Abstract
Background:
Type 2 diabetes mellitus (T2DM) is associated with higher risk for heart failure (HF). The impact of a lifestyle intervention and changes in cardiorespiratory fitness (CRF), and body mass index (BMI) on risk for HF is not well-established.
Methods:
Participants from the Look AHEAD (Action for Health in Diabetes) trial without prevalent HF were included. Time to event analyses were used to compare the risk of incident HF between the intensive lifestyle intervention (ILI) vs. diabetes support and education (DSE) groups. The associations of baseline measures of CRF estimated from a maximal treadmill test, BMI, and longitudinal changes in these parameters with risk of HF were evaluated using multivariable adjusted Cox models.
Results:
Among the 5,109 trial participants, there was no significant difference in the risk of incident HF (n = 257) between the ILI vs. DSE groups [HR (95% CI) = 0.96 (0.75 to 1.23)] over a median follow-up of 12.4 years. In the most adjusted Cox models, the risk of HF was 39% and 62% lower among moderate fit [Tertile 2: HR (95% CI) = 0.61 (0.44 to 0.83)] and high fit [Tertile 3: HR (95% CI) = 0.38 (0.24 to 0.59)] groups, respectively (referent group: low fit, Tertile 1). Among HF subtypes, after adjustment for traditional CV risk factors and interval incidence of MI, baseline CRF was not significantly associated with risk of incident HFrEF. In contrast, the risk of incident HFpEF was 40% lower in moderate fit and 77% lower in the high fit groups. Baseline BMI was also not associated with risk of incident HF, HFpEF, or HFrEF after adjustment for CRF and traditional CV risk factors. Among participants with repeat CRF assessments (n = 3,902), improvements in CRF and weight loss over 4-year follow-up was significantly associated with lower risk of HF [HR (95% CI) per 10% increase in CRF = 0.90 (0.82 to 0.99), per 10% decrease in BMI = 0.80 (0.69 to 0.94)].
Conclusions:
Among participants with T2DM in the Look AHEAD trial, the ILI did not appear to modify the risk of HF. Higher baseline CRF and sustained improvements in CRF and weight loss were associated with lower risk of HF.
Keywords: heart failure, risk, type 2 diabetes mellitus, overweight, obesity, body mass index, cardiorespiratory fitness
INTRODUCTION
Among adults with type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD) is the leading cause of death and heart failure (HF) accounts for 14% of the initial presentations of CVD 1–6. In contrast to risk for myocardial infarction, optimal control of traditional cardiovascular (CV) risk factors such as blood pressure (BP), cholesterol, glycated hemoglobin (HbA1c), albuminuria, and smoking has not been proven to mitigate the risk of hospitalization for HF in T2DM 7. These findings suggest that novel approaches, beyond targeting and managing traditional CV risk factors, are needed for prevention of HF among patients with T2DM.
Low cardiorespiratory fitness (CRF) and obesity are important risk factors for HF. Prior studies have demonstrated a consistent, graded association between lower CRF, higher body mass index (BMI), and increased risk of HF in the general population 8–12. However, the independent associations of CRF and obesity with the risk of incident HF among those with T2DM, who have a higher burden of traditional CV risk factors and are at a higher baseline risk, are not well characterized. Furthermore, it is not known if lifestyle interventions and improvements in CRF and weight loss may modify risk of HF among patients with T2DM.
The Look AHEAD (Action for Health in Diabetes) randomized trial evaluated whether an intensive lifestyle intervention (ILI) would affect the risk of atherosclerotic cardiovascular disease (ASCVD) outcomes among patients with T2DM who are overweight or obese compared with diabetes support and education (DSE) and demonstrated no significant effect on the risk of ASCVD events with ILI 13, 14. Of note, hospitalizations for new onset or worsened HF were adjudicated as part of a secondary composite outcome in the Look AHEAD trial. However, the original trial did not adjudicate events as HF with preserved ejection fraction (HFpEF) or HF with reduced ejection fraction (HFrEF). Accordingly, in this study, we re-adjudicated incident HF events into its subtypes, HFpEF and HFrEF, and extended the follow-up through December 2015 to evaluate the association of ILI with risk of incident HF and its subtypes15. We hypothesize that ILI (vs. DSE) would be associated with a lower risk of HF, particularly HFpEF, among participants of the Look AHEAD trial. We also evaluated the associations of baseline and longitudinal changes in CRF and BMI with risk of incident HF and its subtypes among Look AHEAD participants.
METHODS
The data and materials from the present study will not be made available by the authors for the purpose of reproducing the results.
Look AHEAD trial design and population
Look AHEAD trial design has been reported previously and the primary results were originally published in 2013 13, 14. From 2001 to 2004, the Look AHEAD trial enrolled overweight and obese adults (BMI ≥25 kg/m2 or ≥27 kg/m2 if taking insulin), aged 45 to 76 years, with T2DM (n = 5,145) who could complete a maximal exercise test and evaluated whether an ILI focused on weight loss would affect the risk for CV events compared with DSE. T2DM status was defined according to physician report, prevalent use of antihyperglycemic medication, or measured plasma glucose level. Participants who did not have a history of HF prior to enrollment and had available data on CRF and BMI at baseline were included in the present study (n = 5,109) (Supplemental Figure 1). Individuals unable to complete a maximal exercise test at baseline were excluded. The associations between change in CRF and BMI from baseline to 1- and 4-year follow-up with risk of incident HF were assessed among Look AHEAD participants who were free of HF at the time of the follow-up assessment and had follow-up CRF and BMI data (n = 4,380 and 3,902 for 1- and 4-year follow-up, respectively). The institutional review board at each participating site approved the study protocol. All participants provided written informed consent.
Treatment groups
Look AHEAD participants were randomly assigned to either an ILI or DSE group. As previously described, the ILI focused on achieving and maintaining at least 7% weight loss through group and individual counseling sessions (weekly for the initial 6 months followed by less frequent meetings), diet prescriptions, and encouragement to achieve physical activity goals 13, 14. Participants in the ILI arm of the trial were prescribed a restricted caloric diet (1200 to 1800 kcal/day) and encouraged to achieve ≥ 175 minutes/week of moderate-intensity physical activity. Participants randomized to the DSE group received three educational group sessions per year during the first 4 years followed by an annual meeting focused on diet, exercise, and social support. Both the ILI and DSE interventions were stopped after a median follow-up of 9.6 years in September 2012.
Exposure variables of interest
Cardiorespiratory fitness:
Prior to randomization, all eligible participants underwent a symptom-limited graded maximal treadmill exercise test. The details of the exercise test protocol have been published previously and are described in further detail in the Supplemental Methods 16–18. Briefly, trial participants performed a treadmill-based exercise stress test at a constant speed while grade was incrementally increased. Heart rate and rating of perceived exertion (RPE) were assessed throughout the trial. Exercise testing was terminated according to either standard stopping criteria or voluntary exhaustion. The American College of Sports Medicine metabolic equation for estimating peak oxygen consumption was used to estimate CRF 19. Participants performed subsequent submaximal exercise treadmill tests at years 1 and 4. Changes in CRF at 1- and 4-years were calculated as the difference between estimated peak metabolic equivalents (METs) at baseline and the corresponding follow-up assessment as previously described 17, 20.
