Abstract
Lack of triose phosphate isomerase activity (TIM) is of special interest because this enzyme works at an important branch point of glycolytic flux. In this paper, we report the cloning and sequencing of the Kluyveromyces lactis gene encoding TIM. Unlike Saccharomyces cerevisiae ΔTPI1 mutants, the K. lactis mutant strain was found to be able to grow on glucose. Preliminary bioconversion experiments indicated that, like the S. cerevisiae TIM-deficient strain, the K. lactis TIM-deficient strain is able to produce glycerol with high yield.
The interaction between glycolysis, the pentose-phosphate pathway, ethanol fermentation, and respiration in Saccharomyces cerevisiae has been extensively studied, and, in recent years, this interaction has also been investigated in Kluyveromyces lactis. For the latter yeast, several genes encoding glycolytic enzymes have been isolated, and sequenced, and deletion mutants have been constructed (8, 13, 15, 20, 22). Among these, deletion mutants in the genes encoding phosphoglucose isomerase (KlPGI1) (22) and phosphofructokinase subunits (KlPFK1 and KlPFK2) (13) revealed that, unlike S. cerevisiae, K. lactis can grow on glucose medium lacking these enzymes. This seems to indicate that the pentose-phosphate pathway in K. lactis is sufficient to partially substitute the glycolysis flux in the carbohydrate metabolism (14). However, growth is supported only if respiration is not blocked. In fact, the addition of respiratory inhibitors, such as antimycin A, prevented the growth of these mutants (13, 22). Accordingly, both KlPGI1 and KlPFK2 have been found within a set of previously isolated rag mutants and have been screened for their lack of ability to grow in the presence of antimycin A (23). Some rag mutants carry mutations in glycolytic structural genes (10, 13, 15, 21, 22), while others carry mutations in genes encoding the transcriptional regulation of glucose metabolism (2, 3, 16).
Lack of triose phosphate isomerase activity (TIM) is of special interest because this enzyme works at an important branch point of glycolytic flux. In S. cerevisiae, the absence of TIM is known to cause an accumulation of only one of the two trioses, dihydroxyacetone phosphate (DHAP), due to the sufficient drainage of the glyceraldehyde 3-phosphate through the glycolysis (4). Two pathways in living cells initiate from DHAP: one leads to the production of glycerol, and the other is a system developed by all living cells to detoxify the methylglyoxal arising from DHAP (the so-called methylglyoxal pathway [7]). In our laboratory, we previously developed different S. cerevisiae strains deleted in the TPI1 gene (ΔTPI1), coding for TIM. We used these mutants to obtain elevated productions of glycerol (i.e., 80 g/liter) with high yield on the carbon source (molar ratio, 80 to 90%) (5, 6). With the aim to compare the physiological relevance of TIM and glycerol production in the Crabtree-positive S. cerevisiae and in the Crabtree-negative K. lactis yeast strains, we decided to characterize the TPI1 gene from K. lactis. In this paper we report the cloning and sequencing of the K. lactis gene encoding TIM. Unlike the S. cerevisiae mutant, the deleted mutant strain was found to be able to grow on glucose. Preliminary experiments of bioconversion indicated that, similar to the S. cerevisiae TIM-deficient strain, the K. lactis TIM-deficient strain is able to produce glycerol with high yield.
Cloning and sequence analysis of the K. lactis TPI1 gene.
A 1.5-kb 32P-labelled fragment of the S. cerevisiae TPI1 gene (1) was used as a probe to screen a K. lactis genomic library (kindly supplied by M. Wésolowski-Louvel) by colony hybridization (18). After hybridization (overnight at 43°C in 30% formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 10% dextran sulfate, 4× Denhardt’s solution, and 100 μg of salmon sperm DNA per ml), the membranes were washed at 48°C twice with 2× SSC, once with a solution containing 0.5× SSC and 0.1% sodium dodecyl sulfate (SDS), and once with 0.1× SSC–0.1% SDS. A DNA fragment of about 10 kb was isolated. Different subclones were constructed in the shuttle vector YEplac 112 (9) and were used to complement the TIM-deficient phenotype of the S. cerevisiae strain W303ΔTPI1 (MATα, tpi1::ura3, ade2-1, can1-100, trp1-1, his3-11, 15) (5). The BglII-Csp45 I DNA fragment, which restored both the TIM activity and growth on glucose medium to ΔTPI1 S. cerevisiae, was sequenced on both strands (Primm srl, Milan, Italy). An open reading frame (ORF) of 553 bp encoding a protein of 184 amino acids was identified. The molecular mass of the product, calculated from the predicted amino acid sequence, is 20,293 Da. In the upstream region of the gene, an AT-rich segment was present around position −384, followed by a TATAAA at position −360. The sequence AATAAA, thought to be a polyadenylation signal and/or transcription terminator (17), is present at position +633. The deduced protein sequence of the K. lactis TIM was compared to the sequence of other TIMs reported in GenBank by using the BLAST program. The K. lactis enzyme represents the shortest known TIM sequence. The identity of the segments from position 61 to the end of the K. lactis polypeptide and from position 124 to the end of the S. cerevisiae protein reaches 82%.
