Abstract
Obstructive sleep apnea (OSA) plays an important role in the development of hypertension. Thus, this review summarizes pharmacological and non-pharmacological approaches to blood pressure (BP) control in patients with OSA. Current treatments for OSA, such as continuous positive airway pressure, are effective at lowering BP. However, they only provide a modest BP reduction, and pharmacological treatment remains important for achieving optimal BP control. Furthermore, current guidelines for the treatment of hypertension do not make specific recommendations on pharmacological treatment protocols for controlling BP in patients with OSA. Moreover, the BP-lowering effects of various classes of antihypertensives may be different in hypertensive patients with OSA than in those without OSA due to the underlying mechanisms that promote hypertension in OSA. The acute and chronic increase in sympathetic nerve activity in patients with OSA explain the effectiveness of beta blockers in controlling BP in these patients. As activation of the renin–angiotensin–aldosterone system may also promote hypertension in OSA, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers have generally been found effective for lowering BP in hypertensive patients with OSA. The aldosterone antagonist spironolactone also produces a good antihypertensive response in patients with OSA and resistant hypertension. However, there are limited data available that compare the effects of various classes of antihypertensive medication on BP control in those with OSA, and most data have been obtained from small-scale studies. This demonstrates the need for large-scale randomized controlled trials to evaluate a range of BP-lowering regimens in patients with OSA and hypertension.
Keywords: Blood pressure, Sleep apnea, Hypertension, Antihypertensives, Continuous positive airway pressure, Apnea Hypopnea Index
Abbreviations: AHI, apnea hypopnea index; BP, blood pressure; CPAP, continuous positive airway pressure; MAD, mandibular advancement device; OSA, obstructive sleep apnea
Graphical abstract
1. Introduction
The number of people with hypertension has doubled from 648 million in 1990 to 1278 million in 2019 [1]. Hypertension currently affects more than 25% of the adult population globally and is the leading cause of cardiovascular disease, cerebrovascular disease, and premature death [2]. Moreover, approximately 50% of all ischemic heart disease and strokes are attributable to hypertension [3]. Recent clinical trials, meta-analysis, and guidelines have highlighted the importance of intensive blood pressure (BP) lowering regimens for reducing the risk of cardiovascular events [4], [5], [6].
Obstructive sleep apnea (OSA) is a highly prevalent and underdiagnosed condition [7,8]. It is characterized by recurrent collapse of the upper airway during sleep, leading to hypoxemia, sleep fragmentation, sympathetic activation, and BP surge. OSA is diagnosed by an overnight sleep study which measures the apnea-hypopnea index (AHI) — the number of apnea and hypopnea events per hour of sleep. Based on the AHI, OSA is conventionally classified into mild (AHI 5 to <15 event/hour), moderate (AHI 15–30 event/hour), or severe (AHI >30 event/hour). Most studies investigating the association between OSA and cardiovascular events dichotomized the patients into OSA group versus non-OSA group using an AHI cut-off of 15 events/hour.
In 2019, it was estimated that approximately one billion of the world's adult population have OSA [9], and approximately half of all patients with OSA have coexisting hypertension [10]. Patients with OSA also have a three-fold increased risk of developing new-onset hypertension, as shown in the Wisconsin Sleep Study Cohort [7]. A 2018 meta-analysis of data from 20 original studies confirmed there is a dose–response relationship between essential hypertension and mild OSA (odds ratio [OR] = 1.184, 95% confidence interval [CI] 1.093 to 1.274), moderate OSA (OR = 1.316, 95% CI 1.197 to 1.433), and severe OSA (OR = 1.561, 95% CI 1.287–1.835) [11]. In particular, OSA appears to be especially common in patients with resistant hypertension, with one study showing that 83% of patients with resistant hypertension have concomitant OSA [12].
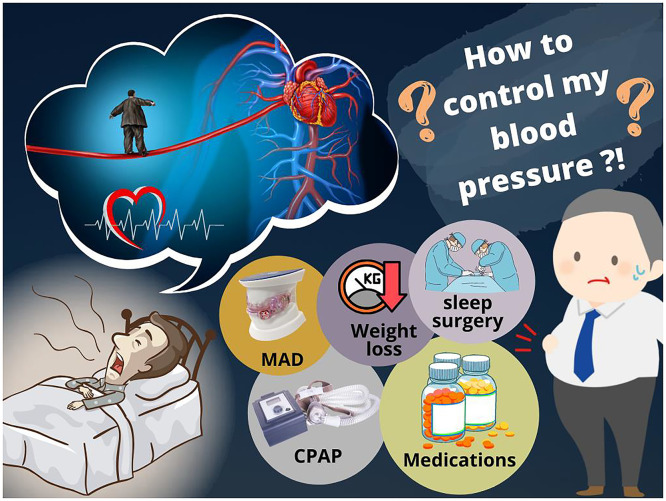
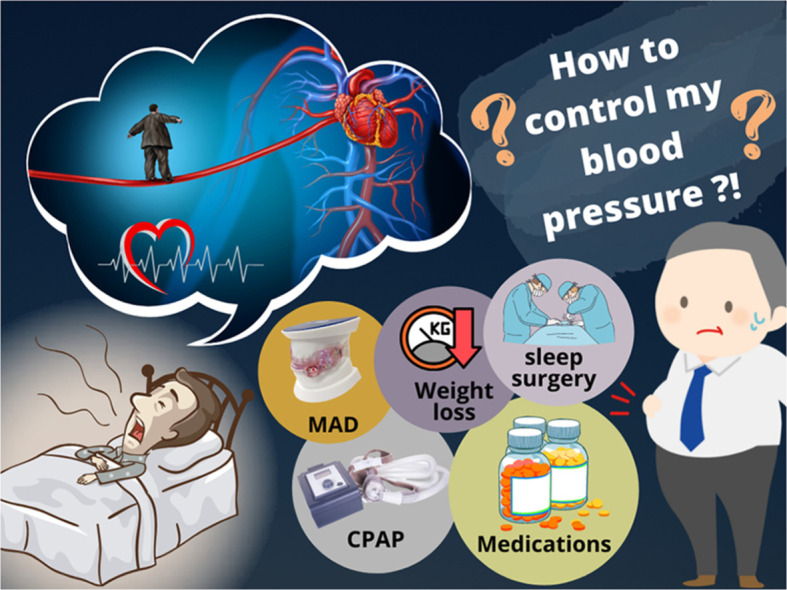
Therefore, the importance of optimal BP control in patients with OSA has been increasingly recognized [13]. However, there are few data on effective BP control in patients with OSA. While current treatments for OSA, such as continuous positive airway pressure (CPAP) and mandibular advancement device (MAD) are effective at lowering BP, the magnitude of the decrease in BP they achieve is relatively small, and patients often need to also take antihypertensive medications to achieve optimal BP control. However, current guidelines do not specify what type of antihypertensive therapy should be offered to patients with OSA and concomitant hypertension [4,14]. This review aims to summarize the various modalities of BP control in patients with OSA and thereby assist clinicians to optimize the management of hypertension in patients with OSA (Fig. 1).