Body mass index:
Research personnel blinded to participant group assignment measured baseline body weight and height in duplicate with a digital scale and stadiometer, respectively. Weight was evaluated annually during follow-up. BMI was calculated using the standard formula: [weight (kg)] / [height (meters)]2.
Outcome of interest
The primary outcomes of interest were incidence of overall HF, HFpEF, and HFrEF. The original trial excluded individuals with New York Heart Association class III or IV HF. In the present study, we excluded Look AHEAD participants with any HF at baseline, and, therefore, adjudicated incident HF. As part of a Look AHEAD HF ancillary study, follow-up was extended with a median follow-up of 12.4 years and we further adjudicated incident HF hospitalizations into HFpEF and HFrEF using a previously validated approach to analyze longer-term HF outcomes and evaluate HF subtypes 15. HF cases were first identified based on self-report and available ICD-9 codes from hospitalization records of participants on follow-up. Two physicians masked to trial-group assignment adjudicated HF hospitalizations. After clinical data (history, physical examination, test results, and medications) were reviewed, each case was classified into one of the following groups: definite or possible acute decompensated HF, chronic stable HF, HF unlikely, or unclassifiable. Incident HF was defined as definite or possible acute decompensated HF and only the first HF hospitalization was adjudicated. HF subtype (HFpEF and HFrEF) was based on left ventricular ejection fraction (LVEF) identified on echocardiography or cardiac ventriculography (catheterization or radionuclide) measured at the time of the incident HF hospitalization. HFpEF and HFrEF were defined as LVEF ≥50% or <50%, respectively.
Statistical analysis
The incidence of HF outcomes across the two randomized trial arms (ILI and DSE) were compared using cumulative incidence plots and log-rank tests. The risk of incident overall HF and its subtypes, HFpEF and HFrEF, associated with ILI (vs. DSE, referent group) were evaluated using Cox proportional hazard models.
Participants from both trial arms were pooled to evaluate the associations of baseline CRF and BMI with risk of incident HF. Baseline characteristics of trial participants were compared across tertiles of CRF and BMI using Jonckheere-Terpstra test for continuous variables and Cochran-Armitage test for categorical variables. The associations between categorical and continuous measures of baseline CRF, BMI, and risk of HF were assessed using multivariable-adjusted Cox proportional hazards models. Separate models were constructed for each outcome (overall HF, HFpEF, and HFpEF) with inclusion of the following covariates: Model 1 included demographic characteristics (age, sex, ethnicity, education level, income), treatment group, and the exposure variable of interest (CRF or BMI in separate models); Model 2 included variables in model 1 plus traditional CV risk factors (history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, glomerular filtration rate), and both exposure variables of interest (CRF and BMI in the same model); Model 3 included variables in model 2 and interval myocardial infarction (MI) on follow-up as a time-updated covariate. Mortality and the other HF subtype (for HFrEF and HFpEF models) were treated as censoring events.
The associations between changes in CRF and BMI over short-term (1-year) and intermediate-term (4-year) follow-up and risk of incident HF were assessed in a subset of participants with available repeated measures of CRF and BMI who were free of HF at the time of repeat assessment. Baseline and follow-up characteristics of these participants were compared across categories of change in CRF and BMI over the specified follow-up period. Change in CRF from baseline to 1- or 4- year follow-up was categorized according to tertiles of change in METs over the specified follow-up period. For BMI change, previously described categories of change in BMI were used: gain (>2% gain), stable (≤2% gain to <5% loss), medium loss (≥5% loss to <10% loss), large loss (≥10% loss) 20. Multivariable adjusted Cox models were constructed to evaluate the associations between longitudinal changes in CRF, BMI, and risk of incident overall HF, HFpEF, and HFrEF with sequential adjustment for covariates: Model 1 included baseline demographic characteristics (age, sex, ethnicity, education level, income), treatment group, baseline CV risk factors (history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, glomerular filtration rate), baseline CRF, baseline BMI, and the exposure variable of interest (change in CRF or BMI in separate models); Model 2 included the same covariates as Model 1 with both changes in CRF and BMI in the same model; Model 3 included variables in Model 2 plus changes in HbA1c and systolic BP from baseline to the year of follow-up (year 1 or 4). Interaction tests were performed to determine if the association between the study intervention and risk of HF were modified by baseline levels of CRF and BMI. Additional interaction tests were performed to evaluate if race (white vs. non-white) modified the associations of the study intervention, baseline CRF, and BMI with the risk of HF.
RESULTS
Intensive lifestyle intervention & risk of incident HF
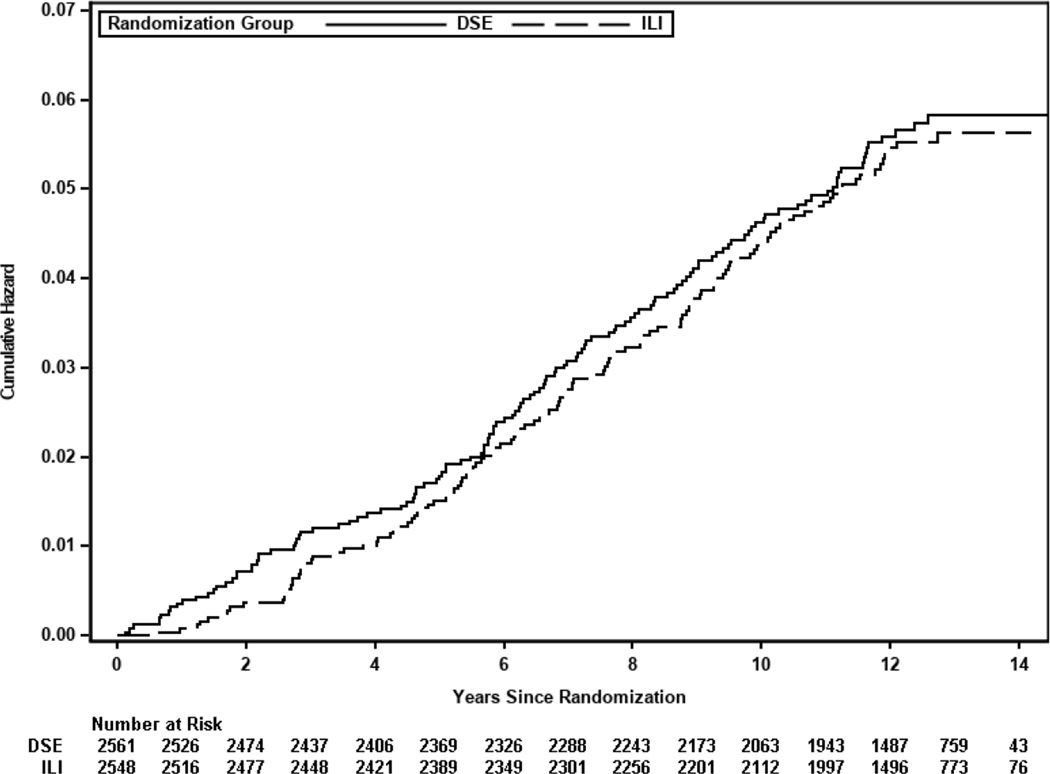
The present study included 5,109 participants from the Look AHEAD trial who were randomized to ILI vs. DSE. Over a median follow-up of 12.4 years (58,094 person-years), 257 incident HF events occurred [event rate per 1,000 person years (PY): 4.42], of which 50.2% (n = 129) were HFpEF (event rate per 1,000 PY: 2.23), 40.5% (n = 104) were HFrEF (event rate per 1,000 PY: 1.79), and 9.3% (n = 24) were HF with missing LVEF. There was no significant difference in the risk of incident HF between the ILI vs. DSE groups [HR (95% CI) = 0.96 (0.75 to 1.23)] (Figure 1). The risk of incident HF subtypes, HFpEF and HFrEF, were also not significantly different between the two randomized trial arms (Supplemental Table 1). The association between study intervention (ILI vs. DSE) and risk of HF, HFpEF, and HFrEF was not different among white vs. non-white participants (study intervention * race for risk of HF p-interaction = 0.38). Furthermore, the association between ILI and risk of HF was not modified by baseline CRF or BMI levels (study intervention * CRF p-interaction >0.5; study intervention * BMI p-interaction >0.5).
