Abstract
Objective:
Data regarding diabetic ketoacidosis (DKA) at diagnosis of type one diabetes (T1D) in developing countries are scarce. The aim of this study was to describe the frequency of DKA at the onset of T1D in children and adolescents in Jordan and to compare the clinical and biochemical characteristics between the group that presented with DKA and the group that did not.
Methods:
The records of 341 children and adolescents, less than sixteen years of age, who were diagnosed with T1D between 2015 and 2019 were evaluated retrospectively.
Results:
Of all the children diagnosed with T1D, 108 (31.7%) presented with DKA. The majority had mild or moderate DKA (38% and 33.3% respectively). Higher paternal education levels were associated with a lower probability of presenting with DKA (p=0.043). A family history of T1D had a protective effect on the occurrence of DKA (Odds ratio=2.138; 95% confidence interval=1.167-3.917, p=0.014). Patients with celiac disease and higher HbA1c levels were more likely to experience recurrent episodes of DKA, (p=0.004 and 0.011, respectively).
Conclusion:
In Jordan, the rate of DKA at presentation of T1D remains high. Prevention campaigns are needed to increase diabetes awareness among the public and healthcare providers.
Keywords: Type one diabetes, diabetic ketoacidosis, Jordan
What is already known on this topic?
Rates and predictors of diabetic ketoacidosis (DKA) at onset of type one diabetes (T1D) vary worldwide. Data from developing countries are scarce.
What this study adds?
The frequency of DKA at diagnosis of T1D in Jordan is relatively high at 31.7%. In this study, being aged less than two years and lower paternal education and employment levels were associated with DKA at diagnosis of T1D. A family history of T1D was protective against presenting with DKA at onset of T1D.
Introduction
Type one diabetes (T1D) is one of the most common chronic endocrine disorders which affects children and adolescents worldwide (1,2). Diabetic ketoacidosis (DKA) is a known acute complication of T1D which can be present at time of diagnosis or occur afterwards. It results from a deficiency of circulating insulin and increased levels of the counter regulatory hormones: catecholamines, glucagon, cortisol, and growth hormone (3). Despite the recent reported decrease in all-cause mortality in some populations with T1D, DKA remains the most common cause of death in children and adolescents with T1D (4,5). Moreover, DKA results in significant morbidity and is considered as a predictor of poor glycemic control (6,7). Overall mortality for children with DKA varies from 0.15 to 0.35% in developed countries (8,9) and from 3.4 to 13.4% in developing countries (10,11). In addition, there are parts of the world, such as some countries in Africa, where mortality rates at onset of T1D are under-reported and might be much higher. This could be due to the inability of families to promptly reach medical care for reasons related to unavailability or remote access. Globally, reported DKA rates at the time of T1D diagnosis vary from 14.7% to 79.8% (12). Data from the middle eastern region are scarce and a systematic review by Zayed (13) showed DKA rates between 17% and 100% at the time of T1D diagnosis in various middle eastern countries. In Jordan, we previously reported a DKA rate of 40.7% at the time of T1D diagnosis (14).
The aim of our study was to describe the frequency of DKA at the onset of T1D in children in Jordan in comparison with earlier data, to compare the clinical and biochemical characteristics of children and adolescents who presented with DKA at diagnosis of T1D and those who did not, to compare children who had recurrent episodes of DKA after diagnosis with the rest of the cohort, and to identify the risk factors associated with DKA development.
Methods
Subjects and Study Design
This was a retrospective cohort study of all children and adolescents who were less than 16 years of age at diagnosis of T1D at Jordan University Hospital from January, 2015 to December, 2019. The electronic medical records of 341 children who presented to our service were reviewed and their data were retrieved after obtaining approval from Institutional Ethics Committee of Jordan University Hospital, Amman, Jordan (approval no.: 99/2021, dated: 14/03/2021). Any type of diabetes other than T1D was excluded from this study.
Socio-demographic data included: birth date, sex, date of T1D diagnosis, presenting symptoms, family history of T1D and type 2 diabetes (T2D) in first and second-degree relatives and the levels of education and the occupations of both parents. Families with missing data were contacted by phone. Laboratory investigations at diagnosis were collected including: venous blood gas results, electrolytes, creatinine, blood glucose levels, glycosylated hemoglobin (HbA1c), glutamic acid decarboxylase antibodies (GAD Ab), islet cell antibodies, thyroid peroxidase antibodies (TPO), thyroglobulin antibodies, tissue transglutaminase IgA antibodies, thyroid stimulating hormone and free thyroxine.
