Abstract
Purpose:
This work aims to review the principles of optical coherence tomography angiography (OCTA), to survey its clinical utility, and to highlight the strengths of this technology as well as barriers to adoption.
Methods:
A literature review with editorial discussion of the current applications for OCTA is presented.
Results:
There have been recent advances in multiple domains in OCTA imaging, including devices, algorithms, and new observations pertaining to a range of pathologies. New devices have improved the scanning speed, signal-to-noise ratio, and spatial resolution and offer an increased field of view. New algorithms have been proposed to optimize image processing and reduce artifacts. Numerous studies employing OCTA have been published describing changes to the microvasculature in diabetic retinopathy, age-related macular degeneration, central serous chorioretinopathy, retinal vein occlusion, and uveitis.
Conclusions:
OCTA provides noninvasive, high-resolution volumetric scans of the retinal and choroidal vasculature. OCTA can provide valuable data to augment traditional dye-based angiography in a range of chorioretinal diseases.
Keywords: optical coherence tomography angiography
Introduction
Optical coherence tomography angiography (OCTA) is a noninvasive imaging modality that uses amplitude or phase decorrelation to detect blood flow without intravenous (IV) dye administration. 1 Before the advent of OCTA, visualization of the retinal or choroidal microvasculature required IV delivery of a dye for fluorescein angiography (FA) or indocyanine green angiography (ICGA). Although the images acquired by traditional angiography methods have certain advantages, such as the ability to detect leakage, OCTA is differentiated as a clinical tool by its generation of depth-resolved images of the retinal capillary plexuses, rather than 2-dimensional en face views. 2 Furthermore, OCTA is quicker and obviates the potential side effects associated with IV dye administration. Despite an ever-increasing body of research demonstrating the clinical utility of OCTA, the technology has not been universally adopted. This article reviews the strengths and weaknesses of OCTA, highlights its clinical value in various retinal and uveitic pathologies, and delineates its future opportunities and the barriers to its adoption.
Methods
The literature search for this review was conducted using PubMed and Google Scholar. The primary search included papers published from 2015 to 2021, with other select articles of high relevance added for completeness. The following search terms were included: optical coherence tomography angiography, diabetic retinopathy, age-related macular degeneration, central serous chorioretinopathy, macular telangiectasia, retinal vein occlusion, and uveitis. Original articles and reviews were included.
Results
Principles of Optical Coherence Tomography Angiography
OCTA was developed from optical coherence tomography (OCT) imaging, a technology that has revolutionized ophthalmology over the past 30 years. 3 OCT uses low-coherence interferometry to produce structural images from the optical scattering of back-reflected light. A-scans (or axial scans) are produced by the interference patterns of a single beam of light, and provide information regarding the depth of a structure within the eye as well as its propensity to reflect or scatter light. 4 B-scans (or cross-sectional scans) are generated by the sequential acquisition of numerous A-scans along a linear path. Volumetric data scans are acquired by performing multiple B-scans along the plane perpendicular to the B-scan image, thereby providing a raster scan.
The speed and resolution of OCT imaging have improved significantly over the years. Spectral-domain OCT (SD-OCT) and swept-source OCT (SS-OCT) are examples of Fourier-domain OCT, whereby interference data undergo a Fourier transformation process in which all light echoes are measured simultaneously. SD-OCT employs a spectrometer, a charge-coupled device camera, and a broad-bandwidth light source (800-900 nm) to achieve speed ranges of 40 to 100 kHz. 5 SS-OCT uses a tunable laser with longer wavelengths (typically > 1000 nm) that are less prone to attenuation and scattering and functions at speeds in excess of 100 kHz. 6 By improving the scanning speed, signal-to-noise ratio, and spatial resolution, these technologies permit a detailed depiction of ocular structures with relatively short scanning times. 7
The functional premise of OCTA is that in repeated B-scans of the same location, stationary tissue will not change substantially from frame to frame, while erythrocytes moving through the blood vessels will be in flux. This dynamic flow of erythrocytes can be analyzed to generate 3-dimensional visualization of the microvasculature. Numerous OCTA algorithms have been developed to identify blood flow and differentiate it from bulk eye motion, with these algorithms having variable reliance on different parts of the OCT signal. 5
Strengths of Optical Coherence Tomography Angiography
OCTA provides in vivo depth-resolved imaging of the retinal vasculature. Unlike the 2-dimensional images provided by FA, OCTA permits visualization of blood flow within distinct layers of the retina and choroid. 5 Indeed, while FA visualizes the superficial retinal capillary vasculature, OCTA provides high-resolution, cross-sectioned, depth-resolved images of superficial, middle, and deep capillary plexuses.8–11 These networks would be obscured by overlying areas of leakage in FA. In this respect, OCTA can be understood as complementing the information provided by dye-based angiography.
As a noninvasive imaging modality, OCTA has other advantages relative to dye-based techniques. The IV administration of FA or ICG is relatively invasive and is not without risk of adverse effects, ranging from common symptoms of nausea and pruritis to vomiting, injection site pain, tissue necrosis, and allergic reactions (including anaphylaxis in rare cases). 12 Patients with relative contraindications to IV FA or ICG administration (challenging IV access, allergy to shellfish in regard to ICG) have no restrictions with respect to OCTA. Dye-based imaging is relatively time-consuming and expensive. These disadvantages are particularly apparent in patients who need frequent vascular imaging (eg, patients being treated with antivascular endothelial growth factor [VEGF] agents for diabetic macular edema or wet age-related macular degeneration [AMD]). For these patients, the benefit of a rapid noninvasive technique to evaluate retinal and choroidal vasculature is clear.
Weaknesses of Optical Coherence Tomography Angiography
There are several limitations of OCTA. Foremost among these is that the technology is prone to a range of artifacts. 13 Blood flow in the superficial vasculature can interfere with light propagation to deeper structures, resulting in projection artifacts. Projection artifacts can be identified by the evaluation of sequential en face images at various depths, and OCTA software algorithms are designed to subtract these artifacts accordingly. 14 Since the functional premise of OCTA is that the eye is static, movement or loss of fixation can result in motion artifact. 15 Decorrelation signals across the entire B-scan cause white lines in the OCTA image. Motion correction can be employed that estimates eye motion between A-scans and aligns the A-scans accordingly. However, this process is computationally intense and prone to introducing its own artifacts (eg, stretching defects and the doubling of vessels). 14 Indeed, motion artifact is a major limiting factor for use of OCTA in eyes with low vision that might not be able to maintain fixation. 16 Errors can also occur when automatic segmentation strategies incorrectly identify different retinal layers. Healthy eyes are less susceptible to segmentation artifacts; however, high myopia as well as pathologies such as atrophy, edema, and hemorrhage pose major challenges for segmentation algorithms. 14 Indeed, studies comparing diseased eyes with fellow eyes found decreased image quality in eyes with pathology, with lower signal strength and increased artifacts.17,18
Although OCTA successfully images blood flow in active choroidal neovascular membranes (CNVM), as shown in Figures 1 and 2, its identification of slow blood-flow lesions (including fibrotic CNVM as well as microaneurysms and polypoidal choroidal vasculopathy) is limited. The time between 2 sequential OCT B-scans determines the slowest detectable flow rate below which blood flow will not be detected. Accordingly, those lesions with blood flow below this threshold might be missed by OCTA. The potential solution—prolonging the interval between consecutive scans—comes at the significant cost of increased motion artifact. 19 When a slow-flow lesion is not evident by analysis of the first and second B-scans, high-speed OCTA systems that use variable interscan time analysis might be able to decrease the minimum threshold by comparing the first and third B-scans without increasing motion artifact. 15
Figure 1.
