Abstract
It has previously been found that, compared with cigarette smoke, the aerosols generated by heated tobacco products contain fewer and lower harmful and potentially harmful constituents (HPHCs) and elicit lower biological activity in in vitro models and lower smoking-related exposure biomarker levels in clinical studies. It is important to accumulate such scientific evidences for heated tobacco products with a novel heating system, because different heating system may affect the quantitative aspect of the amount of HPHCs and the qualitative aspect of the biological activity of the aerosol generated. Here, the chemical properties of, and toxicological responses to aerosols emitted by DT3.0a, a new heated tobacco product with a novel heating system, and cigarette smoke (CS) were compared, using chemical analyses, in vitro battery (standardized genotoxicity and cytotoxicity) assays, and mechanistic (ToxTracker and two-dimensional cell culture) assays. Regular- and menthol-flavored DT3.0a and standard 1R6F reference cigarettes were tested. Selected HPHC yields were lower in DT3.0a aerosol than 1R6F CS. The genotoxicity-related assays indicated that DT3.0a aerosol was not genotoxic, regardless of metabolic activation. The other biological assays indicated that less cytotoxicity induction and oxidative stress response were elicited by DT3.0a aerosol compared with 1R6F CS. Similar results were found for both regular and menthol DT3.0a. Like previous reports for heated tobacco products with other heating systems, the results of this study indicated that DT3.0a aerosols have chemical and biological properties less likely to be harmful than 1R6F CS.
Keywords: Heated tobacco product, HPHCs, In vitro, Cytotoxicity, Genotoxicity, Oxidative stress
Abbreviations: ACM, aerosol collected mass; AqE, aqueous extract; ARE, anti-oxidant responsive element; BDL, below detection limit; CMF-PBS, calcium- and magnesium-free phosphate buffered saline; CS, cigarette smoke; DT3.0a, Direct Heating Tobacco System Platform 3 generation 3 version a; GFP, green fluorescent protein; GVP, gas-vapor phase; HPHCs, harmful and potentially harmful constituents; HTP, heated tobacco product; IC50, half maximal inhibitory concentration; ISO, International Organization for Standardization; LOD, limit of detection; LOQ, limit of quantification; mES, mouse embryonic stem; MLA, mouse lymphoma assay; MN, micronucleus; MRTP, modified risk tobacco product; NRU, neutral red uptake; NQ, not quantified; ROS, reactive oxygen species; THS, tobacco heating system; THP, tobacco heating product; TPM, total particulate matter
Highlights
-
•
A heated tobacco product (HTP) with a novel heating system, DT3.0a, was investigated.
-
•
Selected HPHC yields were significantly lower in HTP aerosol than cigarette smoke.
-
•
HTP aerosol was not genotoxic and less cytotoxic compared to cigarette smoke.
-
•
Less oxidative stress response was detected in ToxTracker and 2D cell culture assays.
1. Introduction
Heated tobacco products (HTPs), a potential alternative to cigarettes, have become popular around the world in recent years. Fewer and lower levels of cigarette smoke-related harmful and potentially harmful constituents (HPHCs) are typically present in HTP aerosols compared with cigarette smoke (CS) because HTPs emit aerosols by heating but not combusting tobacco, which is the main mechanism through which HPHCs in conventional CS are produced [1], [2]. HPHCs such as aldehydes, polycyclic aromatic hydrocarbons, and tobacco-specific nitrosamines have toxicological properties that can cause adverse health effects such as cardiovascular diseases, respiratory diseases, and cancer [3], [4]. Although quantitative relationships between reducing exposure to such toxicants and reduction in risks have not yet been established, reducing exposure to such toxicants has been recommended by various health authorities, including the World Health Organization [5].
The US FDA issued a draft guidance document [6] containing a framework for assessing next generation tobacco and nicotine products, called modified risk tobacco products (MRTPs). A specific HTP, i.e., the IQOS Tobacco Heating System, was the first HTP authorized through the MRTP framework with “exposure modification order” [7]. Preclinical and clinical assessments of the authorized Tobacco Heating System (THS), especially THS2.2, were performed using the MRTP framework, and the results have been published [8], [9], [10], [11], [12]. Other tobacco companies have also developed and marketed HTPs. It has been found in studies of such HTPs that using a temperature low enough not to burn tobacco decreases the amounts of toxicants emitted [10], [13], [14], [15]. Lower in vitro biological activities have also been found for aerosols emitted from HTPs than CS [10], [14], [16], [17]. In clinical studies, lower smoking-related exposure biomarker levels have been seen in the subjects who switched to HTPs than in the subjects who continued to use combustible cigarettes, and the exposure biomarker levels were similar in the subjects who switched to HTPs and the subjects who ceased smoking [12], [18], [19]. This suggests that switching HTPs from combustible cigarettes can reduce exposure to HPHCs.
Various aerosol generation systems are used in HTPs, and the tobacco heating mechanisms are different for the different aerosol generation systems. Currently available HTPs use what can be roughly classed as indirect and direct heating systems. In an HTP with an indirect heating system currently available, an aerosol that may or may not contain nicotine is generated by heating at < 40 °C and passed through a tobacco capsule before being inhaled [15], [20]. In an HTP with a direct heating system, tobacco sticks are directly heated using a blade or periphery heater. The THS2.2 is an HTP with a direct heating system in which an electrically heated metal blade directly heats tobacco to a maximum temperature of 350 °C [1], [10]. Another in-market HTP, glo tobacco heating products (THPs) called THP1.0 and THP1.4, heat the periphery of a tobacco rod to < 250 °C [2], [13], [21]. We (Japan Tobacco Inc.) have developed a new HTP Direct heating Tobacco System Platform Generation 3 version a (DT3.0a), which heats a tobacco stick in a different temperature range (the maximum temperature is approximately 295 °C). As shown schematically in Fig. 1, the DT3.0a peripherally heats a tobacco stick.
Fig. 1.
Components of a DT3.0a system.
The aim of the study was to confirm the potential for DT3.0a with regular- and menthol-flavored consumable sticks to reduce health risks posed by continued tobacco use and to strengthen our understanding of HTPs as the potentially Reduced Risk Products. The main constituents and selected HPHCs, so-called “Hoffmann analytes”, in DT3.0a aerosol were analyzed. Genotoxic, cytotoxic, and oxidative stress-related responses to the particulate phase (aerosol collected mass (ACM) and total particulate matter (TPM)) and gas-vapor phase (GVP) were then assessed. Based on the results obtained in the present study, the results for exposure to CS and DT3.0a aerosol were then compared to assess lower yields of certain biologically-active constituents as well as lower in vitro toxicological responses by DT3.0a aerosol.
2. Materials and methods
2.1. Tobacco products investigated
This study used regular- and menthol-flavored DT3.0a (Japan Tobacco Inc., Tokyo, Japan). DT3.0a consists of a tobacco stick and a device housing a heater and a battery, as shown in Fig. 1. During use, the heater heats the tobacco stick and the maximum temperature is approximately 295 °C. Tests were also performed using 1R6F reference cigarettes (Kentucky Tobacco Research & Development Center, Lexington, KY, USA), which are king-sized cigarettes blended in the USA, to provided data as a comparator. Chemical and toxicological analyses were performed at contract research organizations, as shown in Supplementary Table 1.
2.2. Smoke and aerosol generation
The DT3.0a sticks, in sealed packets, and 1R6F cigarettes were conditioned for at least 48 h at 22 ± 1 °C and 60% ± 3% relative humidity before use, as recommended in the International Organization for Standardization (ISO) 3402 [22]. The battery of a DT3.0a was fully charged before the HTP was used to generate aerosol. DT3.0a aerosol was generated by machine smoking using a modified ISO 20778 smoking regime (55 mL puff volume, 30 s puff interval, 2 s puff duration, bell-shaped puff profile, unblocked ventilation holes). We chose not to block the ventilation holes, because the position of the ventilation holes inside the device precludes possibility of blocking ventilation holes under intended conditions of use. The total puff number for DT3.0a was set as 11 based on the product specification. 1R6F CS was generated during machine smoking using the ISO 20778 smoking regime (55 mL puff volume, 30 s puff interval, 2 s puff duration, bell-shaped puff profile, 100% blocked ventilation holes) [23]. Information about the smoking machines that were used is given in Supplementary Table 1.
2.3. Chemical analyses
The yields of main constituents (ACM/TPM, carbon monoxide, glycerol, nicotine, propylene glycol, and water), menthol, and the other 43 Hoffmann analytes were determined in aerosols produced by DT3.0a and CS from 1R6F cigarettes. The analyses were performed using the validated methods in accordance with the testing laboratory’s ISO 17025 accreditation [24] described in Supplementary Table 2. Room air blank samples were also collected for each analyte to identify contaminants in the ambient air, analytical reagents, or equipment. The mean and 95% confidence interval for each analyte, determined by making five independent measurements, were reported. A yield was considered below the detection limit (BDL) if the mean for five replicate analyses was below the limit of detection (LOD). A yield was classed as not quantified (NQ) if the mean for five replicate analyses was below the limit of quantification (LOQ) but above the LOD.
The yields of Hoffmann analytes in regular- and menthol-flavored DT3.0a aerosols and 1R6F CS were calculated for as many constituents as possible by replacing BDL and NQ results with specified values. Each BDL result was replaced with half the LOD. Each NQ result was replaced with (LOD + LOQ) / 2. If the results for both the 1R6F and DT3.0a were NQ or BDL (i.e., for chromium, nickel, and selenium) the analyte was excluded from the yield comparison.