Figure 1. Cumulative incidence plot of overall incident HF risk according to treatment group.
Abbreviations: DSE = diabetes support and education; HF = heart failure; ILI = intensive lifestyle
Baseline CRF & risk of incident HF
The ILI and DSE groups were pooled together to study the association of baseline and changes in CRF and BMI with risk of incident HF. Participants with higher CRF levels were younger, more commonly men, more likely white, and had lower burden of traditional CV risk factors and prevalent CVD (Table 1). Mean diastolic BP was higher among participants with higher CRF although within normal range and low-density lipoprotein cholesterol was similar across CRF groups.
Table 1.
Baseline and follow-up characteristics stratified by baseline BMI and CRF tertiles
| Cardiorespiratory fitness | Body mass index | |||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 3.3–6.1 METs (n = 1,694) | Tertile 2 6.2–7.8 METs (n = 1,786) | Tertile 3 7.9–16.7 METs (n = 1,629) | Tertile 1 24.53–32.60 kg/m2 (n = 1,703) | Tertile 2 32.61–37.77 kg/m2 (n = 1,703) | Tertile 3 37.78–63.53 kg/m2 (n = 1,703) | P value for CRF tertiles | P value for BMI tertiles | |
| Baseline variables | ||||||||
| Estimated CRF, METs | 5.2 (0.6) | 7.0 (0.5) | 9.5 (1.4) | 8.1 (2.2) | 7.3 (1.8) | 6.2 (1.5) | <0.001 | <0.001 |
| BMI, kg/m2 | 38.8 (6.7) | 36.0 (5.1) | 32.9 (4.0) | 30.1 (1.8) | 35.0 (1.5) | 42.7 (4.2) | <0.001 | <0.001 |
| Age, years | 60.8 (6.9) | 58.3 (6.6) | 57.1 (6.4) | 60.2 (6.9) | 58.8 (6.8) | 57.2 (6.5) | <0.001 | <0.001 |
| Female, % | 75.7 | 61.8 | 40.5 | 54.6 | 58.0 | 66.2 | <0.001 | <0.001 |
| White, % | 60.7 | 62.7 | 66.6 | 62.2 | 63.0 | 64.7 | <0.001 | <0.001 |
| Education, % | <0.001 | <0.001 | ||||||
| <13 years | 25.1 | 19.9 | 14.1 | 19.4 | 21.4 | 18.6 | ||
| 13–16 years | 41.2 | 37.8 | 32.7 | 33.9 | 36.5 | 41.5 | ||
| >16 years | 31.5 | 39.8 | 51.1 | 44.3 | 39.6 | 38.2 | ||
| Missing | 2.2 | 2.5 | 2.0 | 2.4 | 2.5 | 1.8 | ||
| Income, % | <0.001 | 0.007 | ||||||
| <$20,000 | 15.6 | 11.7 | 6.8 | 11.7 | 11.2 | 11.2 | ||
| $20,000 to $39,999 | 26.6 | 18.3 | 12.4 | 18.0 | 19.8 | 19.7 | ||
| $40,000 to $59,999 | 19.1 | 19.2 | 17.3 | 18.1 | 18.0 | 19.4 | ||
| $60,000 to $79,999 | 12.9 | 15.0 | 15.9 | 12.5 | 14.6 | 16.6 | ||
| ≥$80,000 | 14.6 | 25.9 | 39.7 | 29.5 | 26.3 | 23.8 | ||
| Missing | 11.3 | 10.0 | 8.0 | 10.0 | 10.1 | 9.3 | ||
| Weight, kg | 105.4 (21.1) | 100.8 (18.9) | 95.6 (16.1) | 85.2 (11.2) | 98.5 (12.3) | 118.3 (16.8) | <0.001 | <0.001 |
| Systolic BP, mm Hg | 132 (18) | 129 (17) | 126 (16) | 126 (17) | 129 (17) | 132 (17) | <0.001 | <0.001 |
| Diastolic BP, mm Hg | 69 (10) | 70 (10) | 72 (9) | 70 (10) | 70 (10) | 70 (10) | <0.001 | 0.14 |
| History of hypertension, % | 89.9 | 84.1 | 75.0 | 78.1 | 83.2 | 88.0 | <0.001 | <0.001 |
| History of CVD, % | 16.9 | 12.0 | 10.7 | 14.2 | 14.3 | 11.2 | <0.001 | 0.008 |
| Insulin use, % | 20.7 | 16.7 | 9.9 | 12.4 | 16.4 | 18.9 | <0.001 | <0.001 |
| Smoking, % | 0.23 | 0.37 | ||||||
| Never | 50.7 | 51.4 | 48.6 | 50.3 | 49.4 | 51.0 | ||
| Past | 44.4 | 44.3 | 47.5 | 45.4 | 45.4 | 45.2 | ||
| Current | 4.9 | 4.4 | 3.9 | 4.3 | 5.2 | 3.8 | ||
| Alcohol, % | <0.001 | <0.001 | ||||||
| None / week | 75.9 | 68.6 | 58.7 | 63.3 | 68.1 | 72.1 | ||
| 1–3 / week | 16.1 | 19.7 | 22.5 | 19.5 | 19.8 | 18.9 | ||
| 4+ / week | 8.1 | 11.8 | 18.8 | 17.3 | 12.2 | 9.0 | ||
| HbA1c, % | 7.4 (1.2) | 7.3 (1.2) | 7.1 (1.1) | 7.2 (1.1) | 7.2 (1.2) | 7.4 (1.2) | <0.001 | <0.001 |
| GFR, mL/min per 1.73 m2 | 86.9 (17.5) | 90.8 (15.6) | 91.5 (14.3) | 88.1 (15.3) | 89.6 (16.0) | 91.4 (16.5) | <0.001 | <0.001 |
| LDL-C, mg/dL | 111 (33) | 113 (32) | 113 (32) | 112 (33) | 111 (31) | 113 (33) | 0.28 | 0.58 |
| ILI treatment group, % | 48.8 | 50.5 | 50.3 | 51.3 | 49.2 | 49.2 | 0.57 | 0.34 |
| Follow-up variables | ||||||||
| Interval MI, % | 1.1 | 0.4 | 0.2 | 0.7 | 0.7 | 0.3 | <0.001 | 0.18 |
Data presented as mean (standard deviation) or percentage. Comparison across groups performed using Cochran-Armitage test for categorical variables and Jonckheere-Terpstra test for continuous variables. Follow-up variables were assessed after the baseline visit.
BMI = body mass index; BP = blood pressure; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; GFR = glomerular filtration rate; HbA1c = glycated hemoglobin; ILI = intensive lifestyle intervention; LDL-C = low density lipoprotein cholesterol; METs = metabolic equivalents; MI = myocardial infarction.
