Abstract
Introduction
Despite the association of vitamin D deficiency with incident dementia, the role of supplementation is unclear. We prospectively explored associations between vitamin D supplementation and incident dementia in 12,388 dementia‐free persons from the National Alzheimer's Coordinating Center.
Methods
Baseline exposure to vitamin D was considered D+; no exposure prior to dementia onset was considered D−. Kaplan–Meier curves compared dementia‐free survival between groups. Cox models assessed dementia incidence rates across groups, adjusted for age, sex, education, race, cognitive diagnosis, depression, and apolipoprotein E (APOE) ε4. Sensitivity analyses examined incidence rates for each vitamin D formulation. Potential interactions between exposure and model covariates were explored.
Results
Across all formulations, vitamin D exposure was associated with significantly longer dementia‐free survival and lower dementia incidence rate than no exposure (hazard ratio = 0.60, 95% confidence interval: 0.55–0.65). The effect of vitamin D on incidence rate differed significantly across the strata of sex, cognitive status, and APOE ε4 status.
Discussion
Vitamin D may be a potential agent for dementia prevention.
Highlights
In a prospective cohort study, we assessed effects of Vitamin D on dementia incidence in 12,388 participants from the National Alzheimer's Coordinating Center dataset.
Vitamin D exposure was associated with 40% lower dementia incidence versus no exposure.
Vitamin D effects were significantly greater in females versus males and in normal cognition versus mild cognitive impairment.
Vitamin D effects were significantly greater in apolipoprotein E ε4 non‐carriers versus carriers.
Vitamin D has potential for dementia prevention, especially in the high‐risk strata.
Keywords: apolipoprotein E ε4 status, clinical cognitive diagnosis, Cox proportional hazards model, dementia, modifiable risk factors, sex, survival analysis, vitamin D deficiency, vitamin D supplementation
1. BACKGROUND
Currently, more than 50 million people around the world live with dementia and this number will nearly triple by 2050. 1 At present, there is a dearth of highly effective medication for dementia that can stop or reverse the progression of the disease. 2 Interventions based on modifiable risk factors for dementia have been explored as a potential avenue to slow disease progression. 3 , 4 Vitamin D deficiency may be a modifiable risk factor and has been recognized as a widespread health problem, with a worldwide prevalence of up to 1 billion. 5 , 6 Vitamin D is known to participate in the clearance of amyloid beta (Aβ) aggregates, 7 , 8 one of the hallmarks of Alzheimer's disease (AD), and may provide neuroprotection against Aβ‐induced tau hyperphosphorylation. 9 Low levels of serum vitamin D have been associated with a greater risk of dementia and AD. 10 Yet, the role of vitamin D supplementation as a potential intervention has been a subject of debate, and remains in equipoise. 11 , 12
Previous clinical trials of vitamin D supplementation and cognition have resulted in conflicting findings, with some reporting that vitamin D improved cognitive function while others reported no effect. 13 A recent systematic review and meta‐analysis of nine randomized clinical trials (RCTs) on AD prevention found insufficient evidence to support the use of vitamin D supplementation to improve cognitive performance as a proxy for AD prevention. 14 However, dosing variability, small sample sizes, short exposure duration, or short follow‐up times in some of these studies may have contributed to the observed inconsistencies.
Past clinical trials have also varied in terms of the vitamin D formulation. 15 The most used formulation in clinical trials is cholecalciferol, 16 , 17 , 18 , 19 , 20 followed by ergocalciferol. 21 , 22 While some have regarded these two formulations as interchangeable, recent evidence has shown that cholecalciferol may be more effective than ergocalciferol at raising and maintaining serum vitamin D levels. 23 , 24 Another common formulation is calcium–vitamin D, in which the addition of vitamin D (often cholecalciferol) improves calcium absorption. 25 Different vitamin D formulations may have different associations with dementia risk and therefore require further investigation.