Fig. 1.
Different strategies of blood pressure control in patients with hypertension and obstructive sleep apnea. Abbreviations: MAD, mandibular advancement device; CPAP, continuous positive airway pressure.
2. Mechanisms promoting hypertension in OSA
2.1. Sympathetic activation
The acute and chronic increase in sympathetic activation due to intermittent hypoxia is one of the key mechanisms responsible for the increase in BP in OSA. Patients with OSA experience a marked increase in BP during sleep due to sympathetic vasoconstriction in response to repeated hypoxic events [15]. This hypoxemic state stimulates arterial chemoreceptors, resulting in further activation of sympathetic activity [16], [17], [18]. Nocturnal arousal results from intermittent hypoxia, leading to an increase in plasma and urine concentrations of norepinephrine. This results in a change in chemoreceptor sensitivity, an increase in the release of circulating hormones, such as renin, angiotensin II, and endothelin-1; and a downregulation of nitric oxide synthesis, all of which further stimulate sympathetic nervous system activity [19]. Moreover, the elevation in sympathetic activity continues throughout the day due to long-term facilitation, which leads to an increase in vascular resistance and vascular remodeling [20]. All these factors eventually contribute to the development of hypertension.
2.2. Renin–angiotensin–aldosterone system
Another possible mechanism that explains the pathophysiology of hypertension in OSA is the activation of the renin–angiotensin–aldosterone system (RAAS). Renin release is stimulated by an increase in sympathetic nervous activity and the loss of sodium due to spontaneous nocturnal diuresis and an elevated nocturnal B-type natriuretic peptide concentration, which results from excessive negative intrathoracic pressure generated during apneic events [16,21]. Moreover, angiotensin II and aldosterone concentrations were found to be higher in OSA patients than in control subjects [22]. Angiotensin II has a strong vasoconstrictive effect, which can increase BP. This explains why medications that block the effect of angiotensin II effectively lower BP [23], [24], [25]. RAAS plays a role in the development of hypertension in OSA. This is especially so in resistant hypertension and severe OSA, in which both angiotensin II and aldosterone concentrations are elevated [16]. Aldosterone regulates sodium re-uptake, and elevated aldosterone concentrations lead to fluid retention, which increases BP and exacerbates OSA due to pharyngeal edema [26]. Treatment with the aldosterone antagonist spironolactone is an effective therapy for treatment-resistant hypertension in OSA, especially in severe OSA [27,28]. However, more studies are needed to determine the correlation between angiotensin/aldosterone concentrations, OSA severity, and hypertension.
2.3. Endothelial dysfunction
Sympathetic overstimulation as a result of intermittent hypoxia triggers a pro-inflammatory state that leads to endothelial dysfunction [20,29] and impairs vasodilation. This process may be mediated by oxidative stress and a reduction in nitric oxide synthesis, which triggers vasoconstriction and results in hypertension.
3. Current guidelines for the treatment of primary hypertension
Optimal BP control is of paramount importance for reducing the risk of cardiovascular disease. However, optimal BPs are a topic of debate, and BP targets may change with the emergence of new data. The American College of Cardiology/American Heart Association (ACC/AHA) 2017 guidelines maintain that anyone with BP greater than 130/80 mmHg has hypertension, and that BP should be lowered to less than 130/80 mmHg [14]. In contrast, the European Society of Cardiology (ESC) 2018 and 2021 guidelines define hypertension as BP greater than 140/90 mmHg and recommend a BP of less than 140/90 mmHg for most people; they only recommend a BP of less than 130/80 mmHg for those with a high risk of cardiovascular disease [4,30].
At present, the recommended classes of first-line antihypertensive medication are thiazide-type diuretics, dihydropyridine-type calcium channel blockers, and angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs). Moreover, both the ACC/AHA and ESC guidelines recommend against the use of beta-blockers as first-line therapy unless patients have comorbidities or compelling indications (coronary artery disease or heart failure with reduced ejection fraction).
