Abstract
Purpose:
We report a case of multiple extramedullary plasmacytoma (EMP) of the choroid and conjunctiva as a sign of multiple myeloma (MM) relapse.
Methods:
An observational case report with clinicopathologic correlation is presented.
Results:
A 45-year-old man with a 2.5-year history of MM presented with left eye pain. Examination revealed a conjunctival nodule and choroidal infiltrate in the left eye. Excisional biopsy demonstrated plasmacytoma of the conjunctiva and choroid. Despite radiotherapy and chemotherapy, multiple EMP recurred and the patient died 8 months after initial presentation.
Conclusions:
Multiple EMP of the choroid and conjunctiva has a poor prognosis and can present as a sign of MM relapse. Our imaging demonstrates the clinical manifestations of this tumor and can aid future diagnoses. Our radiation dose (20 Gy) was inadequate, which is an important reportable factor for future patients.
Keywords: choroid, conjunctiva, multiple, myeloma, extramedullary, plasmacytoma
Introduction
Multiple myeloma (MM) is a malignant proliferation of plasma cells that accounts for 10% of all hematopoietic malignant neoplasms. 1 It most commonly causes lytic bone lesions, anemia, azotemia, hypercalcemia, and recurrent infections. 2 About 7% to 18% of patients with MM may present with extramedullary plasmacytoma (EMP). 1 Rarely, they can occur in the choroid, ciliary body, iris, eyelid, or orbit. 3,4 Very few cases have been reported in the literature, making management particularly challenging. Here, we report a case of multiple EMP of the choroid and conjunctiva as a sign of recurrence in MM.
Methods
Case Description
A 45-year-old man presented with acute pain in the left eye in January 2017. He had a 2.5-year history of stage III (Durie-Salmon) immunoglobulin G lambda MM. Genetic studies revealed translocation of chromosomes 4 and 14 [t(4:14)] and monosomy 13. The patient was previously treated with chemotherapy and autologous stem cell transplantation (ASCT) but never achieved full remission.
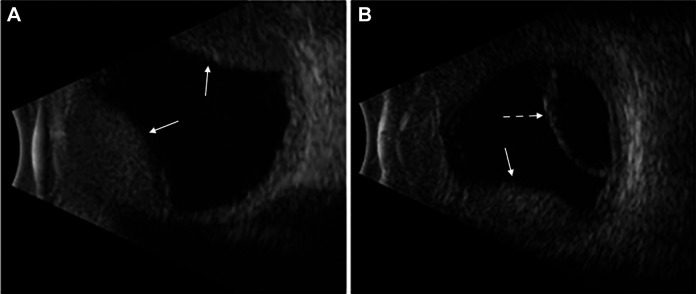
On examination, his visual acuity in the left eye was counting fingers at 4 feet (1.2 m), and intraocular pressure was 23 mm Hg. The anterior segment demonstrated a pink, vascularized superior bulbar conjunctival nodule with engorged scleral vessels and a shallow anterior chamber. The posterior segment demonstrated a 360-degree choroidal detachment or infiltration with a secondary exudative retinal detachment. B-scan ocular echography showed a high internally reflective nonserous choroidal detachment vs diffuse extensive choroidal infiltration sparing the macula and associated exudative retinal detachment inferiorly (Figure 1). The patient was treated with topical steroid, cycoplegic, and antiglaucoma treatment along with sub-Tenon triamcinolone injection. The patient’s ocular finding deteriorated within 1 week. The conjunctival lesion increased in size (Figure 2), as did as the choroidal infiltrate (Figure 3).
Figure 1.
B-scan ocular ecography, left eye. (A) High internally reflective nonserous choroidal detachment. (B) Exudative retinal detachment inferiorly. Straight arrows are directed toward choroidal detachments. Dashed arrow is directed toward retinal detachment.
Figure 2.
