Abstract
Purpose:
This work reviews ocular, systemic, and demographic factors contributing to presentation of choroidal neovascular membrane (CNVM)–associated macular hemorrhage after the New York City coronavirus disease 2019 (COVID-19) lockdown.
Methods:
A retrospective, consecutive case series was conducted of all established patients presenting with macular hemorrhage between March 22, 2020, and August 10, 2020.
Results:
Fourteen patients (mean age 82.2 years) were evaluated. Ten patients had active CNVMs, 1 had an inactive lesion that was last injected 2 years prior, and 3 had new conversions from nonexudative age-related macular degeneration. In the actively treated CNVM group there was a delay in expected follow-up from 50.4 days to 125 days. Eight patients with previously active CNVM (73%) had a history of prior macular hemorrhage. Eight patients (57%) were on some form of antiplatelet or anticoagulation therapy. Twelve patients (86%) had COVID-19–specific risk factors besides age, and all but 1 patient (93%) delayed care without discussion with a physician. Ten patients (71%) had more than 1 week of symptoms prior to presentation. Twelve patients (86%) had signs of CNVM on prior optical coherence tomography.
Conclusions:
Adequate documentation of potential risks for hemorrhage (particularly prior hemorrhage or presence of subclinical type 1 CNVM), as well as COVID-19–specific risk factors, would aid triage of clinic appointments in future lockdowns. High-risk patients would likely benefit from direct physician communication discussing their individual risk profiles to alleviate anxiety over clinic visits and communicate their risk of severe vision loss.
Keywords: age-related macular degeneration, choroidal neovascular membrane, macular hemorrhage, coronavirus, COVID-19
Introduction
Age-related macular degeneration (AMD) is one of the most encountered conditions in the 21st century retinal practice. A subretinal hemorrhage in the setting of untreated or undertreated choroidal neovascular membranes (CNVMs) can be a devastating consequence leading to irreversible vision loss. 1 Fortunately, with current treatment regimens with antivascular endothelial growth factor (anti-VEGF) injections, hemorrhages occur less frequently. However, there is strong literature about the risk of poor visual outcomes (often manifested in the form of hemorrhage) with delayed presentation, delayed treatment, or undertreatment. 2,3
Recently, owing to the spread of coronavirus disease 2019 (COVID-19), the world has undergone a dramatic shift in human-to-human interaction. 4 Medical clinics have undergone rapid practice pattern changes to minimize risk and exposure to patients, staff, and practitioners and have increased telemedicine services. Driven in large part by anxiety over exposure to the virus, patients have also avoided routine and even urgent medical care, leading to a drop in presentation for emergency conditions such as acute coronary syndrome. 5
Exposure concerns have had profound effects on the nature of retinal care, with practitioners and patients alike struggling with the balance of systemic and ocular risks. Data in the aftermath of the Italian COVID-19 lockdown showed a marked increase in the submacular hemorrhage rate in the setting of fewer clinic visits and injections. 6 Data from the Weill Cornell Department of Ophthalmology, and other practices outside New York City, demonstrated a dramatic drop in clinic visits for active neovascular AMD and injections between March 16, 2020, and May 8, 2020 (87% decline in clinic encounters and 58% decline in injections). 7 If patients are delaying care even in the setting of acute coronary syndrome, what steps can be taken to mitigate fear and continue to deliver effective retinal care? Already other subspecialties, specifically glaucoma, have attempted to develop metrics to find a balance between systemic risk of COVID-19 exposure during clinic visits and risk of ocular disease progression without monitoring. 8 It would behoove the retinal field to be prepared for such occurrences in the future.
In light of persistent COVID-19 cases currently in the United States and the possibility of a second wave, we sought to analyze outcomes of our patients during New York City’s previous lockdown to better anticipate similar public health crises in the future. In the present study we analyzed all established patients that presented to our service after the formal start of the COVID-19 lockdown in New York City on March 22, 2020, with submacular hemorrhages. The goals of the study were to (1) determine possible ocular and systemic risk factors for hemorrhage; (2) assess the duration of new ocular symptoms prior to presentation and geographic proximity to our clinic; (3) assess age and presence of COVID-19–specific risk factors in this cohort; and (4) review administrative records of patient communication regarding rescheduling or delaying appointments. By reviewing the combination of these factors, we hope to aid in more effective triage or advisement in the event of future similar episodes.
