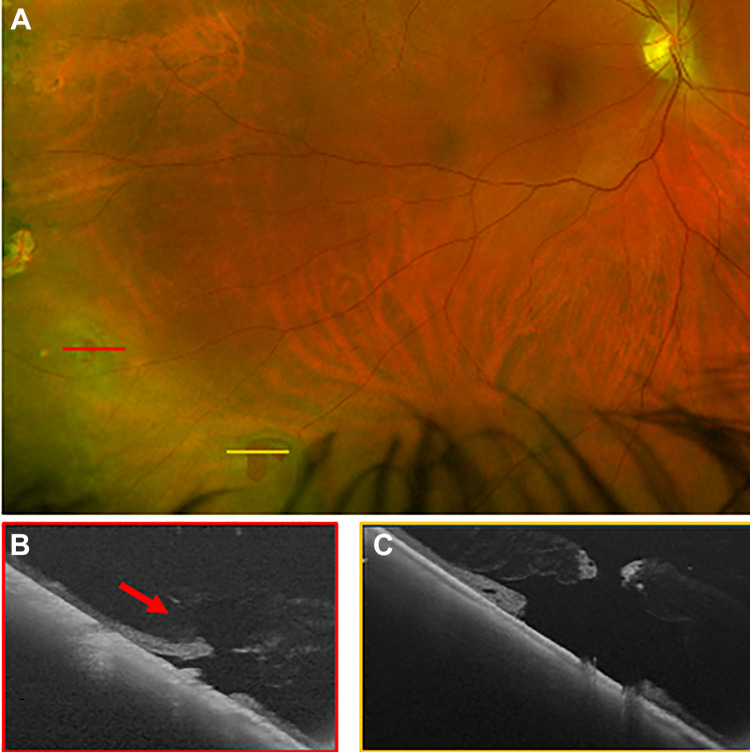
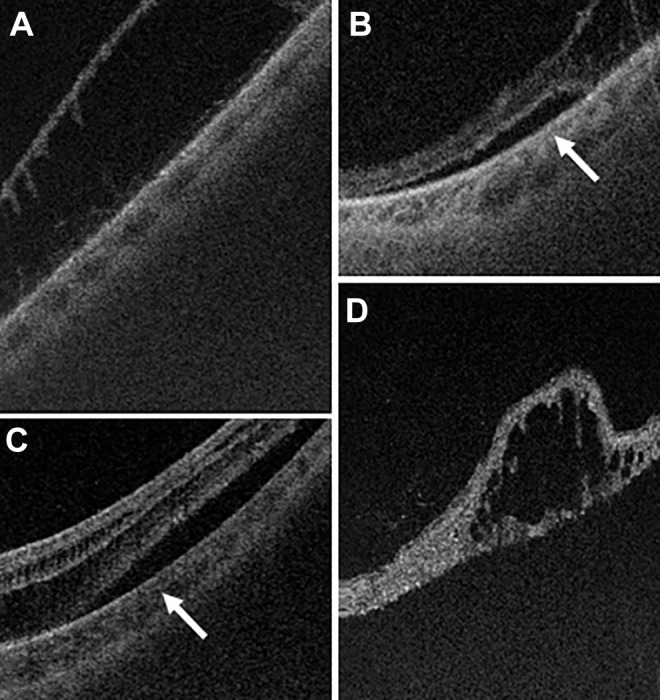
Figure 3.
(A) A scanning laser ophthalmoscope image demonstrating inferior lattice and retinal holes. The orange line shows the location of the (B) ultra-widefield swept-source optical coherence tomography raster through an atrophic hole, which confirmed the hole had subretinal fluid and vitreous traction (orange arrow). The blue line in (A) shows the area of (C) an ultra-widefield swept-source optical coherence tomography through a temporal retinal hole, which also confirmed vitreous traction with associated subretinal fluid. The decision to perform prophylactic laser barrier retinopexy was made based on the clinical appearance of holes with fluid in a patient with symptoms and was supported by the findings that confirmed vitreous traction on the holes’ margins on imaging.
Figure 4.