2.4. In vitro studies
Ames assay, in vitro micronucleus, neutral red uptake, and ToxTracker assays were performed in accordance with the principles of Good Laboratory Practice. Cell viability and antioxidant response element reporter assays in BEAS-2B cells were conducted as non-GLP studies but were scrutinized by the quality assurance unit at the testing facility. Ames, in vitro micronucleus, and neutral red uptake assays were performed generally in line with the corresponding Organisation for Economic Co-operation and Development (OECD) guidelines and Health Canada Official methods as summarized in Supplementary Table 3.
2.5. Sample preparation for in vitro studies
Regular- and menthol-flavored DT3.0a aerosols were generated using linear smoking machines and mainstream CS from 1R6F cigarettes was generated using rotary smoking machines, as described in Supplementary Table 1. ACM from DT3.0a aerosol or TPM from 1R6F CS was collected on a 92 mm or 44 mm (ToxTracker only) Cambridge filter pad. Particle phase samples were extracted with dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO, USA) to give DT3.0a ACM and 1R6F CS TPM extracts, respectively. The ACM/TPM samples used in the in vitro assays were stored at ≤ −70 °C in a cryofreezer until they were tested. The aerosol or smoke fraction that passed through the filter pad was bubbled through an appropriate volume of ice-cold calcium- and magnesium-free phosphate buffer saline (CMF-PBS) to give a GVP sample less than 1 h before an assay was performed. The maximum test sample concentrations used in the toxicological assays are shown in Supplementary Table 3.
2.6. Ames assay
The bacterial strains were Salmonella typhimurium TA98, TA100, TA1535, and TA1537 kindly donated by Dr. B.N. Ames (California University, Berkeley, CA, USA) and Salmonella typhimurium TA102 obtained from the Japan Bioassay Research Center (Kanagawa, Japan). A reaction mixture containing > 1 × 108 bacteria cells, the selected serially diluted test sample, a rat liver S9 which was induced by phenobarbital and 5,6-benzoflavone (IEDA Trading, Tokyo, Japan), and co-factors (Cofactor-I; Oriental Yeast, Tokyo, Japan) was incubated for 20 min at 37 °C with shaking. Sodium phosphate buffer (0.1 mol/L) was used instead of the S9-mix in tests without metabolic activation. After incubation, the reaction mixture was mixed with molten top agar and over-layered on a minimal glucose agar plate. The plate was incubated at 37 °C for 48 h, then the number of revertants per plate was manually counted. Treatment doses were selected based on the evidence of toxicity (thinning of the background bacterial lawn or a reduction in bacteria) if any. Positive controls were performed, as shown in Supplementary Table 4.
For each test, three independent experiments were performed using triplicate plates. A product was classed as mutagenic if the result for any strain ± S9 combination gave a reproducible dose-dependent increase in the number of revertants and at least twice the number of revertants in the concurrent solvent control at one test concentration or more [25].
2.7. In vitro micronucleus assay
Chinese hamster lung cell line (CHL/IU) was obtained from KAC (Tokyo, Japan). Cells (1 ×105 cells/mL) were incubated in Eagle’s minimum essential medium (Nissui Pharmaceutical, Tokyo, Japan) containing 10% (v/v) fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) in an incubator kept at 37 °C with a 5% CO2 atmosphere for 24 h before they were used in tests. The ACM/TPM and GVP samples were tested using three treatments, short-term (3 h) exposure without and with metabolic activation and long-term (24 h) exposure without metabolic activation. Metabolic activation was achieved by adding 5% (v/v) of S9-mix containing phenobarbital and 5,6-benzoflavone-induced rat liver homogenate (IEDA Trading). The positive controls are described in Supplementary Table 4.
After exposure, the cells were detached using trypsin and the aliquot of cell suspension was subjected to cytotoxicity measurements using a Coulter Counter Z2 automatic cell counter (Beckman Coulter, Brea, CA, USA) to calculate relative population doubling [26], [27]. The remaining cells were incubated in 0.075 mol/L potassium chloride for 9 min at 37 °C and then fixed using ice-cold Carnoy’s solution (a 3:1 (v/v) mixture of methanol and acetic acid). The cells were then washed with Carnoy’s solution, resuspended in ethanol containing 3% (v/v) acetic acid, spread on a microscope slide, allowed to dry at room temperature, stained with acridine orange solution (25 µg/mL), and inspected by fluorescence microscopy at 600 × magnification. The frequency of micronucleated cells was determined by counting the number of micronucleated cells per 2000 interphase cells (1000 cells/slide, duplicate cultures). Mitotic cells and multinucleated cells were excluded from the analysis. The maximum MN diameter was defined as half the diameter of the nucleus.
Triplicate independent tests were performed using each sample, and genotoxicity was assessed using a previously published method [28]. The Cochran–Armitage trend test was used to assess the dose-dependency of the MN frequency. Fisher’s exact tests were performed to identify significant increases in MN frequency over the concurrently analyzed solvent controls at one concentration or more. The MN frequency was also compared with the range for historical solvent controls [29]. The test sample was classed as genotoxic if both statistical tests indicated a significant difference, the MN frequency was higher than the historical solvent control range, and the triplicate results were similar. Cochran–Armitage trend tests and Fisher’s exact tests were performed using EXSUS (version 8.1.0, CAC Croit, Tokyo, Japan). A test result was considered significant if p < 0.05.
2.8. Neutral red uptake assay
Chinese hamster ovary K1 cell line was obtained from DS Pharma Biomedical (Osaka, Japan). Cells in 96-well plates (5 ×104 cells/mL) were incubated in Ham’s F-12 nutrient mixture medium (Thermo Fisher Scientific) containing 10% (v/v) FBS (Sigma-Aldrich) in an incubator at 37 °C with a 5% CO2 atmosphere for 24 h before being exposed to a test sample. The cells were then treated with an ACM/TPM or GVP test sample for 24 h. Sodium dodecyl sulfate was used as a positive control. After exposure, the cells were washed with CMF-PBS and incubated with medium containing 24.2 µg/mL of neutral red dye for 3 h. The cells were then fixed with 1% (v/v) formalin in water for 1–2 min. The fixative was removed, and the neutral red dye taken up by the viable cells was extracted by adding 50% ethanol containing 1% (v/v) acetic acid, then absorbance at 540 nm was measured using a microplate reader.
Triplicate independent experiments were performed using each test sample. Cytotoxicity was assessed from the absorbance relative to the absorbance of the concurrent solvent control. The half maximal inhibitory concentration (IC50) for each product was estimated by performing a non-linear regression analysis of the relationship between the relative absorbance and concentration using the least squares method using a logistic function in JMP (version 16, SAS Institute Japan, Tokyo, Japan).
2.9. ToxTracker assay
The ToxTracker assay was performed as described in detail previously [30]. Briefly, mouse embryonic stem (mES) cells in gelatin-coated dishes were cultured in KnockOut Dulbecco’s modified Eagle medium (Toxys, Leiden, The Netherlands) with 200 µL/mL of G418 (Thermo Fisher Scientific) added in an incubator at 37 °C with a 5% CO2 atmosphere. Six mES cell lines were seeded into gelatin-coated 96-well plates with 200 µL of mES cell culture medium (4 ×104 cells/well) in each well, then the plates were incubated at 37 °C with a 5% CO2 atmosphere for 24 h. The cells were then treated with an ACM/TPM or GVP sample for 24 h in the presence or absence of Aroclor 1254-induced rat liver S9 (Moltox, Boone, NC, USA) and co-factors (RegenSys A + B and Moltox). The positive controls are described in Supplementary Table 4. After exposure, induction of green fluorescent protein (GFP) reporters was measured using a BD FACSCanto flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The median GFP fluorescence value was used to calculate GFP reporter induction in the test sample relative to the concurrent solvent control cultures. Cytotoxicity in the ToxTracker assay was assessed using mean relative cell survival for the six different reporter cell lines.
Triplicate independent tests were performed using each test sample. A positive ToxTracker assay response was defined as a test sample inducing at least a doubling of GFP expression of any of the reporters when the treatment cytotoxicity was ≤ 75%.
2.10. Cell viability and anti-oxidant responsive element reporter assay using BEAS-2B cells
Two-dimensional culture assays were performed following a previously published method [31] using an immortalized normal human bronchial epithelial BEAS-2B cell line (American Type Culture Collection, Manassas, VA, USA). BEAS-2B cells were transfected with anti-oxidant responsive element (ARE) luciferase reporter vector (E3641; Promega, Madison, WI, USA) as previously reported [32]. Cells (5 ×103 cells/well) were seeded into a 96-well plate. Each well contained 100 µL of Dulbecco’s modified Eagle medium containing 25 mmol/L glucose, 4 mmol/L L-glutamine (Thermo Fisher Scientific), and 10% (v/v) FBS (MP Biomedicals, Carlsbad, CA, USA). The plate was incubated at 37 °C with a 5% CO2 atmosphere for 24 h. The cells were then treated with an ACM/TPM or GVP test sample for 24 h. After exposure, cell viability and ARE reporter activity were determined using a Cell Titer-Fluor cell viability assay (Promega) and a Luciferase Assay System (Promega), respectively. Fluorescence and luminescence were measured using an Infinite 200 Pro plate reader (Tecan Group, Männedorf, Switzerland).
Each test was performed in triplicate, and statistical analyses were performed using EXSUS (version 8.1.0, CAC Croit). A Bartlett’s test was performed to confirm the homogeneity of variance of each group, then a one-way analysis of variance followed by a Dunnett’s multiple comparison test against the control condition values were performed. If homogeneity of variance was not confirmed, a Kruskal–Wallis test was performed. A difference was considered significant when p < 0.05. The data obtained from the ARE reporter assays were log-transformed before the statistical analyses were performed.
