Abstract
Objective:
To investigate the effects and mechanisms of microRNA 206 (miRNA-206) on neurological recovery through Notch receptor 3 (Notch3).
Methods:
The sciatic functional index (SFI), nerve conduction velocity (NCV), tricipital muscle wet weight (TWW) and cross-sectional area of the muscular fiber, and grip strength of posterior limbs were detected by establishing a model of the sciatic nerve to evaluate the effect of sciatic nerve injury model. miRNA-206 expression in the model was detected by real-time quantitative polymerase chain reaction (qRT-PCR), to regulate the effects of miRNA-206 on the proliferation of gastrocnemius myocytes by Cell Counting Kit-8 (CCK-8).
Results:
SFI of the model established by immediate epineurium suture after sciatic nerve resection was in the range of -150% to -100% and TWW, the average area of gastrocnemius myocytes, the NCV, and the grasping power of the hind limbs in the model were all lower than those in the normal group. And in the model, TWW, the average area of gastrocnemius myocytes, NCV, and grip strength of posterior limbs were lower in the normal group, which verified the successful establishment of the model.
Conclusion:
Over-expression of miRNA-206 can down-regulate Notch3 expression, and then stimulate brain-derived neurotrophic factor (BDNF) activity to promote the repair and functional recovery of sciatic nerve injury.
Keywords: miRNA-206, Nerve Function Recovery, Notch3, Sciatic Nerve Injury
Instruction
Peripheral nervous system includes nerve components other than cranial and spinal cord, including trunk nerves of the body surface system and motor system[1]. Peripheral nerve is particularly vulnerable[2], caused by penetrating injury, laceration, stretching, ischemia, or compression[3], which will produce inflammation in the injury site, to accelerate the nerve degeneration[4]. Peripheral nerve injury can lead to distal neurodegeneration[5] and motor or sensory impairment[6], which is a common clinical problem of trauma, tumor, and treat injury. Different from the central nerve, peripheral nerve can regenerate and achieve certain function recovery after injury[7], which can promote the repair of nerve function completed in a variety of ways. For example, giving neurotrophic factors can stimulate nerve regeneration, while it does not ensure the complete repair of nerve function, which also needs to be completed by promoting axon regeneration and myelin regeneration together[8]. However, there is still an unmet demand for new treatments or adjuvant strategies to promote the recovery of function in patients with nerve injuries[9], therefore, new technologies must be developed to restore function for more patients[10]. MicroRNA (miRNA) is important for the epigenetic regulation of genes. Studies have shown that miRNA plays an important role in the growth of the nervous system. Studies have also reported that miRNA-206 plays an important role in the developmental differentiation of skeletal muscles. For example, early miRNA-206 expression may delay the development of spinal muscular atrophy (SMA) neurodegenerative disease[11], and miR-206 is a new potential therapeutic target for the treatment of skeletal muscle atrophy caused by early denervation[12]. However, the molecular mechanism of miRNA-206 in the recovery of sciatic function remains unclear.
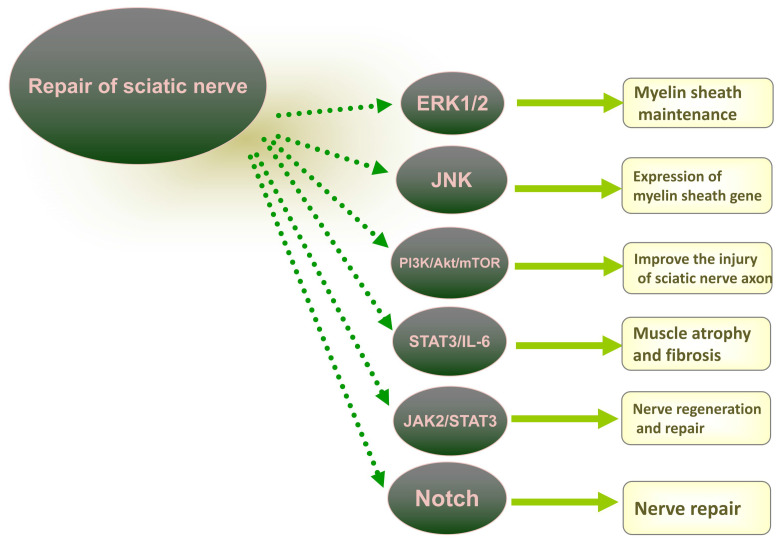
In addition, many signaling molecular pathways are also involved in the repair of the sciatic nerve, and ERK signaling pathway plays a clear role in the recovery of neural function. For example, ERK1/2 pathway is crucial for myelination and myelin maintenance[13], acetyl-11-keto-β-lactate acid (AKBA) can improve the phosphorylation of ERK signaling pathway to promote the repair of rat sciatic nerve injury[14], JNK pathway is rapidly activated after nerve injury and is involved in the expression of myelin gene[15], and taurine can improve the injury in the sciatic nerve axons in diabetic rats by activating the PI3K/Akt/mTOR signaling pathway[16]. The inhibitory effect of STAT3 on the recovery of nerve function is also relatively clear. For example, the inactivation of STAT3/IL-6 signaling alleviates muscle atrophy and fibrosis in denervated mice[17], and highly expressed neurotrophic factors (NFs) can promote the regeneration and repair of nerves by inhibiting the activation of JAK2/STAT3 signaling[18], and the activation of JAK2/STAT3 signaling can accelerate the proliferation and differentiation of myocytes and alleviate muscle atrophy[19]. The role of Notch signaling pathway in neural function remains to be explored in more experimental data. Literature shows that Notch signaling down-regulates and inhibits myelination during the process of myelination. There are reports also showing that the addition of Notch activator (Jagged1) to injured rat nerves can enhance nerve repair, therefore, the relationship between Notch and nerve repair remains to be confirmed by more studies. The pathways involved in the repair of the sciatic nerve are summarized in Figure 1.
Figure 1.
Map of signaling pathways in the repair of sciatic nerve
Nerve repair problem has been a need to find more treatments in the field of regenerative medicine, therefore, it is important to develop new therapeutic strategies to improve peripheral nerve repair after injury, especially those that can be targeted by detecting molecular changes. This study aimed to elucidate the relationship between miR-206 and sciatic nerve repair, and the molecular mechanism of miR-206 by targeting Notch3 on nerve repair.
Materials and Methods
Laboratory animals
In this study, a total of 24 male Sprague Dawley (SD) rats were purchased from Shanghai Lab Animal Research Center, and raised in the light and dark cycle environment with 12 h:12 h, with the conditions as follows: the feeding temperature of 20-22°C, and the indoor air humidity of 50%. Laboratory animals were free access to normal rodent food and acidified sterile water during raising, and were experimented after a week of adaptive feeding.
