Abstract
Purpose:
This work aimed to analyze the association of obstructive sleep apnea (OSA) with choroidal thickness (CT) in patients with central serous chorioretinopathy (CSC).
Methods:
We identified patients in the Stanford Research Repository with a diagnosis of CSC and OSA. Age- and sex-matched controls with either CSC or OSA only were also identified. CT was measured at 5 points (subfoveal, and 1500 and 3000 µm nasal and temporal) by 2 graders. In addition to OSA treatment and severity, we also investigated the association of Oxygen Desaturation Index and nocturnal oxygen saturation nadir with subfoveal CT (SFCT).
Results:
A total of 57 patients and 72 eyes met the study inclusion criteria. The mean SFCT was significantly different across the 3 groups: OSA-only was the thinnest, followed by CSC with OSA, and CSC-only was the thickest (194.2 μm, 295.1 μm, and 357.8 μm, respectively, P < .001). SFCT was thicker in CSC with OSA compared with those with only OSA (P < .05). OSA treatment status and OSA severity did not show a significant difference in SFCT in multivariable modeling. Nocturnal oxygen saturation nadir was positively associated with SFCT, but this did not reach significance..
Conclusions:
SFCT is significantly different in patients with OSA alone, CSC with OSA, and CSC alone. While OSA treatment status did not demonstrate a significant difference in SFCT in this study, future studies should evaluate patients for OSA in patients known to have CSC and atypically thin CT to further investigate the novel metrics leveraged in this study.
Keywords: central serous chorioretinopathy, choroidal thickness, obstructive sleep apnea, optical coherence tomography, subfoveal thickness
Introduction
Central serous chorioretinopathy (CSC) is characterized by localized neurosensory retinal or retinal pigment epithelial detachment secondary to serous exudation from the choroid that can result in permanent vision loss. Although the pathophysiology of CSC remains incompletely understood, it is thought to derive from abnormal choroidal vascular congestion and leakage into the subretinal space. 1,2 Recent studies have supported this theory with evidence of increased choroidal thickness (CT), as seen on enhanced depth imaging (EDI) spectral-domain ocular coherence tomography (SD-OCT), in patients with CSC. 3
Prior studies have found associations between the vascular and mechanical effects of obstructive sleep apnea (OSA) and a number of eye conditions, including CSC, with some studies suggesting that OSA treatment might alleviate the sympathetic insult incurred by the apneic and hypoxic events. 4 -7 Moreover, a recent national database study from our group that looked at more than 59 million insured patients between 2007 and 2016 confirmed the association between CSC and OSA diagnoses, which had been reported in prior studies. 8,9 The incidence of CSC was found to be 17.2 per 100 000 in female and 40.8 per 100 000 in male patients. 9
From a pathophysiologic standpoint, CSC and OSA are known to have opposing effects on CT, with CSC being associated with increased CT and OSA being associated with decreased CT. 10 -14 Prior studies have investigated CT in patients with CSC or OSA but have not addressed OSA treatment status and OSA severity as measured by overnight in-laboratory polysomnography, nor have important variables such as Oxygen Desaturation Index (ODI) or nocturnal oxygen saturation (SpO2) nadir been evaluated. 15 -17 The ODI is a measure of hypoxemic burden during sleep secondary to OSA, and is defined as a 3% or more change in the number of oxygen desaturation events per hour of sleep (eg, a drop in nocturnal SpO2 nadir from 99% to 95% would be considered an oxygen desaturation event). The use of these novel biometric features allows for a detailed characterization of OSA disease severity not previously leveraged in the ophthalmology literature but often described in the sleep medicine literature.
In the present study, we hypothesize that OSA severity and treatment status independently affect CT in patients with both CSC and OSA, based on EDI SD-OCT imaging. We further hypothesize that nocturnal SpO2 nadir and ODI measured during polysomnography, which are novel indicators of OSA severity not previously evaluated in studies on this subject, have an association with subfoveal CT (SFCT).
