Abstract
The prevalence of obesity is rapidly increasing and poses serious health risks accompanied by a decrease in life expectancy and quality of life. Therefore, the therapeutic potential of natural-derived nutraceuticals against obesity and its comorbidities needs to be explored. Molecular inhibition of lipase enzymes and fat mass and obesity-associated (FTO) protein has attracted some recent interest in efforts to find antiobesity agents. This study aims to innovate a fermented drink from Clitoria ternatea kombucha (CTK), find out their metabolites profile, and determine the antiobesity potential through a molecular docking study. The CTK formulation refers to previous research while the metabolites profile was determined using HPLC-ESI-HRMS/MS. Major compounds were selected based on best match value > 99.0% of the M/Z cloud database. A total of 79 compounds were identified in CTK, and 13 ideal compounds were selected to be simulated in the molecular docking study against human pancreatic lipase, α-amylase, α-glucosidase, porcine pancreatic lipase, and FTO proteins. The study found that Kaempferol, Quercetin-3β-D-glucoside, Quercetin, Dibenzylamine, and α-Pyrrolidinopropiophenone showed the best potential as functional antiobesity compounds since their affinity value ranked high in each respective receptor. In conclusion, the major compounds of CTK metabolites have the potential to be promising functional foods against obesity. However, further in vitro and in vivo studies should validate these health benefits.
Keywords: Obesity, Kombucha, Clitoria ternatea, Metabolites, Molecular docking, Lipase, α-Amylase, α-Glucosidase
Graphical abstract
Highlights
-
•
Clitoria ternatea can be incorporated into a kombucha drink.
-
•
Clitoria ternatea kombucha as a functional food with promising major metabolites.
-
•
Clitoria ternatea kombucha metabolites are promising as antiobesity agents.
1. Introduction
Obesity prevalence has increased considerably over the five decades and poses a serious health risk since it significantly raises the risk of type 2 diabetes mellitus, fatty liver disease, hypertension, myocardial infarction, stroke, dementia, osteoarthritis, and cancers, all of which have been linked to a decline in life expectancy and quality of life (Blüher, 2019). Updated systematic reviews and meta-analyses reported pooled prevalence of obesity in adults and older adults of 21.17% and 17.8%, respectively (Okati-Aliabad et al., 2022; Hajek et al., 2022). Obesity is primarily brought on by a long-term energy imbalance between calories taken (nutrients) and calories expended, as well as genetics, lifestyle, and gut microbiota (Lin and Li, 2021). Change in behavior, decrease in energy intake, and increase in energy expenditure – especially from the dietary pattern – is necessary for managing obesity and body weight (Wiechert and Holzapfel, 2021). Potential therapy for obesity and its comorbidities (such as diabetes and metabolic syndrome) especially functional foods made from natural ingredients still needs to be explored.
Functional food will always become the main topic in discussing food consumption to manage obesity. Probiotics have become an efficient and all-encompassing method for altering the microbiome and reversing the microbial dysbiosis brought on by an obesity phenotype (Green et al., 2020). Bioactive compounds in the food product, especially polyphenols, flavonoids, and dietary fibers may attenuate inflammation and exhibit a significant role in preventing obesity (Ramírez-Moreno et al., 2022a). Interestingly, fermented foods have been reported to have higher bioactive compound availability and health benefits, further improving the aspect of functional food (Hussain et al., 2016). One example is kombucha which is currently a research trend for functional food.
Catechins, flavonoids, and other polyphenols are abundant in kombucha, a fermented beverage made with SCOBY (symbiotic culture of bacteria and yeast) (Abaci et al., 2022). The base beverage of kombucha mainly consists of plant-based tea and fruit juice. The application of butterfly pea flower (Clitoria ternatea) as a coloring agent and garnish component has been familiar in both Indonesia and globally. Moreover, butterfly pea flower extract showed beneficial antioxidant and obesity-amelioration effects (Thilavech et al., 2021). However, in addition to extracts, it is necessary to innovate butterfly pea flower (Clitoria ternatea) into various forms of functional food products to make it easier to consume and accept, such as in the form of fermented drinks. Seeing the potential of the butterfly pea flower, we aim to innovate it into a fermented drink and determine the metabolites profile of Clitoria ternatea kombucha (CTK) while also validating their antiobesity potential through computational molecular docking or in silico study. The selected target proteins were human pancreatic lipase, α-amylase, α-glucosidase, porcine pancreatic lipase, and fat mass and obesity-associated (FTO) proteins which in previous studies became potential markers for the discovery of new obesity inhibitors (Permatasari et al., 2022a; Cerk et al., 2018; Lunagariya et al., 2014; Zhou et al., 2021).
2. Materials and methods
2.1. Formulation of Clitoria ternatea kombucha (CTK)
The butterfly pea flower or Clitoria ternatea was collected from Yogyakarta, Indonesia (Wirokerten, Banguntapan, Bantul Regency; coordinates: 7.8484152, 110.3993969). The Biology Department at the State Islamic University of Sunan Kalijaga Yogyakarta, Indonesia carried out the identification and authentication of botanical specialties (Taxonomy ID: 43366; NCBI:txid43366). Samples were gathered for feature comparison. The double petals of butterfly pea flowers were cleaned and then dried in a drying oven 55 at 50 °C for 4 h, resulting in dried butterfly pea flowers with a 10% moisture content (Martini et al., 2020).