In multivariable adjusted analysis, there was a significant, graded, inverse association between baseline CRF and risk of incident HF after adjustment for potential confounders including BMI, traditional CV risk factors, and interval MI on follow-up. Compared with low fit participants (Tertile 1, referent group), the risk of incident HF was 39% lower in moderate fit [Tertile 2: HR (95% CI) = 0.61 (0.44 to 0.83)] and 62% lower in the high fit groups [Tertile 3: HR (95% CI) = 0.38 (0.24 to 0.59)] (Table 2). Similar findings were observed when CRF was modeled as a continuous variable with 20% lower risk of incident HF per 1-MET higher CRF level [HR (95% CI) = 0.80 (0.72 to 0.88), Table 2]. The association between baseline CRF and risk of HF was similar among white vs. non-white participants (p-interaction = 0.86).
Table 2.
Multivariable adjusted association of categories and continuous measures of baseline CRF with risk of incident overall HF, HFpEF, and HFrEF
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Overall HF | |||||||
| CRF categories (referent group: tertile 1) | |||||||
| Tertile 2 | 0.47 (0.35, 0.64) | <0.001 | 0.54 (0.40, 0.73) | <0.001 | 0.61 (0.44, 0.83) | <0.001 | |
| Tertile 3 | 0.22 (0.15, 0.32) | 0.31 (0.20, 0.48) | 0.38 (0.24, 0.59) | ||||
| Continuous CRF measure | |||||||
| Per 1 unit higher CRF | 0.70 (0.64, 0.76) | <0.001 | 0.76 (0.69, 0.83) | <0.001 | 0.80 (0.72, 0.88) | <0.001 | |
| HFpEF | |||||||
| CRF categories (referent group: tertile 1) | |||||||
| Tertile 2 | 0.50 (0.34, 0.75) | <0.001 | 0.57 (0.38, 0.87) | <0.001 | 0.60 (0.39, 0.91) | <0.001 | |
| Tertile 3 | 0.14 (0.07, 0.27) | 0.21 (0.10, 0.42) | 0.23 (0.11, 0.46) | ||||
| Continuous CRF measure | |||||||
| Per 1 unit higher CRF | 0.68 (0.60, 0.77) | <0.001 | 0.74 (0.64, 0.86) | <0.001 | 0.75 (0.65, 0.87) | <0.001 | |
| HFrEF | |||||||
| CRF categories (referent group: tertile 1) | |||||||
| Tertile 2 | 0.56 (0.35, 0.91) | 0.007 | 0.63 (0.38, 1.04) | 0.14 | 0.71 (0.43, 1.19) | 0.41 | |
| Tertile 3 | 0.44 (0.26, 0.77) | 0.61 (0.33, 1.12) | 0.75 (0.40, 1.41) | ||||
| Continuous CRF measure | |||||||
| Per 1 unit higher CRF | 0.80 (0.71, 0.91) | <0.001 | 0.85 (0.74, 0.98) | 0.03 | 0.89 (0.77, 1.03) | 0.12 | |
Hazard ratio refers to the association of CRF categories / continuous measures of CRF with risk of incident overall HF, HFpEF, and HFrEF. Separate models were constructed for each HF outcome (overall, HFpEF, and HFrEF). Tertile 1 was the referent group in the categorical analysis.
Model 1 included age, sex, ethnicity, education level, income, treatment group, baseline CRF
Model 2 included Model 1 covariates plus baseline BMI, history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, GFR
Model 3 included Model 2 covariates plus interval MI on follow-up.
BP = blood pressure; BMI = body mass index; CI = confidence interval; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; GFR = glomerular filtration rate; HbA1c = glycated hemoglobin; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; MI = myocardial infarction.
Among HF subtypes, there was graded inverse association between baseline CRF and risk of incident HFpEF with 40% and 77% lower risk of incident HFpEF among moderate fit [Tertile 2: HR (95% CI) = 0.60 (0.39 to 0.91)] and high fit [Tertile 3: HR (95% CI) = 0.23 (0.11 to 0.46)] individuals, respectively (referent group: Tertile 1, low fit; Table 2). In contrast, baseline CRF was not significantly associated with risk of incident HFrEF in the most adjusted model. Similar patterns of results were obtained when CRF was modeled as a continuous variable with a significant association between CRF and HFpEF but not HFrEF in the most adjusted model (Table 2).
Baseline BMI & risk of incident HF
Baseline characteristics of trial participants across categories of BMI are shown in Table 1. Participants with higher BMI at baseline were younger, more commonly women, more commonly white, had lower CRF levels, higher hypertension prevalence and systolic BP, higher HbA1c, and lower history of CVD.
In multivariable adjusted analyses, higher baseline BMI was significantly associated with higher risk of incident HF after adjustment for demographic characteristics and the treatment arm [HR (95% CI) Tertile 2 vs.1 = 1.64 (1.19 to 2.27), Tertile 3 vs.1 = 2.13 (1.53 to 2.95)]. However, this association was attenuated and no longer significant after further adjustment for CRF and traditional HF risk factors (Table 3). Similar findings were also observed when BMI was modeled as a continuous variable. There was no significant interaction between race (white vs. non-white) and baseline BMI for the risk of HF (p-interaction = 0.57). Among HF subtypes, continuous and categorical measures of BMI were not significantly associated with risk of incident HFpEF or HFrEF in the most adjusted models (Table 3).
Table 3.
Multivariable adjusted association of categories and continuous measures of baseline BMI with risk of incident overall HF, HFpEF, and HFrEF
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Overall HF | ||||||
| BMI categories (referent group: tertile 1) | ||||||
| Tertile 2 | 1.64 (1.19, 2.27) | <0.001 | 1.30 (0.94, 1.81) | 0.29 | 1.36 (0.97, 1.90) | 0.12 |
| Tertile 3 | 2.13 (1.53, 2.95) | 1.20 (0.84, 1.72) | 1.42 (0.99, 2.06) | |||
| Continuous BMI measure | ||||||
| Per 1 unit higher BMI | 1.05 (1.03, 1.07) | <0.001 | 1.00 (0.98, 1.03) | 0.81 | 1.01 (0.99, 1.04) | 0.27 |
| HFpEF | ||||||
| BMI categories (referent group: tertile 1) | ||||||
| Tertile 2 | 1.43 (0.89, 2.29) | 0.001 | 1.04 (0.64, 1.69) | 0.79 | 0.99 (0.61, 1.61) | 0.64 |
| Tertile 3 | 2.28 (1.45, 3.60) | 1.18 (0.71, 1.94) | 1.21 (0.73, 2.00) | |||
| Continuous BMI measure | ||||||
| Per 1 unit higher BMI | 1.06 (1.03, 1.09) | <0.001 | 1.01 (0.97, 1.04) | 0.75 | 1.01 (0.98, 1.04) | 0.62 |
| HFrEF | ||||||
| BMI categories (referent group: tertile 1) | ||||||
| Tertile 2 | 1.75 (1.07, 2.85) | 0.06 | 1.56 (0.94, 2.57) | 0.20 | 1.66 (0.99, 2.76) | 0.16 |
| Tertile 3 | 1.71 (1.01, 2.88) | 1.23 (0.69, 2.19) | 1.43 (0.79, 2.59) | |||
| Continuous BMI measure | ||||||
| Per 1 unit higher BMI | 1.03 (0.997, 1.07) | 0.07 | 1.01 (0.97, 1.05) | 0.81 | 1.01 (0.97, 1.06) | 0.51 |
Hazard ratio refers to the association of BMI categories / continuous measures of BMI with risk of incident overall HF, HFpEF, and HFrEF. Separate models were constructed for each HF outcome (overall, HFpEF, and HFrEF). Tertile 1 was the referent group in the categorical analysis.