4. Non-pharmacological approach
4.1. CPAP
In the ACC/AHA 2017 hypertension guidelines, OSA is recognized as a cause of 25–50% of cases of secondary hypertension. The guidelines recommend that patients with resistant hypertension, snoring, interrupted sleep, breathing pauses during sleep, and daytime sleepiness undergo OSA screening and treatment. CPAP is an efficacious treatment for OSA and there is robust evidence for its effectiveness in reducing BP (especially in resistant hypertension) [31,32], OSA severity [33], and self-reported daytime sleepiness, and increasing quality of life [34] (Fig. 2).
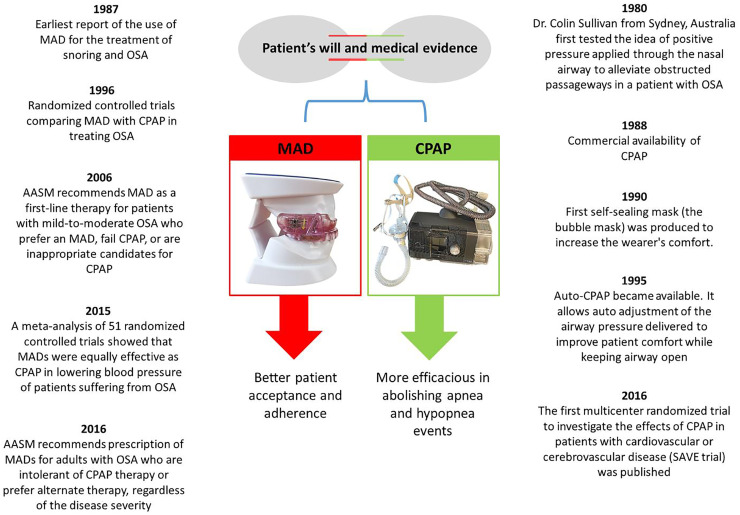
Fig. 2.
Landmarks of continuous positive airway pressure and mandibular advancement device for obstructive sleep apnea. Abbreviations: MAD, mandibular advancement device; CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea; AASM, American academy of sleep medicine; SAVE, the Sleep Apnea Cardiovascular Endpoints.
However, the effects of CPAP on BP are generally modest, as most systemic reviews and meta-analyses have shown that it results in a mean BP reduction of only 2–3 mmHg [35,36]. Moreover, the CPAP-mediated reduction in nocturnal systolic BP is greater than its reduction in diurnal systolic BP [37,38]. However, this evidence may have been confounded by the fact that several studies have recruited patients who had controlled BP at baseline [29,39,40]. This is supported by a recent meta-analysis showing that compared with patients with controlled BP at study entry, those with uncontrolled BP at study entry exhibited a greater reduction in BP (4.14 mmHg) in response to CPAP treatment [41]. Furthermore, the effectiveness of CPAP for BP control is closely linked to CPAP compliance, which is generally poor [42].
CPAP-adherence rates of approximately 5–5.5 h/night are needed to achieve BP reduction, especially for those with non-sleepy OSA phenotypes [43]. However, many studies have reported low adherence rates, with only ∼30% to 80% of CPAP-treated OSA patients using CPAP for more than 4 h/night [44], [45], [46], [47], although compliance is generally higher in those with severe OSA than in those with moderate or mild OSA [45]. Therefore, given the limited effect of CPAP on BP control, effective treatment of hypertension in OSA patients relies on pharmacological therapy.
4.2. MAD
MAD is an alternative treatment for patients with OSA and has also been found to be effective at BP reduction (Fig. 2). In a large meta-analysis of 68 randomized controlled trials that compared CPAP or MADs with either passive or active treatment [41], MAD treatment showed a reduction in both systolic BP (Δ 1.27 mmHg, 95% CI 2.34 to 0.20) and diastolic BP (Δ 1.11 mmHg, 95% CI 1.82 to 0.41) compared to passive control treatment. MADs and CPAPs also show similar effectiveness at BP reduction in patients with OSA [36,41]
4.3. Sleep surgery
Surgical modifications of the upper airway may decrease BP in patients with OSA. A recent meta-analysis of 26 studies that investigated the effect of sleep surgery on BP in adults with OSA found that sleep surgery led to a significant reduction in office systolic BP (Δ 5.6 mmHg, 95% CI 2.9 to 8.3) and office diastolic BP (Δ 3.9 mmHg, 95% CI 1.8 to 6.0) [48]. This meta-analysis used a broad definition for sleep surgery and included studies using various procedures, such as nasal surgical procedures, uvulopalatopharyngoplasty, hyoid suspension, tongue surgery, tongue base surgery, radiofrequency ablation, maxillomandibular advancement, tracheostomy, multilevel surgery, upper airway stimulation, and hypoglossal nerve stimulation. However, most of the studies included in the meta-analysis were case series and none of the studies compared the BP reduction effect of surgery with the BP reduction effect of CPAP or a MAD.
4.4. Weight loss
There is a strong association between OSA and obesity [49]. However, very few studies have compared the effect of weight loss on BP reduction with the effects of other methods of OSA treatment on BP reduction. A randomized controlled trial recruited 181 participants with OSA (AHI ≥ 15 events/h) and obesity (body mass index [BMI] ≥ 30 kg/m2). The trial compared the BP reduction in patients receiving CPAP, a weight-loss intervention (via control of diet and exercise levels), and combined weight-loss and CPAP treatment [50]. In the modified intention-to-treat population, there were no significant between-group differences in systolic BP reduction at 24 weeks. In the per-protocol population, the combined intervention group (Δ 14.1 mmHg, p < 0.001) showed a significantly larger reduction in systolic BP than the weight-loss group (Δ 6.8 mmHg, p = 0.02) and the CPAP group (Δ 3.0 mmHg, p < 0.001). In addition, there was no significant difference between the CPAP and weight-loss groups at week 24.
