Abstract
Objective:
Premature adrenarche (PA) has been associated with an increase in adrenal androgens, and the hyperandrogenic hormonal environment is known to lead to increased platelet (PLT) aggregation. Here, we evaluated the effects of PA on PLT aggregation in PLT-rich plasma samples from female patients.
Methods:
The study included 40 female patients diagnosed with PA between February, 2014 and June, 2018 and 30 healthy female individuals as a control group. Adenosine diphosphate (ADP) and collagen-induced PLT aggregation were studied via the photometric aggregometry method.
Results:
There were no significant differences in the PLT count or volume values between those participants with PA and the control group. Additionally, the ADP-induced maximum aggregation time, value, and slope values did not significantly differ between the patient and control groups (p>0.05). However, the collagen-induced maximum aggregation time, value, and slope values were significantly higher in the studygroup (p<0.001).
Conclusion:
Increased collagen-induced PLT aggregation was detected in female patients with PA. As PA is associated with a higher risk of cardiovascular events later in life, close follow-up of PA in this respect may be beneficial.
Keywords: Premature adrenarche, cardiovascular diseases, hyperandrogenism, platelet aggregation, ADP, collagen
What is already known on this topic?
Premature adrenarche (PA) has been associated with an increase in adrenal androgens. A hyperandrogenic hormonal environment is known to lead to increased platelet (PLT) aggregation.
What this study adds?
We have shown increased collagen-induced PLT aggregation in girls with PA. This is significant as PA may cause increased cardiovascular event risks later in life due to increased PLT aggregation.
Introduction
Premature adrenarche (PA) has been associated with an increase in adrenal androgens due to the premature maturation of the zona reticularis layer of the adrenal cortex before the age of 8 in girls and 9 in boys (1). The most secreted androgens from the adrenal gland are dehydroepiandrosterone (DHEA) and androstenedione, which are weak androgens. DHEA undergoes sulfation in the liver and becomes DHEA-sulfate (DHEAS), and it is considered a marker of adrenal androgenic activity (2).
Girls with PA are at higher risk of developing symptoms of metabolic syndrome, including obesity and type 2 diabetes, and cardiovascular disease later in life (3). The mechanisms underlying these relationships remain unclear but have been partially associated with excess adipose tissue in adulthood (4). Indeed, earlier puberty is predictive of a higher adult body mass index (BMI) and a greater risk of obesity in women (5).
Cardiovascular diseases cause significant morbidity and mortality worldwide. Risk scoring systems have been developed to identify people at high risk of developing an adverse cardiovascular event by evaluating known risk factors (6). However, many patients whose risk assessments for cardiovascular diseases are determined to be at low or moderate levels may experience a cardiovascular event. Platelet (PLT) activity, which is not routinely found in current risk score algorithms, may also be a factor increasing cardiovascular risk (7).
The relationship between PLT aggregation and cardiovascular events has been evaluated in several studies (8). Different measures of PLT separation and purification methods and PLT aggregation measurements with different agonists at varying concentrations have been used in most of these studies (8). Although the data on increased PLT aggregation leading to cardiovascular events are far from conclusive, some significant results have been reported (8). A moderate increase in spontaneous PLT aggregation was detected in vascular events (8,9). It was recently shown that having an increased PLT aggregation response is associated with future arterial thrombosis, and the incidence of coronary heart disease-related mortality may increase significantly in these individuals (10).
Although there are various studies in children and adolescents regarding PLT counts and coagulation factors, as well as various PLT aggregation studies regarding non-hematological diseases (11,12,13,14), there are no studies on PLT counts and PLT aggregation in girls with PA.
In this case-control study, we investigated how PLT counts and aggregation are affected by adenosine diphosphate (ADP) and collagen agonists in girls with PA.