Model 1 included age, sex, ethnicity, education level, income, treatment group, baseline BMI
Model 2 included Model 1 covariates plus baseline CRF, history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, GFR
Model 3 included Model 2 covariates plus interval MI on follow-up.
BP = blood pressure; BMI = body mass index; CI = confidence interval; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; GFR = glomerular filtration rate; HbA1c = glycated hemoglobin; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; MI = myocardial infarction.
Association between longitudinal changes in CRF & risk of incident HF
Association between longitudinal changes in CRF and risk of incident HF was assessed in a subset of participants who were free of HF and had a repeat assessment of CRF and BMI at year 1 (n = 4,380) and year 4 (n = 3,902) visits. Participants with greater improvements in CRF levels over short-term (1-year) or intermediate-term (4-year) follow-up were younger, more commonly assigned to the ILI arm, and had lower history of CVD at baseline (Supplemental Table 2, 3). Greater short-term and intermediate-term improvements in CRF were associated with significantly lower BMI, systolic BP, and HbA1c at follow-up.
In multivariable adjusted analyses, greater increase in CRF levels over short-term (1-year) follow-up was significantly associated with lower risk of incident HF (n = 199 events) after adjustment for baseline confounders [HR (95% CI) per 10% increase in CRF = 0.93 (0.87 to 0.99)]. However, this association was attenuated and no longer significant after further adjustment for changes in BMI [HR (95 % CI) = 0.96 (0.90 to 1.03)] (Table 4). In contrast, longitudinal improvements in CRF levels over intermediate-term follow-up (4-year) was significantly associated with lower risk of incident HF (n = 128 events) independent of baseline confounders as well as changes in BMI and other cardiometabolic parameters [HR (95% CI) per 10% increase in CRF = 0.90 (0.82 to 0.99)] (Table 4). The pattern of associations between changes in CRF and risk of incident HF subtypes were in the same direction as incident overall HF but were not consistently statistically significant.
Table 4.
Multivariable adjusted association of changes in CRF from baseline to 1- and 4-year follow-up with risk of incident overall HF, HFpEF, and HFrEF
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Person-years | Event Rate per 1,000 person years | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Per 10% increase in CRF from baseline to 1-year follow-up | ||||||||
| Overall HF | 51,159 | 3.89 | 0.93 (0.87, 0.99) | 0.02 | 0.96 (0.90, 1.03) | 0.26 | 0.97 (0.90, 1.04) | 0.37 |
| HFpEF | 51,037 | 1.86 | 0.89 (0.81, 0.99) | 0.02 | 0.93 (0.84, 1.03) | 0.16 | 0.94 (0.85, 1.004) | 0.20 |
| HFrEF | 51,037 | 1.69 | 0.95 (0.86, 1.05) | 0.32 | 1.00 (0.90, 1.11) | 0.96 | 1.01 (0.91, 1.11) | 0.92 |
| Per 10% increase in CRF from baseline to 4-year follow-up | ||||||||
| Overall HF | 47,408 | 2.70 | 0.86 (0.79, 0.94) | 0.001 | 0.88 (0.80, 0.97) | 0.009 | 0.90 (0.82, 0.99) | 0.03 |
| HFpEF | 47,324 | 1.23 | 0.85 (0.74, 0.97) | 0.02 | 0.88 (0.77, 1.01) | 0.07 | 0.90 (0.78, 1.03) | 0.14 |
| HFrEF | 47,324 | 1.27 | 0.86 (0.75, 0.98) | 0.02 | 0.87 (0.76, 0.998) | 0.047 | 0.88 (0.77, 1.01) | 0.07 |
HR refers to the association of 10% increase in CRF with risk of incident overall HF, HFpEF, and HFrEF included in separate Cox proportional hazards models. Separate models were constructed for each HF outcome (overall, HFpEF, and HFrEF). Separate models were created with sequential adjustment for confounders.
Model 1 included age, sex, ethnicity, education level, income, treatment group, history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, GFR, baseline BMI, baseline CRF, change in CRF.
Model 2 included age, sex, ethnicity, education level, income, treatment group, history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, GFR, baseline BMI, baseline CRF, change in CRF and change in BMI (both included in the same model).
Model 3 included age, sex, ethnicity, education level, income, treatment group, history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, GFR, baseline BMI, baseline CRF, change in CRF and change in BMI (both included in the same model), % change in A1c, % change in systolic BP.
BP = blood pressure; BMI = body mass index; CI = confidence interval; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; GFR = glomerular filtration rate; HbA1c = glycated hemoglobin; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio.
Association between longitudinal changes in BMI & risk of incident HF
Participants with substantial weight loss over short-term (1-year) and intermediate-term (4-year) follow-up were more commonly white and more commonly assigned to the ILI group (Supplemental Table 4, 5). Greater weight loss over short-term and intermediate-term follow-up was associated with significant improvements in CRF, systolic BP, and HbA1c levels.
In multivariable adjusted analyses, a 10% decrease in BMI over short-term (1-year) follow-up was significantly associated with 31% lower risk of incident HF independent of baseline risk factors and changes in other parameters such as CRF, HbA1c, and systolic BP [HR (95% CI) = 0.69 (0.51 to 0.93)] (Table 5). Similarly, a 10% decrease in BMI over intermediate-term (4-year) follow-up was significantly associated with a 20% lower risk of incident HF in the most adjusted model [HR (95% CI) = 0.80 (0.69 to 0.94)]. The pattern of associations between changes in BMI and risk of incident HF subtypes were in the same direction to incident overall HF but were not consistently statistically significant for HFrEF in adjusted models (Table 5).
Table 5.
Multivariable adjusted association of changes in BMI from baseline to 1- and 4-year follow-up with risk of incident overall HF, HFpEF, and HFrEF
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Person-years | Event rate per 1,000 person years | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Per 10% decrease in BMI from baseline to 1-year follow-up | ||||||||
| Overall HF | 51,159 | 3.89 | 0.61 (0.47, 0.80) | <0.001 | 0.65 (0.48, 0.86) | 0.003 | 0.69 (0.51, 0.93) | 0.01 |
| HFpEF | 51,037 | 1.86 | 0.57 (0.38, 0.84) | 0.005 | 0.63 (0.42, 0.96) | 0.03 | 0.64 (0.42, 0.98) | 0.04 |
| HFrEF | 51,037 | 1.69 | 0.54 (0.36, 0.83) | 0.005 | 0.55 (0.35, 0.85) | 0.008 | 0.59 (0.37, 0.93) | 0.02 |
| Per 10% decrease in BMI from baseline to 4-year follow-up | ||||||||
| Overall HF | 47,408 | 2.70 | 0.76 (0.66, 0.86) | <0.001 | 0.79 (0.68, 0.92) | 0.002 | 0.80 (0.69, 0.94) | 0.006 |
| HFpEF | 47,324 | 1.23 | 0.71 (0.60, 0.84) | <0.001 | 0.74 (0.62, 0.89) | 0.001 | 0.76 (0.63, 0.91) | 0.003 |
| HFrEF | 47,324 | 1.27 | 0.81 (0.64, 1.02) | 0.07 | 0.86 (0.64, 1.15) | 0.30 | 0.89 (0.64, 1.22) | 0.46 |
HR refers to the association of 10% decrease in BMI with risk of incident overall HF, HFpEF, and HFrEF included in separate Cox proportional hazards models. Separate models were constructed for each HF outcome (overall, HFpEF, and HFrEF). Separate models were created with sequential adjustment for confounders.