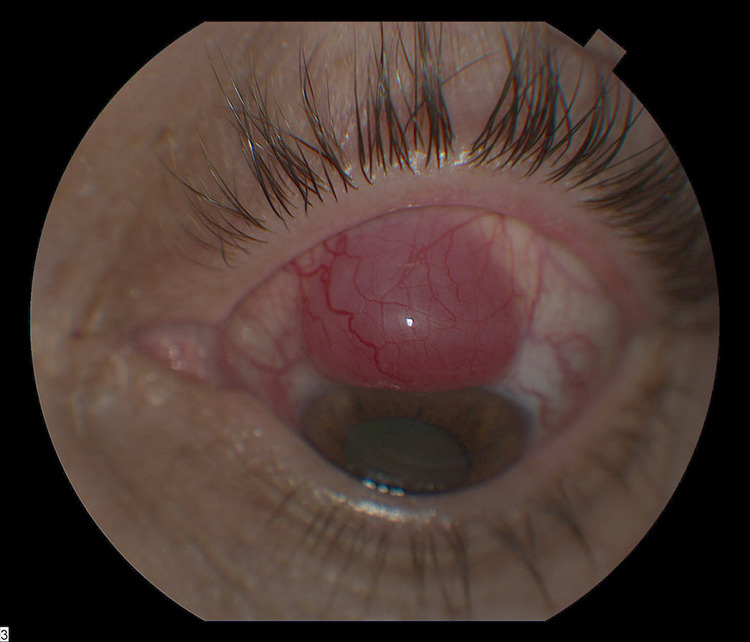
Superior bulbar conjunctival lesion with engorged scleral vessels increased in size.
Figure 3.
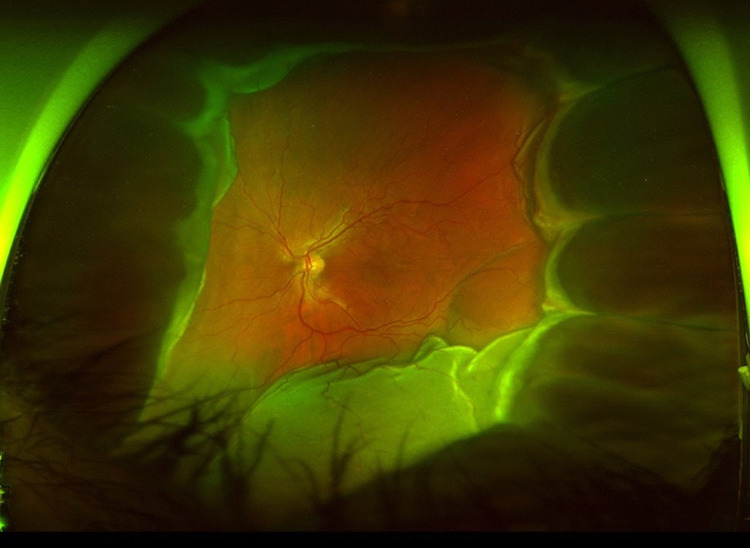
The 360-degree choroidal infiltration increased in size.
The patient also developed a solid, erythematous subcutaneous nodule on the right forearm. Magnetic resonance imaging of the head demonstrated lytic lesions in the calvarium. Excisional biopsy of the conjunctival lesion and fine-needle aspiration biopsy of the choroidal infiltrate demonstrated plasma cells containing an eccentric nucleus, prominent nucleoli, and cytoplasmic perinuclear hof (Figure 4). The findings were consistent with a plasma cell neoplasm. Similarly, the right forearm nodule was found to be a plasmacytoma.
Figure 4.
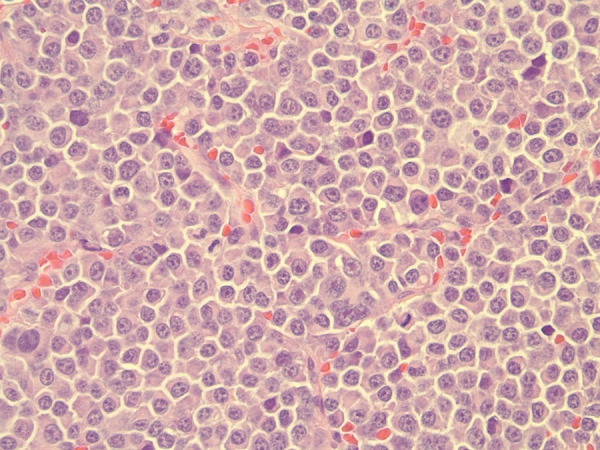
Plasma cells containing an eccentric nucleus, prominent nucleoli, and cytoplasmic perinuclear hof.