DKA with its various levels of severity were defined according to the International Society for Pediatric and Adolescent Diabetes guidelines 2018 as follows: hyperglycemia [blood glucose >11 mmol/L (200 mg/dL)], venous pH <7.3 or serum bicarbonate <15 mmol/L with ketonemia or ketonuria. Mild DKA was defined as venous pH <7.3 or serum bicarbonate <15 mmol/L, moderate DKA as pH <7.2 or serum bicarbonate <10 mmol/L and severe DKA as pH <7.1 or serum bicarbonate <5 mmol/L (15).
The cohort was divided into two groups: the DKA at onset of T1D group and the no DKA at onset of T1D group. Both groups were compared with each other in terms of age at diagnosis, sex, season of presentation, presenting signs and symptoms, family history of T1D and/or T2D, the education levels and the occupations of both parents and their laboratory investigations. The group of patients who developed two or more DKA episodes excluding the one at presentation were termed the recurrent DKA group and they were also compared with the rest of the cohort.
Statistical Analysis
Statistical analysis was performed using IBM Statistical Package for the Social Sciences statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA). Continuous data were presented as mean±standard deviation, and categorical data as frequency (%). Associations between categorical variables were evaluated using chi-squared analysis. Associations between continuous variables were evaluated using the independent samples t-test. Univariate and multivariate logistic regression was used to assess possible predictors of dichotomous dependent variables. Statistical significance was assumed for p values less than 0.05.
Results
A total of 341 children were enrolled in this study, 161 (47.2%) were males. The average age of the children was 11.03±3.88 years and the average duration of T1D was 2.75±1.47 years. Almost one third of the children had DKA at time of diagnosis, 108 (31.7%). Several characteristics and symptoms were compared between the group which presented with DKA at T1D diagnosis and the group that did not; age at diagnosis, sex, polyurea, polydipsia, enuresis, and weight loss were not significantly different in the participants of both groups. However, abdominal pain, vomiting, and rapid breathing were significantly higher in the DKA at onset group, p=0.015, 0.017, 0.008 respectively.
The frequency of DKA in different age groups, different years of diagnosis, and different seasons of the year were all statistically non-significant, p=0.563, 0.578, and 0.654, respectively, Figure 1.
Figure 1.
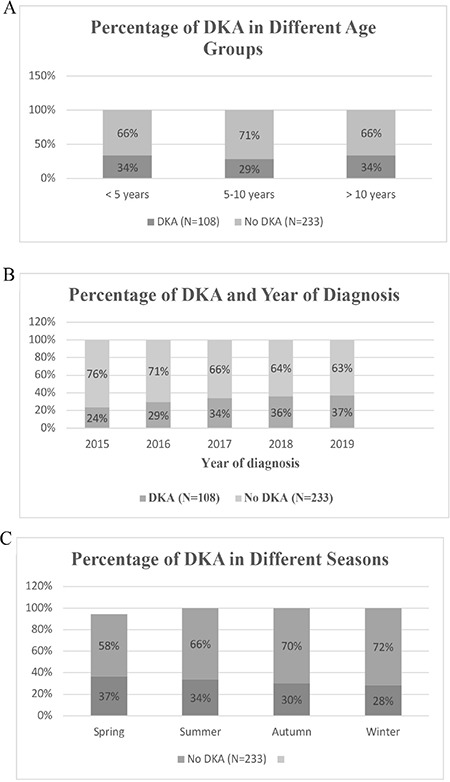
Frequency of DKA according to age at diagnosis (A), year of diagnosis (B), and different seasons (C). All were statistically non-significant
DKA: diabetic ketoacidosis
Further analysis of the years of diagnosis showed that the age at diagnosis, presences of celiac disease, recurrent DKA and HbA1c at diagnosis were all statistically non-significant, p=0.424, 0.325, 0.372, 0.955, respectively.
When the children were categorized into two groups according to age, as two years or younger and older than two years, the difference in the frequency of DKA neared significance with a p value of 0.056. Results of the analysis showed that 50.0% of the children who were two years or younger presented with DKA compared to 30.4% in those children older than two years.