Model 1 included age, sex, ethnicity, education level, income, treatment group, history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, GFR, baseline BMI, baseline CRF, change in BMI.
Model 2 included age, sex, ethnicity, education level, income, treatment group, history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, GFR, baseline BMI, baseline CRF, change in BMI and change in CRF (both included in the same model).
Model 3 included age, sex, ethnicity, education level, income, treatment group, history of hypertension, systolic BP, smoking status, current alcohol use, history of CVD, HbA1c, GFR, baseline BMI, baseline CRF, change in BMI and change in CRF (both included in the same model), % change in A1c, % change in systolic BP.
BP = blood pressure; BMI = body mass index; CI = confidence interval; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; GFR = glomerular filtration rate; HbA1c = glycated hemoglobin; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio.
DISCUSSION
In this study, several important findings were observed. First, in a cohort of adults with T2DM who are overweight or obese from the Look AHEAD trial, the ILI was not associated with lower risk of incident HF or its subtypes on follow-up compared with DSE. Second, higher CRF was significantly associated with lower risk of incident HF independent of traditional risk factors and BMI. Among HF subtypes, a significant, graded inverse association was observed between CRF levels at baseline and risk of incident HFpEF independent of potential confounders. In contrast, baseline CRF was not associated with risk of incident HFrEF after adjustment for potential confounders. Third, significant associations were observed between changes in CRF, BMI and risk of incident HF during the study period such that improvements in CRF as well as greater weight loss over 4-year follow-up were independently associated with lower risk of incident HF. To our knowledge, the present study represents the first and most comprehensive evaluation of the association of ILI and longitudinal changes in CRF with the risk of HF subtypes.
Higher CRF and physical activity levels have been associated with lower risk of HF 21, 22. Prior studies have demonstrated a graded association between CRF levels in young to middle age and risk of HF in older age 8, 11, 23–26. However, most of these studies were limited by inclusion of a referral population with a clinical indication for CRF testing 24, 25 or low-risk participants with a lower burden of traditional HF risk factors such as T2DM 8, 9, 26. Furthermore, these cohorts did not clinically adjudicate incident HF events and thus, it was not clear if the association of low CRF with HF is consistent for both HFpEF and HFrEF 8, 24–26. Findings from the present study add to the existing literature by demonstrating a consistent graded, inverse association between CRF levels and risk of incident HF, particularly HFpEF in a higher risk cohort of patients with prevalent T2DM (Supplemental Table 6).
Prior studies have also evaluated the association of subjective measures of exercise capacity such as self-reported physical activity or walking speed with the risk of HF subtypes 12, 27, 28. In a recent pooled analysis from 3 large cohorts, higher levels of physical activity were more consistently associated with lower risk of HFpEF but not HFrEF 12. However, others have demonstrated consistent and similar patterns of association between physical activity levels and risk of HF subtypes 27, 28. These discordant observations regarding physical activity associated risk of HF subtypes may be related to the subjective nature of the self-reported physical activity levels in these cohorts. Furthermore, physical activity levels are more reflective of the habitual exercise behavior and only modestly associated with peak exercise capacity 29, 30. In the present study, using CRF levels, an objective measure of peak exercise capacity, we demonstrated that higher CRF may modify the risk of HFpEF and HFrEF through potentially different mechanisms. A significant, graded inverse association was observed between CRF and risk of incident HFpEF independent of other risk factors suggesting a more direct effect of CRF on cardiac structure and function. In contrast, the association between CRF and risk of incident HFrEF was largely driven by differences in traditional risk factor burden and antecedent MI events prior to HF development.
The mechanism through which CRF may modify risk of HFpEF is not well established. Prior studies have demonstrated significant associations between low CRF levels and higher burden of diastolic dysfunction, a subclinical cardiac phenotype associated with development of HFpEF 31, 32. Seminal studies have identified increased left ventricular (LV) stiffness, demonstrated by steeper LV pressure-volume loops and higher stiffness constant in invasive hemodynamic studies, as a key pathophysiologic abnormality in HFpEF 33–36. Prior studies have also demonstrated a strong inverse association between lifelong exercise dose and LV stiffness among healthy participants 37. These mechanistic findings corroborate the epidemiological observation of higher HFpEF risk among individuals who are low fit and highlight the independent and direct role of low CRF in development of HFpEF.
The present study also adds to the existing literature on CV effects of lifestyle interventions by evaluating its effects on the risk of HF and its subtypes, HFpEF and HFrEF. Despite the significant association between baseline CRF and risk of incident HF among participants of the Look AHEAD trial, the ILI did not appear to significantly modify the risk of incident HF compared with DSE. This is consistent with the negative results of the Look AHEAD trial for the primary CV outcome and may be related to the overall modest differences in the achieved weight loss and CRF improvements between the ILI vs. DSE groups during follow-up [average between-group difference in weight (ILI vs. DSE): −4 kg; average between-group difference in CRF (ILI vs. DSE): 0.6 METs] 14. However, there were significant associations between changes in CRF and BMI during the trial period and risk of incident HF. Specifically, sustained improvements in CRF and weight loss over 4 years were each significantly associated with lower risk of incident HF independent of baseline confounders as well as changes in other relevant cardiometabolic parameters. In contrast with 4-year CRF changes, the association between short-term improvements in CRF at 1-year follow-up and lower risk of incident HF was largely driven by changes in BMI. It is plausible that early CRF improvements that were observed with ILI in the Look AHEAD trial were largely driven by weight loss and sustained improvements in CRF levels over longer-term follow-up may be more reflective of favorable changes in CV exercise reserve. Along these lines, recent exercise training trials have demonstrated that short-term exercise training (1-year duration) does not significantly modify LV stiffness, the key pathophysiologic abnormality associated with HFpEF, in sedentary, older individuals 38. In contrast, high intensity exercise training over prolonged duration (2-year) in middle-age, sedentary individuals may significantly improve LV stiffness 39. Future studies are needed to determine if implementation of more intense exercise training or weight loss interventions aimed at promoting sustained improvements in CRF and body weight among young to middle-age patients with T2DM may significantly modify HF risk.
In contrast to the observed independent association between CRF and risk of HF, higher BMI associated risk of HF in the Look AHEAD cohort was largely driven by differences in the burden of CV risk factors and CRF levels. The lack of independent association between higher BMI and risk of HF may be related to the cohort characteristics which included only overweight and obese participants with T2DM. Furthermore, consistent with prior observations, adjustment for CRF in the present study may have substantially attenuated the relationship between higher BMI and risk of HF 11.