Methods
Data Source and Sample Selection
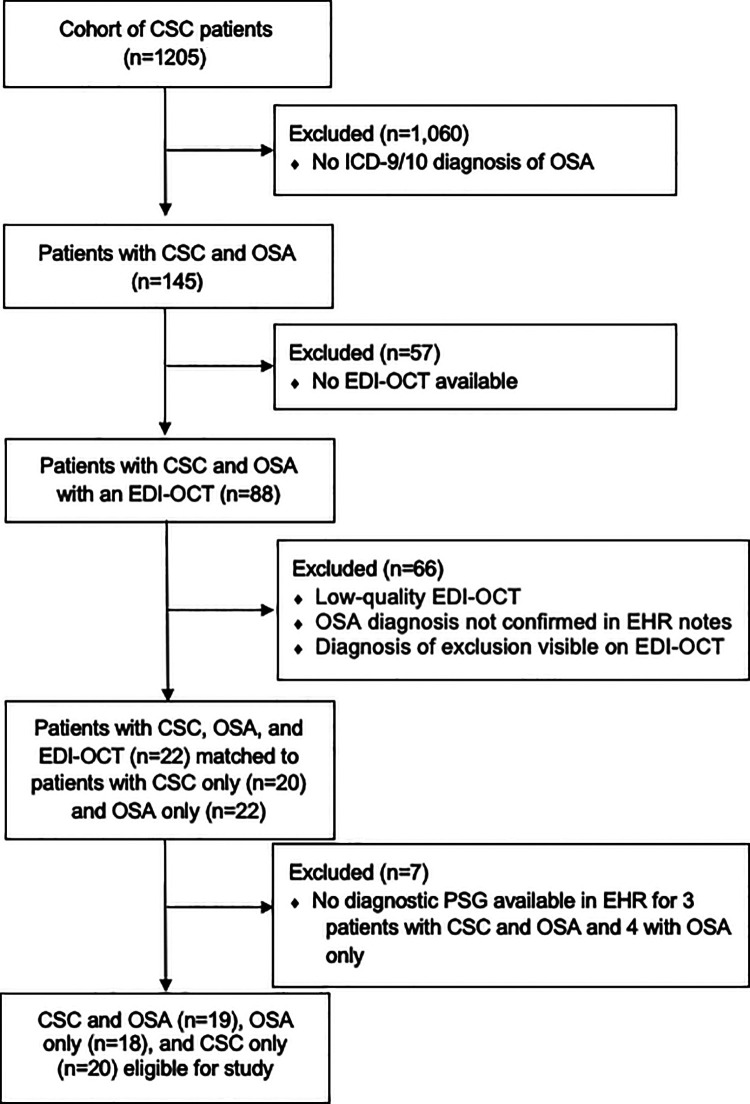
We used data from the Stanford Translational Research Integrated Database Environment (STRIDE), which is an informatics platform that consists of a clinical data warehouse of more than 1.3 million pediatric and adult patients treated at Stanford University Medical Center since 1995. 18 The data source was accessed under approval from the institutional review board at Stanford University. We identified our initial cohort of patients with CSC and OSA using International Classification of Diseases, Ninth and Tenth Revision codes and Current Procedural Terminology codes for EDI SD-OCT. The exclusion criteria were any retinopathy or choroidopathy other than CSC, an inconclusive or poor-quality OCT, as well as a diagnosis of OSA without an in-laboratory polysomnogram (Figure 1). Age- and sex-matched patients, those with only CSC, and those with only OSA served as controls and were also obtained from the STRIDE database.
Figure 1.
Consolidated Standards of Reporting Trials cohort selection diagram. CSC indicates central serous chorioretinopathy; EDI, enhanced depth imaging; EHR, electronic health record; ICD-9/10, International Classification of Diseases, Ninth or Tenth Revisions; OCT, optical coherence tomography; OSA, obstructive sleep apnea; PSG, polysomnography.
The Apnea-Hypopnea Index (AHI; the number of episodes of apneas and hypopneas per hour of sleep), as measured by American Academy of Sleep Medicine’s (AASM) criteria, was obtained from the electronic health records (EHRs) to categorize OSA severity as mild (5 ≤ AHI < 15), moderate (15 ≤ AHI < 30), and severe (AHI ≥ 30). 19 OSA treatment was defined by compliance with positive airway pressure (PAP) therapy for at least 4 or more hours per night and a reduction of AHI to less than 5 on PAP therapy. 20 Additional OSA-relevant variables included the AASM ODI and nocturnal SpO2 nadir during diagnostic polysomnogram.
CT Measurement
The CT was measured using EDI SD-OCT obtained with Zeiss Cirrus versions 2, 3, 4000, or 5000. CT grading started with obtaining the highest definition images available (high-definition 5-line raster scans) through the fovea for each patient. Image quality was graded based on the percentage of choroidoscleral (CS) junction visible in a given scan. Image quality was graded on a 3-point scale: poor quality (<30% of CS junction visible), average quality (30%-70% of CS junction visible), and excellent quality (>70% of CS junction visible). Only images meeting the criteria for average or excellent quality images were included in the study.
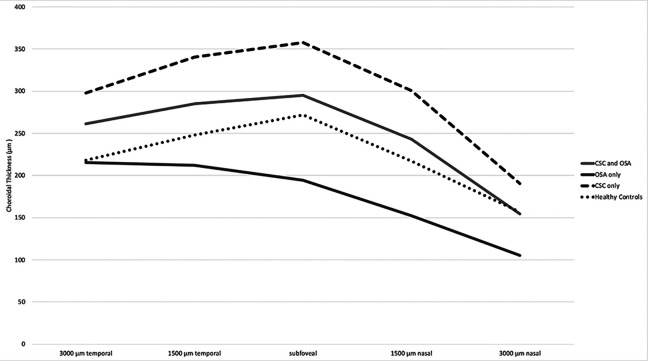
CT was measured independently by 2 masked graders (A.D.A. and J.R.D.) as the axial distance from the outer border of the retinal pigment epithelium layer to the CS junction using the software caliper included in the Zeiss OCT imaging software. These measurements were performed at the fovea and at 1500 and 3000 µm nasal and temporal to the foveal center, as previously described. 21 Measurements differing by greater than 20% were adjudicated by a third grader, a vitreoretinal specialist in training (N.R.). CT grading reached an interclass correlation coefficient of 95% with a less than 10-µm difference for any given measurement. Data for controls were derived from previously published data by Manjunath et al that characterized CT among healthy individuals at the same intervals measured in this study. 22 Figure 2 is a violin density plot that builds on a standard box plot diagram to demonstrate the probability density of the data at different SFCT values smoothed by a kernel density estimator. 23
Figure 2.