The CTK drink formula's components are as follows (Permatasari et al., 2022b): 2000 mL of water; 24 g of dried butterfly pea flowers; 300 g of granulated sugar (sugar cane); 10 g of SCOBY gel; and 166 g v/v of SCOBY starter solution (2500 mL). Two liters of water were heated to between 50 and 80 °C, added with 300 g of sugar cane granules, and mixed with 24 g of dried butterfly pea flowers. After stirring the water until it developed a deep blue hue, the fire stove was turned off and the pan was covered. The mixture was left to cool and then poured into a 3000 mL sterile bottle. 166 g of SCOBY starting solution and 10 g of SCOBY gel were then added to the bottle. The CTK was then kept in anaerobic conditions at 20–25 °C for 12 days. The bottle was wrapped in clean gauze and shut securely. All samples of beverages were kept for further study at a refrigerator temperature of 4–8 °C after 12 days of fermentation (Permatasari et al., 2022b).
2.2. Untargeted metabolomic profiling of Clitoria ternatea kombucha (CTK)
The untargeted metabolomic profiling test on CTK was analyzed at Laboratorium Sentral Ilmu Hayati (LSIH; ISO 9001:2008 and ISO 17025:2005; Central Laboratory of Life Sciences; Brawijaya University, Malang-65145, Indonesia) using a high-performance liquid chromatography system coupled with a high-resolution mass spectrometer (LC-HRMS) with the test number of 040/LSIH-UB/LK, referring to Permatasari et al. (2022) (Permatasari et al., 2022b). A volume of 50 μl of CTK was centrifuged at 6000 rpm for 2 min after being vortexed 30 times with ethanol (96%) at 2000 rpm. Before analysis, supernatants were gathered and filtered using 0.22 m syringe filters. Thermo Scientific Dionex Ultimate 3000 RSLC Nano High-Performance Liquid Chromatography (HPLC) and a micro flow meter comprised the LC-HRMS system. The analytical column was a Hypersil GOLD aQ 50 with a 1 mm x 1.9 particle size that was kept at 30 °C, the solvents A and B contained 0.1% formic acid dissolved in water, and 0.1% formic acid dissolved in acetonitrile, respectively. It was then separated using a linear gradient for 30 min at a flow rate of 40 L/min. With a full scan at 70,000 resolution, data-dependent MS2 at 17,500 resolution, and a 30-min operation period in both positive and negative modes, HRMS utilizes Thermo Scientific Q Exactive.
The data of obtained compounds were sorted according to the Advanced Mass Spectral Database (M/Z cloud; https://www.mzcloud.org) best match criteria with a match rate of >99.0% followed by a molecular docking study.
2.3. Molecular docking simulation
2.3.1. Hardware and software
ASUS Vivobook M413ia – Ek502t with AMD Ryzen 5 4500u (2.3 GHz) processor, 8 GB DDR4 memory, 512 GB SSD M.2 storage, and Windows 10 Home operating system was equipped with ChemDraw Ultra 12.0, AutoDock tools (version 4.2), and BIOVIA Discovery software. The website of Protein Data Bank (https://www.rcsb.org) and PubChem structure database (https://pubchem.ncbi.nlm.nih.gov) were also used in this study. All protocols regarding molecular docking simulation refer to previous research (Permatasari et al., 2022a).
2.3.2. Preparation of ligands and targets
The compounds that were identified as a constituent of the CTK metabolites profile were used as test ligands. ChemDraw Ultra 12.0 was used to sketch the whole structure in 2D, which was then transformed to 3D and optimized using the MM2 algorithm. The selected target proteins were human pancreatic lipase (PDB ID: 1LPB), α-amylase (PBD ID: 2QV4), α-glucosidase (PDB ID: 3L4Y), porcine pancreatic lipase (PDB ID: 1ETH), and Fat mass and obesity-associated (FTO) protein (PDB ID: 3LFM). All proteins were acquired from the Protein Data Bank via the website (https://www.rcsb.org). Kollman charges were applied to the receptors while the ligands were added with a Gasteiger charge.
2.3.3. Validation of molecular docking
Redocking was used as the molecular docking validation approach. Using AutoDock tools (version 4.2), the original ligand was transferred to the target pocket with specific grid coordinates. After the re-docking method, the ligand position's RMSD (root-mean-square deviation) must be less than 2.0 Å.
2.3.4. Molecular docking simulation
The grid and docking parameters were developed using the docking validation findings (Table 1). The outcome was recorded in a *dlg file for each docking's final structure of conformation. Analysis was done on the ligand-receptor interaction using Discovery Studio 2016.
Table 1.