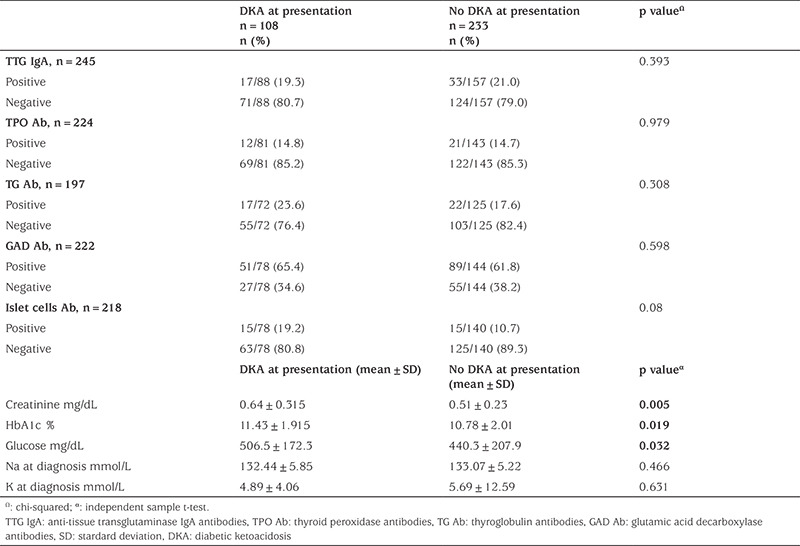
Different laboratory tests were evaluated, and values among children who had DKA at time of diagnosis and those who did not were compared. Creatinine levels, glucose levels, and HbA1c at diagnosis were significantly higher in those children who had DKA at time of diagnosis, Table 1.
Table 1. Laboratory characteristics of children in both groups.
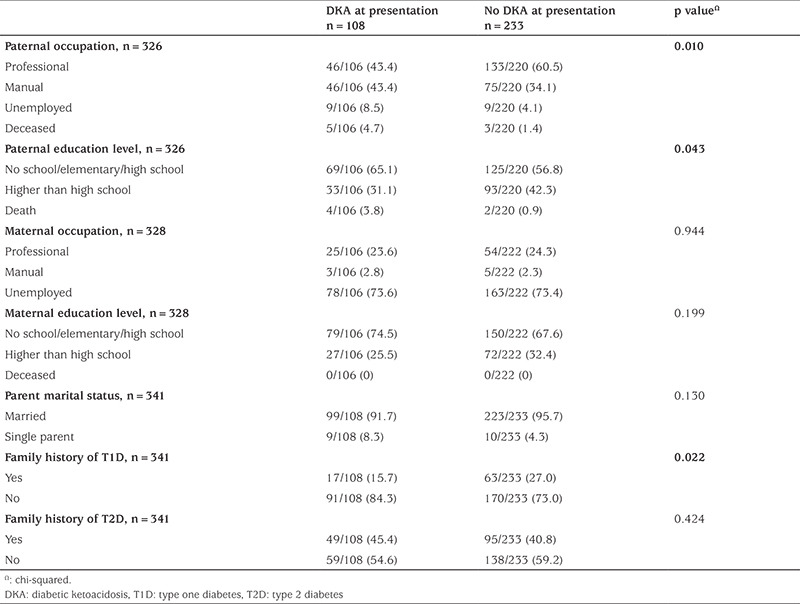
The socioeconomic status of both groups (with/without DKA) was compared. Variables included paternal and maternal occupations and education levels, Table 2.
Table 2. Socio-economic characteristics of children in both groups.
Among the 108 children who had DKA at the time of T1D diagnosis; 41 (38.0%) had mild DKA, 36 (33.3%) had moderate DKA and 31 (28.7%) had severe DKA. Further analysis revealed that the severity of DKA was not associated with sex or age. Degrees of severity of DKA in males and females and in the different age groups were compared, and there were no statistically significant differences, p values=0.857 and 0.998, respectively.