Example of choroidal neovascular membranes before treatment. (A) Infrared and (B) cross-sectional B-scan on spectral-domain optical coherence tomography (OCT) showing subretinal hyperreflective material, subretinal fluid, and retinal pigment epithelium disruption. En face (C) OCT and (D) OCT angiography images as well as (E) cross-sectional B-scan OCT with flow overlay depicting choroidal neovascular membrane.
Figure 2.
Choroidal neovascular membranes after treatment with ranibizumab (same patient as in Figure 1). (A) Infrared and (B) cross-sectional B-scan on spectral-domain optical coherence tomography (OCT). En face (C) OCT and (D) OCT angiography (OCTA) images with (E) cross-sectional B-scan OCT with flow overlay. The images depict resolution of the subretinal hyperreflective material and subretinal fluid along with shrinkage of the vascular network based on OCTA.
In its current form, OCTA provides qualitative data by discriminating between blood vessels with adequate flow and static tissue. Several studies have numerically evaluated this qualitative vascular signal but are not truly quantitative in providing OCT-based measurements of blood flow.20,21 In contrast, OCTA velocimetry has the potential to quantify blood flow in retinal vascular networks. 22 The principle underlying OCTA velocimetry is that an incident OCT beam is partially reflected back by moving erythrocytes within a blood vessel, and evaluation of how the beam is modulated over repeated measurements can provide blood-flow velocity.22,23 Various phase-based, amplitude-based, and complex signal–based algorithms (that use both phase and amplitude data) have been developed to quantify blood flow. 23 In the research setting, quantitative evaluation of blood flow has the potential to deliver new insights into retinal hemodynamics. 24 Although current OCTA devices do not have the processing power to make quantitative velocimetry measurements feasible over large fields of view, it is likely that future generations of devices will increase the clinical applicability of this technology.
Compared with traditional dye-based angiography, OCTA has the significant limitation of not accurately capturing vascular leakage. In contrast, the dynamic appearance of dye leakage, staining, and pooling in 2-dimensional image sets captured by FA and ICGA are well recognized and extensively documented in the literature.15,25,26 OCTA provides a snapshot in time; it does not depict sequential temporal events. Accordingly, the adoption of OCTA as a replacement of dye-based angiography, in its current form, might be limited by its inability to capture leakage in conditions such as diabetic macular edema, neovascular AMD, and retinal vein occlusion. However, because current dye-based imaging is 2-dimensional, vascular leakage has the potential to block visualization of retinal pathology. In contrast, OCTA imaging can resolve and segment different layers of the retina and choroid, thereby permitting identification of the axial location of pathology.
The use of OCTA for screening for disease might be limited by its smaller field of view relative to that of to dye-based angiography. Wide-field imaging is particularly important in vascular conditions that have manifestations in the periphery, including diabetic retinopathy (DR). The standard area imaged by OCTA is 3 mm × 3 mm, although new wide-field swept-source devices permit scanning protocols of up to 23 mm × 20 mm in a single scan.27,28 This increase in field was made possible by increased scanning speeds, yet remains smaller than wide-field FA.
Greater imaging fields can lead to decreased resolution because some software programs use the same number of B-scans across the total area scanned.15,29 Given the progress in the technology over the past 5 years, it is anticipated that hardware and software developments will continue to expand the field of view captured by OCTA while achieving high levels of resolution, for example by using algorithms to montage multiple OCTAs.30,31 Indeed, by montaging multiple images together, areas of 31 mm × 27 mm can be obtained with current devices. 28 There has recently been a move toward integrating multiple imaging technologies, exemplified by the Optos Silverstone device, which combines SS-OCT with the ability to obtain 200° retina pseudocolor fundus images, fundus autofluorescence, FA, and ICGA. It is expected that combination devices that incorporate the ability to perform OCTA will be available in the next few years.
Although the technological innovation of OCTA is expanding the capabilities of this imaging modality, the variability of data generated from different instruments and software is not without issue. 32 After Fourier transformation, OCT signals contain both phase data and magnitude data. OCT algorithms can use either of these data or a combination to identify blood flow. 33 OCT algorithms are numerous, including speckle variance, phase variance, complex difference, correlation mapping, optical microangiography, split-spectrum amplitude decorrelation angiography, and OCT angiography ratio analysis, to name a few. 32 Various artifact-correction and eye-tracking technologies are employed by device manufacturers, further compounding the poor agreement between systems. This lack of consistency between devices has made it challenging to establish robust normative databases.
Finally, it is important to acknowledge the considerable expense associated with purchasing an OCTA platform, in addition to the concomitant data storage and retrieval costs. As discussed, performing FA is a relatively labor-intense process and is expensive for this reason. However, in the United States, practitioners are reimbursed commensurately because FAs have a distinct Current Procedural Terminology (CPT) code. In contrast, OCTA is reimbursed using the same CPT code as OCT imaging, even though OCTA takes longer to interpret (in particular when segmentation artifacts are present and manual adjustment is required). As a result, there is a substantial financial burden associated with the routine use of OCTA rather than the combination of dye-based angiography with traditional OCT imaging. This represents a major factor limiting the adoption of OCTA outside academic practice. Despite these practical limitations, the opportunities for OCTA to deepen our understanding of ocular pathology and to guide treatment decisions in clinical practice have been recognized in numerous conditions.