Methods
This study was a retrospective consecutive case series of 14 patients with macular hemorrhages presenting to the Weill Cornell Medicine Department of Ophthalmology. All were established patients to the practice.
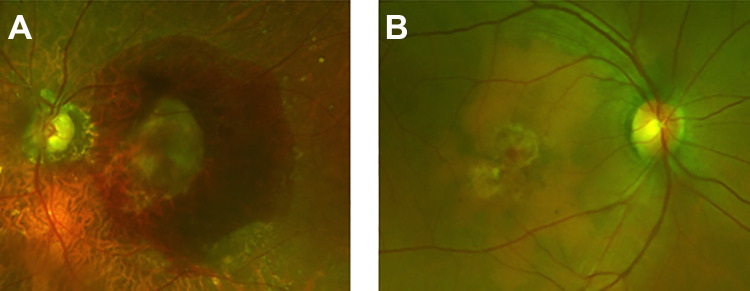
All patients with subretinal hemorrhage in the setting of active CNVM were included starting after March 22, 2020, when the citywide stay at home order went into effect, including clinic visits up to August 10, 2020. Patients included those with known active CNVM, those with nonexudative AMD who were already being monitored by our department, and those with inactive CNVM who had previously elected observation and were being serially followed. Medical records and images were reviewed for all patients, including imaging and clinical characteristics from serial follow-up examinations if available. The presence of hemorrhage as documented in the medical records was confirmed by review of fundus photography, fundus autofluorescence, and optical coherence tomography (OCT). Prior OCT images were reviewed to assess for possible biomarkers predicative of subsequent hemorrhage. Hemorrhages were denoted to be smaller or larger than 1 disc diameter in size (Figure 1). Maximum subretinal hemorrhage thickness was measured on OCT on the date of presentation.
Figure 1.
Color photographs (California, Optos) that demonstrate representative images of patients presenting with subretinal hemorrhages in the setting of delayed visits with active choroidal neovascular membranes. (A) Patient 2 shows a hemorrhage larger than 1 disc diameter, and (B) patient 7 shows a small subretinal bleed less than 1 disc diameter in size.
Ophthalmic examination and history were recorded, as was the duration of any subjective change in patient symptoms. As part of routine care during the current pandemic, patients were asked about any COVID-19–specific risk factors and concerns about their clinic exposure. COVID-19–specific risk factors included cancer, chronic obstructive pulmonary disease, chronic kidney disease, diabetes coronary artery disease, congestive heart failure, smoking, obesity, sickle cell disease, solid organ transplantation, chronic immunosuppressive medications, and asthma. 9 Patients’ geographic location as denoted by their home address was recorded. Emails and administrative records were reviewed for all communication about the scheduling and cancellation of appointments from the outset of the citywide lockdown.
Results
Fourteen eyes from 14 patients were included in the study. Table 1 provides a full description of the cohort. Mean age of presentation was 82.2 years, with only 1 patient younger than 70 years. Five patients were male and 9 were female. All patients had choroidal neovascularization, as opposed to neovascularization of retinal origin. Prior to the lockdown, 10 patients had active exudative AMD being treated with treat-and-extend regimens and 1 patient had inactive exudative AMD without need for injection for more than 2 years. Eight of these 11 patients (73%) had a history of hemorrhage on prior examinations, typically at the time of initial presentation. During the lockdown, 3 patients presented with new conversion from nonexudative AMD to exudative AMD with submacular hemorrhage. Of all 14 patients, 6 patients were not on anticoagulation, 4 patients were on aspirin 81 mg daily, and 4 patients were on aspirin 81 mg and additional anticoagulation.
Table 1.