External beam radiation therapy (EBRT) was performed at the end of January 2017 with a dose of 20 Gy in 10 fractions to the left eye and a dose of 30 Gy in 10 fractions to the right arm lesion. The choroidal infiltrate regressed significantly (Figure 5). Following initial EBRT, whole-body positron emission tomography–computed tomography (PET-CT) with intravenous (IV) contrast material demonstrated a large hypermetabolic mass in the soft tissue of the right arm. After additional EBRT of the right arm and 2 cycles of VDT-PACE (bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide) chemotherapy, a second whole-body PET-CT with IV contrast material demonstrated new soft-tissue lesions in the right medial arm, left abdominal wall, right calf, and perinephric fat. In addition, there was progression of osseous lesions in the right pelvis and humerus and less extensive but more intensive hypermetabolism involving the left globe.
Figure 5.
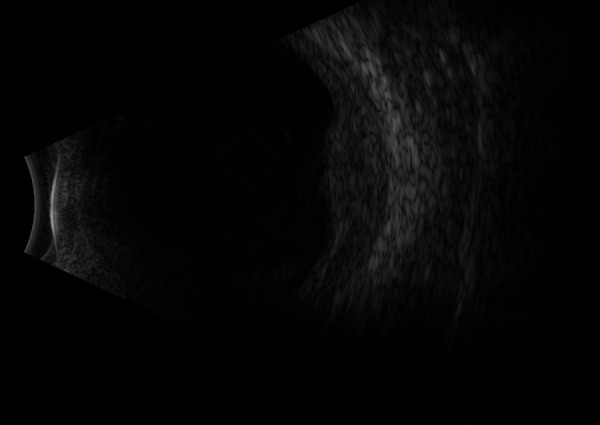
Residual choroidal infiltration nasally after radiation therapy. Subretinal fluid shifts with head positioning and graphic findings consistent with retinal detachment.
By March 2017, a cataract developed precluding any view of the posterior segment. Uneventful cataract surgery was performed later that month. In April 2017, visual acuity in the left eye deteriorated to light perception owing to recurrence of the choroidal plasmacytoma. This prompted treatment with intraocular methotrexate combined with systemic bortezomib and deratumumab. After 2 weeks of treatment, the choroidal infiltrate worsened. Given the extent of choroidal infiltrates, the methotrexate injection could not be safely administered. Moreover, it was clear that the plasmacytoma was not responding to methotrexate. In June, all treatment was halted because of disease progression. The patient died in August 2017.
Results
MM is a malignant proliferation of plasma cells. 2 Diagnosis requires 10% or more monoclonal plasma cells in the bone marrow or extramedullary plasmacytoma with 1 or more myeloma-defining event. 5 Myeloma-defining events include evidence of end organ damage attributable to the underlying plasma cell disorder (eg, hypercalcemia, renal insufficiency, anemia, or bone lesions) or the presence of specific malignancy biomarkers (eg, bone marrow plasma cell percentage ≥ 60%, involved: uninvolved serum free light chain ratio ≥ 100, or > 1 focal lesion on magnetic resonance imaging). 5 Patients with MM have a variable prognosis, with median overall survival ranging from 7 to 10 years. 6 Current treatment involves front-line chemotherapy, followed by consolidative high-dose melphalan and ASCT, then by maintenance therapy. 1
Extramedullary myeloma (EMM) is broadly defined as the presence of plasma cells outside the bone marrow in a patient with MM. 7 This includes EMP, which can arise from focal disruption of cortical bone, hematogenous spread, invasive surgical procedures, or bone fractures. 8 Bone-related plasmacytomas most commonly affect the axial skeleton, whereas hematogenously spread plasmacytomas most commonly affect the skin, liver, breast, kidney, pleura, lymph nodes, and central nervous sytem. 9 Recent studies recommend excluding bone-related plasmacytomas and plasma cell leukemia from the clinical spectrum of EMM due to their distinct histologic and clinical features. 7 Of note, solitary plasmacytoma (SP) is a separate clinic entity defined as a clonal plasma cell proliferation affecting the bone or soft tissue in the absence of MM. 7 It demonstrates markedly different biologic characteristics and an improved response to radiation when compared with EMM arising from preexisting or coexisting bone marrow disease. 10 However, about 10% of patients with SP will develop MM within 3 years. 5 Based on these criteria, we defined our patient’s conjunctival and choroidal infiltrates as multiple extramedullary plasmacytomas.