Methods
Experimental grouping
In this study, 24 SD rats were randomly divided into four groups, including Sham group (had preoperative anesthesia, no nerve dissection was performed), Denervation group (model group, the sciatic nerve was disconnected by surgery), nerve partial anastomosis group (PAG group, the sciatic nerve was disconnected by surgery and partially sutured), and nerve anastomosis group (AG group, the sciatic nerve was disconnected by surgery and fully sutured).
Surgical operation
After rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium 40 mg/kg, the anesthetized rats were fixed on the operating table and then disinfected and clipped. Longitudinal incisions were made on the dorsal side of thigh. After skin incision, biceps femoris and quadriceps femoris muscles were exposed. The muscle space was separated, and the sciatic nerve was dissociated for 15~20 mm (for transection), which was cut off to establish the injury model. Suture was performed according to the suture techniques of Devkota et al[20]. The wound healing was observed within 1 to 2 weeks after surgery, and the nerve function and dynamics were tested for 8 weeks. 10mg of soleus muscle tissue was taken for the detection of relative expression of miRNA-206 and Notch3.
Determination of sciatic functional index (SFI)
SFI is an important evaluation criterion to clarify the correlation between biomechanical function and histomorphology in the rat model of sciatic nerve injury. The specific operation referred to those of Wang et al.[21], in which a black box was made for a footprint testing device, with a blank piece of paper placed at the bottom of the black box. The postoperative rat was dipped its metapedes in inkpad and placed in the black box to drive the rat from one end to the other. 3-4 footprints were made on each foot, and the following parameters were measured: 1) the distance from the heel to the third toe (print length; PL); 2) the distance from the first toe to the fifth toe (toe stretch; TS); and 3) the distance from the second toe to the fourth toe (intermediate toe stretch; ITS). SFI was calculated according to the following formula: SFI= -38.3 ((EPL−NPL)/NPL) + 109.5 ((ETS−NTS)/NTS)+13.3 (EITS−NITS)/NITS) - 8.8, where E is the experimental side and N is the normal side. SFI value= 0±11% represents complete normal nerve function, and SFI value= -100% represents complete loss of nerve function.
Determination of nerve conduction velocity (NCV)
At 8 weeks after surgery, anesthesia (40 mg/kg) was given by intraperitoneal injection of 1% Pentobarbital Sodium, and the growth of nerve tissue in rats was observed through the original incision. NCV was detected by using the BL-420A biomechanical experimental system through the superior and inferior aspects of the thin free nerve anastomosis and the distal nerve. Electrode placement and determination parameters were performed referring to the operation of Devkota et al[20].
Determination of tricipital muscle wet weight (TWW)
The triceps were excised intact up to the Achilles tendon and weighed wet (mg). The maintenance rate of TWW was derived by comparison between operative side and healthy muscles, maintenance rate of TWW = (Right TWW/Left TWW) * 100%.
Detection of cross-sectional area of muscular fiber
Bilateral middle gastrocnemius muscles were cut and fixed in 10% neutral formaldehyde, which was routinely embedded in paraffin and stained with H&E 24 hours later. Four clearer fields were randomly selected and 10 muscle fibers with clear and complete outlines were selected in each field, to measure the cross-sectional area with the Image-Pro Plus 6.0 software. The mean cross-sectional area of 40 muscle fibers was the cross-sectional area of single muscular fiber.
Grip strength test of rats
Rats’ hind limbs were placed on a T-shaped bar for grip testing (BIOGS3, BIOGRIPBR, Bioseb, Vitrolles, France). The grip response of hind limbs was induced by suspending the forelimbs of rats with one hand and pulling the tailback with the other hand. The strength applied to the T-bar was recorded on the instrument by the grip strength sensor, and the mean of three tests was used to evaluate the grip strength change of rats’ hind limbs.
qRT-PCR for miRNA-206 in soleus muscles
Soleus muscle specimens on the operative side were temporarily stored in liquid nitrogen, and after all tissues were removed, they were stored in a -80°C refrigerator for long-term storage. RNA was extracted from the tissues with Trizol Total RNA Reagent (Shanghai Sangon Biotech, China, B511311-0100), and cDNA was synthesized with PrimeScriptTM RT Master Mix (TaKaRa, Japan, DRR036A) and amplified by qRT-PCR. Reaction system: cDNA 10µL, 2µL of each of the upstream and downstream primers, 66µL of ddH2O, and 20µL Of SYBR Green1. Reaction procedures: 95°C for 5min, 94°C for 15s, 55°C for 30s, for a total of 40 cycles, followed by 72°C for 30s and 4°C for 30min. The primer sequences of miRNA-206: Sense: 5′-ATCCAGTGCGTGTCGTG-3′; Anti-sense 5′-TGCTTGGAATGTAAGGAAG-3′. The primer sequences of U6: Sense: 5′-GCTTCGGCAGCACATATACTAAAAT-3′; Anti-sense: 5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Isolation and culture of gastrocnemius myocytes
Male SD rats with different degrees of nerve detachment were selected. The gastrocnemius muscle tissues were cut by micromanipulation, and the gastrocnemius myocytes were isolated and expanded in culture according to the operation of DeRuiter, Gao et al[22,23]. After removing the fascia, the tissues were cut into tissue blocks which were approximately 0.5 mm with scissors, which were then transferred to containers containing trypsin and collagenase for digestion. After digestion, the serum was immediately added to terminate the digestion, and the undigested tissues were separated by centrifugation. The supernatant was transferred to the culture flask, and the remaining tissue continued to digest. All the above operations were repeated for separating cells. Individual cells were isolated and purified according to the differential adherent method, and the cells were used for subsequent experiments when the density was diluted to 3x105/ml when the cells were passaged to 3 passages.
Cell transfection
Lipofectamine 2000 (Invitrogen, CA, USA), the reagent for transfection, and opti-MEM (Invitrogen, CA, USA), the plasmid incubation solution, were all purchased from companies. miRNA-206 mimics (UGGAAUGUAAGGAAGUGUGUGG), Notch3 mimics (UGGGGGGACAGGAUGAGAGGCUGU) and the corresponding controls were designed and synthesized by the company (Invitrogen, CA, USA). When the density was about 75%, the medium was replaced with serum-free medium, and 8 µl of Lipofectamine 2000, 117 µl of plasmid medium opti-MEM, and 10 µl of vectors were mixed at room temperature for 20min. The plasmids were slowly transfected into cells, mixed, and then placed in an incubator for further culture. At 4.5 h after transfection, the medium was replaced with normal 15% FBS and subsequent experiments were performed.