Methods
The patient group of this case-control study included 40 female patients diagnosed with PA between February, 2012 and June, 2018 (Group 1) at the pediatric outpatient clinic of Gülhane Training and Research Hospital (Ankara, Turkey). After the cases were diagnosed with PA, the relevant laboratory studies were conducted prospectively, and the cases and controls were followed up over a 6-year period. Girls with PA who had at least one clinical sign of adrenal androgen action (i.e., adult-type body odor, greasiness of hair and skin, comedones/acne, and axillary or pubic hair) together with increased DHEAS secretion before the age of 8 years, and other sources of hyperandrogenism (including central puberty, congenital adrenal hyperplasia, and androgen-producing tumors) were excluded. DHEAS concentrations of >40 μg/dL were considered adrenarche (15). Thirty healthy female individuals were included as the control group (Group 2). All girls in the control group were healthy, did not use any medication, and did not have any premature signs of androgen action.
Written informed consent from the families of the patients and approval of the Gülhane Training and Research Hospital Local Ethics Committee (date: 30.06.2009, no: 135) were obtained.
The inclusion criteria for both groups of patients were as follows: no use of antiplatelet drugs within the last 30 days, and no hematological diseases, chronic heart, kidney, and/or liver diseases (excluding PA in the patient group).
Complete blood count results, DHEAS, DHEA, luteinizing hormone (LH), 17-hydroxyprogesterone (17-OH progesterone), 11-deoxycortisol, adrenocorticotropic hormone (ACTH) and cortisol hormone levels, ferritin levels, prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen levels, and PLT aggregation were analyzed in both groups. Hormone levels were measured with the chemiluminescence method using the Beckman Coulter DxI® 600 analyzer.
Erythrocyte indices, PLT counts, and mean PLT volume (MPV) values were obtained using an automated device (Technicon H-1 System, Technicon Co, Tournai, Belgium).
Venous blood samples were obtained from the antecubital vein citrate between 8:00 and 9:30 in the morning after 8-12 hours of night fasting and collected into plastic syringes containing 3.8% trisodium by 1/10 volume. PLT-rich and PLT-poor plasma were prepared by centrifugation (16). PLT aggregation was assessed by photometric aggregometry using a complete blood aggregometer (Model 560; Chrono-Log Corporation, Havertown, PA, USA).
Collagen (5 µg/mL, Chrono Par No: 385; Chrono-Log Corporation) and ADP (10 µmol, Chrono Par No: 384; Chrono-Log Corporation) were used as agonists. The maximum aggregation time (s), value (%), and slope (%/min) were determined from the aggregation curves. The effects of ADP and collagen on aggregation were evaluated in both the control and patient groups considering the effect of iron deficiency on aggregation (17,18).
Statistical Analysis
The statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) software (version 22; SPSS Inc., Chicago, IL, USA). The data are presented as mean and standard deviation values. The normally distributed data were compared by independent samples t-test. We analyzed the correlations of DHEAS and DHEA levels with aggregation using the Pearson correlation test, and we used regression analysis for the aggregation values. Differences were considered statistically significant at p<0.05.
Results
Forty female patients diagnosed with PA were included in the patient group (Group 1), and thirty healthy female individuals constituted the control group (Group 2). The demographic characteristics of Groups 1 and 2 are given in Table 1. There were no significant differences between the groups in terms of age, height, weight, or BMI (p>0.05).
Table 1. Demographic characteristics of the study and control groups.
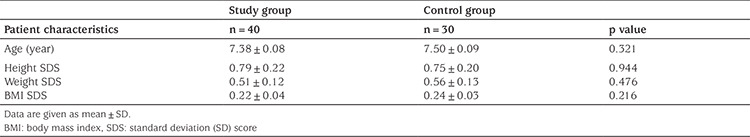
The complete blood count parameters of Groups 1 and 2 are given in Table 2. There were no significant differences between the groups in terms of their white blood cell count, red blood cell count, hemoglobin (HGB) level, hematocrit level, mean erythrocyte volume, mean erythrocyte HGB level, mean erythrocyte HGB concentration, red cell distribution width, PLT count, or MPV values (p>0.05).
Table 2. Complete blood parameters in the study and control groups.