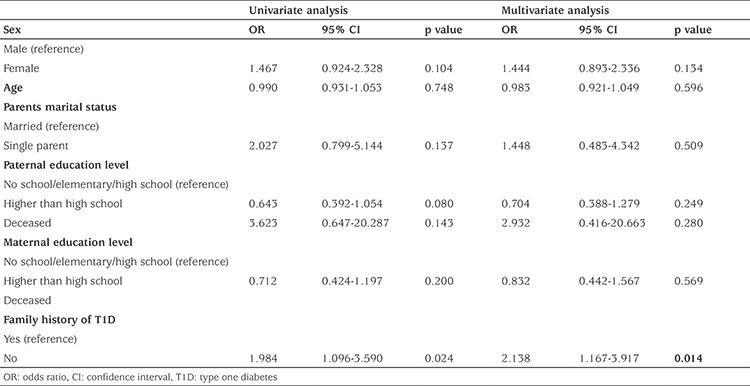
Possible predictors of DKA at diagnosis were analyzed, and family history of T1D was the only statistically significant predictor of DKA. Children with no family history of T1D were two times more likely to present with DKA than those with a family history of T1D (OR=2.138, p=0.014), Table 3.
Table 3. Predictors of diabetic ketoacidosis at diagnosis of type one diabetes.
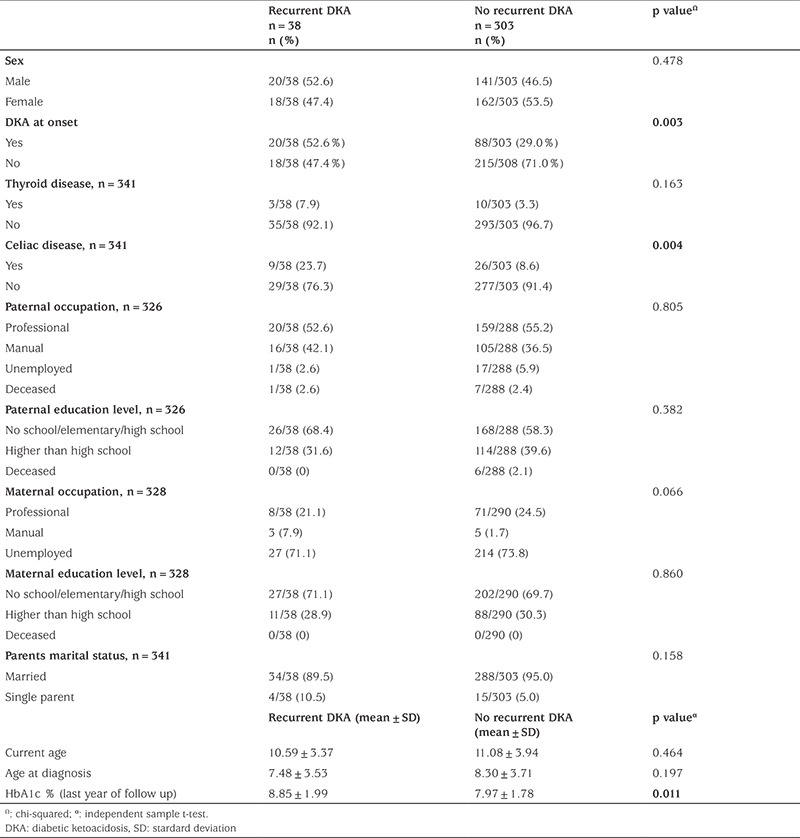
Thirty-eight children (11.1%) had recurrent episodes of DKA. Those with celiac disease had a significantly higher percentage of recurrent DKA, 23.7%, p=0.004. In addition, those children with recurrent DKA had a significantly higher HbA1c than those without recurrent DKA, 8.85% and 7.97%, respectively p=0.011, Table 4.
Table 4. Characteristics of children with and without recurrent DKA.
Discussion
The results from this study identified an association between presentation with DKA at T1D onset and lower paternal education and employment levels. In addition, having a positive family history of T1D was protective against the development of DKA at T1D diagnosis. Furthermore, higher levels of HbA1c and having celiac disease as a comorbidity were associated with recurrent episodes of DKA.