EMM is found in 7% to 18% of patients at the time of MM diagnosis and up to 20% at relapse. 8 EMM at the time of MM diagnosis has a 31% overall survival rate at 5 years, whereas EMM presenting at relapse has an overall survival as low as 5 months. 11,12 Interestingly, EMM has been associated with 3 variables that are poor prognostic indicators for MM: elevated lactate dehydrogenase, t(4;14) or deletion of chromosome 17p [del(17p)], and having the characteristics of International Staging System 3. 7,13 Our patient possessed 2 of these indicators: elevated lactate dehydrogenase and t(4;14).
There is little information about the treatment of EMM. In patients with de novo EMM who are eligible for stem cell transplantations, it has recently been proposed to conduct triplet induction therapy followed by high-dose melphalan and/or ASCT, a triplet consolidation therapy, and a maintenance treatment, consisting of at least lenallidomide. 7 In addition, involved-site radiotherapy of at least 45 Gy using conventional fractionation schedules should be considered for EMP. 14 Further investigation of the treatment of EMM at relapse is needed.
MM produces ocular pathology through a variety of mechanisms, many of which are mediated by increased levels of immunoglobulins in the serum. 15 Most commonly, it produces hemodynamic changes. 15 This includes retinal vein dilation and tortuosity due to hyperviscosity, intraretinal hemorrhage, arteriovenous crossing changes, and retinal vein occlusions. 15 MM can also produce ciliary body cysts that contain eosinophilic material that represent gamma globulin and other proteins. 15 MM can also damage the cranial nerves (CNs), including CN II, CN III, CN IV, and CN VI, via direct infiltration or compression by an adjacent tumor or associated extramedullary hematopoiesis in the epidural space. 15 Because patients with MM have an increased risk of infection, some lose their vision from endogenous endophthalmitis. 15 MM may also result in deposition of myeloma protein crystals within the cornea and conjunctiva. 15 Direct infiltration of the ocular tissues is the rarest mechanism of ocular disease. 15 Nonetheless, ocular infiltration may be the first clinical manifestation of MM or the first sign of disease recurrence. 15
Choroidal manifestations of MM are exceedingly rare. 3 An extensive literature search found 5 reported cases with variable presentations. Palamar and colleagues described a case that presented most similarly to the patient described here. 16 That patient also had a 2-year history of MM with a unilateral EMP of the choroid. However, in that patient, the choroidal infiltrate regressed after 3500 Gy EBRT. Brockmann and colleagues reported a patient who presented with bilateral EMP of the choroid as the first sign of recurrence of MM. 17 That patient was treated with IV injections of bevacizumab, which caused regression in the right eye but not the left after 2 months. Morgan and colleagues presented a case of a unilateral EMP of the choroid that was the first manifestation of MM. 18 It was treated with a 12-mm radioactive iodine 125 plaque, with 80.29 Gy delivered to the apex and 204.59 Gy delivered to the base of the tumor. There was a rapid, complete response in the eye. However, multiple subcutaneous nodules and lytic bone lesions were later discovered.
There are 2 additional reports of unilateral choroidal SP. The first, reported by Honavar et al, was a plasmacytoma confined to the choroid that was treated with 35 Gy. 19 There was no recurrence up to 9 years of follow-up. The second, reported by Coupland et al, was a ciliochoroidal melanoma with coexisting plasmacytoma, known as a “collision” tumor. 20 That patient had previously been treated with radiotherapy; however, the dose and response were not documented.