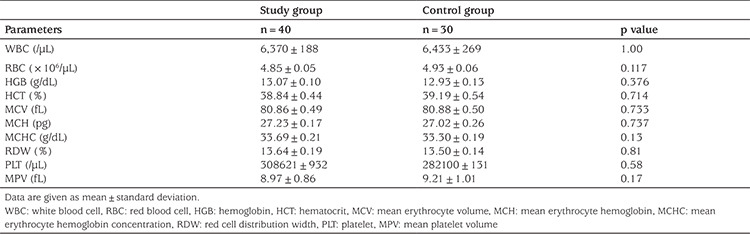
There was no significant difference between Groups 1 and 2 in terms of their plasma ferritin level, PT, aPTT, fibrinogen level, LH, 17-OH progesterone, 11-deoxycortisol, ACTH, or cortisol hormone level values (p>0.05); however, in the patient group, DHEAS and DHEA levels were significantly higher than of those in the control group (p<0.05; Table 3).
Table 3. DHEAS, DHEA, LH, 17-OH progesterone, 11-deoxycortisol, ferritin level, PT, aPTT, and fibrinogen level in the study and control groups.
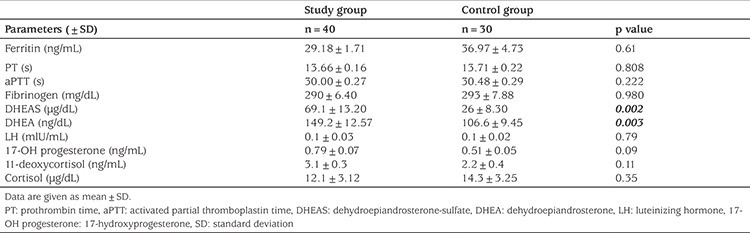
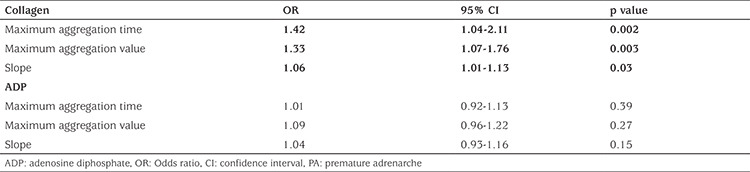
The mean maximum aggregation time, value, and slope induced by 10 µmol ADP and 5 µg/mL collagen in Groups 1 and 2 are shown in Table 4. In the patient group, at 10 µmol ADP, the mean maximum aggregation time, value, and slope did not significantly differ from the values in the control group (p>0.05). However, in the patient group, at a collagen concentration of 5 µg/mL, the mean maximum aggregation time, value, and slope were significantly higher than of those in the control group (p=0.001, 0.002, and 0.04, respectively). DHEAS was positively correlated with the maximum aggregation time (r=0.446, p=0.013), maximum aggregation value (r=0.397, p=0.018), and slope (r=0.263, p=0.034) values in collagen-induced PLT aggregation in the patients with PA. Similarly, DHEA was positively correlated with the maximum aggregation time (r=0.356, p=0.024), maximum aggregation value (r=0.308, p=0.029), and slope (r=0.217, p=0.039) values in collagen-induced PLT aggregation (Table 5). The results of the multivariate logistic regression analysis revealed that the maximum aggregation time [Odds ratio (OR); 95% confidence interval (CI) 1.42 (1.05–2.11); p=0.002], maximum aggregation value [OR; 95% CI 1.33 (1.07–1.76); p=0.003], and slope [OR; 95% CI 1.06 (1.01–1.13); p=0.03] in collagen-induced PLT aggregation were associated with PA in the patient group (Table 6).
Table 4. Platelet aggregation parameters of the study and control groups.
Table 5. Correlation analysis of androgen levels and platelet aggregation parameters in patients with PA.