Western blotting
Referring to the isolation and extraction techniques from Li et al.[24], total protein was extracted from sciatic nerve tissue segments and cells with the protein cell lysis buffer. The lysis products were centrifuged at 12,000 rpm at 4°C for 20 min. After determining the protein concentration, the samples were separated by 8% polyacrylamide gel electrophoresis. The protein was then retransferred to PVDF membrane (Millipore, MA, USA, IPFL00010), and then closed with 5% skim milk and incubated with the following primary antibodies at 4°C: Mouse anti-Notch3 (1:1000, Millipore, MA, USA, APREST76106), Mouse anti-BNDF (1:1000, Sigma-Aldrich, USA, GF029) and Rabbit anti-GAPDH (1:1000, Millipore, MA, USA, G5262).
The protein was incubated with secondary antibodies including Goat anti-rabbit IgG-HRP (1:5000, Millipore, MA, USA, AP112P) and Goat anti-mouse IgG-HRP (1:5000, Millipore, MA, USA, AP126P) at 37°C for 1 h. Finally, protein expression was detected with ECL Western Blotting Substrate Kit (Abnova, KA3725) and chemiluminescence imaging system (Fusion Solo system, Villber Lourmat). All experiments were performed three times.
Dual-luciferase reporter gene assay
The target genes for miRNA-206 were predicted with Starbase V2.0 online database, as well as the potential binding site sequences for both miRNA-206 and the target gene Notch3. The relationship between known genes and target genes was detected with the dual-luciferase reporter gene. The untranslated regions of the two types of known genes were first amplified by PCR, namely, wild-type (WT) and mutant (MUT). Subsequently, both types of untranslated regions were integrated onto psiCHECK-2. After the successful construction of vectors, WT, MUT and target genes (mimics) or NC (ctrl) were co-transformed into isolated nerve gastrocnemius myocytes with Liposome 2000. After 48 hours of co-incubation, the activity could be detected with the Dual-Glo® dual-luciferase reporter gene assay system.
Statistical analysis
Cell statistics and protein grayscale scanning were conducted with Image J (1.4.3.67 Broken symmetry software), and all the data were analyzed with Prism GraphPad 7.0 (GraphPad Software, La Jolla, CA, USA). All the variables in the experiment were expressed as mean ± standard deviation (SD), and one-way ANOVA was conducted to compare the mean difference between two or more samples. P<0.05 was considered statistically significant.
Results
Index detection on the establishment of the rat model of sciatic nerve transection
The rat models of sciatic nerve transection at different degrees established by the suture-occluded method did not die. The degree of sciatic nerve transection was observed at 4-8 weeks after surgery. As shown in Figure 2A, comparing this to 0 weeks, SFI of the Sham group during the 4-8 weeks of molding was in the range of -50% to 0%, indicating normal nerve function. SFI of the model group during the 0-8 weeks was in the range of -150% to 100%, indicating a complete loss of neural function. SFI of the partial neurologic anastomoses rats in the PAG group gradually increased between -120% and -90% over time, indicating that the nerve function was gradually recovering from 0 to 8 weeks after surgery. SFI of the complete anastomotic rats in the AG group gradually increased between -100% and 0% from 0 to 8 weeks after surgery. The final SFI was at a time close to the Sham group around Week 8, indicating that the neural function has returned to normal. Through the SFI measurement of sciatic nerve, the nerve transection model was relatively successful, which could be used in subsequent experiments.
Figure 2.
Index detection on the establishment of the rat model of sciatic nerve transection. Note: A: Statistics of SFI in rats of the four groups 8 weeks after surgery; B-E: Determination of TWW, average area of gastrocnemius myocytes, nerve electrophysiology and grip strength test of hind limbs. Sham: Nerve exposed group not operated; Model: Denervation group; PAG: Nerve partial anastomosis group; AG: Nerve anastomosis group. SFI at 0 ± 11% represents the function was completely normal; -100% or below represents a complete loss of nerve function. *P<0.05; **P<0.01, N=5.
TWW, average area of gastrocnemius myocytes, NCV and grip strength test of hind limbs of SD rats in the four groups were detected to assess the sciatic function. The difference in the results was obvious, and the relevant data are shown in Figure 2B-E. SFI in the model, PAG and AG groups all showed abnormalities compared with the Sham group, and SFI in the model group was significantly lower by 100%, indicating a complete loss of neural function. Also, according to the results of the four indicators, including TWW, average area of gastrocnemius myocytes, NCV and grip strength test of hind limbs, model, PAG and AG groups showed reduced TWW, decreased average area of gastrocnemius myocytes, and lower NCV, and smaller grip strength of hind limbs compared to the Sham group, and the damaging effect in the model group was obvious. According to the detection data, the establishment of the nerve transection model was consistent with the normal occurrence of nerve transection in the body, indicating that the model was relatively well established.
miRNA-206 expression in tissues of middle soleus muscle and regulation
The differences in the average area of gastrocnemius myocytes between various tissues were more obvious under different nerve transection (Figure 3A, *P<0.05; **P<0.01), therefore, isolation of tissues can be performed with reference to this result. The results are shown in Figure 3B, miRNA-206 expression in the middle soleus muscle was different in different groups, which was the highest in the Sham group, gradually decreased as the nerve transection kept going (Figure 3B, *P<0.05; **P<0.01), and was the lowest in the model group, suggesting that miRNA-206 expression may be related to neural integrity and neural function, and the different expression of miRNA-206 in transected and normal nerves may be related to the recovery of neural function.
Figure 3.
qRT-PCR for miRNA-206 expression in tissues of middle soleus muscle. Note: A: Statistics of average area of gastrocnemius muscle tissues of each group; B: qRT-PCR for miRNA-206 expression; C, qRT-PCR for the effect of adding miRNA-206 mimic; D: CCK-28 for the effect of miRNA-206 on the proliferation of gastrocnemius myocytes. Sham: Nerve exposed group not operated; Model: Denervation group; PAG: Nerve partial anastomosis group; AG: Nerve anastomosis group. *P<0.05; **P<0.01, N=5.
To further investigate the role of miRNA-206 on neurological recovery, miRNA-206 mimic and miRNA-206 ctrl were added into gastrocnemius myocytes of different treatment groups. The results showed that miRNA-206 expression of miRNA-206 mimic in the gastrocnemius myocytes of the model group increased compared to the control (miRNA-206-ctrl) group (Figure 3C, *P <0.05), indicating that miRNA-206 mimic can be used to regulate miRNA-206 expression in the gastrocnemius myocytes of the denervation group. Meanwhile, the gastrocnemius myocytes of the denervation group were cultured and added with miRNA-206 mimic and miRNA-206 ctrl to detect the changes in proliferation, and the results are shown in Figure 2D. miRNA-206 mimic treatment gastrocnemius myocytes showed a significantly higher proliferation than the control group (miRNA-206 ctrl) (Figure 3D, **P<0.01), suggesting that regulating miRNA-206 expression can affect the proliferation of denervated gastrocnemius muscles and that adding miRNA-206 mimic can promote the proliferation of denervated gastrocnemius myocytes.