Single chromatogram of 13 major compounds of CTK.
| No | Compounds | Formula | RT (min) | Single Chromatogram | |
|---|---|---|---|---|---|
| 1 | 5-Hydroxymethyl-2-furaldehyde | C6H6O3 | 0.899 |  |
 |
| 2 | Dibenzylamine | C14H15N | 7.286 |  |
 |
| 3 | 3-{[(2S,3R,4S,5R,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-2-yl]oxy}-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one | C27H30O15 | 7.066 |  |
 |
| 4 | Trans-3-Indoleacrylic acid | C11H9NO2 | 3.466 |  |
 |
| 5 | Kaempferol | C15H10O6 | 7.066 |  |
 |
| 6 | Trifolin | C21H20O11 | 7.067 |  |
 |
| 7 | 7-Hydroxycoumarine | C9H6O3 | 14.68 |  |
 |
| 8 | Citral | C10H16O | 6.493 |  |
 |
| 9 | Mauritianin | C33H40O19 | 6.648 |  |
 |
| 10 | Rutin | C27H30O16 | 6.622 |  |
 |
| 11 | Quercetin | C15H10O7 | 6.621 |  |
 |
| 12 | α-Pyrrolidinopropiophenone | C13H17NO | 16.478 |  |
 |
| 13 | Quercetin-3β-D-glucoside | C21H20O12 | 7.098 |  |
 |
FTMS: Fourier transform mass spectrometry. MW: Molecular weight.
3. Results
3.1. Metabolites profile of Clitoria ternatea kombucha (CTK)
This latest research succeeded in innovating a fermented drink from Clitoria ternatea kombucha (CTK), as well as knowing its main metabolite with antiobesity potential via molecular docking studies. A total of 79 compounds were identified in CTK based on the untargeted metabolomic profiling using LC-HRMS (Table Supplementary 1). The majority of these compounds exhibited several health benefits, ranging from antioxidant, antiobesity, hypolipidemic, and protection against cardiometabolic risk factors. Table 1 lists the detected chemical compounds and their respective single chromatograms. 13 compounds were selected as major metabolites as they passed the >99.0% M/Z cloud database criteria and had a major abundance, which is ideal to be tested in the molecular docking or in silico study.
3.2. In silico or molecular docking study antiobesity of CTK major metabolites based on α-amylase, α-glucosidase, lipase, and FTO protein receptor
As shown in Table 2, the targeted receptors for molecular docking assays include human and porcine lipase, a-glucosidase, a-amylase, and FTO protein; which passed the validation based on <2 Å Judgement.
Table 2.
Validation of molecular docking simulation.
| No | Drug Target Receptors | PDB ID | Docking Site (x; y;z) | Docking Area (x.y.z) | RMSD (Å) | ΔG (kcal/mol) | Number in Cluster (/100) | Judgement (<2 Å) |
|---|---|---|---|---|---|---|---|---|
| 1 | Human Pancreatic Lipase | 1LPB | −0.423, 16.723, 26.546 | 42 × 40 x 40 | 1.499 | −4.13 | 26 | Valid |
| 2 | Human Pancreatic α-Amylase | 2QV4 | 12.942, 47.17, 26.2 | 42 × 40 x 40 | 1.072 | −8.99 | 20 | Valid |
| 3 | Human Pancreatic α-Glucosidase | 3L4Y | −1.542, −19.201, −21.043 | 42 × 42 x 42 | 1.186 | −4.27 | 20 | Valid |
| 4 | Porcine Pancreatic Lipase | 1ETH | 54.515, 45.839, 122.106 | 44 × 44 x 44 | 1.984 | −8.45 | 24 | Valid |
| 5 | FTO Protein | 3LFM | 29.043, −6.644, −29.329 | 42 × 42 x 42 | 0.715 | −6.29 | 90 | Valid |
FTO: fat mass and obesity-associated; PDB ID: Protein Data Bank Identity; RMSD: Root-mean-square deviation.
After the validation process was done, the molecular docking simulations against lipase (both human and porcine), α-amylase, α-glucosidase, and FTO protein were performed on 13 selected compounds (considering the spectra database match values), acarbose (as the control for α-amylase and α-glucosidase), and orlistat (as a control for lipase and FTO).
Interestingly, this examination found that almost all major compounds exhibited better binding affinity to all receptors compared to acarbose (standard drug or control positive for α-glucosidase and α-amylase) and orlistat as a positive control in lipase (Table 3). Only 5-Hydroxymethyl-2-furaldehyde (PubChem ID: 237332) showed lower affinity to 2QV4 and 1ETH compared to acarbose and orlistat (−3.20 against −3.21 ΔG (kcal/mol); −3.75 against −4.48 ΔG (kcal/mol)). Quercetin (PubChem ID: 5280343) showed the strongest binding affinity to 1LPB (ΔG = −7.03 kcal/mol) and 3LFM (ΔG = −7.78 kcal/mol). Dibenzylamine (PubChem ID: 7656) had the best affinity to 2QV4 (ΔG = −7.12 kcal/mol) and 3L4Y (ΔG = −7.43 kcal/mol). Kaempferol (PubChem ID: 5280863) exhibited the most potential interaction with 1ETH (ΔG = −8.05 kcal/mol). Furthermore, the molecular docking also found that overall, Kaempferol, Quercetin-3β-D-glucoside (PubChem ID: 5280804), Quercetin, Dibenzylamine, and α-Pyrrolidinopropiophenone (PubChem ID: 209045) showed the best potential as the functional antiobesity major compounds since their affinity value ranked high in each respective receptors. These comparisons are based on the value of ΔG (kcal/mol), and the results of molecular docking tests were listed in Table 3. The visualization of the amino acid interactions of the overall best-docked compounds against selected target proteins was presented in Table 4.