Violin plot of subfoveal choroidal thickness by obstructive sleep apnea (OSA). CSC indicates central serous chorioretinopathy.
Statistical Analysis
Patient demographics and clinical characteristics are reported as mean and SD for continuous measures and as frequencies and percentages for discrete data. The t test and Fisher exact test were used to compare CSC and treated OSA with CSC and untreated OSA. Mean CT was compared using analysis of variance with Tukey post hoc comparisons between groups. Linear regression was used to identify the magnitude of difference between groups. Statistical analysis was performed to detect a statistical significance of the measurements. The statistical software R version 3.5.2 (R Foundation for Statistical Computing) was used to perform all analyses. For all analyses, a P less than .05 was considered statistically significant.
Results
Patient Demographic and Clinical Data
Seventy-two eyes of 57 patients (48 [84.2%] male and 9 [15.8%] female; mean age 58.5 ± 12.9 years) were included. Twenty-six patients (45.6%) were non-Hispanic White and 20 (35.1%) were Asian, and the cohort had an average body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 28.9 (SD, 5.9). There were significant differences of mean BMI at diagnosis between the 3 subgroups; patients with only OSA had the highest average BMI and patients with only CSC had the lowest (P = .006). The majority of the total cohort consisted of never-smokers (73.7%). Among patients with CSC (n = 39), chorioretinopathy was most observed and not treated (24 [61.5%]) (Table 1). Among all patients with OSA (n = 37), the median AHI was 24.7 events per hour (interquartile range [IQR], 15.7-53.9), median nocturnal SpO2 nadir was 85.0% (range, 78.0%-89.0%), and median ODI was 9.8 events per hour (range, 6.5-50.4). AHI, nocturnal SpO2 nadir, and ODI did not significantly differ between the only OSA or CSC with OSA subgroups (see Table 1).
Table 1.
Clinical and Demographic Characteristics of Patients With Only OSA, Only CSC, or Both CSC and OSA, and CSC Treatment for Eyes of Patients with Only CSC or Both CSC and OSA.
| Total patients (N = 57) | Patients with CSC and OSA (n = 19) | Patients with only CSC (n = 20) | Patients with only OSA (n = 18) | P value | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 58.5 (12.9) | 58.4 (13.0) | 59.6 (12.6) | 57.6 (13.8) | .895 |
| BMIa at diagnosis, mean (SD) | 28.9 (5.9) | 28.3 (5.3) | 24.1 (1.8) | 31.3 (6.5) | .006 |
| Sex, No. (%) | .537 | ||||
| Male | 48 (84.2) | 16 (84.2) | 18 (90.0) | 14 (77.8) | |
| Female | 9 (15.8) | 3 (15.8) | 2 (10.0) | 4 (22.2) | |
| Race/Ethnicity, No. (%) | <.0001 | ||||
| Non-Hispanic White | 26 (45.6) | 8 (42.1) | 20 (50.0) | 8 (44.4) | |
| Hispanic | 9 (15.8) | 5 (26.3) | 4 (20.0) | – | |
| Asian | 20 (35.1) | 4 (21.1) | 6 (30.0) | 10 (55.6) | |
| Other | 2 (3.5) | 2 (10.5) | – | – | |
| Smoking history, No. (%) | .499 | ||||
| Never smoker | 42 (73.7) | 14 (73.7) | 16 (80.0) | 12 (66.7) | |
| Prior smoker | 14 (25.9) | 5 (27.8) | 3 (15.8) | 6 (33.3) | |
| Current smoker | 1 (1.9) | – | 1 (5.3) | – | |
| AHI, median (IQR) | 24.7 (15.7-53.9) | 17.0 (14.1-24.5) | – | 31.2 (21.0-54.3) | .320 |
| OSA severity,b mean (SD) | .076 | ||||
| Mild | 11 (19.3) | 8 (42.1) | – | 3 (16.7) | |
| Moderate | 12 (21.1) | 7 (36.8) | – | 5 (27.8) | |
| Severe | 14 (24.6) | 4 (21.1) | – | 10 (55.6) | |
| Nocturnal SpO2 nadir,c median (IQR) | 85.0 (78.0-89.0) | 86.0 (80.3-89.3) | – | 80.0 (73.5-89.0) | .143 |
| ODI,d median (IQR) | 9.8 (6.5-50.4) | 7.9 (3.9-15.7) | – | 15.0 (7.0-63.0) | .236 |
| Total eyes with CSC and OSA or only CSC (n = 43) | Eyes with CSC and OSA (n = 22) | Eyes with only CSC (n = 21) | |||
| CSC treatment | .875 | ||||
| Intravitreal anti-VEGF injection | 3 (7.0) | 1 (4.5) | 2 (9.5) | – | |
| Photodynamic therapy | 10 (23.3) | 6 (27.3) | 4 (16.7) | – | |
| Laser photocoagulation | 1 (2.3) | – | 1 (4.8) | – | |
| Observation | 29 (67.4) | 15 (68.2) | 14 (66.7) | – |
Abbreviations: AHI, Apnea-Hypopnea Index; BMI, body mass index; CSC, central serous chorioretinopathy; IQR, interquartile range; ODI, Oxygen Desaturation Index; OSA, obstructive sleep apnea; SpO2, oxygen saturation; VEGF, vascular endothelial growth factor.