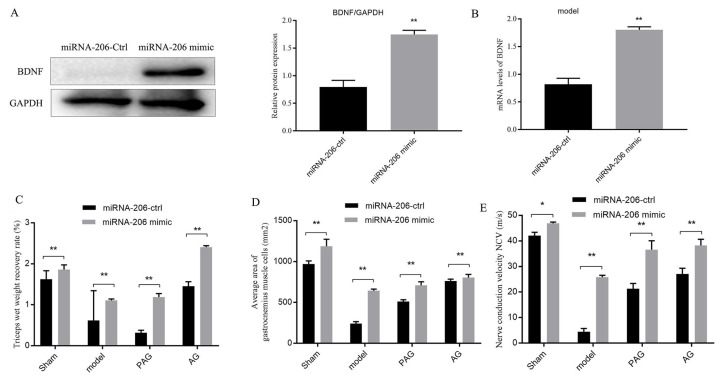
Detection of the effect of miRNA-206 on BDNF expression and neural function
The neurotrophic factor BDNF plays an important role in the recovery of neural function. Therefore, it was determined by Western blotting, with the results shown in Figure 4A, which showed less BDNF expression in gastrocnemius myocytes of the model group (ctrl, *P<0.05). However, the protein expression of BDNF significantly increased after using miRNA-206 mimic (miRNA-206-mimic, **P<0.01). Similarly, qRT-PCR showed that adding miRNA-206 mimic promoted BDNF expression in gastrocnemius myocytes (Figure 4B, **P<0.01), indicating that increasing miRNA-206 expression in denervated gastrocnemius myocytes can stimulate the activity of BDNF expression, which then contributes to the recovery of neural function. On this basis, miRNA-206 was regulated to detect the changes in three indicators related to neural function, as shown in Figure 4C-E. The over-expression of miRNA-206 (miRNA-206 mimic) significantly increased TWW of the model group compared with controls (miRNA-206-ctrl) (Figure 4C, **P<0.01), and the average area of gastrocnemius myocytes and NCV in the model group were also increased by miRNA-206 mimic (Figure 4D, E, **P<0.01), which confirmed that increasing miRNA-206 can improve the performance of neural function and promotes its recovery.
Figure 4.
Effects of miRNA-206 on BDNF expression and neural function. Note: A: Western blotting for the effect of miRNA-206 on BDNF expression; B: qRT-PCR for the effect of miRNA-206 on BDNF expression; C-E: Detection on TWW, average area of gastrocnemius myocytes and nerve electrophysiology. miRNA-206 over-expression control group: miRNA-206-ctrl; miRNA-206 over-expression group: miRNA-206 mimic; Sham: Nerve exposed group not operated; Model: Denervation group; PAG: Nerve partial anastomosis group; AG: Nerve anastomosis group. *P<0.05; **P<0.01, N=5.
Prediction of target molecule for miRNA-206
The effect of miRNA-206 on the recovery of sciatic function has been preliminarily verified. To probe deeply into the specific mechanism of miRNA-206 on the recovery of sciatic function, Starbase V2.0 bioinformatic analysis demonstrated that Notch3 is a direct target gene of miRNA-206 in the sciatic nerve of rats (Figure 5A). To further test the regulatory relationship between miRNA-206 and Notch3, dual-luciferase reporter results showed that compared with the control group (miRNA-206-ctrl), over-expression of miR-206 (miRNA-206 mimic) decreased the luciferase activity of Notch3-3′UTR WT without inhibiting Notch3-3′UTR Mut (Figure 5B, *P<0.05). Notch3 is the target of miRNA-206 in denervation cells, and miRNA-206 can be targeted to regulate mRNA activity of Notch3 (Figure 5C, *P<0.05).
Figure 5.
Prediction of target molecule for miRNA-206. Note: A, Starbase V2.0 online database prediction for the binding site of mirNA-206 at the 3’UTR of Notch3; B, Dual luciferase reporter gene assay for the regulation of miRNA-206 on Notch3; C, qRT-PCR for the regulation of miRNA-206 on Notch3. Notch3 wild type: Notch3-3′UTR WT; Notch3 mutant: Notch3-3’UTR Mut; *P<0.05; **P <0.01, N=5.
Notch3 expression and its effect on BDNF expression
The role of miRNA-206 on nerve repair has been confirmed, and miRNA-206 in turn can target Notch3. Therefore, we speculated that miRNA-206 repairs the transected sciatic nerve by targeting Notch3. To this end, Notch3 expression in the middle soleus muscle was detected in this study. The results are shown in Figure 6A. In the normal tissues of the Sham group, the protein expression of Notch3 was lower, which gradually increased as the nerves were gradually transected, and was the highest in tissues in the model group (Figure 6A, **P<0.01). Meanwhile, qRT-PCR results were consistent with this. mRNA of Notch3 was lower in the normal tissues of the Sham group, and Notch3 expression gradually increased as the nerves were gradually transected (Figure 6B, **P<0.01). Thus, it was speculated that Notch3 may be involved in the recovery of neural function.
Figure 6.
Western blotting for expressions of Notch3 and BDNF. Note: A, Protein expression of Notch3 in different transected nerve groups; B, mRNA expression of Notch3 in different transected nerve groups; C, Detection on Notch3 for BDNF protein expression; D, Detection on Notch3 for mRNA expression of BDNF. Notch3 over-expression control group: Notch3-ctrl; Notch3 over-expression group: Notch3 mimic; Sham: Nerve exposed group not operated; Model: Denervation group; PAG: Nerve partial anastomosis group; AG: Nerve anastomosis group. *P<0.05; **P <0.01, N=5.
Notch3 in different transected nerve tissues has different expression trends. To further verify the effect of Notch3 on nerve function, the effect of Notch3 over-expression on BDNF expression was detected by Western blotting, with the results as shown in Figure 6C, 6D. In the transected gastrocnemius myocytes, BDNF expression in transected nerve cells was reduced by Notch3 over-expression (Notch3 mimic) compared with control group (Notch3-ctrl) (**P<0.01), indicating that the over-expression of Notch3 inhibits neurotrophic factor expression and thus is not conducive to the recovery of neural function, which further verified the relationship between Notch3 and neurological recovery.