Here we assessed dementia‐free older adults longitudinally for the association between vitamin D supplementation and incident dementia, while accounting for demographic, clinical, behavioral, and genetic variables. Three vitamin D formulations were explored: calcium–vitamin D, cholecalciferol, and ergocalciferol. Potential interactions between vitamin D exposure and relevant model covariates were further investigated. We hypothesized that exposure to any type of vitamin D supplement would be associated with lower dementia incidence.
2. METHODS
2.1. Study population: National Alzheimer's Coordinating Center
Data used in the present study were obtained from the National Alzheimer's Coordinating Center (NACC) database (https://naccdata.org), with a December 2021 data freeze (2005–2021), across 40 Alzheimer's Disease Research Centers (ADRCs). NACC was established by the National Institute on Aging (NIA) and consists of multiple NIA‐funded ADRCs collecting data on participants with cognitive function ranging from normal to dementia. The NACC Uniform Data Set (UDS) is a large longitudinal dataset that includes demographic and standardized clinical data collected approximately annually. All test centers administered standardized forms, and informed consent was collected from all participants and their informants. Detailed information on the cohort and neuropsychological tests included in UDS is described elsewhere. 26 , 27 , 28
RESEARCH IN CONTEXT
Systematic Review: PubMed was searched for papers on vitamin D and dementia. The literature revealed that although vitamin D deficiency has been associated with higher risk of dementia, the role of supplementation remains in equipoise. Thus, we explored longitudinal associations between vitamin D supplementation and incident dementia in a sample of 12,388 dementia‐free older adults.
Interpretation: Exposure to vitamin D supplementation was associated with a 40% lower dementia incidence rate than no exposure, providing strong support for supplementation. The results were consistent across three vitamin D formulations. The effect of vitamin D exposure on the rate of incident dementia differed significantly across the strata of sex, cognitive status, and apolipoprotein E (APOE) ε4 status.
Future Directions: Future trials should include a more ethnoracially diverse sample, assess baseline vitamin D levels, and account for sun exposure, in addition to sex, baseline cognitive status, and APOE genotype.
2.2. Participant selection
All NACC participants who were dementia‐free at baseline (i.e., cognitive diagnosis of normal cognition [NC] and mild cognitive impairment [MCI]) with at least one follow‐up visit were initially considered. Study inclusion required baseline status for all covariates of interest (i.e., sex, years of education, race, cognitive diagnosis, depression, and apolipoprotein E [APOE] ε4 status; Figure 1).
FIGURE 1.
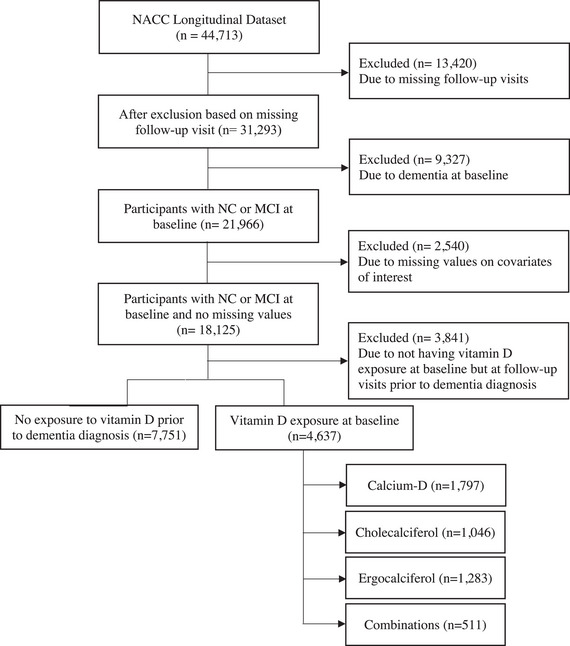
Flowchart illustrating the step‐by‐step process of the participant inclusion/exclusion criteria. MCI, mild cognitive impairment; NACC, National Alzheimer's Coordinating Center; NC, normal cognition.