Table 3.
Molecular docking parameter of identified compounds of CTK.
| No. | Substance | Number in Cluster (/100) |
ΔG (kcal/mol) |
Ki |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1LPB | 2QV4 | 3L4Y | 1ETH | 3LFM | 1LPB | 2QV4 | 3L4Y | 1ETH | 3LFM | 1LPB | 2QV4 | 3L4Y | 1ETH | 3LFM | ||
| control | Orlistat | 6 | 4 | 5 | −2.38 | −4.48 | −3.71 | 5.22 mM | 124.59 uM | 212.83 uM | ||||||
| control | Acarbose | 13 | 11 | −3.21 | −2.3 | 166.89 uM | 1.00 mM | |||||||||
| 1 | Kaempferol | 61 | 57 | 85 | 100 | 95 | −6.84 | −6.61 | −6.32 | −8.05 | −7.41 | 6.97 uM | 5.82 uM | 19.79 uM | 934.24 nM | 2.25 uM |
| 2 | Quercetin | 64 | 82 | 65 | 95 | 95 | −7.03 | −6.77 | −5.77 | −7.85 | −7.78 | 3.99 uM | 6.30 uM | 25.65 uM | 812.39 nM | 1.32 uM |
| 3 | Quercetin-3β-D-glucoside | 83 | 37 | 40 | 91 | 49 | −6.68 | −6.51 | −5.97 | −7.43 | −7.66 | 1.78 uM | 4.00 uM | 12.61 uM | 2.04 uM | 293.55 nM |
| 4 | 7-Hydroxycoumarine | 65 | 100 | 93 | 99 | 98 | −5.44 | −5.05 | −6.09 | −6.1 | −5.92 | 101.30 uM | 193.63 uM | 32.37 uM | 33.09 uM | 42.93 uM |
| 5 | Dibenzylamine | 96 | 79 | 71 | 76 | 79 | −5.96 | −7.12 | −7.43 | −7.49 | −5.85 | 28.25 uM | 3.40 uM | 1.89 uM | 2.18 uM | 43.87 uM |
| 6 | α-Pyrrolidinopropiophenone | 79 | 62 | 63 | 93 | 50 | −5.36 | −6.53 | −7.38 | −7.11 | −5.67 | 88.06 uM | 12.75 uM | 2.21 uM | 3.93 uM | 56.25 uM |
| 7 | Mauritianin | 6 | 9 | 14 | 9 | 9 | −3.67 | −5.52 | −4.33 | −6.09 | −6.57 | 187.98 uM | 1.46 uM | 60.71 uM | 1.36 uM | 606.28 nM |
| 8 | 3-{[(2S,3R,4S,5R,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-2-yl]oxy}-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one | 15 | 24 | 13 | 69 | 30 | −3.09 | −4.83 | −5.97 | −5.25 | −6.94 | 147.51 uM | 73.73 uM | 3.73 uM | 15.23 uM | 2.00 uM |
| 9 | Rutin | 10 | 27 | 8 | 12 | 24 | −5.73 | −4.81 | −3.79 | −5.9 | −6.49 | 2.29 uM | 56.10 uM | 159.09 uM | 2.85 uM | 275.88 nM |
| 10 | 5-Hydroxymethyl-2-furaldehyde | 90 | 41 | 32 | 45 | 45 | −3.7 | −3.2 | −4.39 | −3.75 | −4.12 | 1.56 mM | 3.56 mM | 324.30 uM | 1.54 mM | 572.22 uM |
| 11 | Trans-3-Indoleacrylic acid | 100 | 99 | 44 | 97 | 57 | −5.57 | −4.56 | −3.8 | −5.18 | −6.11 | 73.01 uM | 413.54 uM | 1.23 mM | 124.73 uM | 27.47 uM |
| 12 | Citral | 93 | 80 | 38 | 77 | 37 | −4.28 | −4.24 | −4.74 | −5.46 | −4.38 | 452.25 uM | 561.06 uM | 191.84 uM | 72.61 uM | 391.89 uM |
| 13 | Trifolin | 44 | 47 | 31 | 40 | 52 | −6.14 | −5.51 | −5.78 | −5.74 | −7.72 | 5.17 uM | 32.83 uM | 18.10 uM | 26.31 uM | 592.81 nM |
Table 4.
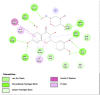
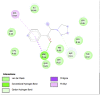
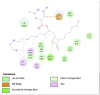
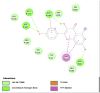
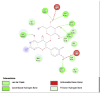
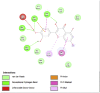
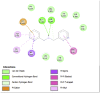
Amino acid interaction of Clitoria ternatea kombucha active compounds against human pancreatic lipase, α-amylase, α-glucosidase, porcine pancreatic lipase, and fat mass and obesity-associated proteins.