Optical Coherence Tomography Angiography in Diabetic Retinopathy
The Early Treatment Diabetic Retinopathy Study group employed FA to identify the vascular anomalies associated with DR and the likelihood of future disease progression. 34 FA remains the gold standard for vessel visualization in DR. 35 However, histological analyses have demonstrated that FA captures incomplete morphologic information about the superficial capillary network and even less detail about the deep capillary network. 36 The observation that FA depicts only a fraction of retinal blood vessels suggests that alternative modalities that provide depth-resolved imaging of the retinal vasculature should have greater utility as screening tools. Indeed, multiple studies have shown that OCTA can identify microvascular changes in diabetic eyes without clinical evidence of retinopathy.9,37
In addition to its potential role as a screening tool for diabetic eye disease, OCTA has been shown to be valuable in following patients with established retinopathy. Figure 3 shows an example of OCTA imaging in DR. The foveal avascular zone (FAZ) is known to expand with increasing DR severity. 38 OCTA effectively evaluates FAZ enlargement over time when using full retinal depth projection or when assessing the superficial or deep capillary plexuses.39,40 Owing to the relative ease of acquisition compared with dye-based angiography, it is feasible to obtain OCTA at every patient visit, thus permitting closer tracking of vascular changes over time in patients with DR. Another OCTA readout that can be used to follow disease severity in DR is vascular perfusion.8,41 Capillary perfusion density values have been shown to correlate closely with clinical grading of DR, suggesting an objective metric for assessing disease progression. 42 This work suggests that OCTA might offer an alternative to FA for the diagnosis of follow-up of DR.
Figure 3.
Example of proliferative diabetic retinopathy. Optical coherence tomography (OCT) angiography en face of (A) macula and (B) nasal retina superficial plexus. (C) Cross-sectional B-scan OCT showing flow overlay with arrows indicating areas of neovascularization at the vitreoretinal interface. The 2 yellow arrows depict the same neovascular frond.
In a retrospective study of 41 eyes comparing FA with OCTA in the evaluation of macular perfusion in diabetic patients, OCTA had close agreement with FAZ analysis but low sensitivity for detecting microaneurysms. 43 Indeed, OCTA has been shown to fail to capture a substantial portion of the microaneurysms identified on FA (~ 40%). 39 The lower sensitivity of OCTA in detecting microaneurysms is believed to be due to blood flow dropping below the slowest detectable rate. 44 Nevertheless, regular evaluation with OCTA can be used to follow the response of microaneurysms to anti-VEGF treatment. 2
OCTA can also be employed in proliferative DR to monitor neovascularization and vessel regression in response to anti-VEGF therapy. 45 A retrospective study of 47 eyes with DR evaluated the ability of structural OCT and OCTA to detect the new onset of, regression of, and reactivation of neovascularization. It found that structural OCT was better at detecting neovascularization; however, OCTA had the best detection rate for regression of neovascularization (100% compared with 45.5% for structural OCT) and reactivation (100% compared with 12.5% for structural OCT). 46 Accordingly, the authors concluded that although posterior pole structural OCT has the best detection rate for neovascularization, OCTA is the superior imaging modality for objective monitoring of proliferative DR after treatment. 46
As the capacity of newer swept-source OCTA devices to capture larger areas of the posterior retina increases, so do opportunities to employ OCTA to monitor more advanced stages of diabetes. For example, wide-angle OCTA imaging might be used to detect peripheral proliferative DR to identify neovascularization, as in Figure 3; to image extramacular capillary nonperfusion; and to follow the response to panretinal photocoagulation.47–50 Indeed, there is evidence that wide-angle OCTA has high sensitivity and specificity when detecting areas of neovascularization and nonperfusion, relative to ultra-widefield FA.50,51 In a prospective observational study of 152 eyes comparing montaged 15 mm × 15-mm OCTA scans with ultra-widefield FA, OCTA was shown to exhibit similar detection rates of microaneurysms, intraretinal microvascular abnormalities, neovascularization, and nonperfusion areas relative to ultra-widefield FA images. 51 Given the invasive nature, the time, and expense associated with dye-based angiography, the ease of acquisition of widefield OCTA might allow for DR changes to be imaged at more regular intervals than is feasible with FA.
Optical Coherence Tomography Angiography in Age-Related Macular Degeneration
Recent studies have employed OCTA to deliver important new insights into the pathophysiology of AMD.6,52–56 There is an intimate and mutual relationship between the health of the retinal pigment epithelium (RPE) and the choriocapillaris.57,58 Although the site of initial insult in AMD remains under debate, there is evidence from histopathological evaluation of AMD eyes that choriocapillaris breakdown precedes RPE and retinal degeneration.59,60 Wherever the primary insult occurs, there is no imaging modality currently available that permits the in vivo evaluation of RPE function, yet OCTA can be used to measure the perfusion of the choriocapillaris, thus providing a proxy metric for function of the RPE–choriocapillaris complex. 61 In advanced nonexudative AMD, OCTA has been particularly valuable for its capacity to evaluate the choriocapillaris. 61
Individual vessels in the choriocapillaris complex cannot be resolved by current OCTA instruments; however, flow voids (areas of absent flow signal) can be identified. Multiple studies have demonstrated decreased choriocapillaris perfusion manifesting as increased flow voids in advanced AMD.52,62 Indeed, impairment of choriocapillaris flow has been detected beyond the margins of areas of geographic atrophy.52,53 It has been suggested that OCTA could be employed as a tool to predict geographic atrophy progression 54 and to quantify and monitor areas of atrophy. 56
OCTA imaging has been adopted in the clinic for the diagnosis of neovascular AMD. In capturing depth-resolved images, OCTA permits differentiation between type 1 (sub-RPE), type 2 (between the RPE and the retina), and type 3 (within the retina) neovascularization.63–66 Indeed, OCTA has been demonstrated to have sensitivity and specificity similar to that of FA for the detection of neovascularization.67,68
In a multicenter, retrospective cohort study of 115 eyes with type 1 neovascular AMD, the sensitivity of the combination of en face OCTA and structural OCT was compared with the reference standard of combined FA and structural OCT. 68 It was found that 90 eyes (86%) could be detected with en face OCTA and structural OCT, compared with 105 eyes detected with the reference standard. Notably, the combination of en face OCTA and structural OCT demonstrated superior detection of type 1 neovascularization than FA alone or en face OCTA alone. 68
Furthermore, OCTA has the capacity to capture nonexudative neovascularization that would otherwise have been undetectable.69,70 Subclinical macular neovascularization detected via OCTA has been shown to be associated with a greater risk of future exudation, suggesting a role for OCTA in guiding decisions about follow-up intervals. 71 The longitudinal response of macular neovascularization to anti-VEGF treatment has been characterized, and it is feasible that tracking changes to vascular networks on OCTA might inform decisions regarding retreatment in the future.72-74