3. Results and discussion
3.1. Characterization of aerosols and smoke
The yields of the main constituents (ACM/TPM, carbon monoxide, nicotine, and water) are shown in Table 1. The yields of glycerol and propylene glycol, added to the tobacco sticks to act as humectants, in the DT3.0a aerosol and 1R6F CS and the yields of menthol (a characteristic chemical in the menthol-flavored DT3.0a aerosol) are also shown in Table 1.
Table 1.
Yields of the main constituents in the regular- and menthol-flavored DT3.0a aerosols and 1R6F cigarette smoke.
| Parameter | Unit | DT3.0a (Regular) |
DT3.0a (Menthol) |
1R6F |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | 95CI | Mean | ± | 95CI | LOD | LOQ | Mean | ± | 95CI | LOD | LOQ | ||||||
| Puff count | puff | 11 | 11 | 8.75 | ||||||||||||||
| ACM/TPM | mg | 42.5 | ± | 1.1 | 43.2 | ± | 1.5 | NA | NA | 47.3 | ± | 5.1 | NA | NA | ||||
| Nicotine | mg | 1.33 | ± | 0.06 | 1.38 | ± | 0.07 | 0.001 | 0.004 | 2.01 | ± | 0.09 | 0.002 | 0.007 | ||||
| Water | mg | 23.1 | ± | 1.1 | 20.8 | ± | 0.7 | 0.038 | 0.128 | 17.7 | ± | 2.4 | 0.064 | 0.213 | ||||
| Propylene glycol | mg | 0.448 | ± | 0.017 | 0.508 | ± | 0.019 | 0.002 | 0.008 | 0.291 | ± | 0.020 | 0.004 | 0.013 | ||||
| Glycerol | mg | 6.35 | ± | 0.30 | 6.31 | ± | 0.33 | 0.014 | 0.048 | 1.35 | ± | 0.09 | 0.024 | 0.080 | ||||
| Menthol | mg | < 0.008 | 2.61 | ± | 0.12 | 0.002 | 0.008 | < 0.004 | 0.004 | 0.014 | ||||||||
| Carbon monoxide | mg | < 0.223 | < 0.223 | 0.067 | 0.223 | 27.5 | ± | 2.1 | 0.159 | 0.530 | ||||||||
The yields per tobacco stick for DT3.0a and per cigarette for 1R6F are given. Each value is the mean ± 95CI for five independent measurements. Abbreviations: ACM aerosol collected mass, 95CI 95% confidence interval, LOD limit of detection, LOQ limit of quantification, NA not applicable, TPM total particulate matter.
The ACM yields of the regular- and menthol-flavored DT3.0a (42.5 and 43.2 mg, respectively) when 11 puffs were taken per tobacco stick were comparable to the TPM yields of the 1R6F cigarettes (47.3 mg) when 8.75 puffs were taken per cigarette. The nicotine yield was ∼30% lower for both regular- and menthol-flavored DT3.0a aerosol than 1R6F smoke. From the major constituent analysis, it was revealed that the ACM from the DT3.0a aerosol was predominantly comprised of water, propylene glycol, and glycerol, which together accounted for > 60% (w/w) of the ACM. However, those contributed ∼40% (w/w) of the TPM from 1R6F CS. Propylene glycol and glycerol are widely used in food, pharmaceuticals, cosmetics, and tobacco products. Additionally, it was demonstrated that the whole-body exposure of aerosol from the base formulation containing these two humectants to A/J mice produced no significant mortality or in-life clinical findings in 5-week study [33]. Therefore, these liquid constituents were yielded higher in DT3.0a aerosol than in 1R6F CS, suggesting less toxic property of DT3.0a aerosol compared with 1R6F CS. Menthol was detected above the LOQ only in the menthol-flavored DT3.0a aerosol, as expected. The carbon monoxide yields in the regular- and menthol-flavored DT3.0a aerosol were below the LOQ, but the carbon monoxide yield in the 1R6F CS was 27.5 mg/cigarette. Carbon monoxide is considered as an important marker of tobacco combustion, so these results are consistent with DT3.0a aerosol being generated without combustion.
3.2. Hoffmann analytes
The Hoffmann analyte yields for the regular- and menthol-flavored DT3.0a aerosols, 1R6F CS, and air blanks are shown (grouped by chemical class) in Table 2. The target analytes were also determined in laboratory air to allow background contamination of the air, analytical equipment, and reagents to be excluded (i.e., to distinguish between background contaminants and the actual constituents of the aerosols or CS). The LOD and LOQ for each analyte are shown in Table 2. The LODs and LOQs for different test items were different because different numbers of test items were used and/or different volumes of extraction solutions were used.
Table 2.
Yields of Hoffmann analytes in regular- and menthol-flavored DT3.0a aerosols, 1R6F cigarette smoke, and air blanks.
| Analytes | Unit | DT3.0a (Regular) |
DT3.0a (Menthol) |
Air Blank |
1R6F |
Air Blank |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | 95CI | Mean | ± | 95CI | Mean | ± | 95CI | LOD | LOQ | Mean | ± | 95CI | Mean | ± | 95CI | LOD | LOQ | ||||||||
| Aromatic amines | ||||||||||||||||||||||||||
| 1-aminonaphthalene | ng | < 0.027 | 0.115 | ± | 0.011 | <0.008 | 0.008 | 0.027 | 21.9 | ± | 0.8 | 0.358 | ± | 0.574 | 0.027 | 0.091 | ||||||||||
| 2-aminonaphthalene | ng | < 0.012 | < 0.012 | <0.004 | 0.004 | 0.012 | 15.4 | ± | 1.3 | 0.384 | ± | 0.606 | 0.012 | 0.040 | ||||||||||||
| 3-aminobiphenyl | ng | < 0.004 | 0.006 | ± | 0.003 | <0.001 | 0.001 | 0.004 | 3.67 | ± | 0.27 | 0.074 | ± | 0.127 | 0.004 | 0.013 | ||||||||||
| 4-aminobiphenyl | ng | 0.008 | ± | 0.001 | 0.017 | ± | 0.003 | <0.001 | 0.001 | 0.005 | 2.54 | ± | 0.19 | 0.052 | ± | 0.082 | 0.005 | 0.016 | ||||||||
| Carbonyls | ||||||||||||||||||||||||||
| Formaldehyde | µg | 4.67 | ± | 0.42 | 3.42 | ± | 0.29 | 2.04 | ± | 0.24 | 0.361 | 1.20 | 85.6 | ± | 5.1 | 2.6 | ± | 0.2 | 0.361 | 1.20 | ||||||
| Acetaldehyde | µg | 93.7 | ± | 4.3 | 98.8 | ± | 5.1 | <3.24 | 0.973 | 3.24 | 1406 | ± | 75 | 6.07 | ± | 1.89 | 0.973 | 3.24 | ||||||||
| Acetone | µg | 10.3 | ± | 0.4 | 12.1 | ± | 0.3 | <2.82 | 0.846 | 2.82 | 571 | ± | 31 | 4.42 | ± | 0.92 | 0.846 | 2.82 | ||||||||
| Acrolein | µg | 2.82 | ± | 0.22 | < 2.38 | <0.713 | 0.713 | 2.38 | 137 | ± | 7 | < 0.713 | 0.713 | 2.38 | ||||||||||||
| Propionaldehyde | µg | 4.51 | ± | 0.37 | 4.66 | ± | 0.48 | <1.00 | 1.00 | 3.34 | 98.9 | ± | 5.2 | < 1.00 | 1.00 | 3.34 | ||||||||||
| Crotonaldehyde | µg | < 0.988 | < 0.988 | <0.988 | 0.988 | 3.29 | 44.2 | ± | 4.3 | < 0.988 | 0.988 | 3.29 | ||||||||||||||
| Methyl Ethyl Ketone | µg | < 3.66 | < 3.66 | <1.10 | 1.10 | 3.66 | 145 | ± | 11 | < 3.66 | 1.10 | 3.66 | ||||||||||||||
| Butyraldehyde | µg | < 0.812 | < 0.812 | <0.812 | 0.812 | 2.71 | 70.2 | ± | 3.9 | < 0.812 | 0.812 | 2.71 | ||||||||||||||
| Phenolics | ||||||||||||||||||||||||||
| Hydroquinone | µg | 3.97 | ± | 0.10 | 3.82 | ± | 0.08 | <0.062 | 0.062 | 0.207 | 110 | ± | 6 | < 1.35 | 1.35 | 4.51 | ||||||||||
| Resorcinol | µg | 0.071 | ± | 0.034 | 0.063 | ± | 0.033 | <0.016 | 0.016 | 0.055 | 1.75 | ± | 0.18 | < 0.395 | 0.395 | 1.32 | ||||||||||
| Catechol | µg | 10.2 | ± | 0.5 | 11.4 | ± | 0.5 | <0.026 | 0.026 | 0.086 | 98.2 | ± | 5.4 | < 1.21 | 1.21 | 4.03 | ||||||||||
| Phenol | µg | 0.403 | ± | 0.010 | 0.397 | ± | 0.017 | <0.026 | 0.026 | 0.086 | 14.3 | ± | 1.8 | < 1.43 | 1.43 | 4.78 | ||||||||||