2.3. Vitamin D supplements
Exposure to vitamin D supplementation was based on the NACC A4 medication form. Three formulations were considered: calcium–vitamin D, cholecalciferol, and ergocalciferol. Participants with baseline exposure to any vitamin D supplement were considered the vitamin D‐exposed group (D+), while those without any exposure throughout all visits prior to dementia diagnosis were considered non‐exposed (D−). Participants who had no baseline exposure but were exposed to vitamin D in follow‐up visits were excluded. The exposed group was further divided based on the formulation taken. The final sample consisted of 12,388 participants, with 4,637 in the D+ group and 7,751 in the D− group.
2.4. Statistical analyses
Baseline demographic, clinical, and genetic variables across the vitamin D exposure groups included age, sex, years of education, race, clinical cognitive diagnosis, depression, and APOE ε4 status. Racial categories included White, Black, or Other. The Other race category included Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islanders, or other races as specified in the NACC UDS, merged due to the small sample size per race. Depressive symptoms were assessed using total score on the Geriatric Depression Scale (GDS). Participants with a GDS total score ≥5 were considered positive for depression. APOE ε4 status was categorized as carrier and non‐carrier, with carriers having one or two copies of the ε4 allele. Between‐group differences across each variable were analyzed using a two‐sample t test for continuous variables and Chi‐squared test for categorical variables. Kaplan–Meier (KM) survival curves were generated to compare 10‐year dementia‐free survival probability in D+ versus D− participants. The sample was further stratified by baseline cognitive status (MCI vs. NC). Individual KM curves were produced for NC and MCI, each stratified by vitamin D exposure. Log‐rank tests were performed to test for statistically significant between‐group differences in survival. A Cox proportional hazards model was implemented to assess the risk of dementia over 10 years across vitamin D exposure groups, while controlling for baseline age, sex, education, race, cognitive diagnosis, depression, and APOE ε4 status. Wald test was used to test for statistical significance. All hazard ratios (HR) were accompanied by their associated 95% confidence interval (CI) and p‐value.
Potential interactions between vitamin D exposure and sex, race, cognitive diagnosis, depression, and APOE ε4 status were further explored in the model, to investigate stratum‐specific differences for each covariate. A reference level was set for each covariate: D− female for sex, D− Black for race, D− NC for cognitive diagnosis, D− non‐depressed for depression, and D− non‐carrier for APOE ε4 status. Then, the relative HR associated with vitamin D exposure was determined within each stratum of a covariate. Adjusted Cox models were implemented to test the interactions. A multiplicative test of interaction was performed to compare the stratum‐specific effects of vitamin D across the levels of each covariate.
To distinguish the individual associations of each vitamin D formulation with dementia risk, sensitivity analyses examined the effect of each formulation (calcium–vitamin D, cholecalciferol, ergocalciferol) on its own, with an additional analysis group for participants with more than one formulation at baseline. Adjusted Cox models were implemented for each formulation to assess the associated risk of dementia. Similar interaction analyses were implemented for each vitamin D formulation to assess whether the effect of exposure differed within the strata of each relevant covariate.
All statistical analyses were performed in RStudio v1.3.1093. The survival package v3.2.7 was used to run Cox regression models, while ggplot2 v3.3.3 and survminer v0.4.8 packages were used to generate the KM curves and forest plots of HRs. Proportional hazard assumptions were tested using the cox.zph function.
3. RESULTS
3.1. Participant demographics
Baseline demographic, clinical, behavioral, and genetic variables of the dementia‐free sample across the vitamin D exposure groups are presented in Table 1. The final sample comprised 12,388 participants, with 4,637 D+ (age = 71.2 ± 8.5; 70.5% female) and 7,751 D− (age = 71.2 ± 11.2; 46.9% female). The exposure groups were significantly different in terms of sex (p < 0.001), years of education (p < 0.001), race (p < 0.001), cognitive diagnosis (p < 0.001), and depression (p < 0.001). Compared to D−, the D+ group was more educated, had more females, and fewer Black participants. MCI and depression were both more frequent in the D− group, compared to D+. There were no significant between‐group differences in terms of age or APOE ε4 status.
TABLE 1.