Optical Coherence Tomography Angiography in Central Serous Chorioretinopathy
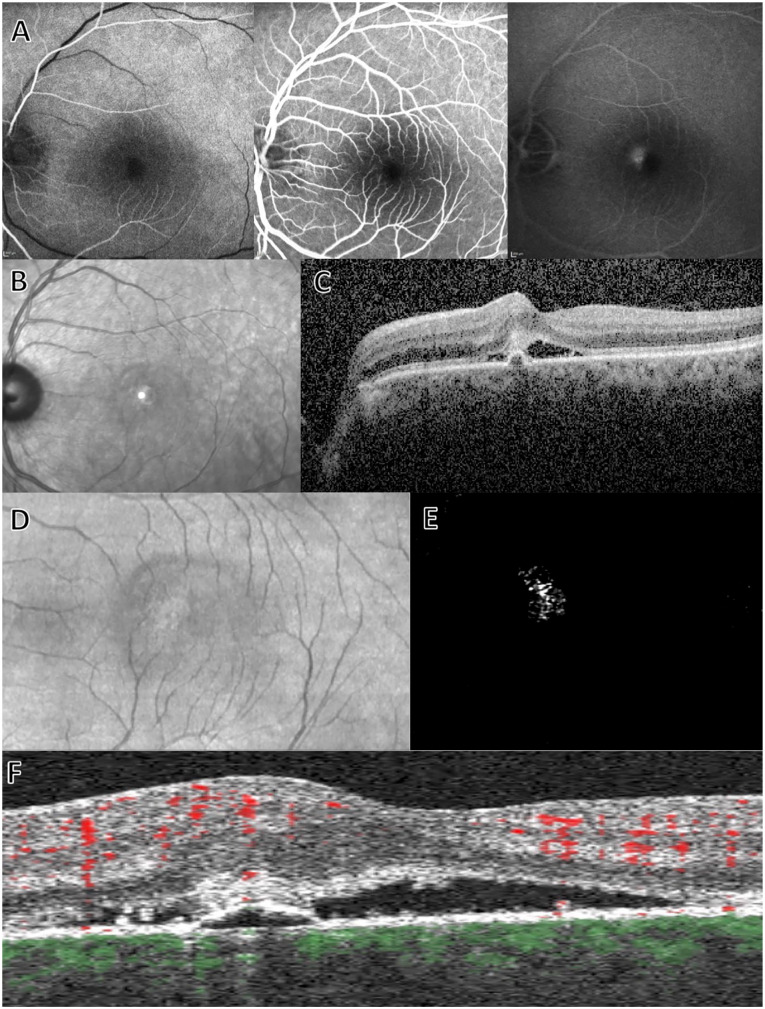
CNV is a relatively rare sequela of chronic central serous chorioretinopathy (CSCR), yet it is strongly associated with a poor visual prognosis. 75 Differentiating between eyes with chronic CSCR with or without CNVM is often challenging because they share several features, including RPE detachment, subretinal and intraretinal fluid, retinal atrophy, and cystoid macular degeneration.76,77 Figure 4 shows an example of OCTA imaging in CSCR. Multiple studies have demonstrated the superiority of OCTA over FA in the detection of CNVM in eyes with chronic CSCR, with OCTA exhibiting significantly higher sensitivity and specificity than FA.67,78–80 The timely diagnosis of CNVM allows for the prompt initiation of anti-VEGF therapy and might ultimately result in superior visual outcome for these patients.
Figure 4.
Example of central serous chorioretinopathy. (A) Fluorescein angiography at early, mid, and late phases demonstrating parafoveal leakage. (B) Infrared and (C) cross-sectional B-scan on spectral-domain optical coherence tomography (OCT). Spectral-domain OCT shows subretinal fluid and hyperreflective material as well as retinal pigment epithelium detachment. En face (D) OCT and (E) OCT angiography images with (F) cross-sectional B-scan OCT showing flow overlay, consistent with a choroidal neovascular membrane.
Optical Coherence Tomography Angiography in Macular Telangiectasia
OCTA has been demonstrated to be superior to FA in identifying rarefaction of the capillary plexus and altered microvascular morphology in macular telangiectasia type 1. 81 Furthermore, OCTA imaging has been employed to demonstrate that telangiectasias are limited to the deep capillary plexus in macular telangiectasia type 1 and identify a reduction in capillary network density in both the deep and superficial plexuses in this condition when compared with fellow eyes and normal controls. 81 OCTA has also shed light on structural changes that occur in macular telangiectasia type 2.82–85 Figure 5 shows an example of OCTA imaging in macular telangiectasia type 2.
Figure 5.
Example of macular telangiectasia type 2. (A) Infrared and (B) cross-sectional B-scan on spectral-domain optical coherence tomography (OCT). En face (C) OCT and (D) OCT angiography images from superficial vascular plexus with (E) cross-sectional B-scan OCT showing flow overlay.
Volume-rendered images of eyes with macular telangiectasia type 2 have shown that vessels occurring posterior to the outer limit of the deep retinal plexus occur in the setting of retinal thinning, vascular invasion, or an amalgam. 82 Visualization of the vascular abnormalities in macular telangiectasia type 2 using OCTA has led investigators to hypothesize that retinal thinning associated with loss of Müller cell density results in deeper vessels being exposed to the relatively hypoxic environment of the inner segments of photoreceptors, thus promoting intraretinal vascular proliferation. 82 OCTA has also been used to identify an association between the origins of right-angled veins and contractile features of tissue in the temporal macula, suggesting that tensile stress across the macula may be implicated in the development of right-angled veins. 83 OCTA studies of macular telangiectasia type 2 have demonstrated that mean capillary density is significantly reduced both in the superficial and deep capillary plexuses and have shown increased intervascular spaces and abnormal capillary anastomoses, relative to controls.84,85 OCTA has also been used to demonstrate that retinal–choroidal anastomoses occur earlier in the pathogenesis of macular telangiectasia type 2 than previously thought, occurring in association with right-angle veins before proliferation laterally above the Bruch membrane occurs. 86 OCTA studies support the concept of capillary proliferation in the outer retina in advanced macular telangiectasia type 2 and have shown a significant association between hyperreflective material–containing vessels visualized on OCTA with increasing disease severity.87,88 By enabling the visualization and quantification of patterns of vascular changes in macular telangiectasia type 2, OCTA offers potential additional parameters to monitor in future treatment trials.87,88
Optical Coherence Tomography Angiography in Retinal Vein Occlusion
OCTA has been shown to detect microvascular changes both in the superficial and deep capillary networks following retinal vein occlusion.18,89,90 These changes include a reduction in foveal and parafoveal vessel densities, increased areas of nonperfusion, the engorgement of capillary networks, increased vascular tortuosity, increased collateral vessels, and the development of telangiectasias.91–94 Notably, the best-corrected visual acuity (VA) in eyes with retinal vein occlusion has been shown to be negatively correlated with the area of the superficial foveal avascular zone and positively correlated with the parafoveal vessel density.89,95 In a case series of 144 eyes with retinal vein occlusion comparing the capacity of OCTA vs FA in delineating areas of retinal capillary nonperfusion, OCTA was found to be more precise. 96 Moreover, superficial and deep capillary plexus ischemia on OCTA have been identified as significant predictors of diminished VA. 96
There are certain challenges associated with the use of OCTA to evaluate retinal vein occlusions. In particular, macular edema might give rise to segmentation errors. 18 Macular edema can also lead to the degree of nonperfusion being overestimated because of shadowing artifacts from fluid in cystoid spaces obscuring the detection of capillaries. 97 To address these potential sources of error, some investigators have used nonsegmented images to evaluate the FAZ, while others have included only eyes without macular edema.95,98
Optical Coherence Tomography Angiography in Uveitis
OCTA can detect changes to the superficial and deep capillary plexuses in uveitic disease. 99 Superficial retinal plexus parafoveal capillary density is significantly decreased in eyes with retinal vasculitis relative to healthy eyes, suggesting that OCTA might be used to index inflammatory changes secondary to uveitis. 100 Although OCTA is not able to detect vascular leakage secondary to retinal vessel inflammation, it offers the advantage of superior imaging of the deep capillary plexus. This is particularly relevant in uveitic diseases that demonstrate markedly deficient capillary flow in the deep retinal capillary plexus, such as birdshot chorioretinopathy. 101 OCTA might also offer superior visualization of perifoveal microvascular changes in eyes with active uveitis than FA, such as has been reported in Behçet disease. 102 OCTA can also be useful in detecting secondary complications of vasculitis that might be missed by FA, such as neovascularization blocked by retinal hemorrhage, telangiectasias, and early peripapillary vascular proliferation. 103
Conclusions
In recent years, OCTA has emerged as a new technology that permits depth-resolved and volumetric imaging of the retinal microvasculature. This noninvasive imaging modality has been employed in research studies to deepen our understanding of numerous vascular and inflammatory diseases of the eye.