Our patient’s conjunctival nodule may either represent an extrascleral nodule emanating from the choroidal plasmacytoma or an entirely separate plasmacytoma. Intraoperative findings did not see an obvious extension from the choroid; however, pathology of the entire eye was not performed postmortem. There are few documented cases of conjunctival plasmacytomas. In a retrospective series of 5002 conjunctival tumors, only 4 were plasmacytomas. 21 In an international multicenter, retrospective study of 263 conjunctival tumors, only 5 were plasmacytomas. 22 All 5 cases were primary disease treated with EBRT with or without chemotherapy. Overall survival at 10 years was 0%. Jiang et al reported a case of SP of the conjunctiva. 23 The lesion was resected and the patient received a dose of 40 Gy in 10 administrations. There was no recurrence at 12 months. Bradley and colleagues documented a case of extramedullary epibulbar plasmacytoma in a patient with MM. 24 Treatment and response were not reported.
Conclusions
In summary, multiple EMP of the choroid and conjunctiva are rare tumors with a poor prognosis that can present as a sign of recurrence of MM. More than half the reported choroidal plasmacytomas were EMP, all but 2 were unilateral, and most responded well to radiotherapy. Of the 11 previously reported conjunctival plasmacytomas, only 1 was an EMP. To the best of our knowledge, this is the first reported multiple EMP of the choroid and conjunctiva. The imaging presented here illustrates clinical manifestations of this rare disease that can aid in the diagnosis of future cases. Although the choroidal infiltrate in our patient rapidly regressed following radiotherapy, disease recurrence occurred within 4 months. Our prescribed dose of radiation (20 Gy) was inadequate for our patient and is an important reportable factor for future patients.
Footnotes
Ethical Approval: This observational case report was conducted in accordance with HIPAA (the Health Insurance Portability and Accountability Act) and the tenets of the Declaration of Helsinki.
Statement of Informed Consent: Informed consent was provided by the patient.
Thomas Aaberg Jr, MD, is a consultant for Alcon, Bausch + Lomb, and Castle Pharmaceuticals. The other authors have nothing to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This case report is funded by the Foundation for Vision Research.
References
- 1. Rajkumar SV, Dispenzieri A. Multiple myeloma and related disorders. In: Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE, eds. Abeloff’s Clinical Oncology, 5th ed. Philadelphia, PA: Elsevier; 2014. [Google Scholar]
- 2. Lee HC, Patel K, Kongtim P, et al. Multiple myeloma and other plasma cell dyscrasias. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 3rd ed. New York, NY: McGraw-Hill; 2016. [Google Scholar]
- 3. Shields JA, Shields CL. Conjunctival lymphoid, leukemic, and metastatic tumors. In: McGowan K, Moyer E, eds. Eyelid, Conjunctival, and Orbital Tumors and Intraocular Tumors: An Atlas and Text. 2nd ed. Philadelphia, PA: Wolters Kluwer Health; 2016. [Google Scholar]
- 4. Shields JA, Shields CL. Intraocular lymphoid tumors and leukemias. In: McGowan K, Moyer E, eds. Intraocular Tumors: An Atlas and Textbook. 3rd ed. Philadelphia, PA: Wolters Kluwer Health; 2016. [Google Scholar]
- 5. Rajkumar SV, Dimopouls MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi:10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 6. DeVita V, Lawrence T, Rosenberg S. Plasma cell neoplasms. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Devita, Hellman, and Rosenberg’s Principles & Practice of Oncology. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 7. Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127(8):971–976. doi:10.1182/blood-2015-07-635383 [DOI] [PubMed] [Google Scholar]
- 8. Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29(28):3805–3812. doi:10.1200/JCO.2011.34.9290 [DOI] [PubMed] [Google Scholar]