| p-cresol | µg | < 0.034 | 0.036 | ± | 0.010 | <0.010 | 0.010 | 0.034 | 8.21 | ± | 0.77 | < 0.207 | 0.207 | 0.691 | ||||||||||||
| m-cresol | µg | < 0.019 | < 0.019 | <0.006 | 0.006 | 0.019 | 3.23 | ± | 0.29 | < 0.451 | 0.451 | 1.50 | ||||||||||||||
| o-cresol | µg | 0.051 | ± | 0.004 | 0.035 | ± | 0.003 | <0.008 | 0.008 | 0.026 | 4.02 | ± | 0.41 | < 0.184 | 0.184 | 0.614 | ||||||||||
| PAH | ||||||||||||||||||||||||||
| Benzo(a)pyrene | ng | 0.519 | ± | 0.051 | 1.18 | ± | 0.08 | <0.106 | 0.106 | 0.354 | 13.4 | ± | 0.5 | < 0.177 | 0.177 | 0.590 | ||||||||||
| Nitrogen oxides | ||||||||||||||||||||||||||
| NO | µg | 2.65 | ± | 0.35 | 2.84 | ± | 0.24 | <0.176 | 0.176 | 0.588 | 346 | ± | 36 | < 3.63 | 3.63 | 12.2 | ||||||||||
| NOx | µg | 3.35 | ± | 0.32 | 3.54 | ± | 0.17 | <0.421 | 0.421 | 1.40 | 386 | ± | 37 | < 7.01 | 7.01 | 18.2 | ||||||||||
| Cyanic compound | ||||||||||||||||||||||||||
| HCN | µg | < 1.75 | < 1.75 | <0.525 | 0.525 | 1.75 | 377 | ± | 18 | < 1.31 | 1.31 | 4.37 | ||||||||||||||
| Amine | ||||||||||||||||||||||||||
| Ammonia | µg | 4.94 | ± | 0.38 | 14.9 | ± | 1.6 | 1.02 | ± | 0.29 | 0.184 | 0.613 | 28.6 | ± | 2.8 | < 1.46 | 1.46 | 4.88 | ||||||||
| Volatile organic compounds | ||||||||||||||||||||||||||
| 1,3-butadiene | µg | < 0.095 | < 0.095 | <0.029 | 0.029 | 0.095 | 98.8 | ± | 2.3 | < 0.190 | 0.190 | 0.633 | ||||||||||||||
| Isoprene | µg | < 0.135 | < 0.135 | <0.041 | 0.041 | 0.135 | 794 | ± | 27 | < 0.901 | 0.270 | 0.901 | ||||||||||||||
| Acrylonitrile | µg | < 0.032 | < 0.032 | <0.032 | 0.032 | 0.107 | 19.5 | ± | 0.9 | < 0.213 | 0.213 | 0.711 | ||||||||||||||
| Benzene | µg | 0.128 | 0.008 | 0.107 | ± | 0.018 | <0.017 | 0.017 | 0.056 | 77.3 | ± | 3.8 | < 0.373 | 0.112 | 0.373 | |||||||||||
| Toluene | µg | 0.233 | 0.033 | 0.237 | ± | 0.020 | <0.061 | 0.061 | 0.204 | 124 | ± | 3 | < 1.36 | 0.408 | 1.36 | |||||||||||
| PQS | ||||||||||||||||||||||||||
| Pyridine | µg | 2.93 | ± | 0.29 | 3.35 | ± | 0.26 | 0.153 | ± | 0.104 | 0.027 | 0.090 | 29.3 | ± | 6.5 | 0.376 | ± | 0.064 | 0.090 | 0.300 | ||||||
| Quinoline | µg | < 0.011 | < 0.011 | <0.003 | 0.003 | 0.011 | 0.506 | ± | 0.039 | < 0.011 | 0.011 | 0.036 | ||||||||||||||
| Styrene | µg | 0.074 | ± | 0.014 | 0.094 | ± | 0.007 | <0.012 | 0.012 | 0.039 | 13.7 | ± | 4.2 | < 0.129 | 0.039 | 0.129 | ||||||||||
| Tobacco specific nitrosamines | ||||||||||||||||||||||||||
| NNN | ng | 18.8 | ± | 0.3 | 37.2 | ± | 1.2 | <0.098 | 0.098 | 0.328 | 185 | ± | 13 | < 0.164 | 0.164 | 0.547 | ||||||||||
| NAT | ng | 40.5 | ± | 1.7 | 65.9 | ± | 1.5 | <0.195 | 0.195 | 0.650 | 269 | ± | 83 | < 0.325 | 0.325 | 1.08 | ||||||||||
| NAB | ng | 5.74 | ± | 0.57 | 10.0 | ± | 0.8 | <0.054 | 0.054 | 0.179 | 24.2 | ± | 4.4 | < 0.089 | 0.089 | 0.298 | ||||||||||
| NNK | ng | 10.8 | ± | 0.3 | 17.5 | ± | 0.7 | <0.151 | 0.151 | 0.502 | 211 | ± | 24 | < 0.251 | 0.251 | 0.836 | ||||||||||
| Metals | ||||||||||||||||||||||||||
| Mercury | ng | 1.62 | ± | 0.11 | 1.44 | ± | 0.13 | <0.348 | 0.104 | 0.348 | 3.97 | ± | 0.54 | < 0.857 | 0.857 | 2.86 | ||||||||||
| Cadmium | ng | < 0.150 | < 0.150 | <0.150 | 0.150 | 0.500 | 99.8 | ± | 15.09 | < 0.953 | 0.953 | 3.18 | ||||||||||||||
| Lead | ng | < 1.00 | < 1.00 | <1.00 | 0.300 | 1.00 | 27.5 | ± | 2.1 | < 7.70 | 7.70 | 25.7 | ||||||||||||||
| Chromium | ng | < 5.00 | < 5.00 | <5.00 | 1.50 | 5.00 | < 11.9 | < 11.9 | 11.9 | 39.6 | ||||||||||||||||
| Nickel | ng | < 10.0 | < 10.0 | <10.0 | 3.00 | 10.0 | < 12.9 | < 12.9 | 12.9 | 43.1 | ||||||||||||||||
| Arsenic | ng | < 1.50 | < 1.50 | <1.50 | 0.450 | 1.50 | 8.62 | ± | 1.01 | < 2.25 | 2.25 | 7.49 | ||||||||||||||
| Selenium | ng | < 0.240 | < 0.800 | <0.240 | 0.240 | 0.800 | < 4.42 | < 4.42 | 4.42 | 14.7 | ||||||||||||||||
The yields per tobacco stick for DT3.0a (11 puffs) and per cigarette for 1R6F (9–12 puffs) are presented. The yields of the air blank samples are also given. The mean ± 95CI values for five independent measurements are shown. The 95CI was not calculated if the mean for five replicate analyses was below LOD or if that was LOQ but above LOD. Abbreviations: 95CI 95% confidence interval, HCN hydrogen cyanide, LOD limit of detection, LOQ limit of quantification, NAB nitrosoanabasine, NAT nitrosoanatabine, NNK 4‐(methylnitrosoamino)‐1‐(3‐pyridyl)‐1‐butanone, NNN nitrosonornicotine, NO nitric oxide, NOx nitrogen oxides, PAH polycyclic aromatic hydrocarbon, PQS pyridine, quinoline, and styrene.
Of the 43 Hoffmann analytes, 24 and 26 were quantifiable in the regular- and menthol-flavored DT3.0a aerosols, respectively, and 40 were quantifiable in the 1R6F CS, as shown in Table 2. Although chromium, nickel, and selenium were not quantitatively detected in either DT3.0a aerosols or 1R6F CS, the amounts of these analytes in DT3.0a aerosols were suggested to be lower than in 1R6F CS considering the respective LOD and LOQ values. The ratios between the yields for the regular- and menthol-flavored DT3.0a aerosols and the 1R6F CS are shown in Fig. 2. The yield (per mg-TPM) of each analyte for the 1R6F CS was given a value of 1.0. On a per mg-TPM basis, ammonia, catechol, mercury, nitrosoanabasine, nitrosoanatabine, nitrosonornicotine, and pyridine had ratios of 0.1–0.5 for both regular- and menthol-flavored DT3.0a, as shown in Fig. 2. The ratios for four aromatic amines (1-aminonaphthalene, 2-aminonaphthalene, 3-aminobiphenyl, and 4-aminobiphenyl), nitrogen oxides, two volatile organic compounds (benzene and toluene), and styrene were much lower for both regular- and menthol-flavored DT3.0a aerosols (≤0.01) than 1R6F CS, as shown in Table 2 and Fig. 2. Similar to carbon monoxide, nitric oxide and nitrogen oxides are also considered as representative markers of tobacco combustion, thus the low yield of these constituents also supported DT3.0a aerosol being generated through heating without combustion. Three carbonyl compounds (butyraldehyde, crotonaldehyde, and methyl ethyl ketone), m-cresol, hydrogen cyanide, three metals (arsenic, cadmium, and lead), quinoline, and three volatile organic compounds (acrylonitrile, 1,3-butadiene, and isoprene) were below the detection limit or not quantified in the DT3.0a aerosols, as shown in Table 2 and Fig. 2. The yields of the Hoffmann analytes in all of the chemical classes were generally significantly lower in the DT3.0a aerosols than the 1R6F CS.
Fig. 2.
Yields of Hoffmann analytes for regular- and menthol-flavored DT3.0a aerosols relative to the yields for 1R6F cigarettes smoke. The yields of 43 chemicals in cigarette smoke classed as priority constituents that require or are proposed for regulation were determined. Yields of chromium, nickel, and selenium were excluded since their yields were not quantified or below the detection limit in both DT3.0a aerosol and 1R6F CS.