Baseline demographics of dementia‐free NACC participants with baseline exposure to vitamin D versus those without any exposure prior to dementia diagnosis.
| No vitamin D (N = 7,751) | Vitamin D (N = 4637) | Estimate a | p‐value | |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 71.2 (11.2) | 71.2 (8.5) | 0.28 | 0.782 |
| Median [min, max] | 72.0 [18.0, 104] | 71.0 [29.0, 100] | ||
| Sex | ||||
| Female | 3632 (46.9%) | 3269 (70.5%) | 656.14 | <0.001 |
| Male | 4119 (53.1%) | 1368 (29.5%) | ||
| Years of education | ||||
| Mean (SD) | 15.5 (3.22) | 16.2 (2.80) | −12.10 | <0.001 |
| Median [min, max] | 16.0 [0, 29.0] | 16.0 [0, 30.0] | ||
| Race | ||||
| White | 6281 (81.0%) | 3824 (82.5%) | 16.39 | <0.001 |
| Black | 1170 (15.1%) | 594 (12.8%) | ||
| Other | 300 (3.9%) | 219 (4.7%) | ||
| Cognitive diagnosis | ||||
| NC | 4748 (61.3%) | 3328 (71.8%) | 140.87 | <0.001 |
| MCI | 3003 (38.7%) | 1309 (28.2%) | ||
| Depression status | ||||
| Negative | 6863 (88.5%) | 4254 (91.7%) | 31.86 | <0.001 |
| Positive | 888 (11.5%) | 383 (8.3%) | ||
| APOE ε4 status | ||||
| Carrier | 2844 (36.7%) | 1620 (34.9%) | 3.80 | 0.051 |
| Non‐carrier | 4907 (63.3%) | 3017 (65.1%) | ||
Abbreviations: APOE, apolipoprotein E; MCI, mild cognitive impairment; NACC, National Alzheimer's Coordinating Center; NC, normal control; SD, standard deviation.
The estimates represent the t‐statistic value for continuous variables and the chi‐squared value for categorical variables.
3.2. Vitamin D exposure and dementia‐free survival
Exposure to vitamin D was associated with significantly higher dementia‐free survival, compared to no exposure (Figure 2A). The 5‐year survival for D− was 68.4% (95% CI: 67.1%–69.7%), while for D+ it was 83.6% (95% CI: 82.3%–84.9%). MCI was associated with lower dementia‐free survival than NC, as expected. In both NC and MCI, exposure to vitamin D was associated with higher dementia‐free survival. In NC, 5‐year survival for D− was 89.1% (95% CI: 87.9%–90.2%), while for D+ it was 95.3% (95% CI: 94.4%–96.3%). In MCI, 5‐year survival for D− was 34.5% (95% CI: 32.3%–36.9%), while for D+ it was 49.6% (95% CI: 46.1%–53.4%).
FIGURE 2.