Prior studies in patients with T2DM have demonstrated the phenomenon of the obesity paradox whereby higher BMI in the overweight to obese range is associated with lower risk of mortality 40, 41. It is noteworthy that the phenomenon of obesity paradox was not observed in the Look AHEAD cohort for development of HF. Furthermore, reduction in BMI was associated with lower risk of HF, highlighting the importance of weight loss to lower the risk of HF in overweight and obese adults with T2DM. These findings may suggest that the obesity paradox may not be applicable to non-fatal incident HF outcomes. However, patients with normal BMI were not included in the Look AHEAD trial limiting the evaluation of HF risk across a broader distribution of BMI.
The present study has several important public health and clinical implications. The burden of HF, particularly HFpEF, continues to increase in the community highlighting the need for novel approaches to its prevention 42. Findings from our study demonstrate that low CRF may identify individuals with T2DM who are at increased risk for development of HF, particularly HFpEF. Furthermore, the low CRF associated risk of HF in this high-risk cohort of individuals with T2DM and overweight/obesity was modifiable with sustained improvements in CRF levels and weight loss. It is noteworthy that the ILI used in the Look AHEAD trial, which led to only modest improvements in CRF and weight loss as compared with the control arm, was not associated with significant reductions in HF risk. Similarly, prior studies of interventions that achieved modest improvement in functional capacity and/or weight loss have not demonstrated reductions in the risk of HF 43–45. In contrast, therapeutic strategies such as bariatric surgery, which are associated with substantial weight loss, have been associated with lower risk of HF development in observational cohort studies (Supplemental Table 7) 46, 47. Taken together, these findings highlight the need to test novel and effective interventions aimed at achieving substantial and sustained improvements in CRF levels and weight loss to modify the risk of HF, particularly HFpEF.
The strengths of the present study include the large sample size of the cohort, availability of adjudicated outcome events with HF subtype information, and availability of objective measures of CRF levels at baseline and follow-up. These analyses are not without limitations. First, there is a potential for unmeasured confounding and selection bias in this secondary analysis. This is particularly relevant for the associations between changes in CRF, BMI and risk of HF. Second, there is also a potential for reverse causation such that presence of subclinical heart disease at baseline may have contributed to lower CRF and observed associations between CRF and risk of incident HF. As a result, these findings do not establish a causal association between CRF and risk of incident HF. Third, serum biomarkers with important prognostic implications for the risk of HF such as high-sensitivity cardiac troponin and/or N-terminal pro-B-type natriuretic peptide were not measured in the overall Look AHEAD cohort. Thus, we could not asses the association of ILI, CRF, and longitudinal changes in CRF with changes in these biomarkers. Finally, the present study findings may not be generalizable to patients with T2DM who would not have qualified for participation in the Look AHEAD trial owing to inability to participate in the ILI.
In conclusion, among individuals with T2DM who are overweight or obese, lower CRF is an independent and potentially modifiable risk factor for incident HF, particularly HFpEF. ILI implemented in the Look AHEAD trial did not significantly lower the risk of incident HF compared with DSE. However, intentional weight loss and sustained improvements in CRF may significantly lower the risk of incident HF. Future studies with more intense interventions targeting substantial weight loss and CRF improvement are needed to evaluate the role of lifestyle interventions in modifying HF risk.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
In the Look AHEAD trial, an intensive lifestyle intervention among adults who are overweight or obese and have type 2 diabetes mellitus did not lower the risk of heart failure on follow-up.
Among individuals with type 2 diabetes mellitus, high cardiorespiratory fitness was associated with lower risk of developing heart failure, particularly heart failure with preserved ejection fraction, independent of traditional risk factors.
Sustained, long-term improvements in cardiorespiratory fitness and weight loss were associated with lower risk of heart failure development among adults with type 2 diabetes mellitus.
What are the clinical implications?
Low cardiorespiratory fitness may identify individuals with type 2 diabetes mellitus who are at higher risk for developing heart failure and may benefit from strategies targeting substantial improvements in cardiorespiratory fitness and weight loss.
Lifestyle intervention strategies with modest improvements in cardiorespiratory fitness and weight loss may not be sufficient to lower the risk of heart failure.
Acknowledgements
The authors would like to thank the participants, study staff, and investigators of the Look AHEAD study.
Funding and Support
Dr. Pandey is supported by the Texas Health Resources Clinical Scholars Program. Dr. Patel is supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training grant (5T32HL125247-03).
Look AHEAD Trial was funded by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General
Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene
ABBREVIATIONS
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CVD
cardiovascular disease
- CRF
cardiorespiratory fitness
- DSE
diabetes support and education
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- ILI
intensive lifestyle intervention
- T2DM
type 2 diabetes mellitus
Footnotes
Disclosures:
Dr. McGuire reports honoraria for trial leadership from Astra Zeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline, Esperion; and honoraria for consulting for Astra Zeneca, Sanofi Aventis, Lilly US, Astra Zeneca, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk, Metavant.
Dr. Kitzman has been a consultant for Abbvie, AstraZeneca, Merck, Novartis, Corvia Medical, Bayer, CinRx, Boehringer-Ingleheim, and St. Luke’s Medical Center in Kansas City, Kansas; received grant support from Novartis, Bayer, AstraZeneca, and St. Luke’s Medical Center in Kansas City, Kansas; and owns stock in Gilead Sciences.
Other authors report no relevant disclosures for this study.
Clinical Trial Registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT00017953.
REFERENCES
- 1.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, et al. of the Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjornsdottir S, Wedel H, Clements M, Dahlqvist S and Lind M. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373:1720–1732. [DOI] [PubMed] [Google Scholar]
- 3.Baena-Diez JM, Penafiel J, Subirana I, Ramos R, Elosua R, Marin-Ibanez A, Guembe MJ, Rigo F, Tormo-Diaz MJ, Moreno-Iribas C, et al. on behalf of the FRESCO Investigators. Risk of Cause-Specific Death in Individuals With Diabetes: A Competing Risks Analysis. Diabetes Care. 2016;39:1987–1995. [DOI] [PubMed] [Google Scholar]
- 4.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A and Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Braunwald E., heart failure, and renal dysfunction: The vicious circles. Prog Cardiovasc Dis. 2019;62:298–302. [DOI] [PubMed] [Google Scholar]
- 6.Murtaza G, Virk HUH, Khalid M, Lavie CJ, Ventura H, Mukherjee D, Ramu V, Bhogal S, Kumar G, Shanmugasundaram M, et al. Diabetic cardiomyopathy - A comprehensive updated review. Prog Cardiovasc Dis. 2019;62:315–326. [DOI] [PubMed] [Google Scholar]
- 7.Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379:633–644. [DOI] [PubMed] [Google Scholar]