List of All Patients Included in the Study.
| Patient | Age, y | Sex | Scheduled follow-up, d | Actual follow-up, d | Pre- BCVA | BCVA after hemorrhage | Baseline BCVA, fellow eye | CNVM status pre–COVID-19, last injected agent |
Size >1 disc diameter | Hemorrhage thickness, μm | Prior OCT risk features | Prior hemorrhage | Duration of subjective change, d | Anticoagulation | COVID-19 systemic risks | Systemic vascular comorbidities | Spoke with physician about delaying follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | F | 84 | 159 | 20/150 | 20/300 | 20/100 | Active, aflibercept | Yes | 944 | SHRM adjacent to active PED-CNVM | Yes | 60 | None | Asthma | HTN | No |
| 2 | 78 | M | 84 | 132 | 20/25 | CF 3 ft | 20/70 | Nonexudative | Yes | 1110 | Irregular PED with pigment | No | 90 | Asa 81 | COPD | HTN, OSA | No |
| 3 | 90 | M | 28 | 55 | 20/250 | CF 2 ft | 20/60 | Active, aflibercept | Yes | 46 | GA, shallow irregular PED | Yes | 3 | Asa 81, apixaban | Smoking/COPD | CAD/Afib | No |
| 4 | 97 | F | 240 | 268 | 20/30 | 20/70 | 20/70 | Nonexudative | No | 420 | Drusen, no signs of CNVM | No | 90 | Asa 81 | Asthma | HTN, heart block | No |
| 5 | 78 | M | 28 | 120 | 20/20 | 20/125 | 20/25 | Active, ranibizumab | No | 119 | Shallow irregular PED with pigment | Yes | 28 | Asa 81 | Diabetes | HTN, CAD | Yes |
| 6 | 83 | F | 63 | 103 | 20/25 | 20/40 | 20/100 | Active, aflibercept | No | 96 | Irregular PED adjacent to GA | Yes | 14 | Asa 81 | Mycophenolate mofetil for AI hepatitis | No | |
| 7 | 53 | F | 70 | 92 | 20/25 | 20/25 | 20/20 | Active, aflibercept | No | 90 | Active FCE-associated CNVM with SRF | Yes | 14 | None | None | No | |
| 8 | 86 | F | 42 | 137 | 20/70 | HM | 20/60 | Active, ranibizumab | Yes | 374 | Active CNVM, PED with SRF | No | – | Asa 81, clopidogrel | CHF, CKD4 | HTN, CAD | No |
| 9 | 96 | M | 63 | 116 | 20/100 | 10/125 | 20/300 | Active, aflibercept | Yes | 190 | Shallow irregular PED with pigment | No | 14 | Asa 81, clopidogrel | Smoking/COPD | Hx of AA s/p repair | No |
| 10 | 88 | M | 112 | 208 | CF 1 ft | CF 1 ft | 20/25 | Inactive | Yes | 360 | Disciform scar, irregular PED adjacent | Yes | – | Rivaroxaban | Smoking/CHF | CAD/Afib | No |
| 11 | 78 | F | 35 | 70 | 20/40 | 20/300 | 20/40 | Active, aflibercept | Yes | 528 | SHRM adjacent to active PED | No | 7 | None | Age only | No | |
| 12 | 86 | F | 180 | 201 | 20/100 | 20/150 | 20/200 | Nonexudative | No | 119 | Drusen, GA, no signs of CNVM | No | 1 | None | Smoking/COPD | No | |
| 13 | 70 | F | 42 | 210 | 20/150 | 20/150 | 20/25 | Active, aflibercept | No | 227 | Large, irregular PED (460-µm height) | Yes | 60 | None | Diabetes | No | |
| 14 | 90 | F | 49 | 188 | 20/50 | 20/200 | 20/25 | Active, aflibercept | No | 236 | Shallow, irregular PED | Yes | 30 | None | Smoking/COPD | No | |
| Mean | 82.2 ± 11.3 | 86.9 ± 66.8 | 147.1 ± 61.0 | ||||||||||||||
Abbreviations: AA, aortic aneurysm; Afib, atrial fibrillation; AI, autoimmune hepatitis; Asa, aspirin; BCVA, best-corrected visual acuity; CAD, coronary artery disease; CF, counting fingers; CHF, congestive heart failure; CKD, chronic kidney disease; CNVM, choroidal neovascular membrane; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; F, female; FCE, focal choroidal excavation; GA, geographic atrophy; HM, hand motion; HTN, hypertension; Hx, history; M, male; OCT, optical coherence tomography; OSA, obstructive sleep apnea; PED, pigment epithelial detachment; SHRM, subretinal hyperreflective material; s/p, status post; SRF, subretinal fluid.