Rate of DKA at Diagnosis of T1D with Regional and International Comparison
In our analysis, the DKA rate at manifestation of T1D in children and adolescents under 16 years of age was 31.7%. This rate is almost 10% less than our previously reported rate of 40.7% (14). This could be due to the fact that we are reporting from a tertiary hospital with an established pediatric diabetes practice which resulted in good awareness and the prompt recognition of diabetes symptoms. This is supported by a study from Kuwait which showed that DKA at onset of T1D was significantly more common in hospitals lacking a structured diabetes team (p<0.002) (16). Studies from the Middle East region showed almost similar rates of DKA at onset of T1D ranging between 33.6% in Kuwait, 31% in Oman and 37.7% in Saudi Arabia (17,18,19). In Sudan, however, a recent study reported a DKA rate of 17.6% at diagnosis of T1D (20). This variation in the rates of DKA at diagnosis of T1D was also seen in developed countries ranging from 19.5% and 19.8% in Sweden and Germany to 41.2% and 43.8% in Italy and Luxembourg respectively (21,22). In the USA and the UK, these rates were 36.9% and 25% respectively (22). Countries with higher incidence of T1D and hence more awareness of this disease were reported to have lower rates of DKA at T1D diagnosis due to prompt diagnosis and early treatment initiation (23,24). Unfortunately, many Middle Eastern countries, including Jordan, lack T1D registries and both the incidence and prevalence rates are unknown. Many other factors were studied as contributors to presentation with DKA at the time of T1D diagnosis, such as age, sex, family history of T1D, ethnic background and the socioeconomic status of the families (25).
Factors Associated with the Development of DKA at Diagnosis of T1D
Several studies investigated the effect of the level of education and parental employment on the possibility of having DKA at T1D diagnosis with variable findings. In our study, we found that having a father with a higher educational level and/or working in a professional job was associated with a lower probability of presenting with DKA at onset of T1D. This is in support of other studies which linked higher educational and employment levels of at least one of the parents to a decreased likelihood of presenting with DKA at diagnosis of T1D (25). A study which was conducted in Italy showed significantly higher DKA frequencies (both overall and severe) in children of 0.5-4 years of age, with both a low level of mother’s education and parents’ occupation (26). An explanation to this could be that having a higher educational level might prompt the family to seek medical advice earlier upon recognition of symptoms suggestive of diabetes. In addition, having a professional job is usually linked to being medically insured.
As for the age at T1D diagnosis, we found an association between young age (below two years) and presentation with DKA with a near statistically significant p value of 0.056. This association has been reported by many other studies from different parts of the world (25,27). This could be due to many factors such as a lower index of suspicion of diabetes in this age group where the classical symptoms are not very clear to clinicians. In addition, this may be due to these children having a stronger humoral autoimmunity and aggressive destruction of beta cells compared to older age groups (28). This highlights the importance of raising awareness among healthcare professionals on the different patterns of presentation of diabetes in different age groups.
In our cohort, having a family history of T1D was the only protective factor against presenting with DKA at diagnosis of T1D. This is probably due to increased awareness among families with prior experience of diabetes or having a family history of diabetes alerting clinicians to an increased possibility of T1D (16,25). This fact emphasizes the importance of awareness among parents and clinicians about the early symptoms of diabetes in preventing delays in diagnosis and hence the development of DKA. Studies have demonstrated that awareness campaigns were successful in reducing the percentage of children presenting with DKA at diagnosis of T1D. In Parma, Italy, during the 8 years of their campaign, the cumulative frequency of DKA dropped from 78% to 12.5% (29). This was replicated with a more modest impact in Saudi Arabia where, after launching a diabetes awareness campaign, DKA rates at diagnosis of T1D dropped from 48% in 2010 to 39% in 2014 (30).
Factors Associated with the Development of Recurrent DKA
Recurrent episodes of DKA were associated with an increase in mortality rates up to 23.3% in people who have had more than five episodes of DKA compared to 5.2% in people with one episode (31). Many modifiable and non-modifiable risk factors for recurrent DKA have been identified and it is of high importance to recognize patients at risk and work on preventing further episodes of DKA (32). During the follow-up of our cohort, recurrent episodes of DKA were seen in those patients with higher HbA1c concentrations. There is strong evidence in the literature that elevated HbA1c level is a risk factor for recurrent DKA in children and adolescents with T1D (33,34). It is a marker of poor metabolic control which might be due to general problems with diabetes management, such as non-adherence or lack of knowledge. Hence, addressing these issues might contribute to a reduction in further episodes of DKA in this group of patients. The second association with recurrent DKA in our cohort was the diagnosis of celiac disease as a comorbidity with T1D. In a study from the DPV registry, 608 patients with biopsy proven celiac disease and T1D were followed for three years, and their celiac disease specific antibody status was observed. The group which reached antibody negative status had better HbA1c and lesser rates of DKA in comparison to the group which continued to have positive antibodies and in comparison to the general T1D group which did not have celiac disease. The authors suggested that the gluten free diet-adherent, antibody-negative patients were more compliant to therapy, in general, and hence, had better metabolic control (35). This might indicate worse adherence to management in our patients who have celiac disease in addition to T1D.