Effects of miRNA-206 targeting Notch3 on the recovery of neural function
In conclusion of the demonstration of the above-mentioned effects on miRNA-206 and Notch3 on the recovery of neural function and the verification of miRNA-206 targeting Notch3 in denervation, it was speculated that miRNA-206 may affect the recovery of neural function by targeted and regulating Notch3 expression. To test this conjecture, miR-206 mimic was added simultaneously with Notch3 mimic to a component of gastrocnemius myocytes, further verifying the regulatory relationship and its effect on the neurotrophic factor BDNF expression. The results are shown in Figure 7A-D. Upon over-expression of miRNA-206 in gastrocnemius myocytes of the model group, Notch3 was inhibited, and BDNF expression was up-regulated. When over-expressing Notch3, BDNF expression was significantly suppressed. Meanwhile, when miRNA-206 and Notch3 were over-expressed, the regulation of BDNF by miRNA-206 was blocked due to the up-regulation of Notch3, which was due to the over-expression of Notch3. Even if Notch3 was over-expressed, Notch3 was targeted and regulated by miRNA-206, and over-expression of miRNA-206 inhibits Notch3 expression. At the same time, the expression changes of Notch3 and BDNF when overexpressing miRNA-206 in denervated gastrocnemius myocytes were detected by qRT-PCR. And the results are shown in Figure 7B. Compared with the control group (miRNA-206-ctrl), over-expression of miRNA-206 inhibited Notch3 expression, while the expression of BDNF was up-regulated. This result indicated that miRNA-206 may activate the BDNF activity by specifically inhibiting Notch3 expression to restore the neural function.
Figure 7.
miRNA-206 targeting Notch3 affects the recovery of neural function. Note: A: Western blotting for miRNA-206 regulating BDNF expression by Notch3; B-D: qRT-PCR for miRNA-206 regulating BDNF expression by Notch3; miRNA-206 over-expression control group: miRNA-206-ctrl, miRNA-206 over-expression group: miR-206 mimic; *P<0.05; **P <0.01, N=5.
Discussion
Sciatic nerve injury disorders are the most common neurological problem[25,26], with complications including numbness in the legs and loss of motor control. Although conservative treatments can alleviate minor trauma neural function may not restore after severe trauma[27], which will lead to a significant decline in patients’ quality of life[26]. In the meantime, the peripheral nerve injury is the main cause of permanent disability and is often accompanied by other skeletal muscle damage[28]. Human peripheral nerve has a high regenerative and repair capacity after trauma[29], however, a variety of problems during nerve repair may lead to impaired neurological recovery, for example, the regeneration and proliferation rate of nerve cells is slow and the expression level of neurotrophic factors is low[30,31]. To this end, the various factors affecting neurological recovery are deeply studied, to find treatments that can accelerate neurological recovery.
Repair methods for nerves include ultra-micro suture technique[32], reverse autologous transplantation (RA) surgical technology[33], local and intravenous injection of mesenchymal stem cells to stimulate nerve regeneration[34], etc., with different effects, therefore, the level of treatments needs to be further improved. Given the complexity and controversy of in vivo operation in studies on the recovery of sciatic function, the construction of nerve transection model in vitro can highly mimic the in vivo nerve injury situation[35]. In this study, the rat models of sciatic nerve transection at different degrees were established by the suture-occluded method for experiments. However, because the nerve is long and cylindrical, dislocation of suture, axon growth error and unsatisfactory repair are easy to occur during the repair of epineural suture, and it is visible that the technology still has limitations, which needs to be further improved. Nonetheless, the neurophysiological models provided by rodents are still accepted as transformation models, which can also provide better research methods for neural repair. microRNAs (miRNAs) is a non-coding RNA molecule of about 22 nucleotides, which has a crucial role in many biological processes[36]. And the regeneration and survival of neuronal cells during nerve repair can be achieved by regulating miRNA expression[37]. MicroRNAs are involved in nerve injury[38]. For example, miR-142a-5p is activated in the denervated gastrocnemius muscle[39], and direct injection of miR-30c stimulates myelin formation, and promotes the regenerative of peripheral nerves[40]. Most miRNAs are highly conserved, cell-specific, and have a strong capacity to regulate cell proliferation and apoptosis[41]. Among them, miR-206 plays a key role in regulating the proliferation, apoptosis, invasion and migration of cells[42,43]. While miRNA-206 highly expresses in skeletal muscles[44], miRNA-206 protects the denervated muscles from atrophic[12]. The expression characteristics of miRNA-206 vary among different species. In this study, miRNA-206 was lower in completely transected tissues and cells than in nerve-exposed tissues and cells. However, it has been shown that miR-206 expression in patients with amyotrophic lateral sclerosis (ALS) increased[45], suggesting that the difference of miRNA-206 in different samples may differ in species and patients. In addition, it was found that miRNA-206 over-expression could regulate miRNA-206 expression in denervated gastrocnemius myocytes, further demonstrating that miRNA-206 expression is regulated in neurotrauma, which is also the first time to find that miRNA-206 expression can be regulated in the sciatic nerve, suggesting that it may have a role on neurological recovery. Studies in agreement with our conjecture show that effective regeneration of neuromuscular synapses after acute nerve injury requires miRNA-206[11], and miR-206 may exert some of its partial neuroprotective effects, to alleviate the severity of spinal muscular atrophy (SMA)[46]. These studies are consistent with the findings of the present study, showing the importance of miRNA-206 for the treatment and prevention of neurological diseases. Neurotrophic factors are a family of proteins, which can regulate neuronal survival, synaptic function and neurotransmitter release, and initiate the plasticity and growth of axons within the adult central and peripheral nervous system[47]. BDNF belongs to the neurotrophic factor family synthesized in the central and peripheral nervous system[48]. It has been shown that the accelerated BDNF expression can stimulate the increase of cAMP levels, and accelerated the up-regulation of growth-related genes, tubulin, actin and growth-associated protein43 (GAP-43)[49]. BDNF can be used for the regenerative of the peripheral axons after injury[50]. In the present study, the over-expression of miRNA-206 was found to stimulate BDNF expression in the denervated cells, this implies that the over-expression of miRNA-206 can increase the expression of neurotrophic factors and contribute to the recovery of neural function. In addition, some studies have shown that miRNA-206 specifically regulates BDNF expression in sensory neurons[51], and there is evidence that miRNA-206 participates in the pathogenesis of AD by inhibiting BDNF expression in the brain[52]. All of these studies suggest a possible relationship between miRNA-206 with neurotrophic factors and neural function. The effect of miRNA-206 on sciatic function was confirmed by examining the assessment of SFI, TWW, the average area of gastrocnemius myocytes, and nerve electrophysiology, Moreover, Notch3 was found as a target molecule of miRNA-206, and miRNA-206 expression can affect the luciferase activity of Notch3, regulating Notch3 expression. There is a study showing that Notch3 is a direct target gene of miRNA-206 in mice, which inhibits Notch3 expression by binding to the 3′UTR of Notch3[53]. This report is consistent with the findings of this study. In the occurrence of disease, Notch3 and miRNA-206 cooperate to regulate each other, while the regulatory mechanism of signaling pathways between the two needs to be explored. Besides, Notch3 expression was found to be higher in the denervation group possibly due to the expression of inflammatory cytokines associated with Notch3[54], as it has been shown that Notch3 can control the developmental process of inflammation by increasing p65 phosphorylation with p38 MAP kinase[55]. And the over-expression of Notch3 inhibited the activity of BDNF, indicating that Notch3 expression is detrimental to the recovery of neural function. It has been demonstrated that BDNF expression activates inhibition of Notch3 activity by over-expressing miRNA-206, thus causing the expression of neurotrophic factors to promote the recovery of neural function. Neurotrophic factors are not only modulated by miRNA, they in turn regulate miRNA expression[56]. Our results suggested that the over-expression of miRNA-206 promotes BDNF expression. In contrast, BDNF expression during promoting the recovery of neural function will also promote more miRNA-206 expression and gradually restore neural function. It has been reported in the literature that BDNF is a direct target gene of miR-206 in neurons cultured in vitro and, in the hippocampus, in vivo, and miR-206 can regulate BDNF expression which is involved in the treatment of depression[57]. And Notch3 and BDNF can be co-enriched in breast cancer[58]. The above studies suggest that the miR-206, Notch3 and BDNF may be mutually regulated in the development of the disease, suggesting that the mechanism is that miR-206 can regulate Notch signaling pathway to affect BDNF expression and further influence the development of the disease. However, the specific relationship between the three remains to be further confirmed. This study merely addressed the relationship between miR-206 and Notch3 in the denervation, while it was not demonstrated how both specifically affect neurological recovery, and whether there is no difference between miR-206 and normal individuals, which also requires in-depth inquiry at the individual level.