All patients had extensive subretinal hemorrhage (without significant intraretinal hemorrhage). In 7 eyes (50%), there was robust expansion of the size of a preexisting pigment epithelial detachment (PED) with subretinal pigment epithelial hemorrhage. There was a range of subretinal hemorrhage thicknesses (46-1110 µm); however, most eyes had at least 250 µm of subretinal fluid (9 of 14, 64%). Seven patients (50%) had hemorrhages larger than 1 disc diameter in size.
Review of prior OCT images revealed that most patients had evidence of active CNVM or high-risk features in nonexudative eyes. Most eyes had scans suggestive of nonexudative neovascular AMD or controlled exudative AMD with a type 1 CNVM with irregular PED (8 eyes, 57%). Other eyes were actively being treated with anti-VEGF and had persistent subretinal hyperreflective material (2 eyes, 14%) or persistent subretinal fluid (2 eyes, 14%). Only 2 eyes (14%) had no discernable biomarkers at their last examination suggestive of CNVM presence or activity.
Of the 10 patients with CNVM being actively managed with anti-VEGF injections prior to COVID-19, the mean planned injection interval was 50.4 days (range, 28-84 days) and mean actual observed injection interval was 125 days (range, 55-210 days). Of the 10 patients with active CNVM, 8 were injected with aflibercept at their last visit and 2 were injected with ranibizumab. Five of these 10 patients also had active CNVM in the fellow eye being injected with anti-VEGF agents.
Six patients (43%) had 4 or more lines of vision loss. Eleven patients (79%) presented with vision of 20/100 or worse. Twelve patients (86%) had noticed a change in symptoms prior to presentation. Two patients had less than 1 week of symptoms, 4 patients had 1 to 2 weeks of symptoms, and 6 patients had more than 4 weeks of symptoms.
All patients had scheduled follow-up visits between March 22, 2020, and May 29, 2020. All patients called to delay their scheduled visits because of concerns about COVID-19 exposure. Nine patients (64%) lived in Manhattan. Only 1 patient had no COVID-19 systemic risk factors. One other patient had only 1 risk factor (age); however, that patient cited a spouse with multiple systemic risk factors. All 12 other patients (86%) had multiple COVID-19 systemic risk factors and specifically cited their “high-risk” nature as a reason for delaying follow-up and care. Only 1 patient spoke directly with a physician about the risks and benefits of delaying care, still opting to delay the scheduled follow-up. All other patients cancelled or repeatedly rescheduled appointments without direct physician contact until either new symptoms arose or the lockdown abated.
Conclusions
In summary, our study found 14 patients with subretinal hemorrhages secondary to delayed treatment of CNVMs during the New York City COVID-19 citywide lockdown in spring 2020. Most patients had lesions being actively treated with treat-and-extend regimens of anti-VEGF agents, although some were new conversions and 1 was a reactivation of a previously inactive lesion.
Submacular hemorrhage is a devastating diagnosis with profound visual implications. Most patients (64%) in this series had thicker hemorrhages (thickness >250 μm), suggesting significant severity with poor prognoses. Importantly, almost all patients (86%) had some discernable indicator on their most recent prior OCT suggestive of high-risk status. This included evidence of persistent known CNVM activity (subretinal fluid or subretinal hyperreflective material), or the presence of an irregular non-drusenoid PED suggestive of a type 1 CNVM.
All but 1 patient was aged 70 or older, with all but one of these older patients with additional COVID-19 systemic risk factors. These are, of course, representative of the normal cohort of patients with exudative AMD. 10 All patients expressed age and their comorbidities as a reason for delaying follow-up. In fact, despite the lockdown formally ending in May 2020, many of our patients presented months later, indicating their behavior was guided more by fear than government regulation. Our findings emphasize the importance of modifying clinic operations that allow for safe, risk-minimized patient care even during lockdowns given the high-risk patient cohort with exudative AMD. Most patients with hemorrhages were not coming from outside Manhattan, suggesting that distance and geography may not be contributing to delayed evaluation.