| Pancreatic Lipase (1LPB) | ||
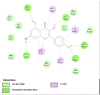 |
 |
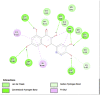 |
| Kaempferol (Ligand Test) | Quercetin-3β-D-glucoside (Ligand Test) | Quercetin (Ligand Test) |
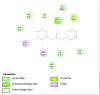 |
 |
 |
| Dibenzylamine (Ligand Test) |
α-Pyrrolidinopropiophenone (Ligand Test) |
Orlistat (Comparison Compound) |
|
Pancreatic α-Amylase (2QV4) | ||
 |
 |
 |
| Kaempferol (Ligand Test) | Quercetin-3β-D-glucoside (Ligand Test) | Quercetin (Ligand Test) |
 |
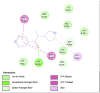 |
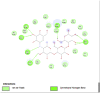 |
| Dibenzylamine (Ligand Test) |
α-Pyrrolidinopropiophenone (Ligand Test) |
Acarbose (Comparison Compound) |
|
Pancreatic α-Glucosidase (3L4Y) | ||
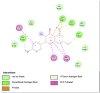 |
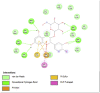 |
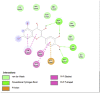 |
| Kaempferol (Ligand Test) | Quercetin-3β-D-glucoside (Ligand Test) | Quercetin (Ligand Test) |
 |
 |
 |
| Dibenzylamine (Ligand Test) |
α-Pyrrolidinopropiophenone (Ligand Test) |
Acarbose (Comparison Compound) |
|
Porcine Pancreatic Lipase (1ETH) | ||
 |
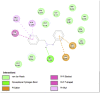
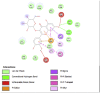 |
 |
| Kaempferol (Ligand Test) | Quercetin-3β-D-glucoside (Ligand Test) | Quercetin (Ligand Test) |
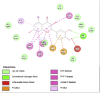
 |
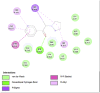 |
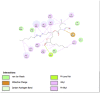 |
| Dibenzylamine (Ligand Test) |
α-Pyrrolidinopropiophenone (Ligand Test) |
Orlistat (Comparison Compound) |
|
Fat Mass and Obesity-Associated Protein (3LFM) | ||
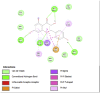 |
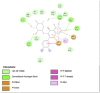 |
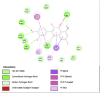 |
| Kaempferol (Ligand Test) | Quercetin-3β-D-glucoside (Ligand Test) | Quercetin (Ligand Test) |
 |
 |
 |
| Dibenzylamine (Ligand Test) | α-Pyrrolidinopropiophenone (Ligand Test) | Orlistat (Comparison Compound) |
4. Discussion
The development of omics technology has led to new disciplines emerging, such as Foodomics which studies the relationship between food and nutrition or health through an omics approach. This study was the first to successfully innovate butterfly pea flowers (C. ternatea) into kombucha drinks through SCOBY fermentation and determine their metabolites profile through HPLC-ESI-HRMS/MS. Of the 79 compounds that were successfully observed, 13 compounds with major abundance were successfully sorted based on the spectral database best match (M/Z Cloud). Another study also found 13 compounds from C. ternatea flower petal extract based on the chromatograms from LC/MS/MS (Chayaratanasin et al., 2019). As part of Foodomics, researchers also conducted a study to determine the antiobesity potential of those 13 compounds against obesity receptors such as human pancreatic lipase (PDB ID: 1LPB), α-amylase (PBD ID: 2QV4), α-glucosidase (PDB ID: 3L4Y), porcine pancreatic lipase (PDB ID: 1ETH), and fat mass and obesity-associated (FTO) protein (PDB ID: 3LFM).
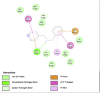
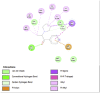
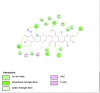
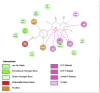
Lipase is the primary enzyme responsible for releasing fatty acids (FAs) from triacylglycerol (TG) stores and generating FAs needed for cellular lipolysis (Cerk et al., 2018) (Graphical Abstract). Dietary TGs are partially hydrolyzed by lipases into free FAs and diacylglycerols that are then emulsified with bile salts to create tiny droplets of fat. This complete breakdown process yields free FAs, mono– and diacylglycerols, bile salts, fat-soluble vitamins, and lysophosphatidic acid, which combine to create mixed micelles that enterocytes can absorb. It has been stated that the most extensively researched mechanism for locating prospective anti-obesity drugs is pancreatic lipase inhibition (Lunagariya et al., 2014). In this study, almost all compounds showed good binding affinity to 1LPB and 1ETH receptors compared to orlistat. Quercetin and kaempferol showed the strongest binding affinity to 1LPB (ΔG = −7.03 kcal/mol) and (ΔG = −8.05 kcal/mol), respectively. Quercetin has been known as a promising antiobesity agent that inhibits pancreatic lipase activity and fat absorption (Zhou et al., 2021). Pancreatic lipase activity was reduced through the binding of quercetin to the non-competitive domain of the enzyme. The rat's postprandial serum TG level was significantly decreased by quercetin as there was an increase in fat excretion through the feces. Strikingly, kaempferol's capability as a pancreatic lipase inhibitor has been proven, along with its synergistic effect with orlistat (Li et al., 2020). Other detected compounds in this study, rutin and citral, also showed therapeutic potential related to obesity (Yuan et al., 2017; Sharma et al., 2021). These mechanisms may explain and support the results of this study that CTK's metabolites inhibit the activity of pancreatic lipase.