Gene disruption.
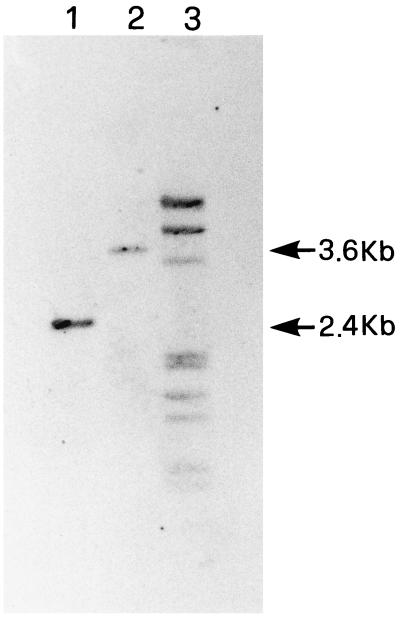
Different strategies were used for disruptions of the KlTPI1 gene. A deletion extending from the KpnI site (+319) to the EcoRI site (+523) inside the ORF was replaced by the S. cerevisiae URA3 gene or by the kanMX module containing the kanamycin resistance ORF of the Escherichia coli transposon Tn903 fused to transcriptional and translational control sequences of the TEF gene of Ashbya gossypii, conferring on yeast the resistance against G418 (size of the fragment, 1,400 bp) (21). The obtained plasmids were opportunely digested in order to obtain a linear fragment containing the disrupted copy of the gene. In order to obtain homologous flanking regions of different lengths, Bal31 digestions were used. K. lactis PM6-7A (MATα, uraA1-1, adeT-600) (10) cells were collected at the late logarithmic phase of growth, and were washed and resuspended in 1 M sorbitol at a cell density of a 1010 cells ml−1. The cells were then transformed at 7.5 kV cm−1, 200 Ω, and 25 μF (Bio-Rad). The transformants were selected for uracil prototrophy on minimal medium plates (0.67% [wt/vol] yeast nitrogen base [YNB; Difco]) containing 1% (wt/vol) ethanol–0.1% (wt/vol) glucose or, after an overnight incubation on YEP (1% [wt/vol] yeast extract [Biolife]–2% [wt/vol] peptone [Biolife]) containing 1% (wt/vol) ethanol–0.1% (wt/vol) glucose and 1 M sorbitol, on plates with the same YEP-ethanol-glucose medium plus G418 (200 mg liter−1). Transformed cells were transferred onto 5% glucose plates and 5% glucose-plus-antimycin A plates at a final concentration of 5 μM. One hundred fifty clones were obtained (40 with the URA3 gene and 110 with G418). All clones grew on glucose, but only one, resistant to G418 and showing a rag phenotype, did not grow on glucose-antimycin A. Cell extracts of this clone were prepared as described (5). Protein content of cell extracts was determined with Bio-Rad kit 500-002 with bovine serum albumin as a standard. The specific activity of the TIM was determined on cell extracts in a solution containing a buffer of 50 mM triethanolamine and 10 mM MgCl2 (pH 7.4), 0.3 mM NADH, and 1 U of glycerol 3-phosphate dehydrogenase ml−1 (Boehringer). The reaction was started by the addition of 0.4 mM glyceraldehyde 3-phosphate (Sigma). The complete absence of TIM in cell extracts of this clone indicated that the disruption of the TPI1 gene had occurred. The correct integration by gene replacement of the disrupted copy of the gene into the TPI locus was confirmed by Southern analysis (Fig. 1). Yeast DNA was extracted from cells by a standard procedure using zymolyase and SDS for cell lysis (19). The DNA was digested with restriction endonuclease Csp45 I, separated on agarose gel, and blotted onto a nylon membrane (Hybond-N; Amersham), as suggested by suppliers. Hybridization was performed by using as a probe the BglII-Csp45 I fragment, Dig-labelled (Random Primed DNA Dig-labelling kit; Boehringer Mannheim) at 48°C in 50% formamide, 5× SSC, 0.2% SDS, and 2.5% blocking solution. The filter was washed once at 50°C with 5× SSC, once at 50°C with 1× SSC, once at 50°C with 1× SSC–0.1% SDS, and once at 50°C with 0.1× SSC–0.1% SDS. The size of the hybridizing band in the DNA of the mutant strain corresponds exactly with the expected size of the fragment obtained after the insertion into the corresponding locus of the disrupted copy of the KlTPI1 gene. The complete absence of TIM in cell extracts of this clone and the presence of only one hybridizing band in the DNA of the wild-type strain indicate that the gene encoding TIM in K. lactis is present in one copy.