Another form of weight-loss intervention is bariatric surgery. In a recently published randomized controlled trial conducted in Brazil, a higher percentage of patients in the bariatric surgery (31%) than the medical therapy (0%, p < 0.001) group has achieved target BP at 3 years follow-up. The number of BP lowering medication was lower in the bariatric surgery than the medical therapy group [51]. A meta-analysis of five observational studies investigated the BP-lowering effect of gastric banding or gastric bypass surgery in patients with moderate-to-severe OSA. It determined that there was a clinically significant decrease in post-surgery systolic BP (Δ 9.3 mmHg, 95% CI, –14.3 to –4.2) and diastolic BP (Δ 6.9 mmHg, 95% CI, –10.2 to –3.6) compared with baseline systolic and diastolic BP, respectively [52].
Weight-loss intervention can also be achieved using pharmacological approach such as the glucagon-like peptide-1 receptor agonists. A recently published randomized controlled trial studied effect of liraglutide, a type of glucagon-like peptide-1 receptor agonist on BP reduction in patients with type 2 diabetes mellitus, severe OSA and currently on CPAP treatment. A total of 90 patients were recruited. The baseline 24-h systolic BP in the liraglutide group was 130 ± 12 mmHg, and 132 ± 13 mmHg in the controlled group (p < 0.509). After 3 months of treatment, the liraglutide group had a significantly larger reduction in 24-h systolic BP (Δ 5.6 mmHg, p < 0.001) than the controlled group (Δ 1.2 mmHg, p < 0.636) [53].
5. Pharmacological approaches
Due to the modest BP-lowering effects of OSA therapy, pharmacological approaches are crucial for achieving optimal BP control in patients with OSA. Table 1 summarizes the most relevant studies that have investigated the efficacy of various classes of antihypertensive medications for lowering BP in those with in OSA.
Table 1.
Studies that investigated the efficacy of various classes of antihypertensive medications for lowering blood pressure in those with in OSA.
| Study Design | n | CPAP (Y/N) | Antihypertensives; dosage (mg) | BP Measurement | BP outcome | Refs. |
|---|---|---|---|---|---|---|
| RCT; double blinded; balanced incomplete block design (6 weeks each drug + 3 weeks washout) | 40 | No | Atenolol (50); Amlodipine (5); Enalapril (20); Hydrochlorothiazide (25); losartan (50) | Office BP 24 h ABPM | ↓ in office SBP and daytime ABPM is not significant for all drugs; Atenolol ↓ night time 24 h SBP & DBP more effectively than amlodipine, enalapril or losartan | Kraiczi et al. [62] |
| RCT; double-blinded; crossover schedule (8 weeks each drug + 2–3 weeks washout) | 15 | No | Atenolol (50); Isradipine (2.5); Hydrochlorothiazide (25); Spirapril (6) | Office BP | Slight ↓ in BP for all drugs; only atenolol affected BP variability | Salo et al. [63] |
| RCT; double blinded; crossover (8 weeks each drug +2–3 weeks washout) | 18 | No | Atenolol (50); Isradipine (2.5); Hydrochlorothiazide (25); Spirapril (6) | 24 h ABPM | ↓ mean 24 h SBPM (except for hydrochlorothiazide); ↓ mean 24 h DBP for all drugs; no significant ↓ in mean night time SBP and DBP for all drugs; Atenolol reduced both SBP and DBP most effectively | Pelttari et al. [61] |
| Retrospective multicenter; cohort study | 5818 | NA | Monotherapy: − Beta blockers − Diuretics − Calcium channel blockers − Rennin- angiotensin blockers − Centrally-acting antihypertensives Combination therapy: − Beta blockers + diuretics − Diuretics + Renin-angiotensin blockers − Beta blockers + Renin-angiotensin blockers − Beta blockers +calcium channel blockers − Diuretics +calcium channel blockers − Calcium channel blockers + Renin-angiotensin blockers |
Office BP | Beta blocker monotherapy or beta blocker + diuretic combination therapy was associated with the lowest SBP | Svedmyr et al. [54] |
| RCT; crossover (8 weeks each treatment +4 weeks washout) | 23 | Yes | Valsartan (160) | Office BP 24 h ABPM |
CPAP: ↓2.1 mmHg MBP & 1.2 mmHg night time MBP Valsartan: ↓ 9.1 mmHg 24 h MBP & 6.1 mmHg night time MBP |
Pepin et al. [64] |
| RCT; double-blinded; crossover (2 weeks each treatment +3 weeks washout) | 16 | No | Doxazosin (4–8); enalapril (10–20) | 24 h ABPM | Doxazosin has proportionally poor effect on nocturnal BP control No difference in 24 h MBP |
Zou et al. [24] |
| RCT; double-blinded; parallel group; single center (6 weeks) | 31 | No | Nebivolol (5); Valsartan (80) | Office BP | No differences between treatment on BP control Nebivolol ↓ heart rate to a greater extent |
Heitmann et al. [23] |
| RCT; double blind; crossover (6 weeks each treatment) | 31 | No | Nebivolol (5–10); Hydrochlorothiazide (12.5–25) | 24 h ABPM | Nebivolol ↓ clinic BP and 24 h diastolic BP more than hydrochlorothiazide | Ziegler et al. [60] |
| RCT; open trial; blank control; parallel group; single center (12 weeks) | 30 | Nil | Spironolactone (20–40) | Office BP, 24 h ABPM | clinic SBP and DBP, 24 h SBP and 24 h DBP, SBP and DBP during the day showed significant ↓ in spironolactone group compared with control | Yang et al. [27] |
| RCT; single center; crossover (2–8 weeks each drug + 2 weeks washout) | 11 | No | Nifedipine (40), Carvedilol (20) | Trigger sleep BP | Nifedipine ↓ mean and minimum Sleep SBP more than Carvedilol Carvedilol ↓ sleep SBP surge more significantly |
Kario et al. [57] |
| RCT; single center; parallel group (12 weeks) | 20 | No | Metoprolol (47.5), Amlodipine (5) | 24 h ABPM | Both significantly ↓ 24 h BP, daytime BP and nighttime BP with no significant difference between the two | Shi et al. [56] |
| Uncontrolled open trial; single center (8 weeks) | 12 | No | Spironolactone (25–50) | Office BP, 24 h ABPM | Spironolactone ↓ clinic SBP and DBP, 24 h SBP and DBP, daytime SBP, nighttime SBP and DBP significantly | Gaddam et al. [28] |
| Uncontrolled, open trial; single center (12 weeks) | 31 | NA | Eplerenone (50) | Office BP, 24 h ABPM | Eplerenone significantly ↓ clinic, 24 h, daytime and night time SBP and DBP | Krasinaska et al. [55] |
Abbreviations: RCT, randomized controlled trial; CPAP, continuous positive airway pressure; ABPM, ambulatory blood pressure monitoring; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure.