- 8.Berry JD, Pandey A, Gao A, Leonard D, Farzaneh-Far R, Ayers C, DeFina L and Willis B. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey A, Patel M, Gao A, Willis BL, Das SR, Leonard D, Drazner MH, de Lemos JA, DeFina L and Berry JD. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: the Cooper Center Longitudinal Study. Am Heart J. 2015;169:290–297 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB and Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 11.Pandey A, Cornwell WK, 3rd, Willis B, Neeland IJ, Gao A, Leonard D, DeFina L and Berry JD. Body Mass Index and Cardiorespiratory Fitness in Mid-Life and Risk of Heart Failure Hospitalization in Older Age: Findings From the Cooper Center Longitudinal Study. JACC Heart Fail. 2017;5:367–374. [DOI] [PubMed] [Google Scholar]
- 12.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol. 2017;69:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ and Look ARG. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. [DOI] [PubMed] [Google Scholar]
- 14.Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G and Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribisl PM, Lang W, Jaramillo SA, Jakicic JM, Stewart KJ, Bahnson J, Bright R, Curtis JF, Crow RS, Soberman JE and Look ARG. Exercise capacity and cardiovascular/metabolic characteristics of overweight and obese individuals with type 2 diabetes: the Look AHEAD clinical trial. Diabetes Care. 2007;30:2679–2684. [DOI] [PubMed] [Google Scholar]
- 17.Jakicic JM, Jaramillo SA, Balasubramanyam A, Bancroft B, Curtis JM, Mathews A, Pereira M, Regensteiner JG, Ribisl PM and Look ASG. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond). 2009;33:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribisl PM, Gaussoin SA, Lang W, Bahnson J, Connelly SA, Horton ES, Jakicic JM, Killean T, Kitzman DW, Knowler WC, Stewart KJ and Look ARG. Lifestyle intervention improves heart rate recovery from exercise in adults with type 2 diabetes: results from the Look AHEAD study. J Obes. 2012;2012:309196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ACSM’s Guidelines for Exercise Testing and Prescription 8th Edition: Lippincott Williams & Wilkins; 2010. Philadelphia, PA, USA. [Google Scholar]
- 20.Look ARG, Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, Mayo HG, de Lemos JA and Berry JD. Dose-Response Relationship Between Physical Activity and Risk of Heart Failure: A Meta-Analysis. Circulation. 2015;132:1786–1794. [DOI] [PubMed] [Google Scholar]
- 22.Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD and Lavie CJ. Physical Activity, Fitness, and Obesity in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 23.Khan H, Kunutsor S, Rauramaa R, Savonen K, Kalogeropoulos AP, Georgiopoulou VV, Butler J and Laukkanen JA. Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fail. 2014;16:180–188. [DOI] [PubMed] [Google Scholar]
- 24.Kokkinos P, Faselis C, Franklin B, Lavie CJ, Sidossis L, Moore H, Karasik P and Myers J. Cardiorespiratory fitness, body mass index and heart failure incidence. Eur J Heart Fail. 2019;21:436–444. [DOI] [PubMed] [Google Scholar]
- 25.Kupsky DF, Ahmed AM, Sakr S, Qureshi WT, Brawner CA, Blaha MJ, Ehrman JK, Keteyian SJ and Al-Mallah MH. Cardiorespiratory fitness and incident heart failure: The Henry Ford ExercIse Testing (FIT) Project. Am Heart J. 2017;185:35–42. [DOI] [PubMed] [Google Scholar]
- 26.Lindgren M, Aberg M, Schaufelberger M, Aberg D, Schioler L, Toren K and Rosengren A. Cardiorespiratory fitness and muscle strength in late adolescence and long-term risk of early heart failure in Swedish men. Eur J Prev Cardiol. 2017;24:876–884. [DOI] [PubMed] [Google Scholar]
- 27.Kraigher-Krainer E, Lyass A, Massaro JM, Lee DS, Ho JE, Levy D, Kannel WB and Vasan RS. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. Eur J Heart Fail. 2013;15:742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaMonte MJ, Manson JE, Chomistek AK, Larson JC, Lewis CE, Bea JW, Johnson KC, Li W, Klein L, LaCroix AZ, et al. Physical Activity and Incidence of Heart Failure in Postmenopausal Women. JACC Heart Fail. 2018;6:983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvie JL, Pandey A, Ayers CR, McGavock JM, Senechal M, Berry JD, Patel KV and McGuire DK. Aerobic Fitness and Adherence to Guideline-Recommended Minimum Physical Activity Among Ambulatory Patients With Type 2 Diabetes Mellitus. Diabetes Care. 2019;42:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R and Lavie CJ. Promoting Physical Activity and Exercise: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:1622–1639. [DOI] [PubMed] [Google Scholar]
- 31.Brinker SK, Pandey A, Ayers CR, Barlow CE, DeFina LF, Willis BL, Radford NB, Farzaneh-Far R, de Lemos JA, Drazner MH, et al. Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC Heart Fail. 2014;2:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey A, Allen NB, Ayers C, Reis JP, Moreira HT, Sidney S, Rana JS, Jacobs DR Jr., Chow LS, de Lemos JA, et al. Fitness in Young Adulthood and Long-Term Cardiac Structure and Function: The CARDIA Study. JACC Heart Fail. 2017;5:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH and Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. [DOI] [PubMed] [Google Scholar]
- 34.Prasad A, Hastings JL, Shibata S, Popovic ZB, Arbab-Zadeh A, Bhella PS, Okazaki K, Fu Q, Berk M, Palmer D, et al. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circ Heart Fail. 2010;3:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata S, Hastings JL, Prasad A, Fu Q, Bhella PS, Pacini E, Krainski F, Palmer MD, Zhang R and Levine BD. Congestive heart failure with preserved ejection fraction is associated with severely impaired dynamic Starling mechanism. J Appl Physiol (1985). 2011;110:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zile MR, Baicu CF and Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. [DOI] [PubMed] [Google Scholar]
- 37.Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B and Levine BD. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick-Ranson G, Palmer D and Levine BD. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol. 2012;590:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, Urey MA, Adams-Huet B and Levine BD. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation. 2018;137:1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kokkinos P, Myers J, Faselis C, Doumas M, Kheirbek R and Nylen E. BMI-mortality paradox and fitness in African American and Caucasian men with type 2 diabetes. Diabetes Care. 2012;35:1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, Konety SH, Rodriguez CJ and Rosamond WD. Trends in Hospitalizations and Survival of Acute Decompensated Heart Failure in Four US Communities (2005–2014): ARIC Study Community Surveillance. Circulation. 2018;138:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohula EA, Wiviott SD, McGuire DK, Inzucchi SE, Kuder J, Im K, Fanola CL, Qamar A, Brown C, Budaj A, et al. for the CAMELLIA-TIMI 61 Steering Committee and Investigators. Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients. N Engl J Med. 2018;379:1107–1117. [DOI] [PubMed] [Google Scholar]
- 44.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, et al. for the LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman AB, Dodson JA, Church TS, Buford TW, Fielding RA, Kritchevsky S, Beavers D, Pahor M, Stafford RS, Szady AD, et al. for the LIFE Study Group. Cardiovascular Events in a Physical Activity Intervention Compared With a Successful Aging Intervention: The LIFE Study Randomized Trial. JAMA Cardiol. 2016;1:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I and Neovius M. Weight Loss and Heart Failure: A Nationwide Study of Gastric Bypass Surgery Versus Intensive Lifestyle Treatment. Circulation. 2017;135:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW and Nissen SE. Association of Metabolic Surgery With Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes and Obesity. JAMA. 2019. DOI: 10.1001/jama.2019.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sillars A, Celis-Morales CA, Ho FK, Petermann F, Welsh P, Iliodromiti S, Ferguson LD, Lyall DM, Anderson J, Mackay DF, et al. Association of Fitness and Grip Strength With Heart Failure: Findings From the UK Biobank Population-Based Study. Mayo Clin Proc. 2019;94:2230–2240. [DOI] [PubMed] [Google Scholar]
- 49.Khan H, Jaffar N, Rauramaa R, Kurl S, Savonen K and Laukkanen JA. Cardiorespiratory fitness and nonfatalcardiovascular events: A population-based follow-up study. Am Heart J. 2017;184:55–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.