Despite the numerous advantages of the technology, OCTA has not yet been adopted for all patients in routine clinical practice but is used to complement traditional dye-based angiography. The barriers to universal adoption include a limited field of view (ie, poor evaluation of retinal periphery relative to ultra-widefield angiography), poor visualization of slow blood-flow lesions, susceptibility to various artifacts, and a lack of standardization across platforms (and thus a lack of robust, extensive normative databases). Nevertheless, the technology is developing rapidly, as evidenced by the increased field of view, resolution, and scanning speeds as well as decreased artifacts available on newer devices. OCTA is well positioned to become a first-line imaging modality over the coming decade as these various challenges are overcome through a combination of research and further technological development.
Footnotes
Ethical Approval: Ethical approval is not applicable to this review.
Statement of Informed Consent: Informed consent is not applicable to this review.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: William Foulsham  https://orcid.org/0000-0002-7088-605X
https://orcid.org/0000-0002-7088-605X
References
- 1. Gorczynska I, Migacz JV, Zawadzki RJ, Capps AG, Werner JS. Comparison of amplitude-decorrelation, speckle-variance and phase-variance OCT angiography methods for imaging the human retina and choroid. Biomed Opt Express. 2016;7(3):911. doi: 10.1364/boe.7.000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greig EC, Duker JS, Waheed NK. A practical guide to optical coherence tomography angiography interpretation. Int J Retina Vitreous. 2020;6(1):55. doi: 10.1186/s40942-020-00262-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181. doi: 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1-55. doi: 10.1016/j.preteyeres.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kashani AH, Chen CL, Gahm JK, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66-100. doi: 10.1016/j.preteyeres.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ploner SB, Moult EM, Choi W, et al. Toward quantitative optical coherence tomography angiography: visualizing blood flow speeds in ocular pathology using variable interscan time analysis. Retina. 2016;36(Suppl 1):S118-S126. doi: 10.1097/IAE.0000000000001328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drexler W, Liu M, Kumar A, Kamali T, Unterhuber A, Leitgeb RA. Optical coherence tomography today: speed, contrast, and multimodality. J Biomed Opt. 2014;19(7):071412. doi: 10.1117/1.jbo.19.7.071412 [DOI] [PubMed] [Google Scholar]
- 8. Nesper PL, Roberts PK, Onishi AC, et al. Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(6):BIO307-BIO315. doi: 10.1167/iovs.17-21787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35(11):2377-2383. doi: 10.1097/IAE.0000000000000849 [DOI] [PubMed] [Google Scholar]
- 10. Hwang TS, Gao SS, Liu L, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134(4):367-373. doi: 10.1001/jamaophthalmol.2015.5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di G, Weihong Y, Xiao Z, et al. A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):873-879. doi: 10.1007/s00417-015-3143-7 [DOI] [PubMed] [Google Scholar]
- 12. Kwan ASL, Barry C, McAllister IL, Constable I. Fluorescein angiography and adverse drug reactions revisited: the Lions Eye experience. Clin Exp Ophthalmol. 2006;34(1):33-38. doi: 10.1111/j.1442-9071.2006.01136.x [DOI] [PubMed] [Google Scholar]
- 13. Coffey AM, Hutton EK, Combe L, Bhindi P, Gertig D, Constable PA. Optical coherence tomography angiography in primary eye care. Clin Exp Optom. 2021;104(1):3-13. doi: 10.1111/cxo.13068 [DOI] [PubMed] [Google Scholar]
- 14. Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163-2180. doi: 10.1097/IAE.0000000000000765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015;1(1):1-15. doi: 10.1186/s40942-015-0005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nobre Cardoso J, Keane PA, Sim DA, et al. Systematic evaluation of optical coherence tomography angiography in retinal vein occlusion. Am J Ophthalmol. 2016;163:93-107.e6. doi: 10.1016/J.AJO.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 17. Say EAT, Ferenczy S, Magrath GN, Samara WA, Khoo CTL, Shields CL. Image quality and artifacts on optical coherence tomography angiography: comparison of pathologic and paired fellow eyes in 65 patients with unilateral choroidal melanoma treated with plaque radiotherapy. Retina. 2017;37(9):1660-1673. doi: 10.1097/IAE.0000000000001414 [DOI] [PubMed] [Google Scholar]
- 18. Tsai G, Banaee T, Conti FF, Singh RP. Optical coherence tomography angiography in eyes with retinal vein occlusion. J Ophthalmic Vis Res. 2018;13(3):315-332. doi: 10.4103/jovr.jovr_264_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chalam KV, Sambhav K. Optical coherence tomography angiography in retinal diseases. J Ophthalmic Vis Res. 2016;11(1):84-92. doi: 10.4103/2008-322X.180709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu Z, Lin J, Gao C, et al. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J Biomed Opt. 2016;21(6):066008. doi: 10.1117/1.jbo.21.6.066008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jia Y, Bailey ST, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015;112(18):E2395-E2402. doi: 10.1073/pnas.1500185112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seidel G, Aschinger G, Singer C, et al. Estimating retinal blood flow velocities by optical coherence tomography. JAMA Ophthalmol. 2016;134(10):1104-1110. doi: 10.1001/jamaophthalmol.2016.2507 [DOI] [PubMed] [Google Scholar]
- 23. Braaf B, Gräfe MGO, Uribe-Patarroyo N, et al. OCT-based velocimetry for blood flow quantification. In: Bille J, ed. High Resolution Imaging in Microscopy and Ophthalmology. Springer; 2019:161-179. [PubMed] [Google Scholar]
- 24. Pechauer AD, Jia Y, Liu L, Gao SS, Jiang C, Huang D. Optical coherence tomography angiography of peripapillary retinal blood flow response to hyperoxia. Investig Ophthalmol Vis Sci. 2015;56(5):3287-3291. doi: 10.1167/iovs.15-16655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Invernizzi A, Pellegrini M, Cornish E, Yi Chong Teo K, Cereda M, Chabblani J. Imaging the choroid: from indocyanine green angiography to optical coherence tomography angiography. Asia Pac J Ophthalmol (Phila.). 2020;9(4):335-348. doi: 10.1097/APO.0000000000000307 [DOI] [PubMed] [Google Scholar]