FIG. 1.
Confirmation of gene disruption by Southern analysis. Lane 1, DNA from the control strain; lane 2, DNA from the disrupted strain; lane 3, Dig-labelled DNA molecular weight marker III (Boehringer Mannheim).
Growth properties of the K. lactis ΔTPI1 mutant strain.
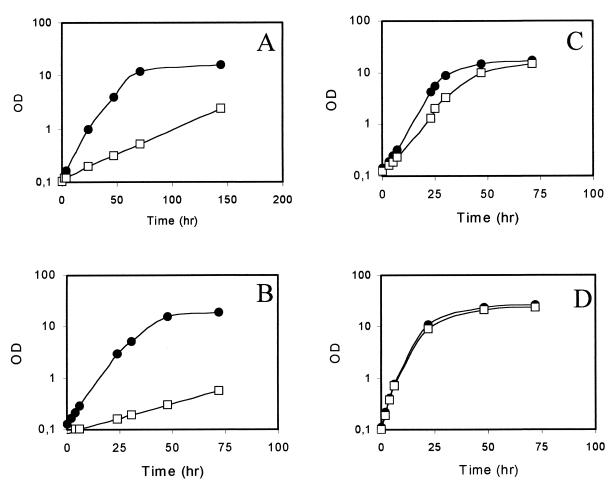
In a mutant strain lacking TIM, a net energy gain from glycolysis alone cannot be obtained, because only half of the glucose flows through the glycolytic pathway. We previously observed that S. cerevisiae ΔTPI1 mutants were unable to grow on minimal YNB medium containing glucose as the sole carbon source. Further, growth was also severely inhibited on YEP-rich media by glucose concentrations higher than 0.2% (5). Further, a S. cerevisiae ΔTPI1 strain is unable to grow on medium containing ethanol as the sole carbon source, because gluconeogenesis is impaired. In K. lactis, the respiratory system does not seem to be glucose-repressed, at least not to the same extent as in S. cerevisiae (11, 12). The kinetics of growth of the K. lactis ΔTPI1 mutant and of the isogenic wild-type strain on minimal medium containing 2% (wt/vol) glucose are reported in Fig. 2A. After an overnight pregrowth period, cells were inoculated in the same fresh medium at an optical density at 600 nm of 0.1 and were incubated with shaking at 30°C. The cell growth was followed by measuring the optical density at 600 nm of a diluted sample of the culture. Unlike the S. cerevisiae ΔTPI1 mutant, the K. lactis mutant was able to grow on YNB with glucose as the sole carbon source, even with a lower specific growth rate than the wild-type strain. The same behavior was observed on YNB medium containing lactose (Fig. 2B). Like S. cerevisiae ΔTPI1, the K. lactis mutant is unable to grow on media containing ethanol as the sole carbon source. The addition of 0.5% (vol/vol) ethanol (Fig. 2C) or growth on YEP-rich media (Fig. 2D) restored the ability to grow on glucose at the same specific rate observed for the wild-type control strain. This seems to suggest that ethanol, as well as other components of the YEP-based medium, might resolve the growth defect of the ΔTPI1 mutant. The presence of ethanol at a much lower level also produces some benefits to the S. cerevisiae ΔTPI1 strains (5); indeed, ethanol restores the growth of S. cerevisiae mutant cells, but at a rate not higher than 25% of that of the wild-type strain. Theoretically, ethanol could be required as a C2 and/or energy (i.e., NADH and ATP) source. Comparative studies are under way to elucidate such requirements for both K. lactis and S. cerevisiae mutant strains.