a Calculated as weight in kilograms divided by height in meters squared.
b Defined by American Academy of Sleep Medicine criteria: mild (5 ≤ AHI < 15), moderate (15 ≤ AHI < 30), and severe (AHI ≥ 30).
c Missing minimum nocturnal SpO2 nadir for 1 patient.
d Missing ODI for 10 patients.
Characteristics of Eyes With CSC and Timing of Diagnoses Relative to OCT
Of the 72 eyes included in the study, 22 (30.6%) were diagnosed with both CSC and OSA, 21 (29.2%) were diagnosed with only CSC, and 29 (40.3%) were diagnosed with only OSA. Among the 43 eyes that carried a CSC diagnosis, the majority (67.4%, n = 29) of eyes were observed and not intervened upon. Of the 14 treated eyes, 3 (7.0%) were treated with intravitreal antivascular endothelial growth factor (anti-VEGF) injection, 10 (23.3%) were treated with photodynamic therapy (PDT), and 1 (2.3%) was treated with laser photocoagulation. There was no statistically significant difference observed in the types of CSC treatment administered to eyes with both CSC and OSA or only CSC (P = .875). The median time from CSC diagnosis to OCT was 415 days (IQR, 0-1129.5 days). Median time from CSC treatment with anti-VEGF to OCT was 595 days (IQR, 493.2-650.8 days) and 63 days (IQR, 0-328 days) from PDT to OCT. The 1 patient treated with laser photocoagulation received treatment 40 years prior to the OCT.
CT in Patients With or Without CSC and OSA
We compared the CT of 22 eyes of patients with both CSC and OSA with 21 CSC-only eyes and 29 OSA-only eyes to determine variation in CT within these diagnostic subgroups. All patients with treated OSA had been given a form of PAP therapy. Mean SFCT was significantly different across the 3 groups. Eyes with only OSA had the thinnest SFCT (194.2 ± 59.2 μm) followed by eyes with both CSC and OSA (295.1 ± 90.6 μm), and eyes with only CSC had the thickest SFCT (357.8 ± 117.0 μm) (P < .001). Tukey post hoc analysis showed that the SFCT in eyes of patients with both CSC and OSA was significantly thicker than SFCT in eyes of patients with OSA only (P < .05), but not significantly different from that of CSC-only eyes. Similar significant differences were observed for CT measurements 1500 µm nasally and temporally and in the same pattern. The pattern was less significant at 3000 µm in either direction although maintained a similar trend (Table 2).
Table 2.
Choroidal Thickness of Patients With Only CSC, Only OSA, or Both CSC and OSA.
| Total (N = 72) | CSC with OSA (n = 22) | Only CSC (n = 21) | Only OSA (n = 29) | P value | |
|---|---|---|---|---|---|
| 3000 µm temporal | 257.7 (93.0) | 261.2 (95.5) | 297.9 (93.2)a | 215.3 (77.2)a | .007 |
| 1500 µm temporal | 271.9 (94.1) | 285.2 (80.8)a | 340.7 (93.8)a | 211.9 (62.3)a | <.001 |
| Subfoveal | 272.7 (111.7) | 295.1 (90.6)a | 357.8 (117.0)a | 194.2 (59.2)a | <.001 |
| 1500 µm nasal | 223.3 (102.6) | 243.3 (92.2)a | 300.5 (104.8)a | 152.1 (49.4)a | <.001 |
| 3000 µm nasal | 144.9 (75.7) | 154.3 (78.9)a | 190.8 (84.7)a | 105.5 (39.9)a | <.001 |
Abbreviations: CSC, central serous chorioretinopathy; OSA, obstructive sleep apnea. aTukey post hoc analysis reached a significance of P ≤ .05.
CT Stratified by Treatment Status
When stratified by treatment status, SFCT was similar in CSC eyes with treated OSA compared with those with untreated OSA (291.6 ± 96.6 vs 301.2 ± 85.1 μm, respectively) (Table 3). Multivariable linear regression did not demonstrate significantly different SFCTs for treated or untreated OSA with CSC eyes compared with those with CSC only.
Table 3.