- 26. Shoughy SS, Kozak I. Selective and complementary use of optical coherence tomography and fluorescein angiography in retinal practice. Eye Vis (Lond). 2016;3:26. doi: 10.1186/s40662-016-0058-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang JC, Miller JB. Optical coherence tomography angiography: review of current technical aspects and applications in chorioretinal disease. Semin Ophthalmol. 2019;34(4):211-217. doi: 10.1080/08820538.2019.1620797 [DOI] [PubMed] [Google Scholar]
- 28. Laíns I, Wang JC, Cui Y, et al. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog Retin Eye Res. 2021;84:100951. doi: 10.1016/J.PRETEYERES.2021.100951 [DOI] [PubMed] [Google Scholar]
- 29. Rodríguez FJ, Staurenghi G, Gale R; Vision Academy Steering Committee. The role of OCT-A in retinal disease management. Graefes Arch Clin Exp Ophthalmol. 2018;256(11):2019-2026. doi: 10.1007/S00417-018-4109-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Carlo TE, Salz DA, Waheed NK, Baumal CR, Duker JS, Witkin AJ. Visualization of the retinal vasculature using wide-field montage optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):611-616. doi: 10.3928/23258160-20150610-03 [DOI] [PubMed] [Google Scholar]
- 31. Mase T, Ishibazawa A, Nagaoka T, Yokota H, Yoshida A. Radial peripapillary capillary network visualized using wide-field montage optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2016;57(9):504-510. doi: 10.1167/iovs.15-18877 [DOI] [PubMed] [Google Scholar]
- 32. Li XX, Wu W, Zhou H, et al. A quantitative comparison of five optical coherence tomography angiography systems in clinical performance. Int J Ophthalmol. 2018;11(11):1784-1795. doi: 10.18240/ijo.2018.11.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang A, Zhang Q, Chen CL, Wang RK. Methods and algorithms for optical coherence tomography-based angiography: a review and comparison. J Biomed Opt. 2015;20(10):100901. doi: 10.1117/1.jbo.20.10.100901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Early Treatment Diabetic Retinopathy Study Research Group. Fluorescein angiographic risk factors for progression of diabetic retinopathy. Ophthalmology. 1991;98(5 Suppl):834-840. [PubMed] [Google Scholar]
- 35. Salz DA, Witkin AJ. Imaging in diabetic retinopathy. Middle East Afr J Ophthalmol. 2015;22(2):145-150. doi: 10.4103/0974-9233.151887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mendis KR, Balaratnasingam C, Yu P, et al. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Investig Ophthalmol Vis Sci. 2010;51(11):5864-5869. doi: 10.1167/iovs.10-5333 [DOI] [PubMed] [Google Scholar]
- 37. Alibhai AY, Moult EM, Shahzad R, et al. Quantifying microvascular changes using OCT angiography in diabetic eyes without clinical evidence of retinopathy. Ophthalmol Retina. 2018;2(5):418-427. doi: 10.1016/j.oret.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984;102(9):1286-1293. doi: 10.1001/archopht.1984.01040031036019 [DOI] [PubMed] [Google Scholar]
- 39. Salz DA, De Carlo TE, Adhi M, et al. Select features of diabetic retinopathy on swept-source optical coherence tomographic angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol. 2016;134(6):644-650. doi: 10.1001/jamaophthalmol.2016.0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samara WA, Shahlaee A, Adam MK, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124(2):235-244. doi: 10.1016/j.ophtha.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 41. Sambhav K, Abu-Amero KK, Chalam KV. Deep capillary macular perfusion indices obtained with OCT angiography correlate with degree of nonproliferative diabetic retinopathy. Eur J Ophthalmol. 2017;27(6):716-729. doi: 10.5301/ejo.5000948 [DOI] [PubMed] [Google Scholar]
- 42. Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35(11):2353-2363. doi: 10.1097/IAE.0000000000000862 [DOI] [PubMed] [Google Scholar]
- 43. La Mantia A, Kurt RA, Mejor S, et al. Comparing fundus fluorescein angiography and swept-source optical coherence tomography angiography in the evaluation of diabetic macular perfusion. Retina. 2019;39(5):926-937. doi: 10.1097/IAE.0000000000002045 [DOI] [PubMed] [Google Scholar]
- 44. Hasegawa N, Nozaki M, Takase N, Yoshida M, Ogura Y. New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Investig Ophthalmol Vis Sci. 2016;57(9):OCT348-OCT355. doi: 10.1167/iovs.15-18782 [DOI] [PubMed] [Google Scholar]
- 45. Hu Z, Su Y, Xie P, et al. OCT angiography-based monitoring of neovascular regression on fibrovascular membrane after preoperative intravitreal conbercept injection. Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1611-1619. doi: 10.1007/s00417-019-04315-0 [DOI] [PubMed] [Google Scholar]
- 46. Schwartz R, Khalid H, Sivaprasad S, et al. Objective evaluation of proliferative diabetic retinopathy using OCT. Ophthalmol Retina. 2020;4(2):164-174. doi: 10.1016/J.ORET.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 47. Russell JF, Shi Y, Hinkle JW, et al. Longitudinal wide-field swept-source OCT angiography of neovascularization in proliferative diabetic retinopathy after panretinal photocoagulation. Ophthalmol Retina. 2019;3(4):350-361. doi: 10.1016/j.oret.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yasukura S, Murakami T, Suzuma K, et al. Diabetic nonperfused areas in macular and extramacular regions on wide-field optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2018;59(15):5893-5903. doi: 10.1167/iovs.18-25108 [DOI] [PubMed] [Google Scholar]
- 49. Belenje A, Rani PK. Role of wide-angle optical coherence tomography angiography in the detection of clinically non-apparent neovascularisation in proliferative diabetic retinopathy. BMJ Case Rep. 2020;13(9):e236836. doi: 10.1136/bcr-2020-236836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sawada O, Ichiyama Y, Obata S, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2018;256(7):1275-1280. doi: 10.1007/s00417-018-3992-y [DOI] [PubMed] [Google Scholar]