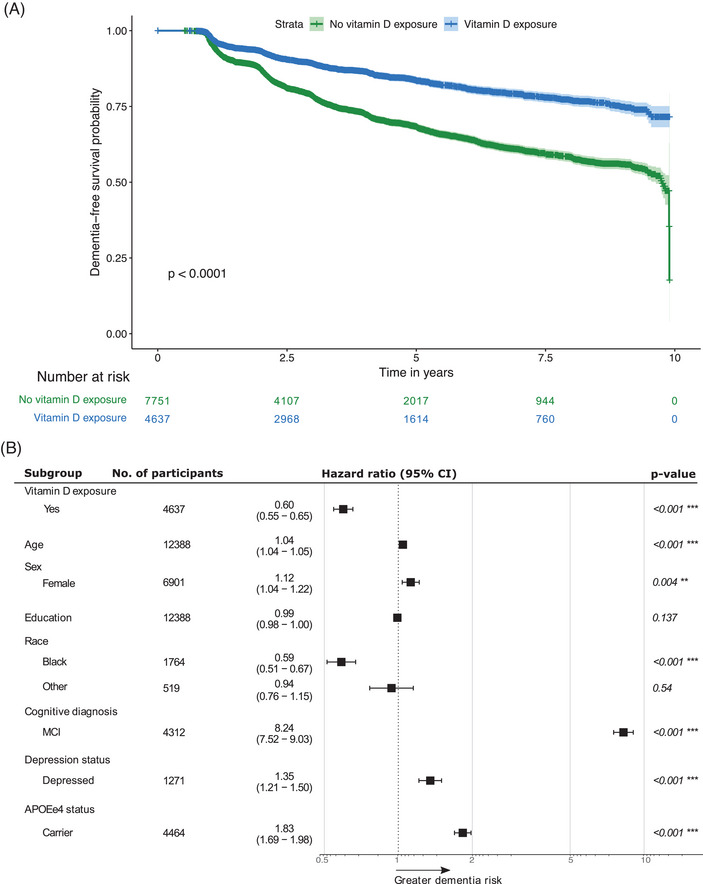
(A) KM curve of dementia‐free survival over 10 years, stratified by exposure to vitamin D. (B) Adjusted HR for dementia across vitamin D exposure groups. The reference groups were the non‐exposed group (N = 7,751) for vitamin D exposure, male (N = 5,487) for sex, White (N = 10,105) for race, NC (N = 8,076) for cognitive diagnosis, non‐depressed group (N = 11,117) for depression status, and non‐carriers (N = 7,924) for APOE ε4 status. Error bars represent the 95% CI. The star notation indicates statistical significance. APOE, apolipoprotein E; CI, confidence interval; HR, hazard ratio; KM, Kaplan–Meier; MCI, mild cognitive impairment; NC, normal cognition.
3.3. Vitamin D exposure and incidence of dementia
Across the entire sample, 2,696 participants progressed to dementia over 10 years and among them, 2,017 (74.8%) had no exposure to vitamin D throughout all visits prior to dementia diagnosis, and 679 (25.2%) had baseline exposure. After adjusting for baseline age, sex, education, race, cognitive diagnosis, depression, and APOE ε4 status, exposure to vitamin D was associated with 40% lower incidence of dementia (HR = 0.60, 95% CI: 0.55–0.65, p < 0.001) compared to no exposure (Figure 2B). Females were at greater dementia risk than males (HR = 1.12, 95% CI: 1.04–1.22, p = 0.004), and Black participants were at lower risk compared to White (HR = 0.59, 95% CI: 0.51–0.67, p < 0.001). Depression was associated with 35% greater incidence of dementia (HR = 1.35, 95% CI: 1.21–1.50, p < 0.001). Of the 4,637 participants with exposure to vitamin D, 14.6% (n = 679) progressed to dementia, consisting of 80.9% AD (n = 549), 4% dementia with Lewy bodies (DLB; n = 27), 2.4% behavioral variant of frontotemporal dementia (bvFTD; n = 16), 0.6% vascular dementia (n = 4), and 12.1% unrecorded dementia subtypes (n = 83). Among the 7,751 participants with no vitamin D exposure, 26% (n = 2,017) progressed to dementia, consisting of 82.6% AD (n = 1,667), 4.8% DLB (n = 97), 2.4% bvFTD (n = 48), 2.1% vascular dementia (n = 42), and 8.1% unrecorded dementia subtypes (n = 163).