Finally, this study provided evidence that miRNA-206 expression is less abundant in sciatic nerve trauma of rats, and that over-expressing miRNA-206 can stimulate BDNF expression and help to restore nerve function-related kinetic indicators. During this regulatory process, miRNA-206 can promote BDNF expression by targeting Notch3, promoting BDNF expression to further promote the repair and functional recovery of sciatic nerve injury (Figure 8). This study provided new insights into the treatments for sciatic nerve injury and the recovery of peripheral nerve function, and the specific mechanism of miRNA-206 in the repair and regeneration of peripheral nerve injury needs further confirmation.
Figure 8.
Plot of recovery mechanism of miRNA-206 affecting the recovery of neural function by targeting Notch3. Note: Over-expression of miRNA-206 inhibited Notch3 expression and activated the BDNF activity, which ultimately caused the recovery of traumatic sciatic function.
Conclusion
In conclusion, miRNA-206 can affect BDNF expression by regulating Notch3 activity and ultimately act in functional recovery of traumatic sciatic nerve.
Ethics approval
All experiments were approved by the Laboratory Animal Ethics Committee of Qiqihar Medical College (Approval No. QMU-AECC-2021-246), and were in accordance with the use and management of laboratory animals.
Funding
This study is funded by Joint Guidance Project of Science and Technology Plan of Qiqihar City (LHYD-2021066).
Authors’ contributions
MW and HG conceived and designed the study, and drafted the manuscript. MW, SW, JW, DF, ZL, ST, SY, HZ and HG collected, analyzed and interpreted the experimental data. SW, JW and HG revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Vallat JM, Tazir M, Calvo J, Funalot B. [Hereditary peripheral neuropathies] Presse Med. 2009;38(9):1325–34. doi: 10.1016/j.lpm.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Caillaud M, Richard L, Vallat JM, Desmoulière A, Billet F. Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res. 2019;14(1):24–33. doi: 10.4103/1673-5374.243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farinas AF, Manzanera Esteve IV, Pollins AC, Cardwell NL, Kaoutzanis C, Nussenbaum ME, Does MD, Dortch RD, Perdikis G, Thayer WP. Diffusion Magnetic Resonance Imaging Predicts Peripheral Nerve Recovery in a Rat Sciatic Nerve Injury Model. Plast Reconstr Surg. 2020;145(4):949–956. doi: 10.1097/PRS.0000000000006638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahromi Z, Mohammadghasemi F, Moharrami Kasmaie F, Zaminy A. Cinnamaldehyde Enhanced Functional Recovery after Sciatic Nerve Crush Injury in Rats. Cells Tissues Organs. 2020;209(1):43–53. doi: 10.1159/000507016. [DOI] [PubMed] [Google Scholar]
- 5.Meng FW, Jing XN, Song GH, Jie LL, Shen FF. Prox1 induces new lymphatic vessel formation and promotes nerve reconstruction in a mouse model of sciatic nerve crush injury. J Anat. 2020;237(5):933–940. doi: 10.1111/joa.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreo L, Soldera CA, Ribeiro BG, de Matos PRV, Sousa PB, de Alcântara Araújo Amorim WW, Horliana ACRT, Bussadori SK, Fernandes KPS, Mesquita-Ferrari RA. Effects of Photobiomodulation on Functionality in Wistar Rats with Sciatic Nerve Injury. Photochem Photobiol. 2019;95(3):879–885. doi: 10.1111/php.13048. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–33. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 8.Kang MS, Lee GH, Choi GE, Yoon HG, Hyun KY. Neuroprotective Effect of Nypa fruticans Wurmb by Suppressing TRPV1 Following Sciatic Nerve Crush Injury in a Rat. Nutrients. 2020;12(9):2618. doi: 10.3390/nu12092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC. Peripheral nerve injury and myelination:Potential therapeutic strategies. J Neurosci Res. 2020;98(5):780–795. doi: 10.1002/jnr.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuffler DP, Foy C. Restoration of Neurological Function Following Peripheral Nerve Trauma. Int J Mol Sci. 2020;21(5) doi: 10.3390/ijms21051808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valsecchi V, Boido M, De Amicis E, Piras A, Vercelli A. Expression of Muscle-Specific MiRNA 206 in the Progression of Disease in a Murine SMA Model. PLoS One. 2015;10(6):e0128560. doi: 10.1371/journal.pone.0128560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang QK, Qiao HY, Fu MH, Li G, Li WB, Chen Z, Wei J, Liang BS. MiR-206 Attenuates Denervation-Induced Skeletal Muscle Atrophy in Rats Through Regulation of Satellite Cell Differentiation via TGF-β1, Smad3, and HDAC4 Signaling. Med Sci Monit. 2016;22:1161–70. doi: 10.12659/MSM.897909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii A, Furusho M, Dupree JL, Bansal R. Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J Neurosci. 2014;34(48):16031–45. doi: 10.1523/JNEUROSCI.3360-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Wang Y, Zhang B, Fei X, Guo X, Jia Y, Yu W. Acetyl-11-keto-β-boswellic acid regulates the repair of rat sciatic nerve injury by promoting the proliferation of Schwann cells. Life Sci. 2020;254:116887. doi: 10.1016/j.lfs.2019.116887. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D'Antonio M, Mirsky R, Jessen KR. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164(3):385–94. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, Shi X, Luo M, Lan Q, Ullah H, Zhang C, Li S, Chen X, Wang Y, Piao F. Taurine ameliorates axonal damage in sciatic nerve of diabetic rats and high glucose exposed DRG neuron by PI3K/Akt/mTOR-dependent pathway. 2021;53(3):395–406. doi: 10.1007/s00726-021-02957-1. [DOI] [PubMed] [Google Scholar]