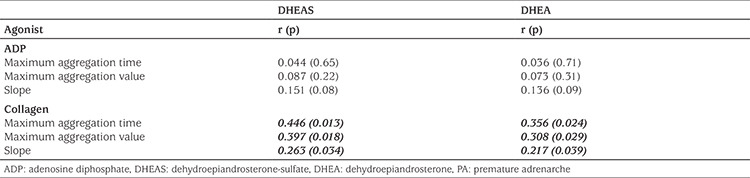
Table 6. Multivariable logistic regression analysis for PA in the study group.
Discussion
To the best of our knowledge, there is no study in the literature concerning PLT counts and PLT aggregation in girls with PA. In this study, we provide evidence of increased collagen-induced PLT aggregation in girls with PA.
Girls with a history of PA display a hyperandrogenic hormonal environment which may lead to increased cardiovascular risk (19,20,21,22,23,24,25). Çelik et al. (20) detected early atherosclerotic changes and subclinical deterioration in cardiac functions in children with PA. However, it was not fully explained why children with PA tend to show early cardiovascular changes. The authors showed that PA increased the risk of coronary heart disease, and this result was attributed to increased carotid intima-media thickness and epicardial adipose tissue measurements in PA patients. The increased risk of coronary heart disease in girls with PA in general was partly associated with the excess adipose tissue found in these patients in adulthood (5). It is thought that a process beginning with childhood obesity may ultimately lead to cardiovascular diseases in later life, in association with PA and adult obesity (21,22,23). The relationship of an atherogenic abnormal lipid profile such as increased serum triglyceride and low-density lipoprotein cholesterol with PA has also been demonstrated (24,25). Moreover, Topaktaş et al. (26) showed that cholesterol and triglyceride-related arterial involvement caused by obesity and metabolic syndrome are effective in the pathogenesis of arterial stiffness in PA, rather than increasing androgens.
Along with obesity and abnormal lipid status, it was found that a hyperandrogenic hormonal environment is also associated with PLT aggregation. It is known that sex steroids are absorbed at PLT membranes, and they modify the surface properties of these membranes. These modifications induce permeability changes (27). Sex steroids may also interact with fibrinogen, plasminogen, or fibrinolytic inhibitors (28). For instance, it was reported that testosterone increases the concentration of plasma prostaglandins (29). Furthermore, it was demonstrated that PLT aggregation induced by arachidonic acid (30) is enhanced after androgens are added under in vitro conditions (31). This effect of testosterone on PLT aggregation leads to an increase in thrombus formation and mortality. In a rat study, arterial thrombosis development was observed after the administration of testosterone (32). Human studies have also demonstrated that increased testosterone levels affect the induction of PLT aggregation. It was speculated that this effect might have resulted via testosterone receptors in PLTs (33). The decrease in the incidence of acute coronary syndrome in men with prostate cancer due to flutamide use was associated with these results (34).
Although sexual maturity has been shown to affect PLT aggregation in pigs, no study has investigated the role of PLT aggregation in the increased risk of cardiovascular disease in later life, either in girls or older women with a history of PA (35). In our study, we demonstrated a relationship between PA and collagen-induced PLT aggregation. In PA patients, mainly DHEA and DHEAS levels are increased, and these androgens are known as weak androgens. However, it is known that the most potent androgens have the greatest aggregation-increasing effect (33). This effect was more significant in collagen-induced PLT aggregation than ADP-induced PLT aggregation (36). In studies with weak androgens, inhibition, rather than an increase in PLT aggregation, has been observed. In a previous study, the in vitro administration of DHEAS showed dose- and time-dependent inhibition in arachidonate-induced PLT aggregation (37). In another study, the administration of DHEAS at physiological doses with thrombin or supraphysiological doses with collagen, thrombin, and TxA2 analog U-46619 also inhibited PLT aggregation (38). The difference in the results of our study may be due to the different potencies of DHEA and DHEAS in children.