3.3. In vitro toxicological testing
3.3.1. Ames assay
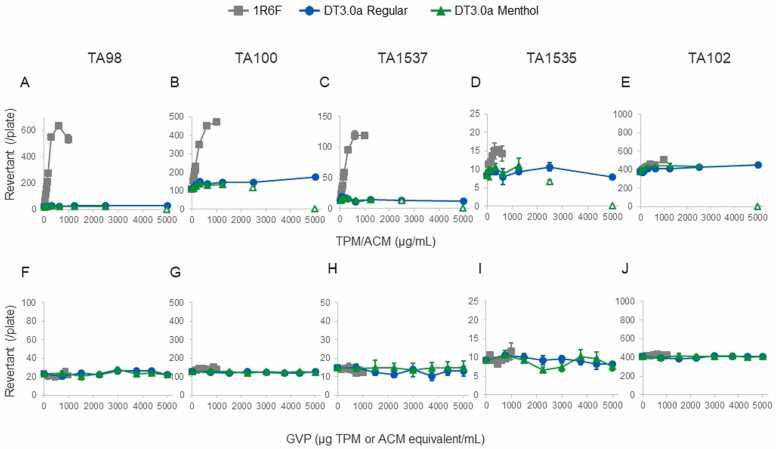
The regular- and menthol-flavored DT3.0a aerosol and 1R6F CS ACM/TPM and GVP samples were subjected to the Ames assay to evaluate mutagenicity. Five tester strains (TA98, TA100, TA102, TA1535, and TA1537) were used with and without metabolic activation during the pre-incubation method. The numbers of revertant colonies in the groups exposed to the ACM/TPM and GVP samples with metabolic activation are shown in Fig. 3. The numbers of revertant colonies in the groups exposed to the ACM/TPM and GVP samples without metabolic activation are shown in Supplementary Fig. 1.
Fig. 3.
Ames mutagenicity assay results for bacterial strains exposed to 1R6F cigarette smoke and regular- and menthol-flavored DT3.0a aerosol collected mass (ACM), total particulate matter (TPM), and gas-vapor phase (GVP) samples. Exposure to the (A–E) ACM/TPM and (F–J) GVP samples was performed using the pre-incubation method with metabolic activation. After 48 h of incubation, the numbers of revertants in (A and F) TA98, (B and G) TA100, (C and H) TA1537, (D and I) TA1535, and (E and J) TA102 were counted. Each result is the mean and standard error for triplicate independent tests. The concentrates where evidence of toxicity was observed as indicated with open marker is excluded in the assessment.
In general, evidence of toxicity (thinning of the background bacterial lawn or a reduction in bacteria) was observed at some higher concentrations in all ACM/TPM samples in the presence and absence of S9 (Fig. 3 A–3 E and Supplementary Fig. 1A–1E, respectively).
Clear and reproducible increases in the numbers of revertant colonies were found in the TA98, TA100, TA1535, and TA1537 tester strain groups exposed to 1R6F TPM with metabolic activation, as shown in Fig. 3 A, 3B, 3C and 3D. In contrast, the DT3.0a ACM samples did not have twice the number of revertants found for the concurrent solvent control group up to the maximum ACM concentration of 5000 µg/plate for any of the tester strains, as shown in Fig. 3A–3E. No clear positive responses to the 1R6F CS or DT3.0a aerosol ACM/TPM and GVP samples were found under the other conditions, as shown in Fig. 3. Similar results were found without metabolic activation except that positive responses to the 1R6F CS GVP sample were found for the TA1537 strain, as shown in Supplementary Fig. 1.
These Ames test results indicate that neither particulate nor gaseous fractions from any-flavored DT3.0a aerosols appear to be mutagenic, whereas mutagenic responses were seen in some bacterial strains exposed to the particulate phase derived from 1R6F CS.
3.3.2. In vitro micronucleus assay
The clastogenic and aneugenic potentials of the regular- and menthol-flavored DT3.0a aerosol and 1R6F CS ACM/TPM and GVP samples were assessed by determining MN induction up to the maximum feasible concentrations.
In the short-term treatments with and without metabolic activation, 1R6F CS TPM significantly induced the %MN in a concentration-dependent manner. However, no statistically significant induction of the %MN was found up to a regular- or menthol-flavored DT3.0a ACM concentration of 2000 µg/mL, as shown in Fig. 4 A and 4B. Similar results were found for the 1R6F CS and DT3.0a aerosol GVP samples, as shown in Figs. 4D and 4E.
Fig. 4.
Results of micronucleus assays using Chinese hamster lung CHL/IU cells exposed to 1R6F cigarette smoke and regular- and menthol-flavored DT3.0a aerosol collected mass (ACM), total particulate matter (TPM), and gas-vapor phase (GVP) samples. Exposure to (A–E) ACM/TPM and (F–J) GVP was performed for 3 h in the (A and D) absence and (B and E) presence of the S9 metabolic activation system or (C and F) for 24 h in the absence of the S9 metabolic activation system. Top concentration of TPM sample for 24-h treatment condition is chosen as the maximum feasible concentration, taking into account the highest TPM concentration that could be prepared and the maximum volume applicable for DMSO preparation. Each result is the mean and standard error for three independent tests.
In the long-term treatments, a positive response was only found for the samples exposed to 1R6F CS GVP and no significant induction was found for the samples exposed to 1R6F CS TPM or any of the DT3.0a aerosol samples up to the highest concentrations that were used, as shown in Fig. 4 C and 4 F.
Based on the in vitro MN assay results, MN-inducing capability of the aerosol emitted from the DT3.0a was not observed in the present experimental condition regardless of the flavor in contrast to the concentration-dependent MN increase by 1R6F CS.
3.3.3. Neutral red uptake assay
The regular- and menthol-flavored DT3.0a aerosol and 1R6F CS ACM/TPM and GVP samples were subjected to the NRU assay to assess cytotoxicity.
The cytotoxicity of the 1R6F CS TPM increased strongly as the concentration increased to 200 µg/mL. The mean IC50 for the 1R6F CS TPM was 85.8 µg/mL. The DT3.0a ACM caused the relative absorbance to decrease gradually up to an ACM concentration of 2000 µg/mL, as shown in Fig. 5A. The mean IC50s for regular- and menthol-flavored DT3.0a ACM were 941 and 838 µg/mL, respectively.
Fig. 5.
Results of neutral red uptake assays using Chinese hamster ovary K1 cells exposed to 1R6F cigarette smoke and regular- and menthol-flavored DT3.0a aerosol collected mass (ACM), total particulate matter (TPM), and gas-vapor phase (GVP) samples. Absorbance was measured after 24 h exposure to the (A) ACM/TPM and (B) GVP samples. Each result is the mean and standard error for three independent tests.
The 1R6F CS GVP cytotoxicity also increased strongly as the GVP concentration increase, as shown in Fig. 5B, and the mean IC50 was 88.1 µg TPM equivalent/mL. No IC50s could be determined even at the maximum test concentration (2000 µg ACM equivalent/mL) for the regular- and menthol-flavored DT3.0a GVP samples.
It has been estimated that 90% of the cytotoxicity of the 1R4F CS GVP fraction can be explained by the presence of some aldehydes [34]. The concentrations of seven aldehydes in the DT3.0a aerosols were < 10% of the concentrations in 1R6F CS, as shown in Table 2 and Fig. 2. This suggests that the low concentrations of these aldehydes contributed to the lower cytotoxicity of the DT3.0a aerosol GVP fraction than the 1R6F CS GVP fraction.
3.3.4. ToxTracker assay
The ToxTracker assay is a reporter assay in which a mES GFP reporter cell line is used to detect the mechanisms involved in genotoxicity (DNA damage (Bscl2-GFP and Rtkn-GFP), oxidative stress (Srxn1-GFP and Blvrb-GFP), protein damage (Ddit3-GFP), and cellular stress (Btg2-GFP)) [30]. Mutagenicity and genotoxicity were found mainly in the groups exposed to 1R6F CS TPM or GVP, so mechanistic investigations using the ToxTracker assay were performed using the 1R6F CS and DT3.0a aerosol ACM/TPM and GVP samples. The relative cell viability (the number of cells) and fold changes determined by GFP fluorescence were evaluated after 24 h treatment with metabolic activation by S9, and the results are shown in Fig. 6. The responses to the ACM/TPM and GVP samples without metabolic activation are shown in Supplementary Fig. 2.
Fig. 6.
Results of ToxTracker assays of green fluorescent protein (GFP)-based mouse embryonic stem reporter cell lines exposed to 1R6F cigarette smoke and regular- and menthol- flavored DT3.0a aerosol collected mass (ACM), total particulate matter (TPM), and gas-vapor phase (GVP) samples. Cell viability after 24 h exposure to the (A) ACM/TPM and (B) GVP samples was calculated from the normalized cell count. The results indicated fold induction occurred compared with vehicle controls for the six reporter cell lines relating to DNA damage ((C and I) Bscl2 and (D and J) Rtkn), oxidative stress ((E and K) Srxn1 and (F and L) Blvrb), protein damage ((G and M) Ddit3), and p53 activation ((H and N) Btg2). Cells were exposed for 24 h in the presence of the S9 metabolic activation system. Each result is the mean and standard error for three independent tests. Each dashed line for reporter activation indicates the GFP induction threshold (a factor of two).
Rather poor cell survival rates of 30% and 60% were found after exposure to the 1R6F CS TPM and GVP samples, respectively, at concentrations of 125 and 500 µg/mL, respectively, but good cell survival rates (>80%) were found after exposure to the regular- and menthol-flavored DT3.0a ACM and GVP samples even at the maximum concentration of 1000 µg/mL, as shown in Fig. 6 A and 6 B.
The 1R6F CS only induced reporter activity (indicating DNA damage) in the Bscl2 reporter cell line at the highest concentration, as shown in Fig. 6C. No significant responses were obtained in the Rtkn reporter cell line using the TPM and GVP samples, as shown in Figs. 6D, 6I, and 6J. The weak but significant ability of 1R6F CS to damage DNA was correlated with the clear mutagenic and genotoxic results of the Ames tests and in vitro MN assays shown in Fig. 3, Fig. 4, respectively. In those tests, the 1R6F CS TPM and GVP samples caused concentration-dependent increases in revertant colonies and MN frequency.