FIG. 2.
Growth curves of the K. lactis control strain (closed circles) and ΔTPI1 (open squares) strains. The values are the average of two independent experiments with a standard deviation lower than 5%. A, YNB–2% glucose; B, YNB–2% lactose; C, YNB–0.5% ethanol–2% glucose; D, YEP–2% glucose. OD, optical density.
Glycerol production by wild-type and mutant strains.
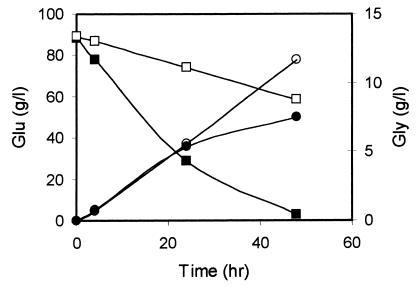
By means of simple bioconversion processes, S. cerevisiae ΔTPI1 mutants can be used to produce glycerol with high yield (5, 6). To test if this also occurs with the K. lactis ΔTPI1 mutant, we performed bioconversion experiments under the same conditions used for S. cerevisiae ΔTPI1 cells. Cells, cultured on medium containing glucose-YEP, were collected at the end of the exponential growth phase and were resuspended at 100 g (wet weight) liter−1 in a buffer containing 10% (wt/vol) glucose and 5% (wt/vol) phosphate. Concentrations of glucose and glycerol were determined by Boehringer enzymatic kits 716251 and 148270, respectively. Data reported in Fig. 3 show glycerol production from both wild-type and ΔTPI1 mutant strains. One can deduce the greater ability of the mutant to produce glycerol by observing the different yields of carbon source, with a value of 74% for the mutant and 17% for the wild type (the theoretical yield of glycerol on glucose being 1 mol/mol). As was observed in S. cerevisiae (5), the K. lactis ΔTPI1 mutant showed a rate of glucose utilization which was reduced in comparison to the wild-type cells.
FIG. 3.
Production of glycerol and glucose utilization by the K. lactis control strain (closed circles and squares, respectively) and the ΔTPI1 strain (open circles and squares, respectively). Experimental results are the average of two independent experiments with a standard deviation lower than 10%. Glu, glucose; Gly, glycerol (g/l).
Nucleotide sequence accession number.
The nucleotide sequence of the KlTPI1 gene has been submitted to the EMBL/GenBank/DDBJ data bank under accession no. AJ012317.
Acknowledgments
This work was supported by CNR PF Biotecnologie Sottoprogetto 4 to B.M.R. and by the project “From gene to product in yeast: a quantitative approach,” which is subsidized by the European Community (DG XII Framework IV Program on Cell Factories to D.P.)