(A) A scanning laser ophthalmoscope image with inferotemporal retinal breaks. The red line shows the location of (B) an ultra-widefield swept-source optical coherence tomography raster through the superior tear, which had vitreous traction (red arrow). The yellow line in (A) shows the location of (C) an ultra-widefield swept-source optical coherence tomography raster through an inferior retinal break that had a flat retina without flap, subretinal fluid, or vitreous traction. Imaging supported the clinical decision for treatment of the symptomatic retinal break with persistent vitreous traction. In this case the second break was also treated at the same time because laser was already being performed and the patient was acutely symptomatic.
Figure 5.
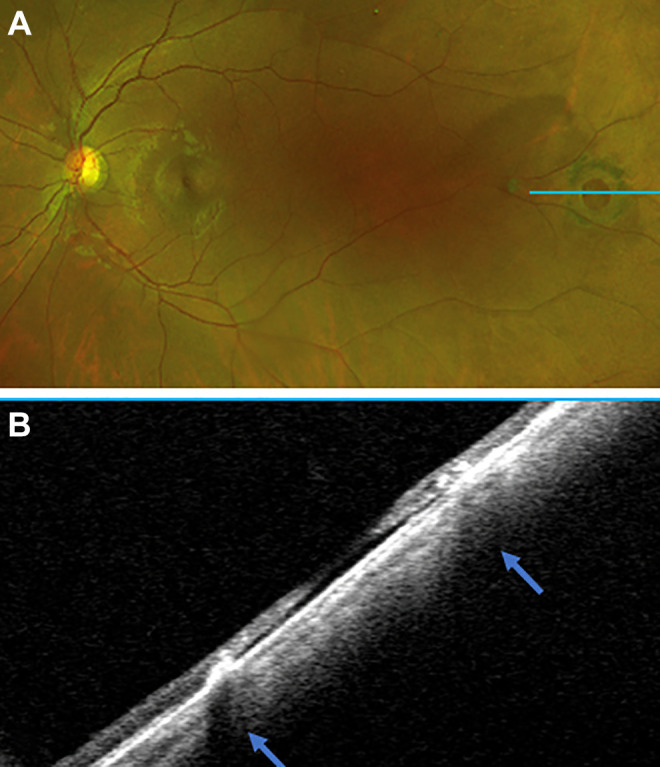
(A) A scanning laser ophthalmoscope image showing an old, scarred down, pigmented retinal hole with an optical coherence tomography scan through the blue line. (B) An ultra-widefield swept-source optical coherence tomography scan demonstrating a flat retinal hole with no traction and scarred down edges (arrows). Supported by the imaging findings, the patient was observed without intervention.
One patient (Figure 4) had laser applied around both new symptomatic breaks, 1 with and 1 without traction on UWF SS-OCT, given the absence of longitudinal studies on such lesions. An interesting variant of the peripheral vitreous traction lesion is that of a peripheral traction retinal detachment in the setting of peripheral neovascularization. One patient with sickle cell retinopathy in our cohort demonstrated a small peripheral traction detachment that aided the clinical decision to barricade around the neovascular frond and area of traction rather than just ablate the peripheral ischemic tissue. Previous studies have attempted to describe some of these peripheral lesions using SD-OCT and time-domain OCT but not with UWF imaging systems nor in a longitudinal fashion. 21 Further natural history studies with UWF SS-OCT are certainly warranted to better understand the prognosticative implications of our findings.
UWF SS-OCT was also used to assess activity of areas of cytomegalovirus (CMV) retinitis by looking for overlying vitreous inflammatory cells or new areas of retinitis adjacent to retinal scars (Figure 6). A prior study has assessed the OCT findings of the choroid, retina, and vitreous with CMV retinitis, but the authors noted that these scans were limited to the posterior pole despite many CMV lesions having been present in the retinal periphery. 22 As with many of the other aforementioned entities, UWF imaging, specifically autofluorescence, can also play a role in monitoring and tracking changes that may be complementary to the ability of OCT to demonstrate subtle overlying inflammatory or intrinsic changes in the retinal anatomy. 23 -25 It is reasonable to expect that UWF SS-OCT may also prove of value in other peripheral inflammatory diseases; however, those were not imaged in the present study.