Treatment-Stratified Choroidal Thickness Measurements.
| Total (N = 72) | CSC with treated OSA (n = 14) | CSC with untreated OSA (n = 8) | Only CSC (n = 21) | Only OSA (n = 29) | P value | |
|---|---|---|---|---|---|---|
| 3000 µm temporal, mean (SD) | 257.7 (93.0) | 283.2 (90.8) | 217.1 (95.5) | 297.9 (93.2)a | 215.3 (77.2)a | .006 |
| 1500 µm temporal, mean (SD) | 271.9 (94.1) | 296.0 (83.8)a | 266.4 (76.7) | 340.7 (93.8)a | 211.9 (62.3)a | <.001 |
| Subfoveal, mean (SD) | 272.7 (111.7) | 291.6 (96.6)a | 301.2 (85.1)a | 357.8 (117.0)a | 194.2 (59.2)a | <.001 |
| 1500 µm nasal, mean (SD) | 223.3 (102.6) | 238.9 (107.2)a | 251.1 (63.8)a | 300.5 (104.8)a | 152.1 (49.4)a | <.001 |
| 3000 µm nasal, mean (SD) | 144.9 (75.7) | 160.6 (90.1) | 139.6 (46.2) | 190.8 (84.7)a | 105.5 (39.9)a | .001 |
Abbreviations: CSC, central serous chorioretinopathy; OSA, obstructive sleep apnea.
aTukey post hoc analysis reached a significance of P ≤ .05.
CT Stratified by OSA Severity
When stratified by OSA severity, SFCT was thickest in eyes in patients with CSC and mild OSA (320.2 ± 80.5), followed by eyes of those with severe OSA (284.1 ± 95.1) and then moderate OSA (273.8 ± 103.1) (Table 4). Post hoc analysis comparing eyes of patients with both CSC and OSA by OSA severity (mild, moderate, or severe) did not reach significance across any comparisons. However, we did observe significantly thicker SFCT in patients’ eyes with CSC and mild OSA compared with patients with OSA only (P < .05). Similar patterns were observed at other measurement locations (see Table 4). Univariate linear regression analysis demonstrated a significant difference for eyes with CSC and moderate OSA (β = –84.01, P = .027) compared with CSC-only eyes (see Table 5). Univariate linear regression for the effect of baseline AHI, nocturnal SpO2 nadir and baseline ODI showed no significant associations with SFCT (see Table 5).
Table 4.
OSA Severity–Stratified Choroidal Thickness Measurements.
| Total (N = 72) | CSC with mild OSA (n = 9) | CSC with moderate OSA (n = 8) | CSC with severe OSA (n = 5) | Only CSC (n = 21) | Only OSA (n = 29) | P value | |
|---|---|---|---|---|---|---|---|
| 3000 µm temporal, mean (SD) | 257.7 (93.0) | 251.0 (66.9) | 238.2 (116.5) | 314.3 (96.0) | 297.9 (93.2)a | 215.3 (77.2)a | .015 |
| 1500 µm temporal, mean (SD) | 271.9 (94.1) | 304.9 (64.5)a | 262.6 (84.4) | 286.0 (107.6) | 340.7 (93.8)a | 211.9 (62.3)a | <.001 |
| Subfoveal, mean (SD) | 272.7 (111.7) | 320.2 (80.5)a | 273.8 (103.1) | 284.1 (95.1) | 357.8 (117.0)a | 194.2 (59.2)a | <.001 |
| 1500 µm nasal, mean (SD) | 223.3 (102.6) | 268.7 (77.0)a | 231.7 (115.9) | 216.4 (81.7) | 300.5 (104.8)a | 152.1 (49.4)a | <.001 |
| 3000 µm nasal, mean (SD) | 144.9 (75.7) | 166.0 (46.4) | 156.2 (112.8) | 127.1 (57.6) | 190.8 (84.7)a | 105.5 (39.9)a | .002 |
Abbreviations: CSC, central serous chorioretinopathy; OSA, obstructive sleep apnea.
aTukey post hoc analysis reached a significance of P ≤ .05.
Table 5.
Association of OSA’s Treatment Status and Severity With Subfoveal Thickness.
| Coefficient | SE | P value | |
|---|---|---|---|
| Univariate linear regression of subfoveal thickness among those with only OSA or both CSC and OSA | |||
| Baseline AHI | –0.53 | 0.50 | .296 |
| Minimum nocturnal SpO2 nadir | 2.98 | 1.78 | .099 |
| ODI | –0.51 | 0.55 | .358 |
| Multivariable linear regression of subfoveal thickness by OSA treatmenta | |||
| CSC only | Reference | ||
| CSC and treated OSA | –74.73 | 47.62 | .121 |
| CSC and untreated OSA | –72.06 | 50.96 | .162 |
| OSA only | –163.56 | 25.78 | <.001 |
Abbreviations: AHI, Apnea-Hypopnea Index; CSC, central serous chorioretinopathy; ODI, Oxygen Desaturation Index; OSA, obstructive sleep apnea; SpO2, oxygen saturation,
a Adjusted for OSA severity.
Conclusions
A recent study from our group demonstrated a significant association between CSC and OSA diagnoses. 9 Therefore, we performed a retrospective case review of patients with CSC and OSA who were evaluated with EDI SD-OCT at our academic center, and we sought to elucidate OSA treatment and severity on CT in patients with dual diagnoses of CSC and OSA.