Significant interaction effects were seen for sex, cognitive status, and APOE ε4. D+ females had lower dementia incidence rate than D+ males (multiplicative interaction test: HR = 0.68, 95% CI: 0.57–0.81, p < 0.001). D+ females had 49% lower dementia incidence rate than D− females (HR = 0.51, 95% CI: 0.45–0.57, p < 0.001), and D+ males had 26% lower rate than D− males (HR = 0.74, 95% CI: 0.65–0.85, p < 0.001). D+ NC participants had lower dementia incidence rate than D+ MCI participants (multiplicative interaction test: HR = 0.66, 95% CI: 0.54–0.81, p < 0.001). D+ NC participants had 56% lower incidence rate than D− NC (HR = 0.44, 95% CI: 0.37–0.53, p < 0.001), and D+ MCI participants had 33% lower incidence rate than D− MCI (HR = 0.67, 95% CI: 0.60–0.74, p < 0.001). D+ APOE ε4 non‐carriers had lower incidence rate for dementia than D+ carriers (multiplicative interaction test: HR = 0.78, 95% CI: 0.66–0.93, p = 0.005). D+ APOE ε4 non‐carriers had 47% lower dementia incidence rate than D− non‐carriers (HR = 0.53, 95% CI: 0.46–0.60, p < 0.001) and D+ APOE ε4 carriers had 33% lower rate than D− carriers (HR = 0.67, 95% CI: 0.60–0.76, p < 0.001). There were no significant interaction effects of vitamin D exposure with race (Black vs. White, P = 0.95; Black vs. Other, p = 0.12, Other vs. White, p = 0.07) or depression (p = 0.17; Table 2).
TABLE 2.
Interaction effects of exposure to vitamin D with sex, cognitive diagnosis, APOE ε4 status, race, and depression on dementia risk.
| D− | D+ | Within‐strata effect of vitamin D | Multiplicative test of interaction | ||
|---|---|---|---|---|---|
| Predictor | Strata | HR [95% CI] p‐value | HR [95% CI] p‐value | HR [95% CI] p‐value | HR [95% CI] p‐value |
| Sex | Female | 1 [Reference] | 0.51 [0.45, 0.57] p < 0.001 | 0.51 [0.45, 0.57] p < 0.001 | 0.68 [0.57, 0.81] p < 0.001 |
| Male | 0.81 [0.74, 0.89] p < 0.001 | 0.60 [0.53, 0.69] p < 0.001 | 0.74 [0.65, 0.85] p < 0.001 | ||
| Cognitive diagnosis | NC | 1 [Reference] | 0.44 [0.37, 0.53] p < 0.001 | 0.44 [0.37, 0.53] p < 0.001 | 0.66 [0.54, 0.81] p < 0.001 |
| MCI | 7.36 [6.62, 8.17] p < 0.001 | 4.9 [4.31, 5.56] p < 0.001 | 0.67 [0.6, 0.74] p < 0.001 | ||
| APOE ε4 | Non‐carrier | 1 [Reference] | 0.53 [0.46, 0.6] p < 0.001 | 0.53 [0.46, 0.6] p < 0.001 | 0.78 [0.66, 0.93] P = 0.0054 |
| Carrier | 1.72 [1.57, 1.88] p < 0.001 | 1.16 [1.02, 1.31] p = 0.02 | 0.67 [0.6, 0.76] P < 0.001 | ||
| Race | Black | 1 [Reference] | 0.61 [0.45, 0.84] p = 0.002 | 0.61 [0.45, 0.84] p = 0.002 | Black versus White: 1.0 [0.73, 1.39] p = 0.95 |
| White | 1.71 [1.47, 1.98] p < 0.001 | 1.04 [0.88, 1.22] p = 0.669 | 0.61 [0.55, 0.67] p < 0.001 | Black versus Other: 0.65 [0.38–1.12] p = 0.12 | |
| Other | 1.84 [1.39, 2.42] p < 0.001 | 0.74 [0.49, 1.09] p = 0.13 | 0.40 [0.26, 0.62] p < 0.001 | Other versus White: 0.66 [0.42–1.03] p = 0.07 | |
| Depression | Non‐depressed | 1 [Reference] | 0.58 [0.53, 0.64] p < 0.001 | 0.58 [0.53, 0.64] P < 0.001 | 1.18 [0.93–1.51] P = 0.165 |
| Depressed | 1.29 [1.15, 1.46] P < 0.001 | 0.89 [0.73, 1.09] p = 0.267 | 0.69 [0.55, 0.86] p < 0.001 |
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; HR, hazard ratio; MCI, mild cognitive impairment; NC, normal control.