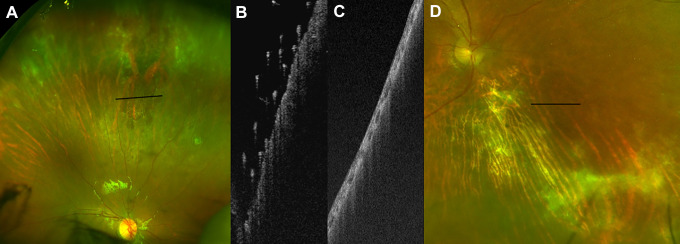
Figure 6.
(A) Color image of retinal periphery demonstrating an active cytomegalovirus lesion. (B) Ultra-widefield swept-source optical coherence tomography of lesion, with preretinal hyperreflective foci suggestive of disease activity. (C) Ultra-widefield swept-source optical coherence tomography and (D) color images of a different patient with an old, inactive cytomegalovirus lesion that had no preretinal hyperreflective foci, suggesting absence of activity.
UWF SS-OCT also demonstrated incidental findings of shallow retinoschisis vs degenerative cystoid degeneration in many of the patients imaged (Figure 7). Often these patients were being imaged for other indications, or in some cases the physicians suspected these mild changes based on a clinical examination or review of color images. Future studies will need to better characterize the incidence of these changes in a normal population and their clinical significance.
Figure 7.
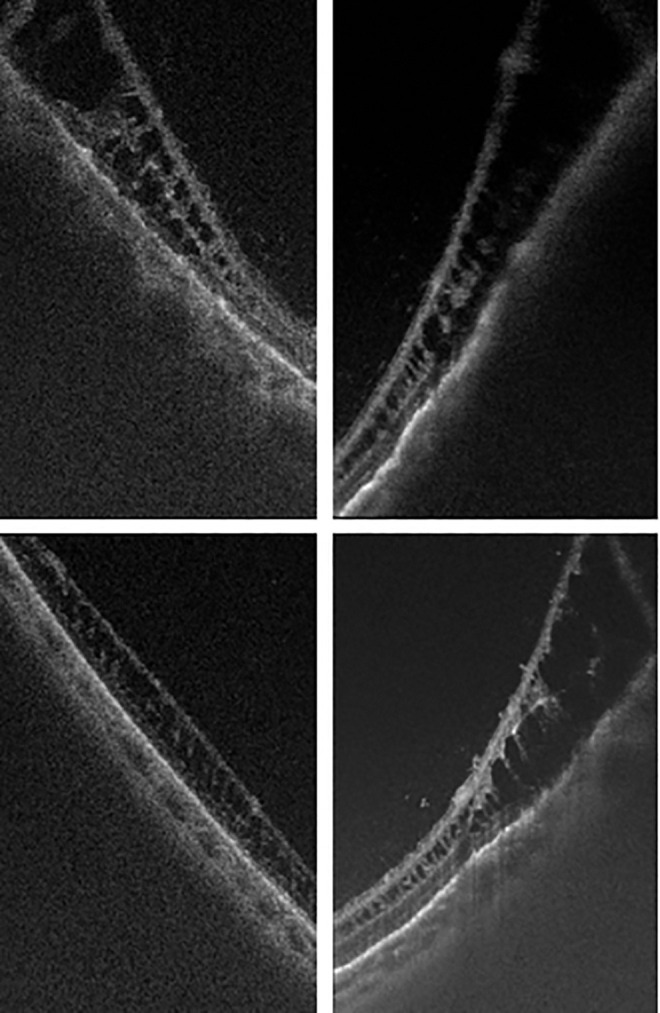
Ultra-widefield swept-source optical coherence tomography images of various peripheral retinoschisis and cystoid degeneration found in asymptomatic patients.
Finally, one other area of use was in screening melanotic choroidal lesions (nevi) for signs indicative of melanoma (Figure 8). Recent recommendations have stressed the importance of imaging in determining choroidal melanoma from nevus, in addition to or in replacement of other traditional markers. 26,27 UWF SS-OCT (in conjunction with other imaging modalities such as UWF autofluorescence) offers the means to determine the presence of overlying drusen or subretinal fluid. Alternatively, it may provide a sensitive means for measuring lesion thickness, as the nevi in our cohort were all noted to be flat on ultrasonography but had some discernable thickness on UWF-OCT. However, it is worth noting that these peripheral thickness measurements have not yet been validated for reliability or repeatability.