- 9. Gertz MA, Rosinol L, Bladé J. Plasma cell leukemia and extramedullary plasmacytoma. In: Dimopoulous M, Facon T, Terpos E, eds. Multiple Myeloma and Other Plasma Cell Neoplasms. Cham, Switzerland: Springer; 2018:157–175. doi:10.1007/978-3-319-25586-6_9 [Google Scholar]
- 10. Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leuk Lymphoma. 2013;54(6):1135–1141. doi:10.3109/10428194.2012.740562 [DOI] [PubMed] [Google Scholar]
- 11. Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease in the era of novel agents. Haematologica. 2012;97(11):1761–1767. doi:10.3324/haematol.2012.065698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pour L, Sevcikova S, Greslikova H, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99(2):360–364. doi:10.3324/haematol.2013.094409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreau P, Cavo M, Sonneveld P, et al. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol. 2014;32(20):2173–2180. doi:10.1200/JCO.2013.53.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen G, Wang W, Zhang Y, Niu S, Li Q, Li Y. Management of extramedullary plasmacytoma: role of radiotherapy and prognostic factor analysis in 55 patients. Chin J Cancer Res. 2017;29(5):438–446. doi:10.21147/j.issn.1000-9604.2017.05.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agarwal MR, Arnold AC, Balcer LJ, et al. Multiple myeloma, plasmacytomas, and related disorders (monoclonal gammopathies). In: Miller NR, Newman NJ, eds. Walsh and Hoyt’s Clinical Neuro-Ophthalmology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 16. Palamar M, Shields CL, Ghassemi F, Ehya H, Shields JA. Choroidal plasmacytoma in a patient with multiple myeloma. Diagnosis by fine-needle aspiration biopsy. Graefes Arch Clin Exp Ophthalmol. 2008;246(8):1195–1197. doi:10.1007/s00417-008-0804-9 [DOI] [PubMed] [Google Scholar]
- 17. Brockmann C, Ingold Heppner B, Joussen AM. Bilateral choroidal lesions as first sign of recurrence in multiple myeloma—histopathological findings and treatment in response to bevacizumab. Acta Ophthalmol. 2014;95(2):e155–e157. doi:10.1111/aos.12928 [DOI] [PubMed] [Google Scholar]
- 18. Morgan AE, Shields JA, Shields CL, Piccone MR, Harrison SA. Presumed malignant plasmacytoma of the choroid as the first manifestation of multiple myeloma. Retina. 2003;23(6):867–868. doi:10.1097/00006982-200312000-00021 [DOI] [PubMed] [Google Scholar]
- 19. Honavar SG, Shields JA, Shields CL, Demirci H, Ehya H. Extramedullary plasmacytoma confined to the choroid. Am J Ophthalmol. 2001;131(2):277–278. doi:10.1016/S0002-9394(00)00706-6 [DOI] [PubMed] [Google Scholar]
- 20. Coupland SE, Dodson A, Hongxiang L, Ming-Qing D, Angi M, Damato BE. Intraocular collision tumor: case report and literature review. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1383–1388. doi:10.1007/s00417-012-2216-0 [DOI] [PubMed] [Google Scholar]
- 21. Shields CL, Alset AE, Boal NS, et al. Conjunctival tumors in 5002 cases. Comparative analysis of benign versus malignant counterparts. The 2016 James D. Allen Lecture. Am J Ophthalmol. 2017;173:106–133. doi:10.1016/j.ajo.2016.09.034 [DOI] [PubMed] [Google Scholar]
- 22. Kirkegaard MM, Rasmussen PK, Coupland SE, et al. Conjunctival lymphoma—an international multicenter retrospective study. JAMA Ophthalmol. 2016;134(4):406–414. doi:10.1001/jamaophthalmol.2015.6122 [DOI] [PubMed] [Google Scholar]
- 23. Jiang J, Liu Y, Wang F, Huang Y. An unusual occurrence of solitary extramedullary plasmacytoma in the conjunctiva. Oncol Lett. 2012;4(2):245–246. doi:10.3892/ol.2012.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradley A, Estes A, Ulrich L, Dilip T, Gay D. Epibulbar plasmacytoma masquerading as subconjunctival hemorrhage in a patient with multiple myeloma. Cornea. 2017;36(2):249–251. doi:10.1097/ICO.0000000000001071 [DOI] [PubMed] [Google Scholar]