5.1. Non-randomized studies
In one study, the European Sleep Apnea Database [54] was used to recruit participants (n = 5818) with concomitant OSA and hypertension to evaluate the association between various classes of antihypertensive medication and BP control. The mean AHI and BMI of the participants were 34 ± 26 events/h and 33 ± 7 kg/m2, respectively. The drugs used by the participants who were undergoing monotherapy were most often renin-angiotensin blockers (55%) or beta-blockers (18%). In the fully adjusted model, systolic BP was lower in participants treated with beta-blockers than in those treated with renin-angiotensin blockers (Δ 2 mmHg, 95% CI 1 to 3, p = 0.007), centrally acting hypertensive medication (Δ 3 mmHg, 95% CI 2 to 5, p = 0.017), or calcium-channel blockers (Δ 3 mmHg, 95% CI 2 to 4, p = 0.008).
The drugs used by the participants who were undergoing combination therapy were most frequently combinations of (i) a renin–angiotensin blocker and a beta-blocker (34%), (ii) a renin-angiotensin blocker and a diuretic (23%), or (iii) a renin–angiotensin blocker and a calcium channel blocker (22%). Systolic BP was lower in patients being treated with a beta-blocker and a diuretic than in those being treated with a renin–angiotensin blocker and a calcium channel blocker (Δ 6 mmHg, 95% CI 4 to 7, p < 0.001), a renin–angiotensin blocker and a beta-blocker (Δ 5 mmHg, 95% CI 4 to 7, p < 0.001), a beta-blocker and a calcium channel blocker (Δ 4 mmHg, 95% CI 2 to 6, p = 0.02), a calcium channel blocker and a diuretic (Δ 4 mmHg, 95% CI 2 to 6, p = 0.087), and a diuretic and a renin–angiotensin blocker (Δ 3 mmHg, 95% CI 2 to 5, p = 0.036). The study concluded that treatment with a beta-blocker alone or in combination with a diuretic was associated with the lowest systolic BP. However, this cross-sectional study did not determine the most effective individual dosages of the drugs.
A pilot study showed that treatment with spironolactone reduces the severity of OSA and improves BP in patients with resistant hypertension and OSA [28]. Twelve patients with OSA (AHI ≥ 15 events/h) were treated with spironolactone in addition to their usual BP treatment regimen. Clinic systolic and diastolic BP, 24-h systolic BP, daytime systolic BP, and nighttime systolic and diastolic BP all decreased following treatment. This result was later supported by a randomized controlled trial involving 30 participants that studied the effect of spironolactone on BP in those with resistant hypertension and OSA (AHI ≥ 15 events/h) [27]. Those in the active treatment group were treated with spironolactone in addition to their usual BP treatment regimen, while those in the control group continued their usual BP treatment regimen. After 12 weeks, office, 24-h, daytime, and nighttime systolic and diastolic BP were significantly reduced in the active treatment group compared with the control group [27]. In the same year as this randomized controlled trial, an uncontrolled open trial examined the antihypertensive effect of eplerenone in OSA patients with resistant arterial hypertension [55]. The addition of eplerenone to standard antihypertensive therapy resulted in a statistically significant reduction in 24-h, daytime, nighttime, and office systolic and diastolic BP. The greatest reduction in BP was observed for daytime systolic BP (15 mmHg, p < 0.05) and 24-h systolic BP (11 mmHg, p < 0.05).
5.2. Randomized trials
5.2.1. Randomized trials comparing two classes of antihypertensive medication
Beta-blocker versus ARB
In a randomized controlled trial, the BP-lowering effect of a beta-blocker (nebivolol 5 mg) was compared with that of an ARB (valsartan 80 mg) in 31 participants with OSA (AHI ≥ 15 events/hour) and hypertension [23]. At baseline, the mean systolic BP in the beta-blocker and ARB groups were 151 ± 10 mmHg and 150 ± 12 mmHg, respectively. A placebo run-in period of at least 14 days was used, and after 6 weeks of treatment, there was no significant difference in BP reduction between the two treatment groups.