In our study, we showed that collagen-induced PLT aggregation was increased in those girls with PA, and this may be due to increased DHEAS levels. Collagen is a strong agonist, but ADP is a weak one (39). In our study, ADP was used at a concentration of 10 µmol, which is sufficient for PLT aggregation (39). We did not detect any changes in aggregation at this level of ADP, but there was an increase in collagen, suggesting selectivity for collagen-induced PLT activation pathways. Similar to our findings, Leng et al. (40) achieved an increase in collagen-induced PLT aggregation, but not thrombin-induced PLT aggregation, by giving different estrogen derivatives to ovariectomized mice, which was done to examine the effects of estrogens on arterial thrombosis. In their study, estrogens acted in an agonist-specific fashion which changed collagen-induced PLT surface glycoprotein-VI expression, which then initiated adhesion followed by aggregation. Collagen, unlike ADP, also stimulates the release of thromboxane A2 (TxA2), a strong aggregation agent (41). Several studies have shown that adrenal androgens increase the number of TxA2 receptors (42,43). This may indicate that the increased collagen-induced PLT aggregation in our study may be due to increased adrenal androgens via glycoprotein-VI and/or TxA2. In order to explain this, studies showing the effects of androgens on PLT surface glycoprotein-VI expression are needed.
There are several studies which revealed normal and increased PLT counts in healthy people using anabolic androgenic steroids (43,44,45). In a study of 25-month-old Sprague-Dawley rats, a slight increase in PLT numbers was found with DHEAS treatment (46). In our study, we found no significant difference between the patient and control groups in terms of PLT counts and volumes.
Although an increased risk of coronary heart disease in girls with PA history is partly associated with the excess adipose tissue and the increased atherogenic lipid profile found in adulthood in these individuals, the increased PLT aggregation we found in girls with PA may ultimately lead to cardiovascular disease.
Study Limitations
Some limitations of our study were 1) the small sample size of our study may have been insufficient in the evaluation of PLT aggregation; 2) other high-risk factors for cardiovascular events were not discussed in detail in our study, and 3) it is not known how long the increase in PLT aggregation detected in these children with PA would last and whether this increase in aggregation would continue when they reach the normal age of puberty as we did not study the results of this test again in these children.
Conclusion
In conclusion, increased collagen-induced PLT aggregation was detected in girls with PA. As PA is associated with a higher risk of cardiovascular events later in life, close follow-up of PA may be beneficial. Repeated studies of PLT aggregation in patients with PA are needed in order to demonstrate whether the increase in collagen-induced PLT aggregation persists later in life.
Footnotes
Ethics
Ethics Committee Approval: The study was approved by the Gülhane Training and Research Hospital Local Ethics Committee (date: 30.06.2009, no: 135).
Informed Consent: Written informed consent from the families of the patients.
Peer-review: Externally peer-reviewed.
Authorship Contributions