- 51. Cui Y, Zhu Y, Wang JC, et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br J Ophthalmol. 2021;105(4):577-581. doi: 10.1136/BJOPHTHALMOL-2020-316245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choi W, Moult EM, Waheed NK, et al. Ultrahigh-speed, swept-source optical coherence tomography angiography in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmology. 2015;122(12):2532-2544. doi: 10.1016/j.ophtha.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kvanta A, De Salles CC, Amrén U, Bartuma H. Optical coherence tomography angiography of the foveal microvasculature in geographic atrophy. Retina. 2017;37(5):936-942. doi: 10.1097/IAE.0000000000001248 [DOI] [PubMed] [Google Scholar]
- 54. Lindner M, Böker A, Mauschitz MM, et al. Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015;122(7):1356-1365. doi: 10.1016/j.ophtha.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 55. Bindewald A, Schmitz-Valckenberg S, Jorzik JJ, et al. Classification of abnormal fundus autofluorescence patterns in the junctional zone of geographic atrophy in patients with age related macular degeneration. Br J Ophthalmol. 2005;89(7):874-878. doi: 10.1136/bjo.2004.057794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Corbelli E, Sacconi R, Rabiolo A, et al. Optical coherence tomography angiography in the evaluation of geographic atrophy area extension. Investig Ophthalmol Vis Sci. 2017;58(12):5201-5208. doi: 10.1167/iovs.17-22508 [DOI] [PubMed] [Google Scholar]
- 57. McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2009;50(10):4982-4991. doi: 10.1167/iovs.09-3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arya M, Sabrosa AS, Duker JS, Waheed NK. Choriocapillaris changes in dry age-related macular degeneration and geographic atrophy: a review. Eye Vis (Lond). 2018;5(1):22. doi: 10.1186/s40662-018-0118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med. 2012;33(4):295-317. doi: 10.1016/j.mam.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35(11):2562-2573. doi: 10.1016/j.neurobiolaging.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 61. Lauermann JL, Eter N, Alten F. Optical coherence tomography angiography offers new insights into choriocapillaris perfusion. Ophthalmologica. 2018;239(2-3):74-84. doi: 10.1159/000485261 [DOI] [PubMed] [Google Scholar]
- 62. Spaide RF. Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am J Ophthalmol. 2016;170:58-67. doi: 10.1016/j.ajo.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 63. Kuehlewein L, Bansal M, Lenis TL, et al. Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Am J Ophthalmol. 2015;160(4):739-748.e2. doi: 10.1016/j.ajo.2015.06.030 [DOI] [PubMed] [Google Scholar]
- 64. Iafe NA, Phasukkijwatana N, Sarraf D. Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Dev Ophthalmol. 2016;56:45-51. doi: 10.1159/000442776 [DOI] [PubMed] [Google Scholar]
- 65. Souied EH, El Ameen A, Semoun O, Miere A, Querques G, Cohen SY. Optical coherence tomography angiography of type 2 neovascularization in age-related macular degeneration. Dev Ophthalmol. 2016;56:52-56. doi: 10.1159/000442777 [DOI] [PubMed] [Google Scholar]
- 66. Phasukkijwatana N, Tan ACS, Chen X, Freund KB, Sarraf D. Optical coherence tomography angiography of type 3 neovascularisation in age-related macular degeneration after antiangiogenic therapy. Br J Ophthalmol. 2017;101(5):597-602. doi: 10.1136/bjophthalmol-2016-308815 [DOI] [PubMed] [Google Scholar]
- 67. De Carlo TE, Bonini Filho MA, Chin AT, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122(6):1228-1238. doi: 10.1016/j.ophtha.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 68. Inoue M, Jung JJ, Balaratnasingam C, et al. A comparison between optical coherence tomography angiography and fluorescein angiography for the imaging of type 1 neovascularization. Investig Ophthalmol Vis Sci. 2016;57(9):OCT314-OCT323. doi: 10.1167/iovs.15-18900 [DOI] [PubMed] [Google Scholar]
- 69. Roisman L, Zhang Q, Wang RK, et al. Optical coherence tomography angiography of asymptomatic neovascularization in intermediate age-related macular degeneration. Ophthalmology. 2016;123(6):1309-1319. doi: 10.1016/j.ophtha.2016.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carnevali A, Cicinelli MV, Capuano V, et al. Optical coherence tomography angiography: a useful tool for diagnosis of treatment-naïve quiescent choroidal neovascularization. Am J Ophthalmol. 2016;169:189-198. doi: 10.1016/j.ajo.2016.06.042 [DOI] [PubMed] [Google Scholar]
- 71. de Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125(2):255-266. doi: 10.1016/j.ophtha.2017.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Spaide RF. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am J Ophthalmol. 2015;160(1):6-16. doi: 10.1016/j.ajo.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 73. Parravano M, Querques L, Scarinci F, et al. Optical coherence tomography angiography in treated type 2 neovascularization undergoing monthly anti-VEGF treatment. Acta Ophthalmol. 2017;95(5):e425-e426. doi: 10.1111/aos.13180 [DOI] [PubMed] [Google Scholar]
- 74. Muakkassa NW, Chin AT, De Carlo T, et al. Characterizing the effect of anti-vascular endothelial growth factor therapy on treatment-naive choroidal neovascularization using optical coherence tomography angiography. Retina. 2015;35(11):2252-2259. doi: 10.1097/IAE.0000000000000836 [DOI] [PubMed] [Google Scholar]
- 75. Loo RH, Scott IU, Flynn HW, Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22(1):19-24. doi: 10.1097/00006982-200202000-00004 [DOI] [PubMed] [Google Scholar]
- 76. Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23(1):1-7; quiz 137-138. doi: 10.1097/00006982-200302000-00001 [DOI] [PubMed] [Google Scholar]
- 77. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103(12):2070-2080. doi: 10.1016/S0161-6420(96)30386-2 [DOI] [PubMed] [Google Scholar]
- 78. Quaranta-El Maftouhi M, El Maftouhi A, Eandi CM. Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. Am J Ophthalmol. 2015;160(3):581- 587.e1. doi: 10.1016/j.ajo.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 79. Bousquet E, Bonnin S, Mrejen S, Krivosic V, Tadayoni R, Gaudric A. Optical coherence tomography angiography of flat irregular pigment epithelium detachment in chronic central serous chorioretinopathy. Retina. 2018;38(3):629-638. doi: 10.1097/IAE.0000000000001580 [DOI] [PubMed] [Google Scholar]