First, we found that eyes of patients with both CSC and OSA had CT that was thinner than those with only CSC and thicker than those with only OSA, but was significantly different from eyes only with OSA only (see Figure 3). Second, patients with CSC and treated or untreated OSA had significantly thicker SFCT than patients with OSA only (see Figure 2), but there was no difference between eyes of patients with CSC and treated OSA compared with those with CSC and untreated OSA (see Tables 3 and 4). Third, we did not find a significant difference in CT of patients based on their diagnostic polysomnogram AHI or ODI, nor in their nocturnal SpO2 nadir—all measures of OSA severity. Even so, the use of these novel biometric features for evaluation of severity of OSA disease is important for future investigations, because these are the variables that sleep medicine specialists use to diagnose and follow OSA; they are more detailed characterizations of disease severity than AHI alone.
Figure 3.
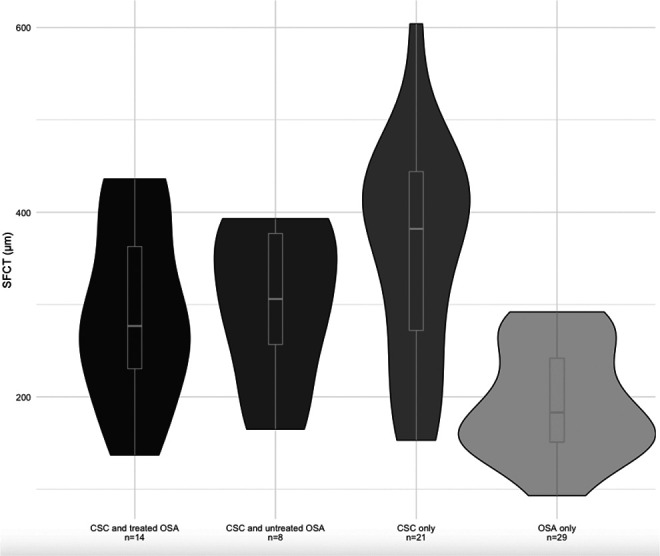
Choroidal thickness of patients with central serous chorioretinopathy (CSC), obstructive sleep apnea (OSA), or both CSC and OSA compared with controls. SFCT indicates subfoveal choroidal thickness.
Our findings of thicker CT in eyes with only CSC and thinner CT in patients with only OSA are consistent with findings from prior studies. 3,15,24 -28 Although the precise mechanisms underlying these changes are unclear, prior hypotheses have posited that systemic vascular congestion and engorgement manifest as thicker CT in eyes with CSC, 29 and that repeated episodes of nocturnal hypoxemia result in microvascular ischemia and atrophy, which leads to thinner CT in patients with OSA. 13,14 Several recent studies have also looked at the effects of both CSC and OSA on CT, as reported in a recent systematic review by Wu et al. 17 The results of these studies indicate that patients with CSC and moderate or severe OSA have significantly thinner CT when compared with healthy controls. In our study sample, eyes of patients with CSC and moderate OSA had a significantly thinner CT compared with CSC-only controls. Although we did not observe a difference among CSC eyes of patients with severe OSA, it is possible our sample was underpowered to detect these differences.
The literature on the effect of OSA treatment on CT changes is sparse and contradictory. 30,31 While a major strength of our study is the use of a strict compliance definition for OSA treatment, we did not find a difference in SFCT among patients with treated and untreated OSA (see Tables 3 and 4). Future studies to reconcile these conflicting results would offer a major contribution to the understanding of eyes in patients with OSA and CSC.
The novel pursuit of our study was to investigate whether SFCT in patients with both CSC and OSA was associated with previously unexplored indicators of sleep-disordered breathing commonly used in the sleep medicine literature, such as AHI, ODI, and nocturnal SpO2 nadir. 32 -34 Based on our analysis of 37 eyes of patients with OSA (22 eyes of patients with both CSC and OSA plus 29 with only OSA), we observed a trend toward significance in the association between nocturnal SpO2 nadir and SFCT (see Table 4). This finding may support the theory that choroidal thinning in OSA is a result of nocturnal hypoxemia; however, more work is needed to definitively confirm or refute this association. We did not find a significant association between AHI and CT or between ODI and CT. Future studies may further explore the utility of these indices given the proposed mechanism that underlies CT changes in CSC and OSA, but our study was underpowered to assess these effects.
As with all retrospective studies, our study has its limitations. While every effort was made to minimize limitations, our study had a relatively small cohort of patients (n = 57). Moreover, all patients were recruited from a single institution, which may limit the generalizability of the study. AHI was used as a measure of OSA severity, but there are data that indicate this may not be the best proxy. 35 Furthermore, we do not have data on the patients' OSA treatment aside from what was documented in the EHR, and important confounders such as OSA duration and choroidal volume were not assessed in this study. Finally, OCTs among the minority of eyes receiving treatment for CSC were predominantly measured posttreatment with intravitreal anti-VEGF agents or PDT, which may have led to greater variation in CT, although the evidence for changes in CT following anti-VEGF or PDT treatment in CSC is not well established. 31,36,37 We aimed to prioritize OCT quality over timing in relation to diagnosis or treatment, but timing is an important consideration for future, prospective studies.