Surgical and Medical Practices: Onur Akın, Concept: Orhan Gürsel, Mehmet Emre Taşçılar, Design: Ahmet Bolat, Cengiz Zeybek, Data Collection or Processing: Ahmet Bolat, Analysis or Interpretation: Ahmet Bolat, Literature Search: Ahmet Bolat, Cengiz Zeybek, Onur Akın, Writing: Ahmet Bolat, Cengiz Zeybek.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Palmert MR, Chan Y-M, Dunkel L. Puberty and its disorders in the male. Sperling Pediatric Endocrinology. Elsevier. 2021:p. 661–694. [Google Scholar]
- 2.Voutilainen R, Jääskeläinen J. Premature adrenarche: etiology, clinical findings, and consequences. J Steroid Biochem Mol Biol. 2015;145:226–236. doi: 10.1016/j.jsbmb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, Reiter EO, Sharpe RM, Skakkebaek NE, Toppari J. Public health implications of altered puberty timing. Pediatrics. 2008;121 Suppl 3:S218–S230. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- 4.Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015;5:11208. doi: 10.1038/srep11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond) 2013;37:1036–1043. doi: 10.1038/ijo.2012.177. [DOI] [PubMed] [Google Scholar]
- 6.Berger JS, Jordan CO, Lloyd-Jones D, Blumenthal RS. Screening for cardiovascular risk in asymptomatic patients. J Am Coll Cardiol. 2010;55:1169–1177. doi: 10.1016/j.jacc.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 7.Eagle KA, Ginsburg GS, Musunuru K, Aird WC, Balaban RS, Bennett SK, Blumenthal RS, Coughlin SR, Davidson KW, Frohlich ED, Greenland P, Jarvik GP, Libby P, Pepine CJ, Ruskin JN, Stillman AE, Van Eyk JE, Tolunay HE, McDonald CL, Smith SC Jr. Identifying patients at high risk of a cardiovascular event in the near future: current status and future directions: report of a national heart, lung, and blood institute working group. Circulation. 2010;121:1447–1454. doi: 10.1161/CIRCULATIONAHA.109.904029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma G, Berger JS. Platelet activity and cardiovascular risk in apparently healthy individuals: a review of the data. J Thromb Thrombolysis. 2011;32:201–208. doi: 10.1007/s11239-011-0590-9. [DOI] [PubMed] [Google Scholar]
- 9.Breddin HK, Lippold R, Bittner M, Kirchmaier CM, Krzywanek HJ, Michaelis J. Spontaneous platelet aggregation as a predictive risk factor for vascular occlusions in healthy volunteers? Results of the HAPARG Study. 1999;144:211–219. doi: 10.1016/s0021-9150(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 10.Puurunen MK, Hwang SJ, Larson MG, Vasan RS, O’Donnell CJ, Tofler G, Johnson AD. ADP Platelet Hyperreactivity Predicts Cardiovascular Disease in the FHS (Framingham Heart Study) J Am Heart Assoc. 2018;7:e008522. doi: 10.1161/JAHA.118.008522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toulon P, Berruyer M, Brionne-François M, Grand F, Lasne D, Telion C, Arcizet J, Giacomello R, De Pooter N. Age dependency for coagulation parameters in paediatric populations. Results of a multicentre study aimed at defining the age-specific reference ranges. Thromb Haemost. 2016;116:9–16. doi: 10.1160/TH15-12-0964. [DOI] [PubMed] [Google Scholar]
- 12.Lohse J, Schweigel J, Naeke A, Lee-Kirsch MA, Siegert G, Bergmann S, Kuhlisch E, Suttorp M, Knöfler R. Platelet function in obese children and adolescents. Hamostaseologie. 2010;30 Suppl 1:S126–S132. [PubMed] [Google Scholar]
- 13.Gursel O, Atay AA, Kurekci AE, Avcu F, Nevruz O, Senses Z, Ozturk E, Hasimi A, Ozcan O. Platelet aggregation in children with Helicobacter pylori infection. Clin Appl Thromb Hemost. 2010;16:637–642. doi: 10.1177/1076029609339747. [DOI] [PubMed] [Google Scholar]
- 14.Kontos A, Willoughby S, Lushington K, Martin J, Wabnitz D, Dorrian J, Kennedy D. Increased Platelet Aggregation in Children and Adolescents with Sleep-disordered Breathing. Am J Respir Crit Care Med. 2020;202:1560–1566. doi: 10.1164/rccm.201911-2229OC. [DOI] [PubMed] [Google Scholar]