Oxidative stress caused by the 1R6F CS TPM and GVP samples in the Srxn1 reporter cells (Figs. 6E and 6 K) and Blvrb reporter cells (Fig. 6F and 6L) corresponded well with the genotoxicity results from the Ames test and in vitro MN assay and the cytotoxicity results from the NRU assay (Fig. 3, Fig. 4, Fig. 5), suggesting that oxidative stress may play a role in the genotoxic and cytotoxic responses of 1R6F CS. Compared with the results found using the 1R6F CS samples, the reporter genes related to oxidative stress were weakly induced by DT3.0a aerosol samples, which may explain the negative results of the Ames and in vitro MN assays and the weak cytotoxicity found in the NRU assay using the DT3.0a aerosol samples.
For the other reporter activities, protein damage was clearly induced by the 1R6F TPM sample only (Fig. 6 G) and p53 activation was slightly increased by the 1R6F GVP sample only (Fig. 6 N). Those reporter responses were similar in the absence of metabolic activation as shown in Supplementary Fig. 2.
The ToxTracker results demonstrated that 1R6F CS induced various toxicological responses relating to DNA damage, oxidative stress, protein damage, and p53 activation, whereas DT3.0a aerosols caused reporter activities derived from the oxidative stress-based mechanism, illustrating the mechanistic differences in toxicity caused by HTP aerosol and combustible CS.
3.3.5. Cell viability and anti-oxidant responsive element reporter assay using BEAS-2B cells
Oxidative responses were also assessed using immortalized normal human bronchial epithelial cells in an ARE reporter assay that detects nuclear factor-erythroid 2-related factor-2-mediated oxidative stress responses [35], [36], like in previous studies [31], [32], [37].
After 24 h of exposure, the 1R6F CS TPM at concentrations > 75 µg/mL statistically significantly decreased cell viability, and the 1R6F CS GVP statistically significantly decreased cell viability at concentrations > 150 µg TPM equivalent/mL. Exposure to the regular-flavored DT3.0a ACM sample at concentrations > 375 µg/mL and to the menthol-flavored DT3.0a ACM sample at concentrations > 250 µg/mL significantly and concentration-dependently decreased cell viability. The regular- and menthol-flavored DT3.0a GVP samples did not significantly decrease cell viability, as shown in Fig. 7 A and 7B.
Fig. 7.
Oxidative stress responses in BEAS-2B cell cultures exposed to 1R6F cigarette smoke and regular- and menthol-flavored DT3.0a aerosol collected mass (ACM), total particulate matter (TPM), and gas-vapor phase (GVP) samples. The (A and B) cell viability and (C and D) anti-oxidant responsive element reporter gene activity were determined after 24 h exposure to the (A and C) ACM/TPM and (B and D) GVP samples. Each result is the mean and standard error for three independent experiments. * p < 0.05 (Dunnett’s multiple comparison test against the control).
The 1R6F CS samples strongly increased ARE reporter activity at any concentration higher than the lowest that were tested (6.25 µg/mL for TPM and 75 µg TPM equivalent/mL for GVP), as shown in Fig. 7 C and 7D. In contrast, the ARE reporter activity only significantly increased at regular-flavored DT3.0a ACM sample concentrations > 250 µg/mL and menthol-flavored DT3.0a ACM sample concentrations > 187.5 µg/mL, as shown in Fig. 7 C. Like for the DT3.0a GVP samples, the ARE reporter activity was significantly increased only by exposure to regular-flavored DT3.0a aerosol at concentrations > 1500 µg/mL, as shown in Fig. 7D. These ARE reporter activity results indicated that oxidative stress was caused in human bronchial epithelial cells by both of 1R6F CS and DT3.0a aerosol but it occurred at lower 1R6F CS concentrations than DT3.0a aerosol concentrations.
The ARE reporter assay results shown in Fig. 7 supported the ToxTracker assay results shown in Fig. 6, particularly the Srxn1 reporter cell line results. This could be related to the Srxn1-GFP reporter cells being strongly activated by cellular oxidative stress and being directly controlled by the nuclear factor (erythroid derived 2)-like 2 transcription factor [38].
3.4. Comparison with heated tobacco products with other heating systems
As mentioned in the Introduction section, there are several kinds of HTPs commercially available on the market. Here, we discuss and compare the summarized chemical and in vitro toxicological properties of DT3.0a with those of two commercially available HTPs, i.e., THS2.2 and THP1.0/THP1.4, by reviewing the relative difference of each HTP to the Kentucky reference cigarette specifically on the quantitative decrease of the selected HPHCs and qualitative reduction of the toxicological response.
In the previous studies reported in literature, chemical analysis was conducted using regular and menthol-flavored THS2.2 [10], and non-mentholated and mentholated THP1.0 [13]. Based on these literatures and present studies, the analyte yields of the main constituents and the selected HPHCs can be compared as shown in Supplementary Table 5. Supplementary Table 5 illustrates that the liquid components including water, propylene glycol, and glycerol contributed to approximately 60% of these HTP aerosols as the main constituents, and that the proportion of liquid components in any HTP aerosol was larger than that in the reference CS. This table also shows that ammonia, catechol, mercury, nitrosoanatabine, and pyridine were relatively high among the three HTPs, which were expected results because these constituents are thought to be formed or transferred from tobacco heated even at low temperatures [39], [40], [41]. Overall, the results of the previous chemical analyses for THS2.2 and THP1.0 HTPs were similar to those for DT3.0a.
As summarized in Supplementary Table 6, the previous mutagenicity studies confirmed that THS2.2 [10] and THP1.0 [16], [42] aerosols did not affect the same five bacteria strains as the present study in the presence and absence of metabolic activation whether particulate or gaseous phases were used. These Ames test results indicate that aerosols produced by HTPs with any direct heating systems appear not to be mutagenic.
The genotoxic assessment using mammalian cells was conducted not only in the in vitro MN assay of the THP1.0 aerosol sample [43], [44] but also in the mouse lymphoma assay (MLA) of the THS2.2 [10] and THP1.0 [16], [42] aerosol samples, as shown Supplementary Table 6. The THP1.0 ACM sample was negative to inducing MN irrespective of metabolic activation using rodent V79 and CHO-K1 cells and human TK6 cells [43]. The negative outcome of the THP1.0 ACM sample was confirmed in another study using V79 cells [44]. In general, MLA is equally appropriate to the in vitro MN assay for assessing the genotoxic potentials of chemicals in mammalian test systems according to the ICH guideline for genotoxicity testing of pharmaceuticals [45]. Unlike in vitro MN assay, MLA data indicated the mutagenicity of the THS2.2 ACM and GVP samples [10] as well as the THP1.0 GVP sample [42] in a concentration-dependent manner. However, those mutagenic responses by THS2.2 ACM and GVP samples and THP1.0 GVP samples were seen at 10 times higher concentrations than those for the concurrent 3R4F CS TPM and GVP samples. Based on the in vitro MN assay results of DT3.0a and THP1.0 and the MLA results of THS2.2 and THP1.0, the genotoxic potential of the aerosol emitted from the HTP was considered to be consistently and substantially lower than CS.
NRU cytotoxicity assessments for DT3.0a, THS2.2, and THP1.0 are summarized in Supplementary Table 6. In a previous study of THS2.2 aerosol, cytotoxic concentrations showing 50% of control were observed around 1754 and 1639 µg/mL (for regular- and menthol-flavored ACM fractions, respectively) and 1075 and 1250 µg ACM equivalent/mL (for regular- and menthol-flavored GVP fractions, respectively) [10]. In their study, 1R6F CS showed IC50 values around 90 µg TPM/mL for the TPM fraction and around 70 µg TPM equivalent/mL for the GVP fraction. Similarly, in the NRU assays sample using the same Balb/c 3T3 (clone A31) cell line for THP1.0 ACM, no significant decrease in cell viability was found up to 500 µg/mL (the highest concentration tested), whereas an IC50 of about 90 µg/mL was found for 3R4F CS TPM [44]. These cytotoxicity results indicated that HTP aerosols have consistently lower cytotoxic potentials than conventional CS.
As shown in Fig. 6, our ToxTracker assay revealed that the 1R6F CS sample increased several reporter activities, whereas oxidative stress was the common response caused by both CS and HTP aerosols even at different exposure concentrations. As depicted in Supplementary Table 6, similar results were seen in the previous ToxTracker assay results of THP1.4 [21], in which significant concentration-dependent increases in oxidative stress were found in both Srxn1 and Blvrb reporter cell lines exposed to aqueous extracts (AqE) prepared from 1R6F CS and THP 1.4 aerosol samples [21]. These ToxTracker assay results also supported that oxidative stress could play a role in the genotoxic and cytotoxic mechanism of CS and the cytotoxic mechanism of HTP aerosols.
Supplementary Table 6 shows that several ARE reporter assays have been used to compare the potentials of reference cigarettes and regular-flavored or non-flavored HTPs to induce oxidative stress. In a previous study, THS2.2 and THP1.0 ACM fractions generated twofold increases of ARE activation at 166 µg/mL and 200 µg/mL, respectively, but 3R4F CS TPM fractions induced concentration-dependent ARE activation at much lower exposure concentration of 7.4 µg/mL [46]. A concentration-dependent increase of ARE reporter activity was also confirmed with AqE test samples prepared from 3R4F CS and THS2.2 (the authors called this HTP a “heat-not-burn product” in their manuscript) in immortalized normal human bronchial epithelial BEAS-2B cells [37] and NCI-H292 human lung epithelial cells [17]. However, substantially higher concentrations of the THS2.2 aerosol AqE than the 3R4F CS AqE were required to increase the ARE reporter activity in these studies. These previous results were consistent with that in the present ARE reporter assay of DT3.0a aerosol samples in which about twofold increases to control were observed at 125 µg/mL for regular-flavored DT3.0a ACM fractions, at 187.5 µg/mL for menthol-flavored DT3.0a ACM fractions, and at 6.25 µg/mL for 1R6F CS TPM fractions as shown in Fig. 7 C. Taken together, these results indicate that HTP aerosols regardless of the heating system produce substantially lower potential of oxidative stress than the 1R6F CS.