The strengths of our study include the use of an EHR to capture all patients with CSC and OSA who were seen at our academic center over a 25-year period and the use of strict definitions of OSA severity and treatment. Furthermore, to our knowledge, this is the largest study to date that analyzes OCT data for patients with CSC and OSA. As stated earlier, all patients with OSA had their diagnoses confirmed by in-laboratory polysomnography. OSA severity grading was defined by the AASM; OSA treatment was defined by a reduced AHI and by the number of hours of PAP therapy per night. We were also able to attain a high degree of interrater reliability for CT measurements, similar to prior published studies.
In summary, we found that patients with only CSC had thicker CT than patients with CSC and treated or untreated OSA and that patients with OSA only had the thinnest CT at all 5 measurement points. We did not observe any differences between OSA treatment status, OSA severity, ODI, or nocturnal SpO2 nadir among patients with CSC and OSA, but this study may be leveraged for future, prospective studies to directly investigate the relationship of OSA treatment in patients with both CSC and OSA or the screening for OSA in patients with CSC and abnormally thin CT. ODI and nocturnal SpO2 nadir are novel biometric features of OSA disease severity that should be used in future studies. Longitudinal studies are needed to confirm these findings, but our results may suggest a role for OSA evaluation in patients with known CSC who also have atypically thin CT.
Footnotes
Authors’ Note: Amee D. Azad, BA, and Jose R. Davila, MD, contributed equally to this work.
Ethical Approval: This study was conducted in accordance with the Declaration of Helsinki. Ethical approval for this study was obtained from the Stanford Institutional Review Board (IRB-3976).
Statement of Informed Consent: Informed consent was not sought for the present study because all data were deidentified and no protected health information is presented.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Stanford University School of Medicine MedScholars Fund (to A.D.A.), an unrestricted grant from the Research to Prevent Blindness, and the National Eye Institute (grant No. P30-EY026877 to the Department of Ophthalmology; P.M. and C.K.P.).
ORCID iD: Amee D. Azad, BA  https://orcid.org/0000-0002-7690-9435
https://orcid.org/0000-0002-7690-9435
Jose R. Davila, MD  https://orcid.org/0000-0001-6402-8027
https://orcid.org/0000-0001-6402-8027
References
- 1. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(3 suppl):1–139. doi:10.1016/0002-9394(67)90026-8 [PubMed] [Google Scholar]
- 2. Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989;96(6):854–859. doi:10.1016/s0161-6420(89)32810-7 [DOI] [PubMed] [Google Scholar]
- 3. Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013;33(2):302–308. doi:10.1097/IAE.0b013e318263d11f [DOI] [PubMed] [Google Scholar]
- 4. Santos M, Hofmann RJ. Ocular manifestations of obstructive sleep apnea. J Clin Sleep Med. 2017;13(11):1345–1348. doi:10.5664/jcsm.6812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huon LK, Liu SY, Camacho M, Guilleminault C. The association between ophthalmologic diseases and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2016;20(4):1145–1154. doi:10.1007/s11325-016-1358-4 [DOI] [PubMed] [Google Scholar]
- 6. Yavaş GF, Küsbeci T, Kaşikci M, et al. Obstructive sleep apnea in patients with central serous chorioretinopathy. Curr Eye Res. 2014;39(1):88–92. doi:10.3109/02713683.2013.824986 [DOI] [PubMed] [Google Scholar]
- 7. Jain AK, Kaines A, Schwartz S. Bilateral central serous chorioretinopathy resolving rapidly with treatment for obstructive sleep apnea. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):1037–1039. doi:10.1007/s00417-009-1257-5 [DOI] [PubMed] [Google Scholar]
- 8. Carvalho-Recchia CA, Yannuzzi LA, Negrão S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834–1837. [DOI] [PubMed] [Google Scholar]
- 9. Pan CK, Vail D, Bhattacharya J, Cao M, Mruthyunjaya P. The effect of obstructive sleep apnea on absolute risk of central serous chorioretinopathy. Am J Ophthalmol. 2020;218:148–155. doi:10.1016/j.ajo.2020.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115(1):169–173. doi:10.1016/j.ophtha.2007.02.032 [DOI] [PubMed] [Google Scholar]
- 11. Liu B, Deng T, Zhang J. Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis. Retina. 2016;36(1):9–19. doi:10.1097/IAE.0000000000000837 [DOI] [PubMed] [Google Scholar]
- 12. Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Retina. 2012;32(suppl 1):709. doi:10.1097/iae.0b013e31823ff9a6 [DOI] [PubMed] [Google Scholar]
- 13. Karaca EE, Ekici F, Yalçın NG, Çiftçi TU, Özdek Ş. Macular choroidal thickness measurements in patients with obstructive sleep apnea syndrome. Sleep Breath. 2015;19(1):335–341. doi:10.1007/s11325-014-1025-6 [DOI] [PubMed] [Google Scholar]