- 15.Guran T, Firat I, Yildiz F, Kaplan Bulut I, Dogru M, Bereket A. Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clin Endocrinol (Oxf) 2015;82:712–718. doi: 10.1111/cen.12612. [DOI] [PubMed] [Google Scholar]
- 16.Munsterhjelm E, Niemi TT, Syrjälä MT, Ylikorkala O, Rosenberg PH. Propacetamol augments inhibition of platelet function by diclofenac in volunteers. Br J Anaesth. 2003;91:357–362. doi: 10.1093/bja/aeg195. [DOI] [PubMed] [Google Scholar]
- 17.Kürekçi AE, Atay AA, Sarící SU, Zeybek C, Köseoğlu V, Ozcan O. Effect of iron therapy on the whole blood platelet aggregation in infants with iron deficiency anemia. Thromb Res. 2000;97:281–285. doi: 10.1016/s0049-3848(99)00150-4. [DOI] [PubMed] [Google Scholar]
- 18.Naimushin YA, Mazurov AV. Von Willebrand factor can support platelet aggregation via interaction with activated GPIIb-IIIa and GPIb. Platelets. 2004;15:419–425. doi: 10.1080/09537100410001721333. [DOI] [PubMed] [Google Scholar]
- 19.Livadas S, Bothou C, Macut D. Premature Adrenarche and its Association with Cardiovascular Risk in Females. Curr Pharm Des. 2020;26:5609–5616. doi: 10.2174/1381612826666201012164726. [DOI] [PubMed] [Google Scholar]
- 20.Çelik N, Alp H, Çamtosun E, Alp E, Çelik S, Berk E. The Association between Premature Adrenarche and Cardiovascular Risk May Be Greater than Expected. Horm Res Paediatr. 2017;87:7–14. doi: 10.1159/000452445. [DOI] [PubMed] [Google Scholar]
- 21.Bjørge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168:30–37. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 22.Freedman DS, Patel DA, Srinivasan SR, Chen W, Tang R, Bond MG, Berenson GS. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 2008;32:749–756. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 23.Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibáñez L, Potau N, Chacon P, Pascual C, Carrascosa A. Hyperinsulinaemia, dyslipaemia and cardiovascular risk in girls with a history of premature pubarche. Diabetologia. 1998;41:1057–1063. doi: 10.1007/s001250051030. [DOI] [PubMed] [Google Scholar]
- 25.Güven A, Cinaz P, Bideci A. Is premature adrenarche a risk factor for atherogenesis? Pediatr Int. 2005;47:20–25. doi: 10.1111/j.1442-200x.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 26.Topaktaş E, Erolu E, Dursun F, Kırmızıbekmez H. Evaluation of metabolic parameters and aortic elasticity in normotensive children with premature adrenarche. J Pediatr Endocrinol Metab. 2021;34:1009–1015. doi: 10.1515/jpem-2021-0160. [DOI] [PubMed] [Google Scholar]
- 27.Florence AT, Rahman R. The effect of some oral contraceptive steroids on platelet electrophoretic mobility in vitro. J Pharm Pharmacol. 1972;24:983–985. doi: 10.1111/j.2042-7158.1972.tb08932.x. [DOI] [PubMed] [Google Scholar]
- 28.Turksoy Rn, Phillips Ll, Southam Al. Influence of ovarian function on the fibrinolytic enzyme system I Ovulatory and anovulatory cycles. Am J Obstet Gynecol. 1961;82:1211–1215. doi: 10.1016/s0002-9378(16)36242-1. [DOI] [PubMed] [Google Scholar]
- 29.Barcikowski B, Saksena SK, Bartke A. Androgenic regulation of plasma prostaglandin F levels in the rat. J Reprod Fertil. 1973;35:549–551. doi: 10.1530/jrf.0.0350549. [DOI] [PubMed] [Google Scholar]
- 30.Sharma HM, Moore S, Merrick HW, Smith MR. Platelets in early hyperacute allograft rejection in kidneys and their modification by sulfinpyrazone (Anturan) therapy An experimental study. Am J Pathol. 1972;66:445–460. [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson M, Ramey E, Ramwell PW. Sex and age differences in human platelet aggregation. Nature. 1975;253:355–357. doi: 10.1038/253355a0. [DOI] [PubMed] [Google Scholar]