Beta-blocker versus calcium channel blocker
In a small randomized controlled trial, the BP-lowering effect of a beta-blocker (metoprolol) was compared with that of a calcium channel blocker (amlodipine) in 20 participants (aged 30–70) with hypertension and newly diagnosed OSA (AHI ≥ 5 events/h) [56]. Participants with other significant comorbid conditions were excluded. The participants underwent 24-h ambulatory BP monitoring at baseline and after 12 weeks. Both the beta-blocker and calcium channel blocker significantly reduced 24-h, daytime, and nighttime BP, while the reduction in 24-h ambulatory BP was not significantly different between the two groups.
Another small-scale randomized controlled trial investigated the BP-lowering effects of a beta-blocker (carvedilol) and a calcium channel blocker (nifedipine) in 11 participants with hypertension and OSA (AHI > 15 events/h) who had declined CPAP therapy [57]. BP was measured using novel equipment developed by the author (Trigger sleep BP [58,59]). The team concluded that the BP-lowering effect of nifedipine was greater than that of carvedilol, where this effect was determined as the mean and minimum systolic BP reduction during sleep
Beta-blocker versus diuretic
Ziegler et al. compared the BP-lowering effect of a beta-blocker (nebivolol) with that of a diuretic (hydrochlorothiazide) in 31 patients with OSA (AHI ≥ 30 events/h) in a 6-week randomized crossover study [60]. Only the beta-blocker (p < 0.001) exhibited a statistically significant reduction in 24-h diastolic BP, which was the primary endpoint. That is, the beta-blocker decreased the 24-h mean BP by 6 mmHg (p < 0.05), but the diuretic had no significant effect on this outcome. Awake mean BP, systolic BP, and diastolic BP were decreased more significantly by the beta-blocker (Δ 6 mmHg, Δ 5 mmHg, Δ 6 mmHg, respectively; p < 0.05) than by the diuretic (Δ 4 mmHg, Δ 1 mmHg, Δ 2 mmHg, respectively; p < 0.05). No significant difference was found between the effects of the beta-blocker and the diuretic on BP during sleep.
ACEI versus alpha blocker
In a small, randomized crossover study, the effect of an ACEI (enalapril) was compared with the effect of an alpha 1 adrenergic receptor antagonist (doxazosin) on nocturnal BP in 16 men with OSA (respiratory disturbance index ≥ 20 events/h) and hypertension [24]. The mean baseline systolic BP was 165 ± 14 mmHg and the diastolic BP was 98 ± 8 mmHg. The nighttime beat-to-beat BP taken from the finger was significantly lower in participants receiving the ACEI than those receiving the alpha 1 adrenergic receptor antagonist (systolic BP: 119 ± 23 mmHg vs. 129 ± 13 mmHg, p = 0.02; diastolic BP 74 ± 14 mmHg vs. 81 ± 12 mmHg, p = 0.04). No statistically significant between-group difference was found in the 24-h BP profile of the participants, although doxazosin had a smaller effect than enalapril on nocturnal BP. However, this result may be difficult to interpret as neither the 24-h BP nor the beat-to-beat BP were taken from the finger at baseline before treatment.
5.2.2. Randomized trials comparing more than two classes of antihypertensive medication
In a randomized, double-blind, crossover clinical trial, 18 obese patients (mean BMI 32 kg/m2) with OSA and hypertension were randomized into each of the four antihypertensive medication classes for 8 weeks (2- to 3-week washout period) [61]: (i) beta-blocker (atenolol), (ii) calcium channel blocker (isradipine), (iii) diuretic (hydrochlorothiazide), and (iv) ACEI (spirapril). The BP-lowering effects of these different classes of antihypertensive medications were compared based on ambulatory BP monitoring. The mean office systolic and diastolic BP at the beginning of the first treatment period were 152 ± 22 mmHg and 108 ± 18 mmHg, respectively. The beta-blocker (−13 mmHg), calcium channel blocker (−10 mmHg), and ACEI (−7 mmHg) were associated with a reduction in 24-h mean systolic BP (p < 0.01 for all). An analysis of variance (ANOVA) showed that the beta-blocker led to the greatest reduction in 24-h mean systolic and diastolic BP.
A single-center, randomized controlled trial, recruited 40 men aged 25 to 70 with OSA (AHI or oxygen desaturation index ≥ 10 events/h) and concomitant hypertension [62]. The participants were either on antihypertensive medication or had documented high office BP. Each of the participants was treated with two of the five different types of antihypertensive medications listed below using a balanced incomplete block design. The treatment duration for each drug class was 6 weeks, and there was a 3-week washout period. The drugs were (i) a beta-blocker (atenolol), (ii) a calcium channel blocker (amlodipine), (iii) a diuretic (hydrochlorothiazide), (iv) an ACEI (enalapril), and (v) an ARB (losartan). There were some unusual features of this study. Diastolic BP instead of systolic or mean BP was used as the primary endpoint, even though systolic and mean BP have been shown to predict adverse cardiovascular events. Moreover, the primary endpoint BP was based on an office recording rather than the more reliable method of ambulatory BP monitoring.
An ANOVA revealed that the effects of these five types of drugs on office diastolic (p = 0.046) differed in magnitude. The beta-blocker was the most effective (−12.1 mmHg), followed by the diuretic (−7.4 mmHg) and calcium channel blocker (−6.8 mmHg). However, the five types of drugs did not generate significantly different office systolic BP, or 24-h ambulatory systolic, or diastolic BP in the participants [62].