3.5. Limitations of the study
In the present study, we performed several in vitro toxicological assays which includes not only traditional genotoxicity and cytotoxicity assays but also non-standardized but mechanistic assays. New approach methodologies (NAMs), which include in silico, in chemico, in vitro, and ex vivo approaches [47], [48], are being rapidly developed because of, while not yet fully validated their potential to reliably reflect human biology [49]. Recently, the 3D bronchial epithelial model has been applied as mechanistic follow-up in vitro toxicity comparison among CS and THS2.2 [50], THP1.0 [51], [52], or the other type of HTP [31], [53], [54]. These assessments using NAMs such as human 3D tissue models, once fully validated, would also contribute to the examination of reduced risk potential of DT3.0a.
The present study did not demonstrate an individual-level reduction in exposure to selected HPHCs. Following MRTP guidance, clinical assessments have also been performed using the THS2.2 [12] and the THP1.0 [18], and these assessments have indicated that exposure to CS toxicants was markedly decreased by a smoker switching to using an HTP for a short period. In some cases the decrease in exposure to CS toxicants was similar to the decrease achieved by ceasing smoking [12], [18], [19]. Therefore, assessments of exposure biomarker levels when the DT3.0a is used in clinical studies would provide further insights that using a DT3.0a could reduce health risks posed relative to using conventional cigarettes.
4. Conclusions
Like in previous studies in which HTPs with direct heating system were compared with combustible cigarettes, we found that DT3.0a aerosols have chemical and biological properties making them likely to be less harmful than 1R6F CS. Chemical analyses indicated that selected HPHC yields were significantly lower in DT3.0a aerosol than 1R6F CS. The low constituent yields led to the negative result of DT3.0a aerosol in the assessment for mutagenicity, genotoxicity, and DNA damage results, while 1R6F CS gave some positive responses. The other biological and mechanistic assays indicated that less cytotoxic and oxidative stress responses were caused by DT3.0a aerosol than 1R6F CS. These in vitro studies provide convincing evidence to support reduced toxicity potential compared to CS, but it's direct relevance to in vivo is beyond the scope.
Funding information
This research was sponsored by Japan Tobacco Inc (JT) and JT International SA (JTI). JT and JTI funded the project, and all authors were employees of Japan Tobacco Group at the time of the study. Japan Tobacco Group is a company that manufactures the heated tobacco products called DT3.0a.
CRediT authorship contribution statement
Tsuneo Hashizume: Conceptualization, Methodology, Investigation, Data curation, Visualization, Writing – original draft, Writing – review & editing, Project administration. Shinkichi Ishikawa: Conceptualization, Methodology, Investigation, Data curation, Visualization, Writing – review & editing. Kazushi Matsumura: Methodology, Investigation, Data curation, Writing – review & editing. Shigeaki Ito: Writing – review & editing. Toshiro Fukushima: Conceptualization, Methodology, Investigation, Data curation, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Dr. Ian Jones and Dr. Hiroki Shikata for critically reading the manuscript and Dr. Takaaki Matsufuji, Ms. Mikiko Tsuboi, Mr. Toru Ishii, Dr. Junichiro Saito, Mr. Hiroshi Ito, Mr. Ryujiro Fujita, Ms. Kyoka Kaiya, Mr. Kazunari Uda, and Ms. Kanae Ishimori for their kind support of this study. The authors would like to thank Dr. Mingliang Bao at Labstat International Inc., Mr. Tooru Fujimoto, and Mr. Akihiko Kajiwara, and Mr. Munehiro Nakagawa at LSIM Safety Institute Corporation, and Dr. Hannah Flockton at Labcorp Early Development Ltd. for conducting and executing the study. The authors also thank Dr. Gareth Thomas, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.02.005.
Contributor Information
Tsuneo Hashizume, Email: tsuneo.hashizume@jt.com.
Shinkichi Ishikawa, Email: shinkichi.ishikawa@jt.com.
Kazushi Matsumura, Email: kazushi.matsumura@jti.com.
Shigeaki Ito, Email: shigeaki.ito@jt.com.
Toshiro Fukushima, Email: toshiro.fukushima@jt.com.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Data will be made available on request.
References
- 1.Cozzani V., Barontini F., McGrath T., Mahler B., Nordlund M., Smith M., Schaller J.P., Zuber G. An experimental investigation into the operation of an electrically heated tobacco system. Thermochim. Acta. 2020;684 [Google Scholar]
- 2.Eaton D., Jakaj B., Forster M., Nicol J., Mavropoulou E., Scott K., Liu C., McAdam K., Murphy J., Proctor C.J. Assessment of tobacco heating product THP1.0. Part 2: product design, operation and thermophysical characterisation. Regul. Toxicol. Pharm. 2018;93:4–13. doi: 10.1016/j.yrtph.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann I., Hoffmann D. Tobacco: Science, policy and public health. Oxford University Press; 2010. The changing cigarette: chemical studies and bioassays. [Google Scholar]
- 4.Fowles J., Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . WHO Technical Report Series 951. World Health Organization; Geneva, Switzerland: 2008. The Scientific Basis of Tobacco Product Regulation, Second Report of a WHO Study Group. [PubMed] [Google Scholar]
- 6.FDA, Modified Risk Tobacco Product Applications, (2012).
- 7.FDA, Modified Risk Orders. IQOS System Holder and Charger, (2020).
- 8.Dayan A.D. Investigating a toxic risk (self-inflicted) the example of conventional and advanced studies of a novel Tobacco Heating System. Regul. Toxicol. Pharm. 2016;81(Suppl 2):S15–S16. doi: 10.1016/j.yrtph.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Smith M.R., Clark B., Ludicke F., Schaller J.P., Vanscheeuwijck P., Hoeng J., Peitsch M.C. Evaluation of the Tobacco Heating System 2.2. Part 1: description of the system and the scientific assessment program. Regul. Toxicol. Pharm. 2016;81(Suppl 2):S17–S26. doi: 10.1016/j.yrtph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Schaller J.P., Keller D., Poget L., Pratte P., Kaelin E., McHugh D., Cudazzo G., Smart D., Tricker A.R., Gautier L., Yerly M., Reis Pires R., Le Bouhellec S., Ghosh D., Hofer I., Garcia E., Vanscheeuwijck P., Maeder S. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharm. 2016;81(Suppl 2):S27–S47. doi: 10.1016/j.yrtph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Wong E.T., Kogel U., Veljkovic E., Martin F., Xiang Y., Boue S., Vuillaume G., Leroy P., Guedj E., Rodrigo G., Ivanov N.V., Hoeng J., Peitsch M.C., Vanscheeuwijck P. Evaluation of the Tobacco Heating System 2.2. Part 4: 90-day OECD 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects compared with cigarette smoke. Regul. Toxicol. Pharm. 2016;81(Suppl 2):S59–S81. doi: 10.1016/j.yrtph.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Haziza C., de La Bourdonnaye G., Skiada D., Ancerewicz J., Baker G., Picavet P., Ludicke F. Evaluation of the tobacco heating system 2.2. Part 8: 5-Day randomized reduced exposure clinical study in Poland. Regul. Toxicol. Pharm. 2016;81(Suppl 2):S139–S150. doi: 10.1016/j.yrtph.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Forster M., Fiebelkorn S., Yurteri C., Mariner D., Liu C., Wright C., McAdam K., Murphy J., Proctor C. Assessment of novel tobacco heating product THP1.0. Part 3: comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharm. 2018;93:14–33. doi: 10.1016/j.yrtph.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi Y., Kanemaru Y., Fukushima T., Eguchi K., Yoshida S., Miller-Holt J., Jones I. Chemical analysis and in vitro toxicological evaluation of aerosol from a novel tobacco vapor product: a comparison with cigarette smoke. Regul. Toxicol. Pharm. 2018;92:94–103. doi: 10.1016/j.yrtph.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Poynton S., Sutton J., Goodall S., Margham J., Forster M., Scott K., Liu C., McAdam K., Murphy J., Proctor C. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): Product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol. 2017;106:522–532. doi: 10.1016/j.fct.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Thorne D., Breheny D., Proctor C., Gaca M. Assessment of novel tobacco heating product THP1.0. Part 7: Comparative in vitro toxicological evaluation. Regul. Toxicol. Pharm. 2018;93:71–83. doi: 10.1016/j.yrtph.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Breheny D., Adamson J., Azzopardi D., Baxter A., Bishop E., Carr T., Crooks I., Hewitt K., Jaunky T., Larard S., Lowe F., Oke O., Taylor M., Santopietro S., Thorne D., Zainuddin B., Gaca M., Liu C., Murphy J., Proctor C. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 2): In vitro biological assessment and comparison with different tobacco-heating products. Food Chem. Toxicol. 2017;106:533–546. doi: 10.1016/j.fct.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 18.McEwan M., Gale N., Ebajemito J.K., Camacho O.M., Hardie G., Proctor C.J., Murphy J. A randomized controlled study in healthy participants to explore the exposure continuum when smokers switch to a tobacco heating product or an E-cigarette relative to cessation. Toxicol. Rep. 2021;8:994–1001. doi: 10.1016/j.toxrep.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuki D., Takeshige Y., Nakaya K., Futamura Y. Assessment of the exposure to harmful and potentially harmful constituents in healthy Japanese smokers using a novel tobacco vapor product compared with conventional cigarettes and smoking abstinence. Regul. Toxicol. Pharm. 2018;96:127–134. doi: 10.1016/j.yrtph.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto Y., Imai R., Nanjo K., Fukai Y., Ishikawa K., Kotaki M. Comparison of the effects of three types of heating tobacco system and conventional cigarettes on indoor air quality. SN Appl. Sci. 2021;4 [Google Scholar]
- 21.Smart D.E., Bozhilova S., Miazzi F., Haswell L.E., Gaca M.D., Thorne D., Breheny D. Application of ToxTracker for the toxicological assessment of tobacco and nicotine delivery products. Toxicol. Lett. 2022;358:59–68. doi: 10.1016/j.toxlet.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 22.ISO, ISO 3402:1999 Tobacco and tobacco products — Atmosphere for conditioning and testing, (1999).