- 32.Uzunova A, Ramey E, Ramwell PW. Effect of testosterone, sex and age on experimentally induced arterial thrombosis. Nature. 1976;261:712–713. doi: 10.1038/261712a0. [DOI] [PubMed] [Google Scholar]
- 33.Johnson M, Ramey E, Ramwell PW. Androgen-mediated sensitivity in platelet aggregation. Am J Physiol. 1977;232:H381–H385. doi: 10.1152/ajpheart.1977.232.4.H381. [DOI] [PubMed] [Google Scholar]
- 34.Boccon-Gibod L, Fournier G, Bottet P, Marechal JM, Guiter J, Rischman P, Hubert J, Soret JY, Mangin P, Mallo C, Fraysse CE. Flutamide versus orchidectomy in the treatment of metastatic prostate carcinoma. Eur Urol. 1997;32:391–395. discussion 395-396.. [PubMed] [Google Scholar]
- 35.Jayachandran M, Okano H, Chatrath R, Owen WG, McConnell JP, Miller VM. Sex-specific changes in platelet aggregation and secretion with sexual maturity in pigs. J Appl Physiol (1985) 2004;97:1445–1452. doi: 10.1152/japplphysiol.01074.2003. [DOI] [PubMed] [Google Scholar]
- 36.Ferenchick G, Schwartz D, Ball M, Schwartz K. Androgenic-anabolic steroid abuse and platelet aggregation: a pilot study in weight lifters. Am J Med Sci. 1992;303:78–82. doi: 10.1097/00000441-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Jesse RL, Loesser K, Eich DM, Qian YZ, Hess ML, Nestler JE. Dehydroepiandrosterone inhibits human platelet aggregation in vitro and in vivo. Ann N Y Acad Sci. 1995;774:281–290. doi: 10.1111/j.1749-6632.1995.tb17388.x-i1. [DOI] [PubMed] [Google Scholar]
- 38.Muñoz YC, Gomez GI, Moreno M, Solis CL, Valladares LE, Velarde V. Dehydroepiandrosterone prevents the aggregation of platelets obtained from postmenopausal women with type 2 diabetes mellitus through the activation of the PKC/eNOS/NO pathway. Horm Metab Res. 2012;44:625–631. doi: 10.1055/s-0032-1309056. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Schmaier AH. Platelet aggregation testing in platelet-rich plasma: description of procedures with the aim to develop standards in the field. Am J Clin Pathol. 2005;123:172–183. doi: 10.1309/y9ec-63rw-3xg1-v313. [DOI] [PubMed] [Google Scholar]
- 40.Leng XH, Zhang W, Nieswandt B, Bray PF. Effects of estrogen replacement therapies on mouse platelet function and glycoprotein VI levels. Circ Res. 2005;97:415–417. doi: 10.1161/01.RES.0000181025.43762.cf. [DOI] [PubMed] [Google Scholar]
- 41.Woulfe D, Yang J, Brass L. ADP and platelets: the end of the beginning. J Clin Invest. 2001;107:1503–1505. doi: 10.1172/JCI13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742–2747. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 43.Zucker TP, Higashiura K, Mathur RS, Halushka PV. Androstenedione increases thromboxane A2 receptors in human erythroleukemia cells. Life Sci. 1996;58:683–690. doi: 10.1016/s0024-3205(96)80007-5. [DOI] [PubMed] [Google Scholar]
- 44.Zitzmann M, Junker R, Kamischke A, Nieschlag E. Contraceptive steroids influence the hemostatic activation state in healthy men. J Androl. 2002;23:503–511. [PubMed] [Google Scholar]
- 45.Severo CB, Ribeiro JP, Umpierre D, Da Silveira AD, Padilha MC, De Aquino Neto FR, Stein R. Increased atherothrombotic markers and endothelial dysfunction in steroid users. Eur J Prev Cardiol. 2013;20:195–201. doi: 10.1177/2047487312437062. [DOI] [PubMed] [Google Scholar]
- 46.Strasser A, Dedoyard A, Lohninger A, Niedermüller H. L-Carnitine L-tartrate (LCLT) and dehydroepiandrosterone sulfate (DHEAS) affect red and white blood cells in aged Sprague-Dawley rats. Arch Gerontol Geriatr. 2007;44:325–336. doi: 10.1016/j.archger.2006.07.003. [DOI] [PubMed] [Google Scholar]