- 80. Bansal R, Dogra M, Mulkutkar S, et al. Optical coherence tomography angiography versus fluorescein angiography in diagnosing choroidal neovascularization in chronic central serous chorioretinopathy. Indian J Ophthalmol. 2019;67(7):1095-1100. doi: 10.4103/ijo.IJO_1238_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Matet A, Daruich A, Dirani A, Ambresin A, Behar-Cohen F. Macular telangiectasia type 1: capillary density and microvascular abnormalities assessed by optical coherence tomography angiography. Am J Ophthalmol. 2016;167:18-30. doi: 10.1016/j.ajo.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 82. Spaide RF, Klancnik JM, Cooney MJ, et al. Volume-rendering optical coherence tomography angiography of macular telangiectasia type 2. Ophthalmology. 2015;122(11):2261-2269. doi: 10.1016/j.ophtha.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 83. Spaide RF, Suzuki M, Yannuzzi LA, Matet A, Behar-Cohen F. Volume-rendered angiographic and structural optical coherence tomography angiography of macular telangiectasia type 2. Retina. 2017;37(3):424-435. doi: 10.1097/IAE.0000000000001344 [DOI] [PubMed] [Google Scholar]
- 84. Chidambara L, Gadde SGK, Yadav NK, et al. Characteristics and quantification of vascular changes in macular telangiectasia type 2 on optical coherence tomography angiography. Br J Ophthalmol. 2016;100(11):1482-1488. doi: 10.1136/bjophthalmol-2015-307941 [DOI] [PubMed] [Google Scholar]
- 85. Toto L, Di Antonio L, Mastropasqua R, et al. Multimodal imaging of macular telangiectasia type 2: focus on vascular changes using optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2016;57(9):OCT268-OCT276. doi: 10.1167/iovs.15-18872 [DOI] [PubMed] [Google Scholar]
- 86. Spaide RF, Yannuzzi LA, Maloca PM. Retinal-choroidal anastomosis in macular telangiectasia type 2. Retina. 2018;38(10):1920-1929. doi: 10.1097/IAE.0000000000002289 [DOI] [PubMed] [Google Scholar]
- 87. Pauleikhoff D, Gunnemann F, Book M, Rothaus K. Progression of vascular changes in macular telangiectasia type 2: comparison between SD-OCT and OCT angiography. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1381-1392. doi: 10.1007/s00417-019-04323-0 [DOI] [PubMed] [Google Scholar]
- 88. Gaudric A, Krivosic V, Tadayoni R. Outer retina capillary invasion and ellipsoid zone loss in macular telangiectasia type 2 imaged by optical coherence tomography angiography. Retina. 2015;35(11):2300-2306. doi: 10.1097/IAE.0000000000000799 [DOI] [PubMed] [Google Scholar]
- 89. Kang JW, Yoo R, Jo YH, Kim HC. Correlation of microvascular structures on optical coherence tomography angiography with visual acuity in retinal vein occlusion. Retina. 2017;37(9):1700-1709. doi: 10.1097/IAE.0000000000001403 [DOI] [PubMed] [Google Scholar]
- 90. Dave VP, Pappuru RR, Gindra R, et al. OCT angiography fractal analysis-based quantification of macular vascular density in branch retinal vein occlusion eyes. Can J Ophthalmol. 2019;54(3):297-300. doi: 10.1016/j.jcjo.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 91. Mastropasqua R, Toto L, Di Antonio L, et al. Optical coherence tomography angiography microvascular findings in macular edema due to central and branch retinal vein occlusions. Sci Rep. 2017;7:40763. doi: 10.1038/srep40763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shiraki A, Sakimoto S, Tsuboi K, et al. Evaluation of retinal nonperfusion in branch retinal vein occlusion using wide-field optical coherence tomography angiography. Acta Ophthalmol. 2019;97(6):e913-e918. doi: 10.1111/aos.14087 [DOI] [PubMed] [Google Scholar]
- 93. Lee H, Kim MA, Kim HC, Chung H. Characterization of microvascular tortuosity in retinal vein occlusion utilizing optical coherence tomography angiography. Sci Rep. 2020;10(1):1-9. doi: 10.1038/s41598-020-74871-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shahlaee A, Hong BK, Ho AC. Optical coherence tomography angiography features of branch retinal vein occlusion. Retin Cases Brief Rep. 2017;11(1):90-93. doi: 10.1097/ICB.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 95. De Salles MC, Kvanta A, Amrén U, Epstein D. Optical coherence tomography angiography in central retinal vein occlusion: correlation between the foveal avascular zone and visual acuity. Investig Ophthalmol Vis Sci. 2016;57(9):OCT242-OCT246. doi: 10.1167/iovs.15-18819 [DOI] [PubMed] [Google Scholar]
- 96. Moussa M, Leila M, Bessa AS, et al. Grading of macular perfusion in retinal vein occlusion using en-face swept-source optical coherence tomography angiography: a retrospective observational case series. BMC Ophthalmol. 2019;19(1):1-13. doi: 10.1186/S12886-019-1134-X/TABLES/9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Novais EA, Waheed NK. Optical coherence tomography angiography of retinal vein occlusion. Dev Ophthalmol. 2016;56:132-138. doi: 10.1159/000442805 [DOI] [PubMed] [Google Scholar]
- 98. Adhi M, Bonini Filho MA, Louzada RN, et al. Retinal capillary network and foveal avascular zone in eyes with vein occlusion and fellow eyes analyzed with optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2016;57(9):486-494. doi: 10.1167/iovs.15-18907 [DOI] [PubMed] [Google Scholar]
- 99. Tranos P, Karasavvidou EM, Gkorou O, Pavesio C. Optical coherence tomography angiography in uveitis. J Ophthalmic Inflamm Infect. 2019;9(1):21. doi: 10.1186/s12348-019-0190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim AY, Rodger DC, Shahidzadeh A, et al. Quantifying retinal microvascular changes in uveitis using spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;171:101-112. doi: 10.1016/j.ajo.2016.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pichi F, Sarraf D, Morara M, Mazumdar S, Neri P, Gupta V. Pearls and pitfalls of optical coherence tomography angiography in the multimodal evaluation of uveitis. J Ophthalmic Inflamm Infect. 2017;7(1):20. doi: 10.1186/s12348-017-0138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Khairallah M, Abroug N, Khochtali S, et al. Optical coherence tomography angiography in patients with Behçet uveitis. Retina. 2017;37(9):1678-1691. doi: 10.1097/IAE.0000000000001418 [DOI] [PubMed] [Google Scholar]
- 103. Abucham-Neto JZ, Torricelli AAM, Lui ACF, Guimarães SN, Nascimento H, Regatieri CV. Comparison between optical coherence tomography angiography and fluorescein angiography findings in retinal vasculitis. Int J Retina Vitreous. 2018;4(1):15. doi: 10.1186/s40942-018-0117-z [DOI] [PMC free article] [PubMed] [Google Scholar]