- 23.ISO, ISO 20778: 2018 Cigarettes -Routine analytical cigarette smoking machine - Definitions and standard conditions with an intense smoking regime, (2018).
- 24.ISO, ISO/IEC 17025:2017 General requirements for the competence of testing and calibration laboratories, (2017).
- 25.Cariello N.F., Piegorsch W.W. The ames test: the two-fold rule revisited, mutation research/genetic. Toxicology. 1996;369:23–31. doi: 10.1016/s0165-1218(96)90044-0. [DOI] [PubMed] [Google Scholar]
- 26.OECD, OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, (2016).
- 27.Lorge E., Moore M.M., Clements J., O'Donovan M., Fellows M.D., Honma M., Kohara A., Galloway S., Armstrong M.J., Thybaud V., Gollapudi B., Aardema M.J., Tanir J.Y. Standardized cell sources and recommendations for good cell culture practices in genotoxicity testing. Mutat. Res. 2016;809:1–15. doi: 10.1016/j.mrgentox.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Matsushima T., Hayashi M., Matsuoka A., Ishidate M., Jr., Miura K.F., Shimizu H., Suzuki Y., Morimoto K., Ogura H., Mure K., Koshi K., Sofuni T. Validation study of the in vitro micronucleus test in a Chinese hamster lung cell line (CHL/IU) Mutagenesis. 1999;14:569–580. doi: 10.1093/mutage/14.6.569. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi M., Dearfield K., Kasper P., Lovell D., Martus H.-J., Thybaud V. Compilation and use of genetic toxicity historical control data. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2011;723:87–90. doi: 10.1016/j.mrgentox.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Hendriks G., Derr R.S., Misovic B., Morolli B., Calleja F.M., Vrieling H. The extended toxtracker assay discriminates between induction of DNA damage, oxidative stress, and protein misfolding. Toxicol. Sci. 2016;150:190–203. doi: 10.1093/toxsci/kfv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori S., Ishimori K., Tanabe I., Ishikawa S. Evaluation of the biological effects of novel tobacco product vapor using two- or three-dimensional culture systems of human bronchial epithelia. Appl. Vitr. Toxicol. 2021;7:24–33. [Google Scholar]
- 32.Sekine T., Hirata T., Mine T., Fukano Y. Activation of transcription factors in human bronchial epithelial cells exposed to aqueous extracts of mainstream cigarette smoke in vitro. Toxicol. Mech. Methods. 2016;26:22–31. doi: 10.3109/15376516.2015.1123788. [DOI] [PubMed] [Google Scholar]
- 33.Wong E.T., Luettich K., Cammack L., Chua C.S., Sciuscio D., Merg C., Corciulo M., Piault R., Ashutosh K., Smith C., Leroy P., Moine F., Glabasnia A., Diana P., Cecilia C., Ching Keong T., Ivanov N., Hoeng J., Peitsch M., Lee M.K., Vanscheeuwijck P. Assessment of inhalation toxicity of cigarette smoke and aerosols from flavor mixtures: 5-week study in A/J mice. J. Appl. Toxicol. 2022 doi: 10.1002/jat.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stabbert R., Dempsey R., Diekmann J., Euchenhofer C., Hagemeister T., Haussmann H.J., Knorr A., Mueller B.P., Pospisil P., Reininghaus W., Roemer E., Tewes F.J., Veltel D.J. Studies on the contributions of smoke constituents, individually and in mixtures, in a range of in vitro bioactivity assays. Toxicol. Vitr. 2017;42:222–246. doi: 10.1016/j.tiv.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Bryan H.K., Olayanju A., Goldring C.E., Park B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharm. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Disco. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 37.Munakata S., Ishimori K., Kitamura N., Ishikawa S., Takanami Y., Ito S. Oxidative stress responses in human bronchial epithelial cells exposed to cigarette smoke and vapor from tobacco- and nicotine-containing products. Regul. Toxicol. Pharm. 2018;99:122–128. doi: 10.1016/j.yrtph.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Soriano F.X., Baxter P., Murray L.M., Sporn M.B., Gillingwater T.H., Hardingham G.E. Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin. Mol. Cells. 2009;27:279–282. doi: 10.1007/s10059-009-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goujon C., Kleinhans S., Maeder S., Poget L., Schaller J.-P. Robustness of HPHC reduction for THS 2.2 aerosol compared with 3r4f reference cigarette smoke under high intensity puffing conditions. Contrib. Tob. Nicotine Res. 2020;29:66–83. [Google Scholar]
- 40.Torikai K., Yoshida S., Takahashi H. Effects of temperature, atmosphere and pH on the generation of smoke compounds during tobacco pyrolysis. Food Chem. Toxicol. 2004;42:1409–1417. doi: 10.1016/j.fct.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 41.McGrath T.E., Brown A.P., Meruva N.K., Chan W.G. Phenolic compound formation from the low temperature pyrolysis of tobacco. J. Anal. Appl. Pyrolysis. 2009;84:170–178. [Google Scholar]
- 42.Godec T.L., Crooks I., Scott K., Meredith C. In vitro mutagenicity of gas-vapour phase extracts from flavoured and unflavoured heated tobacco products. Toxicol. Rep. 2019;6:1155–1163. doi: 10.1016/j.toxrep.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorne D., Whitwell J., Clements J., Walker P., Breheny D., Gaca M. The genotoxicological assessment of a tobacco heating product relative to cigarette smoke using the in vitro micronucleus assay. Toxicol. Rep. 2020;7:1010–1019. doi: 10.1016/j.toxrep.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crooks I., Neilson L., Scott K., Reynolds L., Oke T., Forster M., Meredith C., McAdam K., Proctor C. Evaluation of flavourings potentially used in a heated tobacco product: chemical analysis, in vitro mutagenicity, genotoxicity, cytotoxicity and in vitro tumour promoting activity. Food Chem. Toxicol. 2018;118:940–952. doi: 10.1016/j.fct.2018.05.058. [DOI] [PubMed] [Google Scholar]
- 45.ICH . Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use. 2011. [PubMed] [Google Scholar]
- 46.Taylor M., Thorne D., Carr T., Breheny D., Walker P., Proctor C., Gaca M. Assessment of novel tobacco heating product THP1.0. Part 6: a comparative in vitro study using contemporary screening approaches. Regul. Toxicol. Pharm. 2018;93:62–70. doi: 10.1016/j.yrtph.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 47.ECHA, New approach methodologies in regulatory science: proceedings of a scientific workshop: Helsinki, 19–20 April 2016, European Chemicals Agency, (2016).
- 48.EPA . List of alternative test methods and strategies (or new approach methodologies [NAMs]) Office of pollution prevention and toxics; Washington, DC: 2019. [Google Scholar]
- 49.Stucki A.O., Barton-Maclaren T.S., Bhuller Y., Henriquez J.E., Henry T.R., Hirn C., Miller-Holt J., Nagy E.G., Perron M.M., Ratzlaff D.E., Stedeford T.J., Clippinger A.J. Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front. Toxicol. 2022;4 doi: 10.3389/ftox.2022.964553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iskandar A.R., Mathis C., Martin F., Leroy P., Sewer A., Majeed S., Kuehn D., Trivedi K., Grandolfo D., Cabanski M., Guedj E., Merg C., Frentzel S., Ivanov N.V., Peitsch M.C., Hoeng J. 3-D nasal cultures: systems toxicological assessment of a candidate modified-risk tobacco product. ALTEX. 2017;34:23–48. doi: 10.14573/altex.1605041. [DOI] [PubMed] [Google Scholar]
- 51.Haswell L.E., Corke S., Verrastro I., Baxter A., Banerjee A., Adamson J., Jaunky T., Proctor C., Gaca M., Minet E. In vitro RNA-seq-based toxicogenomics assessment shows reduced biological effect of tobacco heating products when compared to cigarette smoke. Sci. Rep. 2018;8:1145. doi: 10.1038/s41598-018-19627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haswell L.E., Smart D., Jaunky T., Baxter A., Santopietro S., Meredith S., Camacho O.M., Breheny D., Thorne D., Gaca M.D. The development of an in vitro 3D model of goblet cell hyperplasia using MUC5AC expression and repeated whole aerosol exposures. Toxicol. Lett. 2021;347:45–57. doi: 10.1016/j.toxlet.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa S., Matsumura K., Kitamura N., Ishimori K., Takanami Y., Ito S. Application of a direct aerosol exposure system for the assessment of biological effects of cigarette smoke and novel tobacco product vapor on human bronchial epithelial cultures. Regul. Toxicol. Pharmacol. 2018;96:85–93. doi: 10.1016/j.yrtph.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Takanami Y., Kitamura N., Ito S. LC/MS analysis of three-dimensional model cells exposed to cigarette smoke or aerosol from a novel tobacco vapor product. J. Toxicol. Sci. 2020;45:769–782. doi: 10.2131/jts.45.769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.