The salivary glands and pancreas are the main sites of expression for amylase, while α-glucosidase is mainly located in small intestine brush borders. Both α-glucosidase and α-amylase break down starch and disaccharides into glucose as part of their mechanism of action (Etsassala et al., 2020) (Graphical Abstract). A study highlighted that α-amylase was expressed at higher levels in obesity (Afsartala et al., 2016). It is also becoming more evident in pathological conditions like obesity and diabetes that inhibiting α-amylase and α-glucosidase may promote weight loss and improve glycemic control (Mahmood, 2016). Similar to lipase receptors, almost all compounds in this study showed better binding affinity to 2QV4 and 3L4Y compared to acarbose. Dibenzylamine had the best affinity to 2QV4 (ΔG = −7.12 kcal/mol), followed by quercetin and kaempferol. On the other hand, dibenzylamine also had the strongest affinity to 3L4Y (ΔG = −7.43 kcal/mol), followed by α-pyrrolidinopropiophenone (ΔG = −7.38 kcal/mol) and kaempferol (ΔG = −6.32 kcal/mol). No study regarding the antiobesity and antidiabetic potential of dibenzylamine and α-pyrrolidinopropiophenone was found. Quercetin has been studied as α-amylase and α-glucosidase inhibitors, with a synergistic effect with rutin (Oboh et al., 2015). Kaempferol mechanisms in inhibiting α-glucosidase have also been proposed. According to Peng et al. (2016), kaempferol bound to α-glucosidase with a high affinity that was primarily driven by hydrogen bonds and van der Waals forces, and this binding caused α-glucosidase to change conformation (Peng et al., 2016). The amino acid residues in the active site of α-glucosidase may also interact with kaempferol, occupying the catalytic core and ultimately reducing the enzyme's activity. This study also found that citral, rutin, and 7-hydroxycoumarine had antidiabetic and antiobesity properties which are in line with existing literature (Modak and Mukhopadhaya, 2011; Ghorbani, 2017; Li et al., 2017).
A recently discovered genetic contributor to obesity is the fat mass and obesity-associated (FTO) gene. Genetic variants in the FTO gene have been linked to human adiposity and metabolic disorders, according to genome-wide studies. Energy and adipose tissue homeostasis are disturbed by the disruption of FTO enzymatic activity, which dysregulates the genes involved in energy metabolism (Zhao et al., 2014). Interestingly, all major compounds in this study exhibited better binding affinity to 3LFM compared to orlistat, which supported the fact that bioactive compounds – when consumed – contribute clinical implications on obesity (Ramírez-Moreno et al., 2022b). Furthermore, diet also influenced the DNA methylation processes which regulate gene expression (Kadayifci et al., 2018). Quercetin showed the strongest binding affinity to 3LFM (ΔG = −7.78 kcal/mol). We also compared our result with a similar study by Mohammed et al., 2015) (Mohammed et al., 2015). Even though we agreed that quercetin is a potent FTO inhibitor, they found that rutin and kaempferol showed lower binding affinity to orlistat, which contrasts with our study. These flavonoids – which are commonly found in fruits and vegetables – may downregulate the FTO gene expression in obesity (Asuquo et al., 2022).
This early phase of the study used untargeted metabolomic profiling and molecular docking simulations to determine the antiobesity potential of CTK. Compared to other in silico studies, we brought a more detailed assay on potential antiobesity from CTK by adding the 1ETH and 3LFM receptors (Permatasari et al., 2022a). Strong binding affinity to the specified target proteins indicates that CTK's main metabolites possess antiobesity potential. Even though the result of this study highlighted the good capability of CTK and its bioactive compounds as an antiobesity agent, further in vitro and in vivo studies should validate this health benefit. For future direction, studies to assess the efficacy of CTK and their metabolites on obesity and cholesterol-induced rats or animal model are needed to simulate obesity and metabolism similar to humans. Furthermore, the potential bioavailability of major metabolites elucidated from CTK can be further determined by in vitro digestion assays. In addition, it is highly recommended to conduct studies of molecular dynamics simulations of ligands or major metabolites that have an affinity to the obesity receptors, which is a limitation of the results reported in this short communication.
5. Conclusions
Clitoria ternatea can be utilized as an innovative antiobesity functional drink such as CTK which has major functional metabolites especially Kaempferol, Quercetin-3β-D-glucoside, Quercetin, Dibenzylamine, and α-Pyrrolidinopropiophenone. These compounds showed the best potential as functional antiobesity agents since their affinity value ranked high in each respective receptor (compared to the control). In conclusion, major metabolites compounds of CTK have the potential to be promising functional foods against obesity as evidenced by molecular docking while further in vitro and in vivo studies are needed to validate the health benefits of CTK.
Patents
The CTK formulation used in this study has been registered as a patent in Indonesia with Fahrul Nurkolis as the patent holder (Patent number S00202205671; https://pdki-indonesia.dgip.go.id/detail/S00202205671?type=patent&keyword=Kombucha+Telang).
Funding
This research received no external funding.