Figure 8.
(A) A color image demonstrating a peripheral choroidal nevus with overlying drusen. (B) Ultra-widefield swept-source optical coherence tomography of a peripheral choroidal nevus with minimal thickness, overlying drusen, and no subretinal fluid.
There are several limitations to the present study. First, this is a retrospective review of cases from routine clinical practice, and it was not designed to study specific patient cohorts. As such, there are limitations to the conclusions that can be ascertained about specific disease entities and their appearance on UWF SS-OCT. Our sample size is relatively small compared with the overall volume of a retina practice, which may reflect the time required for additional testing or the lack of incorporation into routine practice patterns. Most of the patients were collected from tertiary referral retinal practices, which could lead to ascertainment bias regarding the nature and frequency of the peripheral abnormalities included in this study. There is currently no normative baseline study that delineates findings with this technology, and as such the findings in this study could reflect either underestimation or overestimation of “normal” findings.
Furthermore, although this was a consecutive case series, we did not keep track of patients who had examination findings in areas that were judged by the examiner to be inaccessible by the imaging device. These would likely be superior or inferior lesion locations where the UWF device is more limited because of lid artifacts. While no specific case was recalled by the study providers, there is likely inclusion bias that conditions captured on UWF-SLO were more likely to be included and thus more likely to have interpretable images obtained. Certainly additional studies with larger cohorts and longer follow-up times will be necessary to more accurately delineate findings in specific diseases with UWF SS-OCT. Only 4 patients in our cohort had serial examinations, and there was a wide range of interval periods from 28 to 434 days.
While the time of image acquisition was objectively brief, we measured this based on the time stamps in the image review software between first and last images for each patient. This metric does not include the time that would have been required to position the patient at the device or to generate the UWF-SLO map to begin targeted UWF SS-OCT. As such, actual image acquisition time would be longer than reported in this study. Furthermore, in our practice the physician (who could be the resident, fellow, or attending) did not necessarily need to be present during image acquisition; however, a physician did need to directly indicate to the photographer the target lesion of interest on an UWF-SLO image. Even this type of interaction may not be practical in nonacademic clinical practices. It is worth noting that for some patients (a minority during the early use of the device, but the exact number was not tracked), a physician was present during the image acquisition, which would likely contribute to a higher interpretability and yield compared with a photographer alone.
In summary, there was a wide spectrum of peripheral findings readily imaged with UWF SS-OCT that yielded clinically relevant images that supported and confirmed management decisions. In the present study we sought to (1) describe the cohort of patients who underwent UWF SS-OCT imaging as part of routine clinical practice (ie, what anatomic features providers were seeking to image); (2) assess the logistics of image acquisition and image interpretability in these patients; and (3) determine which indications for imaging offered insightful clinical information about the specific abnormality at hand, either by changing follow-up duration or helping to confirm clinical or surgical decision-making. Our study demonstrated the utility and feasibility of UWF SS-OCT in real-world practice. Images were successfully acquired in all patients and contributed to clinical management and decision-making in 38% of patients. There was a particularly meaningful role for UWF SS-OCT in the clinical management of retinoschisis and retinal breaks.
The study has a number of strengths, including the use of 1 instrument to obtain the UWF SS-OCT images, conservation of image acquisition protocol, and the yield of high-quality interpretable images in all patients. Further studies are needed to monitor peripheral retinal abnormalities, in particular to delineate incidental subclinical findings from those with prognostic implications.
Footnotes
Authors’ Notes: K.D.K. and M.A.M. contributed equally to this manuscript and study, and are co-first authors.
The content of this manuscript was presented at the virtual annual meeting of the American Academy of Ophthalmology on November 14 and 15, 2020.
Ethical Approval: This case report was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and all federal and state laws. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner. The study protocol was reviewed and approved by the institutional review board at Weill Cornell Medical College (New York, New York).