The clinics at Weill Cornell Medicine Department of Ophthalmology were open throughout the citywide lockdown, with a retina service member on site every day and modified clinic operations to streamline visits and minimize exposure. Despite this, as mentioned, the overall clinic volume dropped precipitously around this time (87% decline in clinic encounters and 58% decline in injections, as reported in another study). 7 In our cohort, only 1 patient had a direct discussion with a retina practitioner about the risks of delaying their appointment. All other patients called and rescheduled their appointments (or did so through the online patient portal) without requesting recommendations or a discussion with a physician. Physician communication with other patients with active exudative AMD during this period was frequent and robust. The combination of these findings (high-risk patient cohort with lack of communication) suggests that better physician counseling regarding specific personal risk profiles as well as clinic operation changes might help allay anxiety over injection visits. This seems especially prudent in patients with a history of prior hemorrhage or known active CNVM.
However, patients with nonexudative AMD in this hemorrhage cohort had their scheduled visits intentionally rescheduled by our department to minimize exposure. Perhaps these are the patients that would most benefit from home monitoring devices or hybrid telemedicine visits (imaging only with phone or written message discussing results). Furthermore, there were a considerable number of patients who had OCT findings suggestive of type 1 CNVM presence who may have benefitted from closer monitoring. Although we do not have absolute values of subclinical CNVM prevalence, our findings suggest that in the presence of future lockdowns, these patients with nonexudative neovascular AMD may benefit from closer follow-up.
Certainly, any patients with new symptoms should be having more thorough evaluations with OCT imaging, and within our cohort even these patients delayed evaluation because of COVID-19 exposure concerns. It is worth noting that even in newly converted patients there was a significant delay between onset of new symptoms and presentation to our clinics (1 patient had 1 day of symptoms but 2 others had months of symptoms). In fact, 10 of the 14 patients (71%) had more than 1 week of symptoms before presentation, again likely because of anxiety regarding clinic exposure.
Perhaps most important in prognosticating risk of presenting with a hemorrhage was prior appearance of a CNVM-associated hemorrhage (73%), regardless of the time since prior hemorrhage. While a seemingly obvious risk, this should likely be well noted in the patient’s medical record to aid in real-time conversations when the issue of rescheduling appointments is raised.
Many of the patients in our series were on antiplatelet medications (typically aspirin 81 mg), with only a small subset on more robust anticoagulation. Prior studies demonstrated mixed results in the association of anticoagulation use with hemorrhage. Some studies found no association; 11 however, a subanalysis of CATT (Comparison of Age-related AMD Treatments Trials) participants found increased hemorrhage rates in patients on anticoagulation who were also hypertensive. 12 Our cohort was far too small to draw meaningful implications of hemorrhage extent or presence with anticoagulation; however, like the presence of prior hemorrhage, it is likely prudent to denote concurrent use of anticoagulation in the medical record to facilitate patient-physician discussion and appointment triage.
Our findings also allude to the importance of sustained, long-acting delivery vehicles, many of which are already in development, to minimize the frequency of injection visits, and thus exposure, for our active exudative AMD patients during public health crises. 13 -15
This is obviously a limited study because of the small cohort and retrospective nature of the review, and with such a small number of patients it is difficult to determine statistical significance of hemorrhage risks. However, some broad concerns were identified. Based on our findings, we recommend thorough documentation of possible hemorrhage risk factors (prior presence of hemorrhage, concurrent anticoagulation use, and presence of suspected CNVM even if nonexudative), as well as COVID-19–specific risk factors (present in almost all patients in our cohort) in the medical record to better aid informed discussion in the event of future lockdowns. We further suggest that all patients with the aforementioned ocular risk factors have specific follow-up with physician calls or telemedicine visits to adequately counsel them on their risk for significant vision loss, as was often absent with our cohort. We hope that such recommendations do not become necessary but feel such preparation to be prudent.