CRediT authorship contribution statement
Hardinsyah Hardinsyah: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Validation. William Ben Gunawan: Writing – review & editing, Visualization. Fahrul Nurkolis: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Visualization. Darmawan Alisaputra: Visualization, Software, Writing - review & editing. Rudy Kurniawan: Writing – review & editing, Visualization. Nelly Mayulu: Supervision, Validation, Formal analysis, Writing – review & editing. Nurpudji Astuti Taslim: Supervision, Validation, Formal analysis, Writing – review & editing. Trina Ekawati Tallei: Validation, Formal analysis.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We offer a great thank you to the Chairman of the Indonesian Association of Clinical Nutrition Physicians, Professor Nurpudji Astuti Taslim, MD., MPH., PhD., Sp. GK(K); and Dr. Nelly Mayulu, MD, who have reviewed and provided suggestions, as well as input on the draft article.
Handling Editor: Dr. Yeonhwa Park
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2023.100464.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abaci N., Senol Deniz F.S., Orhan I.E. Kombucha - an ancient fermented beverage with desired bioactivities: a narrowed review. Food Chem. X. 2022 Apr 6;14 doi: 10.1016/j.fochx.2022.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsartala Z., Savabkar S., Nazemalhosseini Mojarad E., Assadollahi V., Tanha S., Bijangi K., Gholami M. Expression of liver alpha-amylase in obese mouse hepatocytes. Gastroenterol. Hepatol. Bed Bench. 2016;9(4):278–285. doi: 10.1016/j.diabres.2019.107974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuquo E.A., Nwodo O.F.C., Assumpta A.C., Orizu U.N., Oziamara O.N., Solomon O.A. FTO gene expression in diet-induced obesity is downregulated by Solanum fruit supplementation. Open Life Sci. 2022 Jun 15;17(1):641–658. doi: 10.1515/biol-2022-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019 May;15(5):288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- Cerk I.K., Wechselberger L., Oberer M. Adipose triglyceride lipase regulation: an overview. Curr. Protein Pept. Sci. 2018 Feb 1;19(2):221–233. doi: 10.2174/1389203718666170918160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayaratanasin P., Caobi A., Suparpprom C., Saenset S., Pasukamonset P., Suanpairintr N., Barbieri M.A., Adisakwattana S. Clitoria ternatea flower petal extract inhibits adipogenesis and lipid accumulation in 3T3-L1 preadipocytes by downregulating adipogenic gene expression. Molecules. 2019 May 17;24(10):1894. doi: 10.3390/molecules24101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etsassala N.G.E.R., Badmus J.A., Marnewick J.L., Iwuoha E.I., Nchu F., Hussein A.A. Alpha-glucosidase and alpha-amylase inhibitory activities, molecular docking, and antioxidant capacities of salvia aurita constituents. Antioxidants. 2020 Nov 19;9(11):1149. doi: 10.3390/antiox9111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017 Dec;96:305–312. doi: 10.1016/j.biopha.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Green M., Arora K., Prakash S. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int. J. Mol. Sci. 2020 Apr 21;21(8):2890. doi: 10.3390/ijms21082890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek A., Kretzler B., König H.H. Prevalence and correlates of obesity among the oldest old. A systematic review, meta-analysis and meta-regression. Geriatr. Gerontol. Int. 2022 May;22(5):373–383. doi: 10.1111/ggi.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Bose S., Wang J.H., Yadav M.K., Mahajan G.B., Kim H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016 Mar 1;81:1–6. doi: 10.1016/j.foodres.2015.12.026. [DOI] [Google Scholar]
- Kadayifci F.Z., Zheng S., Pan Y.X. Molecular mechanisms underlying the link between diet and DNA methylation. Int. J. Mol. Sci. 2018 Dec 14;19(12):4055. doi: 10.3390/ijms19124055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Yao Y., Li L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017 Oct;69(10):1253–1264. doi: 10.1111/jphp.12774. [DOI] [PubMed] [Google Scholar]
- Li S., Pan J., Hu X., Zhang Y., Gong D., Zhang G. Kaempferol inhibits the activity of pancreatic lipase and its synergistic effect with orlistat. J. Funct.Foods. 2020 Sep 1;72 doi: 10.1016/j.jff.2020.104041. [DOI] [Google Scholar]
- Lin X., Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021 Sep 6;12 doi: 10.3389/fendo.2021.706978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunagariya N.A., Patel N.K., Jagtap S.C., Bhutani K.K. Inhibitors of pancreatic lipase: state of the art and clinical perspectives. Excli J. 2014 Aug 22;13:897–921. PMCID: PMC4464291, PMID: 26417311. [PMC free article] [PubMed] [Google Scholar]
- Mahmood N. A review of α-amylase inhibitors on weight loss and glycemic control in pathological state such as obesity and diabetes. Comp. Clin. Pathol. 2016 Nov;25(6):1253–1264. doi: 10.1007/s00580-014-1967-x. [DOI] [Google Scholar]