Statement of Informed Consent: Informed consent for the submission of this report was deferred because the patients were not identifiable from any of the collected images and out of concern for placing undue stress on the patients. This was a retrospective medical record review.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.K. declared consulting and research funding from Optos (research funding was not received for the development of this study). He has also served as a consultant for Adverum, Alcon, Novartis, and Genentech/Roche; received research funding from Allergan, Novartis, Genentech/Roche, and Regeneron; received equity from Adverum, Regenxbio, and Fortress Bio; and intellectual property—gene therapy for age-related macular degeneration, and T cells for CMV retinitis—assigned to Cornell University. K.D.K. has served as a consultant for Regenxbio. D.J.D. has served as a consultant for Alcon, Iveric bio, and Aufbao Holdings; received equity from Iveric bio and Aufbau Holdings; and holds intellectual property for Aufbau Holdings. The other authors have nothing to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported, in part, by an unrestricted department grant (Weill Cornell Medicine Department of Ophthalmology) from Research to Prevent Blindness, Inc.
ORCID iD: Kyle D. Kovacs, MD  https://orcid.org/0000-0001-7568-6703
https://orcid.org/0000-0001-7568-6703
References
- 1. Fujimoto J, Swanson E. The development, commercialization, and impact of optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT1–OCT13. doi:10.1167/iovs.16-19963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujimoto JG, Brezinski ME, Tearney GJ, et al. Optical biopsy and imaging using optical coherence tomography. Nat Med. 1995;1(9):970–972. doi:10.1038/nm0995-970 [DOI] [PubMed] [Google Scholar]
- 3. Fogliato G, Borrelli E, Iuliano L, et al. Comparison between ultra-widefield pseudocolor imaging and indirect ophthalmoscopy in the detection of peripheral retinal lesions. Ophthalmic Surg Lasers Imaging Retina. 2019;50(9):544–549. doi:10.3928/23258160-20190905-02 [DOI] [PubMed] [Google Scholar]
- 4. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: recommendations from the International Widefield Imaging Study Group. Ophthalmol Retina. 2019;3(10):843–849. doi:10.1016/j.oret.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 5. Choudhry N, Golding J, Manry MW, Rao RC. Ultra-widefield steering-based SD-OCT imaging of the retinal periphery. Ophthalmology. 2016;123(6):1368–1374. doi:10.1016/j.ophtha.2016.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nadelmann JB, Gupta MP, Kiss S, et al. Ultra-widefield autofluorescence imaging of retinal detachment compared to retinoschisis. Ophthalmic Surg Lasers Imaging Retina. 2019;50(9):550–556. doi:10.3928/23258160-20190905-03 [DOI] [PubMed] [Google Scholar]
- 7. Fan W, Wang K, Ghasemi Falavarjani K, et al. Distribution of nonperfusion area on ultra-widefield fluorescein angiography in eyes with diabetic macular edema: DAVE study. Am J Ophthalmol. 2017;180:110–116. doi:10.1016/j.ajo.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 8. Nagiel A, Lalane RA, Sadda SR, Schwartz SD. Ultra-widefield fundus imaging: a review of clinical applications and future trends. Retina. 2016;36(4):660–678. doi:10.1097/IAE.0000000000000937 [DOI] [PubMed] [Google Scholar]
- 9. Han IC, Zhang AY, Liu TYA, Linz MO, Scott AW. Utility of ultra-widefield retinal imaging for the staging and management of sickle cell retinopathy. Retina. 2019;39(5):836–843. doi:10.1097/IAE.0000000000002057 [DOI] [PubMed] [Google Scholar]