- 14. Xin C, Wang J, Zhang W, Wang L, Peng X. Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS). Eye (Lond). 2014;28(4):415–421. doi:10.1038/eye.2013.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bayhan HA, Aslan Bayhan S, İntepe YS, Muhafiz E, Gürdal C. Evaluation of the macular choroidal thickness using spectral optical coherence tomography in patients with obstructive sleep apnoea syndrome. Clin Exp Ophthalmol. 2015;43(2):139–144. doi:10.1111/ceo.12384 [DOI] [PubMed] [Google Scholar]
- 16. Kara S, Ozcimen M, Bekci TT, et al. Evaluation of choroidal thickness in patients with obstructive sleep apnea/hypopnea syndrome. Arq Bras Oftalmol. 2014;77(5):280–284. doi:10.5935/0004-2749.20140071 [DOI] [PubMed] [Google Scholar]
- 17. Wu CY, Riangwiwat T, Rattanawong P, Nesmith BLW, Deobhakta A. Association of obstructive sleep apnea with central serous chorioretinopathy and choroidal thickness: a systematic review and meta-analysis. Retina. 2018;38(9):1642–1651. doi:10.1097/IAE.0000000000002117 [DOI] [PubMed] [Google Scholar]
- 18. Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE—an integrated standards-based translational research informatics platform. AMIA Annu Symp Proc. 2009;2009:391–395. [PMC free article] [PubMed] [Google Scholar]
- 19. Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi:10.5664/jcsm.6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi:10.1016/j.smrv.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Entezari M, Karimi S, Ramezani A, Nikkhah H, Fekri Y, Kheiri B. Choroidal thickness in healthy subjects. J Ophthalmic Vis Res. 2018;13(1):39–43. doi:10.4103/jovr.jovr_148_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. Sep 2010;150(3):325–329.e1. doi:10.1016/j.ajo.2010.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hintze JL, Nelson RD. Violin plots: a box plot-density trace synergism. Am Stat. 1998;52(2):181–184. doi:10.1080/00031305.1998.10480559 [Google Scholar]
- 24. Jirarattanasopa P, Ooto S, Tsujikawa A, et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012;119(8):1666–1678. doi:10.1016/j.ophtha.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 25. Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Subfoveal choroidal thickness in central serous chorioretinopathy: a meta-analysis. PLoS One. 2017;12(1):e0169152. doi:10.1371/journal.pone.0169152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leroux les Jardins G, Glacet-Bernard A, Lasry S, Housset B, Coscas G, Soubrane G. Occlusion veineuse retinienne et syndrome d’apnee du sommeil [Retinal vein occlusion and obstructive sleep apnea syndrome]. J Fr Ophtalmol. 2009;32(6):420–424. doi:10.1016/j.jfo.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 27. Karalezli A, Eroglu FC, Kivanc T, Dogan R. Evaluation of choroidal thickness using spectral-domain optical coherence tomography in patients with severe obstructive sleep apnea syndrome: a comparative study. Int J Ophthalmol. 2014;7(6):1030–1034. doi:10.3980/j.issn.2222-3959.2014.06.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He M, Han X, Wu H, Huang W. Choroidal thickness changes in obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Breath. 2016;20(1):369–378. doi:10.1007/s11325-015-1306-8 [DOI] [PubMed] [Google Scholar]
- 29. Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121(1):26–34. doi:10.1016/s0002-9394(14)70531-8 [DOI] [PubMed] [Google Scholar]
- 30. Yuvacı İ, Pangal E, Bayram N, et al. Evaluation of posterior ocular changes using enhanced depth imaging-optical coherence tomography in patients with obstructive sleep apnea syndrome. Arq Bras Oftalmol. 2016;79(4):247–252. doi:10.5935/0004-2749.20160070 [DOI] [PubMed] [Google Scholar]
- 31. Uslu H, Kanra AY, Cetintas G, Tatar MG. Effect of therapy on choroidal thickness in patients with obstructive sleep apnea syndrome. Ophthalmic Surg Lasers Imaging Retina. 2018;49(11):846–851. doi:10.3928/23258160-20181101-05 [DOI] [PubMed] [Google Scholar]
- 32. Lui MM, Lam DC, Ip MS. Significance of endothelial dysfunction in sleep-related breathing disorder. Respirology. 2013;18(1):39–46. doi:10.1111/j.1440-1843.2012.02212.x [DOI] [PubMed] [Google Scholar]
- 33. Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi:10.1378/chest.127.6.2076 [DOI] [PubMed] [Google Scholar]
- 34. Temirbekov D, Güneş S, Yazıcı ZM, Sayın İ. The ignored parameter in the diagnosis of obstructive sleep apnea syndrome: the Oxygen Desaturation Index. Turk Arch Otorhinolaryngol. 2018;56(1):1–6. doi:10.5152/tao.2018.3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weaver EM, Woodson BT, Steward DL. Polysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol Head Neck Surg. 2005;132(2):255–262. doi:10.1016/j.otohns.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 36. Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117(9):1792–1799. doi:10.1016/j.ophtha.2010.01.023 [DOI] [PubMed] [Google Scholar]
- 37. Okamoto M, Matsuura T, Ogata N. Choroidal thickness and choroidal blood flow after intravitreal bevacizumab injection in eyes with central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina. 2015;46(1):25–32. doi:10.3928/23258160-20150101-04 [DOI] [PubMed] [Google Scholar]