6. Limitations to the interpretation of studies
OSA is a major cause of hypertension, and pharmacological treatment plays an important role in BP management. However, only a limited number of studies have compared the effect of different classes of antihypertensive medication on BP control in patients with OSA, and most of these studies have been cohort studies with small sample sizes. Furthermore, most of the studies have only considered the number or class of drugs taken by participants, and thus their results are difficult to interpret, as most of the participants in these studies have already been under treatment for hypertension. This lack of high-quality data has contributed to the difficulty in establishing BP management recommendations for patients with OSA.
7. Conclusions
OSA and hypertension frequently co-exist as they share some common risk factors, such as obesity. There is also evidence that OSA contributes to suboptimal BP control in patients with hypertension. CPAP is the current first-line treatment for OSA, but it is only able to deliver a modest BP reduction (∼2–3 mmHg). The high rate of CPAP non-adherence further limits its ability to control BP. As such, pharmacological therapy remains essential to achieve sufficient BP control, as advocated by the hypertension treatment guidelines. However, limited data are available on the comparative efficacy and effectiveness of various antihypertensive medications in patients with OSA and hypertension. Moreover, most of the evidence has been generated by non-randomized studies of a very small number of participants. As such, more high-quality studies are needed to evaluate various BP-lowering regimens in patients with OSA and hypertension.
Funding
This study was supported by a Clinician Scientist Award from the National Medical Research Council of Singapore (award number: NMRC/CSA–INV/002/2015).
Declaration of Competing Interest
None declared for all the authors.
Footnotes
DisclosureS: none.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar M.H., et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115mm Hg, 1990-2015. JAMA. 2017;317(2):165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 3.Roth G.A., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams B., et al. ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 5.Ettehad D., et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 6.Group S.R., et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan A., et al. Prevalence of sleep-disordered breathing in a multiethnic Asian population in Singapore: a community-based study. Respirology. 2016;21(5):943–950. doi: 10.1111/resp.12747. [DOI] [PubMed] [Google Scholar]
- 8.Heinzer R., et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjafield A.V., et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad M., Makati D., Akbar S. Review of and updates on hypertension in obstructive sleep apnea. Int J Hypertens. 2017;2017 doi: 10.1155/2017/1848375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou H., et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8(1) doi: 10.7189/jogh.08.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peppard P.E., et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 13.Pio-Abreu A., Moreno H., Jr., Drager L.F. Obstructive sleep apnea and ambulatory blood pressure monitoring: current evidence and research gaps. J Hum Hypertens. 2021;35(4):315–324. doi: 10.1038/s41371-020-00470-8. [DOI] [PubMed] [Google Scholar]
- 14.Whelton P.K., et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 15.Venkataraman S., et al. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler M.G., Milic M., Elayan H. Cardiovascular regulation in obstructive sleep apnea. Drug Discov Today Dis Models. 2011;8(4):155–160. doi: 10.1016/j.ddmod.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loredo J.S., et al. Obstructive sleep apnea and hypertension: are peripheral chemoreceptors involved? Med Hypotheses. 2001;56(1):17–19. doi: 10.1054/mehy.2000.1086. [DOI] [PubMed] [Google Scholar]
- 18.Schultz H.D., Li Y.L., Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50(1):6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- 19.Diogo L.N., Monteiro E.C. The efficacy of antihypertensive drugs in chronic intermittent hypoxia conditions. Front Physiol. 2014;5:361. doi: 10.3389/fphys.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips C.L., O'Driscoll D.M. Hypertension and obstructive sleep apnea. Nat Sci Sleep. 2013;5:43–52. doi: 10.2147/NSS.S34841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunze D.L., Kline D., Ramirez-Navarro A. Hypertension in sleep apnea: the role of the sympathetic pathway. Cleve Clin J Med. 2007;74 Suppl(1):S34–S36. doi: 10.3949/ccjm.74.suppl_1.s34. [DOI] [PubMed] [Google Scholar]
- 22.Moller D.S., et al. Abnormal vasoactive hormones and 24-h blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16(4):274–280. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 23.Heitmann J., et al. Comparison of the effects of nebivolol and valsartan on BP reduction and sleep apnoea activity in patients with essential hypertension and OSA. Curr Med Res Opin. 2010;26(8):1925–1932. doi: 10.1185/03007995.2010.497326. [DOI] [PubMed] [Google Scholar]
- 24.Zou D., et al. A double-blind, crossover study of Doxazosin and Enalapril on peripheral vascular tone and nocturnal blood pressure in sleep apnea patients. Sleep Med. 2010;11(3):325–328. doi: 10.1016/j.sleep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Mayer J., et al. Influence of metoprolol and cilazapril on blood pressure and on sleep apnea activity. J Cardiovasc Pharmacol. 1990;16(6):952–961. doi: 10.1097/00005344-199012000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Gonzaga C.C., et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6(4):363–368. [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L., et al. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin Exp Hypertens. 2016;38(5):464–468. doi: 10.3109/10641963.2015.1131290. [DOI] [PubMed] [Google Scholar]
- 28.Gaddam K., et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24(8):532–537. doi: 10.1038/jhh.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drager L.F., Polotsky V.Y., Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140(2):534–542. doi: 10.1378/chest.10-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visseren F.L.J., et al. [2021 ESC guidelines on cardiovascular disease prevention in clinical practice] G Ital Cardiol. 2022;23(6 Suppl 1) doi: 10.1093/eurjpc/zwab154. (Rome)e3-e115. [DOI] [PubMed] [Google Scholar]