Study Limitations
Limitations to our study are its retrospective design and the fact that it might not represent the whole country as it is reporting from a single tertiary center.
Conclusion
Despite the reduction in our reported rate of DKA at T1D diagnosis, it is still considered high. Awareness campaigns for the public and the health care professionals should be implemented and continued in an effort to reduce the rates of DKA whether at T1D diagnosis or thereafter.
Footnotes
Ethics
Ethics Committee Approval: The study was approved by the Institutional Ethics Committee of Jordan University Hospital, Amman, Jordan (approval no.: 99/2021, dated: 14/03/2021).
Informed Consent: Retrospective cohort study.
Peer-review: Externally peer-reviewed.
Authorship Contributions
Concept: Rasha Odeh, Abeer Alassaf, Design: Rasha Odeh, Abeer Alassaf, Data Collection or Processing: Lobna Gharaibeh, Bahaa Ashour, Fatima Al Barakat, Dina Dahabreh, Hiba Hadadin, Tala Melhem, Analysis or Interpretation: Rasha Odeh, Lobna Gharaibeh, Amirah Daher, Jumana Albaramki, Abeer Alassaf, Literature Search: Rasha Odeh, Amirah Daher, Jumana Albaramki, Bahaa Ashour, Fatima al Barakat, Dina Dahabreh, Hiba Hadadin, Tala Melhem, Writing: Rasha Odeh, Lobna Gharaibeh, Amirah Daher, Jumana Albaramki, Bahaa Ashour, Fatima al Barakat, Dina Dahabreh, Hiba Hadadin, Tala Melhem, Abeer Alassaf.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 2.Ogle GD, James S, Dabelea D, Pihoker C, Svennson J, Maniam J, Klatman EL, Patterson CC. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res Clin Pract. 2022;183:109083. doi: 10.1016/j.diabres.2021.109083. [DOI] [PubMed] [Google Scholar]
- 3.Foster DW, McGarry JD. The metabolic derangements and treatment of diabetic ketoacidosis. N Engl J Med. 1983;309:159–169. doi: 10.1056/NEJM198307213090307. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz PLD, Chen L, Morton JI, Salim A, Carstensen B, Gregg EW, Pavkov ME, Mata-Cases M, Mauricio D, Nichols GA, Pildava S, Read SH, Wild SH, Shaw JE, Magliano DJ. Mortality trends in type 1 diabetes: a multicountry analysis of six population-based cohorts. Diabetologia. 2022;65:964–972. doi: 10.1007/s00125-022-05659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan E, Black CR, Abid N, Cardwell CR, McCance DR, Patterson CC. Mortality in type 1 diabetes diagnosed in childhood in Northern Ireland during 1989-2012: A population-based cohort study. Pediatr Diabetes. 2018;19:166–170. doi: 10.1111/pedi.12539. [DOI] [PubMed] [Google Scholar]
- 6.Cameron FJ, Scratch SE, Nadebaum C, Northam EA, Koves I, Jennings J, Finney K, Neil JJ, Wellard RM, Mackay M, Inder TE; DKA Brain Injury Study Group. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. 2014;37:1554–1562. doi: 10.2337/dc13-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duca LM, Wang B, Rewers M, Rewers A. Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes Predicts Poor Long-term Glycemic Control. Diabetes Care. 2017;40:1249–1255. doi: 10.2337/dc17-0558. [DOI] [PubMed] [Google Scholar]
- 8.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990-96. Arch Dis Child. 1999;81:318–323. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis JR, To T, Muirhead S, Cummings E, Daneman D. Recent trends in hospitalization for diabetic ketoacidosis in Ontario children. Diabetes Care. 2002;25:1591–1596. doi: 10.2337/diacare.25.9.1591. [DOI] [PubMed] [Google Scholar]
- 10.Syed M, Khawaja FB, Saleem T, Khalid U, Rashid A, Humayun KN. Clinical profile and outcomes of paediatric patients with diabetic ketoacidosis at a tertiary care hospital in Pakistan. J Pak Med Assoc. 2011;61:1082–1087. [PubMed] [Google Scholar]