Footnotes
Ethical Approval: The study received an exemption from the Weill Cornell Medical College Institutional Review Board and was conducted in accordance with the tenets of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner.
Statement of Informed Consent: Informed consent for the submission of this report was deferred because the patients are not identifiable from any of the collected images, and out of concern for placing undue stress on the patient. This was a retrospective medical-record review.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by an unrestricted department grant from Research to Prevent Blindness.
ORCID iDs: Kyle D. Kovacs, MD  https://orcid.org/0000-0001-7568-6703
https://orcid.org/0000-0001-7568-6703
Donald J. D’Amico, MD  https://orcid.org/0000-0002-4508-635X
https://orcid.org/0000-0002-4508-635X
References
- 1. Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999;213(2):97–102. doi:10.1159/000027400 [DOI] [PubMed] [Google Scholar]
- 2. Chong Teo KY, Saxena N, Gan A, et al. Detrimental effect of delayed re-treatment of active disease on outcomes in neovascular age-related macular degeneration: the RAMPS Study. Ophthalmol Retina. 2020;4(9):871–880. doi:10.1016/j.oret.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 3. Greenlee TE, Wang VY, Kang H, et al. Consequences of lapses in treatment with vascular endothelial growth factor inhibitors in neovascular age-related macular degenerations in routine clinical practice. Retina. Published online July 8, 2020. doi:10.1097/IAE.0000000000002888 [DOI] [PubMed] [Google Scholar]
- 4. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Filippo O, D’Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020;383(1):88–89. doi:10.1056/NEJMc2009166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romano F, Monteduro D, Airaldi M, et al. Increased number of submacular hemorrhages as a consequence of COVID-19 lockdown. Ophthalmol Retina. 2020;4(12):1209–1210. doi:10.1016/j.oret.2020.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Hamichi S, Gold A, Heier J, Kiss S, Murray TG. Impact of the COVID-19 pandemic on essential vitreoretinal care with three epicenters in the United States. Clin Ophthalmol. 2020;14:2593–2598. doi:10.2147/OPTH.S267950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bommakanti NK, Zhou Y, Ehrlich JR, et al. SOURCE Consortium. Application of the Sight Outcomes Research Collaborative Ophthalmology Data Repository for triaging patients with glaucoma and clinic appointments during pandemics such as COVID-19. JAMA Ophthalmol. 2020;138(9):974–980. doi:10.1001/jamaophthalmol.2020.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottlieb M, Sansom S, Frankenberger C, Ward E, Hota B. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad Emerg Med. 2020;27(10):963–973. doi:10.1111/acem.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1991;99(6):933–943. doi:10.1016/s0161-6420(92)31871-8 [DOI] [PubMed] [Google Scholar]
- 11. Buitendijk GHS, Schauwvlieghe ASME, Vingerling JR, Schlingemann RO, Klaver CCW; comparing Bevacizumab to Ranibizumab in Age-related macular degeneration (BRAMD) Trial Research Group. Antiplatelet and anticoagulant drugs do not affect visual outcome in neovascular age-related macular degeneration in the BRAMD trial. Am J Ophthalmol. 2018;187:130–137. doi:10.1016/j.ajo.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 12. Ying GS, Maguire MG, Daniel E, Grunwald JE, Ahmed O, Martin DF; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Association between antiplatelet or anticoagulant drugs and retinal or subretinal hemorrhage in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(2):352–360. doi:10.1016/j.ophtha.2015.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 Ladder clinical trial. Ophthalmology. 2019;126(8):1141–1154. doi:10.1016/j.ophtha.2019.03.036 [DOI] [PubMed] [Google Scholar]
- 14. Rakoczy EP, Magno AL, Lai CM, et al. Three-year follow-up of phase 1 and 2a rAAV.sFLT-1 subretinal gene therapy trials for exudative age-related macular degeneration. Am J Ophthalmol. 2019;204:113–123. doi:10.1016/j.ajo.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 15. Khanani AM, Patel SS, Ferrone PJ, et al. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138(9):964–972. doi:10.1001/jamaophthalmol.2020.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]