- 17.Madaro L, Passafaro M, Sala D, Etxaniz U, Lugarini F, Proietti D, Alfonsi MV, Nicoletti C, Gatto S, De Bardi M, Rojas-García R, Giordani L, Marinelli S, Pagliarini V, Sette C, Sacco A, Puri PL. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol. 2018;20(8):917–927. doi: 10.1038/s41556-018-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu XM, Wang Y, Fu WL, Liu DH, Zhang CY, Wang QL, Tong XJ. The Combination of Adipose-derived Schwann-like Cells and Acellular Nerve Allografts Promotes Sciatic Nerve Regeneration and Repair through the JAK2/STAT3 Signaling Pathway in Rats. Neuroscience. 2019;422:134–145. doi: 10.1016/j.neuroscience.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Cui W, Liu CX, Wang J, Zhang YC, Shen Q, Feng ZH, Wu J, Li JX. An oleanolic acid derivative reduces denervation-induced muscle atrophy via activation of CNTF-mediated JAK2/STAT3 signaling pathway. Eur J Pharmacol. 2019;861:172612. doi: 10.1016/j.ejphar.2019.172612. [DOI] [PubMed] [Google Scholar]
- 20.Devkota P, Lei W, Bing-Fang Z, Jian-Fei T, Cun-Yi F. Effect of tension on force of contraction of muscle and nerve conduction velocity of the repaired nerve in a rat model. Nepal Med Coll J. 2006;8(4):227–9. [PubMed] [Google Scholar]
- 21.Wang T, Ito A, Aoyama T, Nakahara R, Nakahata A, Ji X, Zhang J, Kawai H, Kuroki H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model:A comparison between sciatic functional index and kinematic analysis. PLoS One. 2018;13(12):e0208985. doi: 10.1371/journal.pone.0208985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeRuiter CJ, De Haan A, Sargeant AJ. Fast-twitch muscle unit properties in different rat medial gastrocnemius muscle compartments. J Neurophysiol. 1996;75(6):2243–54. doi: 10.1152/jn.1996.75.6.2243. [DOI] [PubMed] [Google Scholar]
- 23.Gao RQ, Tang CL, Huang SQ, Cao J, Guo QH, Zhang Y, Tian Y, Yuan HZ, Zhao DD, Luo A, Zhang AN. [Effects of Electroacupuncture on Apoptosis-related Protein Expression of Gastrocnemius Muscle Cells in Rats with Denervated Sciatic Nerve] Zhen Ci Yan Jiu. 2017;42(4):302–7. [PubMed] [Google Scholar]
- 24.Li J, Zhang Y, Yang Z, Zhang J, Lin R, Luo D. Salidroside promotes sciatic nerve regeneration following combined application epimysium conduit and Schwann cells in rats. Exp Biol Med (Maywood) 2020;245(6):522–531. doi: 10.1177/1535370220906541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckenmeyer MJ, Meder TJ, Prest TA, Brown BN. Decellularization techniques and their applications for the repair and regeneration of the nervous system. Methods. 2020;171:41–61. doi: 10.1016/j.ymeth.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menorca RM, Fussell TS, Elfar JC. Nerve physiology:mechanisms of injury and recovery. Hand Clin. 2013;29(3):317–30. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glat MJ, Benninger F, Barhum Y, Ben-Zur T, Kogan E, Steiner I, Yaffe D, Offen D. Ectopic Muscle Expression of Neurotrophic Factors Improves Recovery After Nerve Injury. J Mol Neurosci. 2016;58(1):39–45. doi: 10.1007/s12031-015-0648-9. [DOI] [PubMed] [Google Scholar]
- 28.Ciaramitaro P, Mondelli M, Logullo F, Grimaldi S, Battiston B, Sard A, Scarinzi C, Migliaretti G, Faccani G, Cocito D Italian Network for Traumatic Neuropathies. Traumatic peripheral nerve injuries:epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst. 2010;15(2):120–7. doi: 10.1111/j.1529-8027.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 29.Tatagiba M, Rosahl S, Gharabaghi A, Blömer U, Brandis A, Skerra A, Samii M, Schwab ME. Regeneration of auditory nerve following complete sectioning and intrathecal application of the IN-1 antibody. Acta Neurochir (Wien) 2002;144(2):181–7. doi: 10.1007/s007010200022. [DOI] [PubMed] [Google Scholar]
- 30.Jiang B, Zhang P, Jiang B. Advances in small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 2010;38(1):1–4. doi: 10.3109/10731190903495652. [DOI] [PubMed] [Google Scholar]
- 31.Hodgetts SI, Harvey AR. Neurotrophic Factors Used to Treat Spinal Cord Injury. Vitam Horm. 2017;104:405–457. doi: 10.1016/bs.vh.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Orgel MG, Terzis JK. Epineurial vs. perineurial repair. Plast Reconstr Surg. 1977;60(1):80–91. doi: 10.1097/00006534-197707000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Farinas AF, Pollins AC, Stephanides M, O'Neill D, Al-Kassis S, Esteve IVM, Colazo JM, Keller PR, Rankin T, Wormer BA, Kaoutzanis C, Dortch RD, Thayer WP. Diffusion tensor tractography to visualize axonal outgrowth and regeneration in a 4-cm reverse autograft sciatic nerve rabbit injury model. Neurol Res. 2019;41(3):257–264. doi: 10.1080/01616412.2018.1554284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, Wu LW, Sarhane KA, Lopez J, Wallner C, Furtmüller GJ, Yuan N, Pang J, Sarkar K, Lee WP, Brandacher G. Mesenchymal Stem Cells Enhance Nerve Regeneration in a Rat Sciatic Nerve Repair and Hindlimb Transplant Model. Sci Rep. 2016;6:31306. doi: 10.1038/srep31306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PJ, Wu A, Moalem-Taylor G. Moalem-Taylor, Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp. 2012;(61):3393. doi: 10.3791/3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Z, Luo M, Wang L, Yin H, Zhu W, Fu J. MicroRNA-206 Regulation of Skin Pigmentation in Koi Carp (Cyprinus carpio L) Front Genet. 2020;11:47. doi: 10.3389/fgene.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Cui X, Guan G, Dong Y, Zhang Z. microRNA-192-5p is involved in nerve repair in rats with peripheral nerve injury by regulating XIAP. Cell Cycle. 2020;19(3):326–338. doi: 10.1080/15384101.2019.1710916. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Yang X, Xue P, Chen H, Yuan M, Kang Y, Duscher D, Machens HG, Chen Z. Denervation drives skeletal muscle atrophy and induces mitochondrial dysfunction, mitophagy and apoptosis via miR-142a-5p/MFN1 axis. Theranostics. 2020;10(3):1415–1432. doi: 10.7150/thno.40857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi S, Wang QH, Zhao LL, Qin J, Wang YX, Yu B, Zhou SL. miR-30c promotes Schwann cell remyelination following peripheral nerve injury. Neural Regen Res. 2017;12(10):1708–1715. doi: 10.4103/1673-5374.217351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta Y, Möller S, Witte M, Belheouane M, Sezin T, Hirose M, Vorobyev A, Niesar F, Bischof J, Ludwig RJ, Zillikens D, Sadik CD, Restle T, Häsler R, Baines JF, Ibrahim SM. Dissecting genetics of cutaneous miRNA in a mouse model of an autoimmune blistering disease. BMC Genomics. 2016;17:112. doi: 10.1186/s12864-016-2455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan Y, Huang X, Quan X. Expression of miRNA-206 and miRNA-145 in breast cancer and correlation with prognosis. Oncol Lett. 2018;16(5):6638–6642. doi: 10.3892/ol.2018.9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park YR, Seo SY, Kim SL, Zhu SM, Chun S, Oh JM, Lee MR, Kim SH, Kim IH, Lee SO, Lee ST, Kim SW. MiRNA-206 suppresses PGE2-induced colorectal cancer cell proliferation, migration, and invasion by targetting TM4SF1. Biosci Rep. 2018;38(5):BSR20180664. doi: 10.1042/BSR20180664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan D, Dong Xda E, Chen X, Wang L, Lu C, Wang J, Qu J, Tu L. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem. 2009;284(43):29596–604. doi: 10.1074/jbc.M109.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sobuś A, Baumert B, Litwińska Z, Gołąb-Janowska M, Stępniewski J, Kotowski M, Pius-Sadowska E, Kawa MP, Gródecka-Szwajkiewicz D, Peregud-Pogorzelski J, Dulak J, Nowacki P, Machaliński B. Safety and Feasibility of Lin- Cells Administration to ALS Patients:A Novel View on Humoral Factors and miRNA Profiles. Int J Mol Sci. 2018;19(5):1312. doi: 10.3390/ijms19051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valsecchi V, Anzilotti S, Serani A, Laudati G, Brancaccio P, Guida N, Cuomo O, Pignataro G, Annunziato L. miR-206 Reduces the Severity of Motor Neuron Degeneration in the Facial Nuclei of the Brainstem in a Mouse Model of SMA. Mol Ther. 2020;28(4):1154–1166. doi: 10.1016/j.ymthe.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keefe KM, Sheikh IS, Smith GM. Targeting Neurotrophins to Specific Populations of Neurons:NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int J Mol Sci. 2017;18(3):E548. doi: 10.3390/ijms18030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Małczyńska P, Piotrowicz Z, Drabarek D, Langfort J, Chalimoniuk M. [The role of the brain-derived neurotrophic factor (BDNF) in neurodegenerative processes and in the neuroregeneration mechanisms induced by increased physical activity. Postepy Biochem. 2019;65(1):2–8. doi: 10.18388/pb.2019_251. [DOI] [PubMed] [Google Scholar]
- 49.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 50.Wilhelm JC, Xu M, Cucoranu D, Chmielewski S, Holmes T, Lau KS, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012;32(14):5002–9. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shrestha S, Phay M, Kim HH, Pouladvand P, Lee SJ, Yoo S. Differential regulation of brain-derived neurotrophic factor (BDNF) expression in sensory neuron axons by miRNA-206. FEBS Open Bio. 2019;9(2):374–383. doi: 10.1002/2211-5463.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang CN, Wang YJ, Wang H, Song L, Chen Y, Wang JL, Ye Y, Jiang B. The Anti-dementia Effects of Donepezil Involve miR-206-3p in the Hippocampus and Cortex. Biol Pharm Bull. 2017;40(4):465–472. doi: 10.1248/bpb.b16-00898. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z, Chen Y, Li B, Yao Y, Jiang A, Wei W, Liu H, Wu W. Identification of a novel miR-206-Notch3 pathway regulating mouse myoblasts proliferation. Gene. 2019;695:57–64. doi: 10.1016/j.gene.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 54.Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hämmerle M, Esk C, Bagley JA, Lindenhofer D, Chen G, Boehm M, Agu CA, Yang F, Fu B, Zuber J, Knoblich JA, Kerjaschki D, Penninger JM. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565(7740):505–510. doi: 10.1038/s41586-018-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.López-López S, Monsalve EM, Romero de Ávila MJ, González-Gómez J, Hernández de León N, Ruiz-Marcos F, Baladrón V, Nueda ML, García-León MJ, Screpanti I, Felli MP, Laborda J, García-Ramírez JJ, Díaz-Guerra MJM. Notch3 signaling is essential for NF-κB activation in TLR-activated macrophages. Sci Rep. 2020;10(1):14839. doi: 10.1038/s41598-020-71810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keifer J, Zheng Z, Ambigapathy G. A MicroRNA-BDNF Negative Feedback Signaling Loop in Brain:Implications for Alzheimer's Disease. Microrna. 2015;4(2):101–8. doi: 10.2174/2211536604666150813152620. [DOI] [PubMed] [Google Scholar]
- 57.Yang X, Yang Q, Wang X, Luo C, Wan Y, Li J, Liu K, Zhou M, Zhang C. MicroRNA expression profile and functional analysis reveal that miR-206 is a critical novel gene for the expression of BDNF induced by ketamine. Neuromolecular Med. 2014;16(3):594–605. doi: 10.1007/s12017-014-8312-z. [DOI] [PubMed] [Google Scholar]
- 58.Yang M, Li H, Li Y, Ruan Y, Quan C. Identification of genes and pathways associated with MDR in MCF-7/MDR breast cancer cells by RNA-seq analysis. Mol Med Rep. 2018;17(5):6211–6226. doi: 10.3892/mmr.2018.8704. [DOI] [PMC free article] [PubMed] [Google Scholar]