- Martini N.K.A., Ekawati N.G.A., Ina P.T. Pengaruh suhu dan lama pengeringan terhadap karakteristik teh bunga telang (Clitoria ternatea L.) J. Itepa. 2020;9(3):327–340. doi: 10.24843/itepa.2020.v09.i03.p09. 2020 Sep. [DOI] [Google Scholar]
- Modak T., Mukhopadhaya A. Effects of citral, a naturally occurring antiadipogenic molecule, on an energy-intense diet model of obesity. Indian J. Pharmacol. 2011 May;43(3):300–305. doi: 10.4103/0253-7613.81515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A., Al-Numair K.S., Balakrishnan A. Docking studies on the interaction of flavonoids with fat mass and obesity associated protein. Pak. J. Pharm. Sci. 2015 Sep;28(5):1647–1653. PMID: 26408884. [PubMed] [Google Scholar]
- Oboh G., Ademosun A.O., Ayeni P.O., et al. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015 Sep;24:1103–1110. doi: 10.1007/s00580-014-2040-5. [DOI] [Google Scholar]
- Okati-Aliabad H., Ansari-Moghaddam A., Kargar S., Jabbari N. Prevalence of obesity and overweight among adults in the Middle East countries from 2000 to 2020: a systematic review and meta-analysis. J. Obes. 2022 Feb 3;2022 doi: 10.1155/2022/8074837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Zhang G., Liao Y., Gong D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016 Jan 1;190:207–215. doi: 10.1016/j.foodchem.2015.05.088. [DOI] [PubMed] [Google Scholar]
- Permatasari H.K., Nurkolis F., Hardinsyah H., Taslim N.A., Sabrina N., Ibrahim F.M., Visnu J., Kumalawati D.A., Febriana S.A., Sudargo T., Tanner M.J., Kurniatanty I., Yusuf V.M., Rompies R., Bahar M.R., Holipah H., Mayulu N. Metabolomic assay, computational screening, and pharmacological evaluation of caulerpa racemosa as an anti-obesity with anti-aging by altering lipid profile and peroxisome proliferator-activated receptor-γ coactivator 1-α levels. Front. Nutr. 2022 Jul 14;9 doi: 10.3389/fnut.2022.939073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permatasari H.K., Nurkolis F., Gunawan W.B., Yusuf V.M., Yusuf M., Kusuma R.J., Sabrina N., Muharram F.R., Taslim N.A., Mayulu N., Batubara S.C., Samtiya M., Hardinsyah H., Tsopmo A. Modulation of gut microbiota and markers of metabolic syndrome in mice on cholesterol and fat enriched diet by butterfly pea flower kombucha. Curr. Res. Food Sci. 2022 Aug 18;5:1251–1265. doi: 10.1016/j.crfs.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Moreno E., Arias-Rico J., Jiménez-Sánchez R.C., Estrada-Luna D., Jiménez-Osorio A.S., Zafra-Rojas Q.Y., Ariza-Ortega J.A., Flores-Chávez O.R., Morales-Castillejos L., Sandoval-Gallegos E.M. Role of bioactive compounds in obesity: metabolic mechanism focused on inflammation. Foods. 2022 Apr 25;11(9):1232. doi: 10.3390/foods11091232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Moreno E., Arias-Rico J., Jiménez-Sánchez R.C., Estrada-Luna D., Jiménez-Osorio A.S., Zafra-Rojas Q.Y., Ariza-Ortega J.A., Flores-Chávez O.R., Morales-Castillejos L., Sandoval-Gallegos E.M. Role of bioactive compounds in obesity: metabolic mechanism focused on inflammation. Foods. 2022 Apr 25;11(9):1232. doi: 10.3390/foods11091232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Habib S., Sahu D., Gupta J. Chemical properties and therapeutic potential of citral, a monoterpene isolated from lemongrass. Med. Chem. 2021 Jan 1;17(1):2–12. doi: 10.2174/1573406416666191227111106. [DOI] [PubMed] [Google Scholar]
- Thilavech T., Adisakwattana S., Channuwong P., Radarit K., Jantarapat K., Ngewlai K., Sonprasan N., Chusak C. Clitoria ternatea flower extract attenuates postprandial lipemia and increases plasma antioxidant status responses to a high-fat meal challenge in overweight and obese participants. Biology. 2021 Sep 28;10(10):975. doi: 10.3390/biology10100975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechert M., Holzapfel C. Nutrition concepts for the treatment of obesity in adults. Nutrients. 2021 Dec 30;14(1):169. doi: 10.3390/nu14010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Wei G., You Y., Huang Y., Lee H.J., Dong M., Lin J., Hu T., Zhang H., Zhang C., Zhou H., Ye R., Qi X., Zhai B., Huang W., Liu S., Xie W., Liu Q., Liu X., Cui C., Li D., Zhan J., Cheng J., Yuan Z., Jin W. Rutin ameliorates obesity through brown fat activation. Faseb. J. 2017 Jan;31(1):333–345. doi: 10.1096/fj.201600459RR. [DOI] [PubMed] [Google Scholar]
- Zhao X., Yang Y., Sun B.F., Zhao Y.L., Yang Y.G. FTO and obesity: mechanisms of association. Curr. Diabetes Rep. 2014 May;14:1–9. doi: 10.1007/s11892-014-0486-0. [DOI] [PubMed] [Google Scholar]
- Zhou J.F., Wang W.J., Yin Z.P., Zheng G.D., Chen J.G., Li J.E., Chen L.L., Zhang Q.F. Quercetin is a promising pancreatic lipase inhibitor in reducing fat absorption in vivo. Food Biosci. 2021 Oct 1;43 doi: 10.1016/j.fbio.2021.101248. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.