REFERENCES
- 1.Alber T, Kawasaki G. Nucleotide sequence of the triose phosphate isomerase gene of Saccharomyces cerevisiae. J Mol Appl Genet. 1982;1:419–434. [PubMed] [Google Scholar]
- 2.Blaisonneau J, Fukuhara H, Wésolowski-Louvel M. The Kluyveromyces lactis equivalent of casein kinase I is required for the transcription of the gene encoding the low-affinity glucose permease. Mol Gen Genet. 1997;253:469–477. doi: 10.1007/s004380050345. [DOI] [PubMed] [Google Scholar]
- 3.Chen X-J, Wésolowski-Louvel M, Fukuhara H. Glucose transport in the yeast Kluyveromyces lactis. II. Transcriptional regulation of the glucose transporter gene RAG1. Mol Gen Genet. 1992;33:97–105. doi: 10.1007/BF00587566. [DOI] [PubMed] [Google Scholar]
- 4.Ciriacy M, Breitenbach I. Physiological effects of seven different blocks in glycolysis in Saccharomyces cerevisiae. J Bacteriol. 1979;139:152–160. doi: 10.1128/jb.139.1.152-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compagno C, Boschi F, Ranzi B M. Glycerol production in a triose phosphate isomerase deficient mutant of Saccharomyces cerevisiae. Biotechnol Prog. 1996;12:591–595. doi: 10.1021/bp960043c. [DOI] [PubMed] [Google Scholar]
- 6.Compagno C, Boschi F, Ranzi B M. Factors affecting glycerol production by a bioconversion process with a triose phosphate isomerase deficient mutant of Saccharomyces cerevisiae. Biocat Biotransf. 1998;16:135–143. [Google Scholar]
- 7.Cooper R A. Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol. 1984;38:49–68. doi: 10.1146/annurev.mi.38.100184.000405. [DOI] [PubMed] [Google Scholar]
- 8.Fournier A, Fleer R, Yeh P, Mayaux J-F. The primary structure of the 3-phosphoglycerate kinase (PGK) gene from Kluyveromyces lactis. Nucleic Acids Res. 1990;18:365. doi: 10.1093/nar/18.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gietz R D, Sugino A. New yeast-E. coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 10.Goffrini P, Wésolowski-Louvel M, Ferrero I. A phosphoglucose isomerase gene is involved in the Rag phenotype of the yeast Kluyveromyces lactis. Mol Gen Genet. 1991;228:401–409. doi: 10.1007/BF00260633. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales Siso M I, Ramil E, Cerdan M E, Freire-Picos M A. Respirofermentative metabolism in Kluyveromyces lactis: ethanol production and the Crabtree effect. Enzyme Microb Technol. 1996;18:585–591. doi: 10.1016/s0141-0229(00)00161-7. [DOI] [PubMed] [Google Scholar]
- 12.Guyen C N, Bolotin-Fukuhara M, Wésolowski-Louvel M, Fukuhara H. The respiratory system of Kluyveromyces lactis escapes from HAP2 control. Gene. 1995;152:113–115. doi: 10.1016/0378-1119(94)00684-k. [DOI] [PubMed] [Google Scholar]
- 13.Heinish J, Kirchrath L, Liesen T, Vogelsang K, Hollemberg C P. Molecular genetics of phosphofructokinase in the yeast Kluyveromyces lactis. Mol Microbiol. 1993;8:559–570. doi: 10.1111/j.1365-2958.1993.tb01600.x. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby J, Hollemberg C P, Heinisch J J. Transaldolase mutants in the yeast Kluyveromyces lactis provide evidence that glucose can be metabolized through the pentose phosphate pathway. Mol Microbiol. 1993;10:867–876. doi: 10.1111/j.1365-2958.1993.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 15.Prior C, Mamessier P, Fukuhara H, Chen X-J, Wésolowski-Louvel M. The hexokinase gene is required for the transcriptional regulation of the glucose transporter gene RAG1 in Kluyveromyces lactis. Mol Cell Biol. 1993;13:3882–3889. doi: 10.1128/mcb.13.7.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prior C, Tizzani L, Fukuhara H, Wésolowski-Louvel M. RAG3 gene and transcriptional regulation of the pyruvate decarboxylase gene in Kluyveromyces lactis. Mol Microbiol. 1996;20:765–772. doi: 10.1111/j.1365-2958.1996.tb02515.x. [DOI] [PubMed] [Google Scholar]
- 17.Proudfoot N J, Brownlee G G. 3′ Non-coding region sequence in eukaryotic messanger RNA. Nature. 1976;263:211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 20.Shuster J R. Kluyveromyces lactis glyceraldehyde-3-phosphate dehydrogenase and alcohol dehydrogenase-1 genes are linked and divergently transcribed. Nucleic Acids Res. 1990;18:4271. doi: 10.1093/nar/18.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 22.Wésolowski-Louvel M, Goffrini P, Ferrero I. The RAG2 gene of the yeast Kluyveromyces lactis codes for a putative phosphoglucose isomerase. Nucleic Acids Res. 1988;16:8714. doi: 10.1093/nar/16.17.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wésolowski-Louvel M, Prior C, Bornecque D, Fukuhara H. Rag mutation is involved in glucose metabolism in yeast: isolation and genetic characterization. Yeast. 1992;8:711–719. [Google Scholar]