- 10. Kothari A, Narendran V, Saravanan VR. In vivo sectional imaging of the retinal periphery using conventional optical coherence tomography systems. Indian J Ophthalmol. 2012;60(3):235–239. doi:10.4103/0301-4738.95885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chu RL, Pannullo NA, Adam CR, Rafieetary MR, Sigler EJ. Morphology of peripheral vitreoretinal interface abnormalities imaged with spectral domain optical coherence tomography. J Ophthalmol. 2019;2019:3839168. doi:10.1155/2019/3839168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navaratnam J, Salvanos P, Vavvas DG, Bragadóttir R. Ultra-widefield autofluorescence imaging findings in retinoschisis, rhegmatogenous retinal detachment and combined retinoschisis retinal detachment. Acta Ophthalmol. Published online June 29, 2020. doi:10.1111/aos.14521 [DOI] [PubMed] [Google Scholar]
- 13. Thanos A, Todorich B, Pasadhika S, et al. Degenerative peripheral retinoschisis: observations from ultra-widefield fundus imaging. Ophthalmic Surg Lasers Imaging Retina. 2019;50(9):557–564. doi:10.3928/23258160-20190905-04 [DOI] [PubMed] [Google Scholar]
- 14. Francone A, Kothari N, Farajzadeh M, et al. Detection of neurosensory retinal detachment complicating degenerative retinoschisis by ultra-widefield fundus autofluorescence imaging. Retina. 2020;40(5):819–824. doi:10.1097/IAE.0000000000002488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verdaguer P, Nadal J. Intraretinal cyst secondary to longstanding retinal detachment. Eur J Ophthalmol. 2012;22(3):506–508. doi:10.5301/ejo.5000034 [DOI] [PubMed] [Google Scholar]
- 16. Marcus DF, Aaberg TM. Intraretinal macrocysts in retinal detachment. Arch Ophthalmol. 1979;97(7):1273–1275. doi:10.1001/archopht.1979.01020020015003 [DOI] [PubMed] [Google Scholar]
- 17. Labriola LT, Brant AM, Eller AW. Chronic retinal detachment with secondary retinal macrocyst and peripheral neovascularization. Semin Ophthalmol. 2009;24(1):2–4. doi:10.1080/08820530802508561 [DOI] [PubMed] [Google Scholar]
- 18. Byer NE. Long-term natural history of lattice degeneration of the retina. Ophthalmology. 1989;96(9):1396–1401. doi:10.1016/s0161-6420(89)32713-8 [DOI] [PubMed] [Google Scholar]
- 19. Winslow RL, Tasman W. Juvenile rhegmatogenous retinal detachment. Ophthalmology. 1978;85(6):607–618. doi:10.1016/s0161-6420(78)35641-4 [DOI] [PubMed] [Google Scholar]
- 20. Folk JC, Arrindell EL, Klugman MR. The fellow eye of patients with phakic lattice retinal detachment. Ophthalmology. 1989;96(1):72–79. doi:10.1016/s0161-6420(89)32926-5 [DOI] [PubMed] [Google Scholar]
- 21. Kothari A, Narendran V, Saravanan VR. In vivo sectional imaging of the retinal periphery using conventional optical coherence tomography systems. Indian J Ophthalmol. 2012;60(3):235–239. doi:10.4103/0301-4738.95885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Invernizzi A, Agarwal A, Ravera V, Oldani M, Staurenghi G, Viola F. Optical coherence tomography findings in cytomegalovirus retinitis: a longitudinal study. Retina. 2018;38(1):108–117. doi:10.1097/IAE.0000000000001503 [DOI] [PubMed] [Google Scholar]
- 23. Tadepalli S, Bajgai P, Dogra M, et al. Ultra-widefield fundus autofluorescence in cytomegalovirus retinitis. Ocul Immunol Inflamm. 2020;28(3):446–452. doi:10.1080/09273948.2019.1595671 [DOI] [PubMed] [Google Scholar]
- 24. Liscombe-Sepúlveda JP, Alba-Linero C, Llorenç-Belles V, Adán-Civera A. Utility of ultra-widefield retinal imaging in the follow-up and management of patients with cytomegalovirus retinitis. Ocul Immunol Inflamm. 2020;28(4):659–664. doi:10.1080/09273948.2019.1606257 [DOI] [PubMed] [Google Scholar]
- 25. Mudvari SS, Virasch VV, Singa RM, MacCumber MW. Ultra-wide-field imaging for cytomegalovirus retinitis. Ophthalmic Surg Lasers Imaging. 2010;41(3):311–315. doi:10.3928/15428877-20100430-03 [DOI] [PubMed] [Google Scholar]
- 26. Shields CL, Dalvin LA, Ancona-Lezama D, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: the 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39(10):1840–1851. doi:10.1097/IAE.0000000000002440 [DOI] [PubMed] [Google Scholar]
- 27. Dalvin LA, Shields CL, Ancona-Lezama DA, et al. Combination of multimodal imaging features predictive of choroidal nevus transformation into melanoma. Br J Ophthalmol. 2019;103(10):1441–1447. doi:10.1136/bjophthalmol-2018-312967 [DOI] [PubMed] [Google Scholar]