- 11.Kanwal SK, Bando A, Kumar V. Clinical profile of diabetic ketoacidosis in Indian children. Indian J Pediatr. 2012;79:901–904. doi: 10.1007/s12098-011-0634-3. [DOI] [PubMed] [Google Scholar]
- 12.Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of Diabetic Ketoacidosis of New-Onset Type 1 Diabetes in Children and Adolescents in Different Countries Correlates with Human Development Index (HDI): An Updated Systematic Review, Meta-Analysis, and Meta-Regression. Horm Metab Res. 2018;50:209–222. doi: 10.1055/s-0044-102090. [DOI] [PubMed] [Google Scholar]
- 13.Zayed H. Epidemiology of diabetic ketoacidosis in Arab patients with type 1 diabetes: A systematic review. Int J Clin Pract. 2016;70:186–195. doi: 10.1111/ijcp.12777. [DOI] [PubMed] [Google Scholar]
- 14.Odeh R, Alassaf A, Ajlouni K. Clinical and biochemical features at diagnosis of type 1 diabetes in patients between 0 and 18 years of age from Jordan. Pediatr Diabetes. 2018;19:707–712. doi: 10.1111/pedi.12625. [DOI] [PubMed] [Google Scholar]
- 15.Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, Sperling MA, Codner E. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155–177. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Rasoul M, Al-Mahdi M, Al-Qattan H, Al-Tarkait N, Alkhouly M, Al-Safi R, Al-Shawaf F, Mahmoud H. Ketoacidosis at presentation of type 1 diabetes in children in Kuwait: Frequency and clinical characteristics. Pediatr Diabetes. 2010;11:351–356. doi: 10.1111/j.1399-5448.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- 17.Shaltout AA, Channanath AM, Thanaraj TA, Omar D, Abdulrasoul M, Zanaty N, Almahdi M, Alkandari H, AlAbdulrazzaq D, d’Mello L, Mandani F, Alanezi A, AlBasiry E, Alkhawari M. Ketoacidosis at first presentation of type 1 diabetes mellitus among children : a study from Kuwait. Sci Rep. 2016;6:27519. doi: 10.1038/srep27519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Yaarubi S, Ullah I, Sharef SW, Al Shidhani A, Al Hanai S, Al Kalbani R, Al Jamoodi S. Demographic and clinical characteristics of type 1 diabetes mellitus in omani children-single center experience. Oman Med J. 2014;29:119–122. doi: 10.5001/omj.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Shaikh A, Farahat F, Saeedi M, Bakar A, Al Gahtani A, Al-Zahrani N, Jaha L, Aseeri MA, Al-Jifree HM, Al Zahrani A. Incidence of diabetic ketoacidosis in newly diagnosed type 1 diabetes children in western Saudi Arabia: 11-year experience. J Pediatr Endocrinol Metab. 2019;32:857–862. doi: 10.1515/jpem-2018-0548. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed AM, Khabour OF, Ahmed SM, Alebaid IA, Ibrahim AM. Frequency and severity of ketoacidosis at diagnosis among childhood type 1 diabetes in khartoum state, sudan. Afr Health Sci. 2020;20:841–848. doi: 10.4314/ahs.v20i2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segerer H, Wurm M, Grimsmann JM, Karges B, Neu A, Sindichakis M, Warncke K, Dost A, Holl RW. Diabetic Ketoacidosis at Manifestation of Type 1 Diabetes in Childhood and Adolescence Incidence and Risk Factors. Dtsch Arztebl Int. 2021;118:367–372. doi: 10.3238/arztebl.m2021.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherubini V, Grimsmann JM, Åkesson K, Birkebæk NH, Cinek O, Dovč K, Gesuita R, Gregory JW, Hanas R, Hofer SE, Holl RW, Jefferies C, Joner G, King BR, Mayer-Davis EJ, Peña AS, Rami-Merhar B, Schierloh U, Skrivarhaug T, Sumnik Z, Svensson J, Warner JT, Bratina N, Dabelea D. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia. 2020;63:1530–1541. doi: 10.1007/s00125-020-05152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lévy-Marchal C, Patterson CC, Green A; EURODIAB ACE Study Group. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. European and Dibetes. Diabetologia. 2001;44(Suppl 3):B75–80. doi: 10.1007/pl00002958. [DOI] [PubMed] [Google Scholar]