- 31.Gottlieb D.J., et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedrosa R.P., et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144(5):1487–1494. doi: 10.1378/chest.13-0085. [DOI] [PubMed] [Google Scholar]
- 33.Gay P., et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 34.Kushida C.A., et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 35.Durán-Cantolla J., et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 36.Bratton D.J., et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314(21):2280–2293. doi: 10.1001/jama.2015.16303. [DOI] [PubMed] [Google Scholar]
- 37.Hu X., et al. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens. 2015;17(3):215–222. doi: 10.1111/jch.12472. (Greenwich) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Garcia M.A., et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 39.Posadas T., et al. Obstructive sleep apnea and arterial hypertension: implications of treatment adherence. Curr Hypertens Rep. 2020;22(2):12. doi: 10.1007/s11906-020-1015-y. [DOI] [PubMed] [Google Scholar]
- 40.Faccenda J.F., et al. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163(2):344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 41.Pengo M.F., et al. Obstructive sleep apnoea treatment and blood pressure: which phenotypes predict a response? A systematic review and meta-analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.01945-2019. [DOI] [PubMed] [Google Scholar]
- 42.Rotenberg B.W., Murariu D., Pang K.P. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbé F., et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181(7):718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 44.Campos-Rodriguez F., et al. Long-term continuous positive airway pressure compliance in females with obstructive sleep apnoea. Eur Respir J. 2013;42(5):1255–1262. doi: 10.1183/09031936.00165812. [DOI] [PubMed] [Google Scholar]
- 45.Baratta F., et al. Long-term prediction of adherence to continuous positive air pressure therapy for the treatment of moderate/severe obstructive sleep apnea syndrome. Sleep Med. 2018;43:66–70. doi: 10.1016/j.sleep.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 46.Virk J.S., Kotecha B. When continuous positive airway pressure (CPAP) fails. J Thorac Dis. 2016;8(10):E1112–E1121. doi: 10.21037/jtd.2016.09.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolkove N., et al. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365–369. doi: 10.1155/2008/534372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang K.T., et al. Effect of sleep surgery on blood pressure in adults with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2022;62 doi: 10.1016/j.smrv.2022.101590. [DOI] [PubMed] [Google Scholar]
- 49.Wolk R., Shamsuzzaman A.S., Somers V.K. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42(6):1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 50.Chirinos J.A., et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiavon C.A., et al. Three-year outcomes of bariatric surgery in patients with obesity and hypertension: a randomized clinical trial. Ann Intern Med. 2020;173(9):685–693. doi: 10.7326/M19-3781. [DOI] [PubMed] [Google Scholar]
- 52.Kent D., et al. Referral of adults with obstructive sleep apnea for surgical consultation: an American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17(12):2507–2531. doi: 10.5664/jcsm.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang W., et al. Efficacy and safety of liraglutide in patients with type 2 diabetes mellitus and severe obstructive sleep apnea. Sleep Breath. 2022 doi: 10.1007/s11325-022-02768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svedmyr S., et al. Superior hypertension control with betablockade in the European sleep apnea database. J Hypertens. 2021;39(2):292–301. doi: 10.1097/HJH.0000000000002629. [DOI] [PubMed] [Google Scholar]
- 55.Krasinska B., et al. Effect of eplerenone on the severity of obstructive sleep apnea and arterial stiffness in patients with resistant arterial hypertension. Pol Arch Med Wewn. 2016;126(5):330–339. doi: 10.20452/pamw.3410. [DOI] [PubMed] [Google Scholar]
- 56.Shi J., et al. Metoprolol has a similar therapeutic effect as amlodipine on BP lowering in hypertensive patients with obstructive sleep apnea. Sleep Breath. 2019;23(1):227–233. doi: 10.1007/s11325-018-1688-5. [DOI] [PubMed] [Google Scholar]
- 57.Kario K., et al. Effects of nighttime single-dose administration of vasodilating vs sympatholytic antihypertensive agents on sleep blood pressure in hypertensive patients with sleep apnea syndrome. J Clin Hypertens. 2014;16(6):459–466. doi: 10.1111/jch.12327. (Greenwich) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirasaki O., et al. Development and clinical application of a new technique for detecting 'sleep blood pressure surges' in sleep apnea patients based on a variable desaturation threshold. Hypertens Res. 2011;34(8):922–928. doi: 10.1038/hr.2011.52. [DOI] [PubMed] [Google Scholar]
- 59.Shirasaki O., et al. A new technique for detecting sleep apnea-related "midnight" surge of blood pressure. Hypertens Res. 2006;29(9):695–702. doi: 10.1291/hypres.29.695. [DOI] [PubMed] [Google Scholar]
- 60.Ziegler M.G., et al. Effect of obstructive sleep apnea on the response to hypertension therapy. Clin Exp Hypertens. 2017;39(5):409–415. doi: 10.1080/10641963.2016.1259327. [DOI] [PubMed] [Google Scholar]
- 61.Pelttari L.H., et al. Little effect of ordinary antihypertensive therapy on nocturnal high blood pressure in patients with sleep disordered breathing. Am J Hypertens. 1998;11(3 Pt 1):272–279. doi: 10.1016/s0895-7061(97)00469-x. [DOI] [PubMed] [Google Scholar]
- 62.Kraiczi H., et al. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1423–1428. doi: 10.1164/ajrccm.161.5.9909024. [DOI] [PubMed] [Google Scholar]
- 63.Salo T.M., et al. The effect of four different antihypertensive medications on cardiovascular regulation in hypertensive sleep apneic patients–assessment by spectral analysis of heart rate and blood pressure variability. Eur J Clin Pharmacol. 1999;55(3):191–198. doi: 10.1007/s002280050617. [DOI] [PubMed] [Google Scholar]
- 64.Pepin J.L., et al. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182(7):954–960. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]