- 24.Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55:2878–2894. doi: 10.1007/s00125-012-2690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343:d4092. doi: 10.1136/bmj.d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gesuita R, Maffeis C, Bonfanti R, Cardella F, Citriniti F, D’Annunzio G, Franzese A, Iafusco D, Iannilli A, Lombardo F, Maltoni G, Patera IP, Piccinno E, Predieri B, Rabbone I, Ripoli C, Toni S, Schiaffini R, Bowers R, Cherubini V; Network of the Italian Society of Pediatric Endocrinology and Diabetes (ISPED) for DKA Study and Prevention. Socioeconomic Inequalities Increase the Probability of Ketoacidosis at Diagnosis of Type 1 Diabetes: A 2014-2016 Nationwide Study of 2,679 Italian Children. Front Pediatr. 2020;8:575020. doi: 10.3389/fped.2020.575020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souza LCVF, Kraemer GC, Koliski A, Carreiro JE, Cat MNL, Lacerda L, França SN. Diabetic Ketoacidosis As The Initial Presentation Of Type 1 Dıabetes In Children And Adolescents: Epidemiological Study In Southern Brazil. Rev Paul Pediatr. 2020;38:e2018204. doi: 10.1590/1984-0462/2020/38/2018204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle EA. Autoimune Conditions Associated With Type 1 Diabetes. Pediatr Nurs. 2015;41:89–92. [PubMed] [Google Scholar]
- 29.Adler AI, Turner RC. The diabetes prevention program. Diabetes Care. 1999;22:543–545. doi: 10.2337/diacare.22.4.543. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed AM, Al-Maghamsi M, Al-Harbi AM, Eid IM, Baghdadi HH, Habeb AM. Reduced frequency and severity of ketoacidosis at diagnosis of childhood type 1 diabetes in Northwest Saudi Arabia. J Pediatr Endocrinol Metab. 2016;29:259–264. doi: 10.1515/jpem-2015-0077. [DOI] [PubMed] [Google Scholar]
- 31.Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia. 2016;59:2082–2087. doi: 10.1007/s00125-016-4034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrmann D, Kulzer B, Roos T, Haak T, Al-Khatib M, Hermanns N. Risk factors and prevention strategies for diabetic ketoacidosis in people with established type 1 diabetes. Lancet Diabetes Endocrinol. 2020;8:436–446. doi: 10.1016/S2213-8587(20)30042-5. [DOI] [PubMed] [Google Scholar]
- 33.Semenkovich K, Berlin KS, Ankney RL, Klages KL, Keenan ME, Rybak TM, Banks GG, Alemzadeh R, Eddington A. Predictors of Diabetic Ketoacidosis Hospitalizations and Hemoglobin A1c among Youth with Type 1 Diabetes. Health Psychol. 2019;38:577–585. doi: 10.1037/hea0000719. [DOI] [PubMed] [Google Scholar]
- 34.Maahs DM, Hermann JM, Holman N, Foster NC, Kapellen TM, Allgrove J, Schatz DA, Hofer SE, Campbell F, Steigleder-Schweiger C, Beck RW, Warner JT, Holl RW; National Paediatric Diabetes Audit and the Royal College of Paediatrics and Child Health, the DPV Initiative, and the T1D Exchange Clinic Network. Rates of diabetic ketoacidosis: International comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U. S., Austria, and Germany. Diabetes Care. 2015;38:1876–1882. doi: 10.2337/dc15-0780. [DOI] [PubMed] [Google Scholar]
- 35.Nagl K, Bollow E, Liptay S, Rosenbauer J, Koletzko S, Pappa A, Näke A, Fröhlich-Reiterer E, Döring C, Wolf J, Salfeld P, Prinz N. Lower HbA1c in patients with type 1 diabetes and celiac disease who reached celiac-specific antibody-negativity-A multicenter DPV analysis. Pediatr Diabetes. 2019;20:1100–1109. doi: 10.1111/pedi.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]