3.4. Individual effect of each vitamin D formulation on the risk of dementia
Within the sample of participants exposed to vitamin D at baseline, 1,797 had calcium–vitamin D only (age = 71.7 ± 8.4; 78% females), 1,046 had cholecalciferol only (age = 70.2 ± 8.6; 63.2% females), 1,283 had ergocalciferol only (age = 71.1 ± 8.7; 63.2% females), and 511 had at least two supplements together (age = 71.3 ± 8.1; 77.5% females), mostly ergocalciferol plus calcium–vitamin D (n = 268) and cholecalciferol plus calcium–vitamin D (n = 233). Nine participants were on both ergocalciferol and cholecalciferol, and only one participant on all three formulations.
Compared to no exposure, exposure to each formulation on its own had a lower dementia incidence rate (see Figure S1 in supporting information; calcium–vitamin D: HR = 0.56, 95% CI: 0.49–0.64, p < 0.001; cholecalciferol: HR = 0.63, 95% CI: 0.53–0.75, p < 0.001; ergocalciferol: HR = 0.61, 95% CI: 0.53–0.71, p < 0.001; combinations of formulations: HR = 0.50, 95% CI: 0.4–0.64, p < 0.001). Interaction effects were similar to those of the primary analysis. For calcium–vitamin D only (p = 0.003) and ergocalciferol only (p = 0.003), D+ females had lower dementia incidence rate than D+ males. For calcium–vitamin D only (p = 0.0003) and cholecalciferol only (p = 0.003), D+ NC participants had lower incidence than D+ MCI. For participants on more than one vitamin D formulation, the only significant interaction was with depression, such that D+ non‐depressed participants had lower incidence than D+ depressed participants (p = 0.016, Tables S1–S4 in supporting information).
4. DISCUSSION
In this longitudinal study of dementia‐free NACC participants, exposure to vitamin D was associated with higher dementia‐free survival and lower dementia incidence rates over 10 years. These findings were consistent across each vitamin D formulation: calcium–vitamin D, cholecalciferol, and ergocalciferol. Interaction analyses revealed that while exposure to vitamin D was associated with lower dementia incidence across all strata of sex, cognitive diagnosis, and APOE ε4 status, the rates were lower in females versus males, NC versus MCI, and APOE ε4 non‐carriers versus carriers.
Several studies have explored the associations of vitamin D supplementation with cognitive performance and dementia in those with and without cognitive impairment at baseline, but findings have been contradictory. In a systematic review and meta‐analysis of nine RCTs on vitamin D interventions to enhance cognitive performance, no significant differences were reported in global and domain‐specific cognition between the exposure groups. 14 In another systematic review of 20 RCTs on vitamin D interventions and cognition, mixed findings were reported in half of the RCTs, but a quarter of them reported positive effects of vitamin D exposure on cognitive performance. 13 Variability in serum vitamin D levels, supplementation dosage, and cognitive tests administered may explain the observed inconsistencies. A recent RCT in 210 patients with AD assessed the effect of 12‐month vitamin D supplementation on cognition and Aβ levels, reporting improved performance on several cognitive tests and lower Aβ burden. 12 Several observational studies have provided evidence on the association of lower vitamin D with greater risk of AD and dementia, 10 and the positive effect of vitamin D supplementation on performance on neuropsychological tests 29 and risk of developing AD. 30 Our longitudinal findings with a large sample of dementia‐free older adults provide further support for the beneficial effect of vitamin D supplementation on dementia risk.
While exposure to vitamin D was associated with significantly lower dementia incidence in both males and females, the sex‐specific difference was also statistically significant. The effect of vitamin D exposure was greater in females than males. This finding might be explained by the associations of estrogen and activated vitamin D and declining levels of estrogen in aging females. Evidence has shown that estrogen may increase the activity of the enzymes responsible for activating vitamin D. 31 Subsequently, it can be hypothesized that declining levels of estrogen in peri‐ and post‐menopausal stages could contribute to vitamin D deficiency in females. In our sample of participants with mean age of 71.2, most of the female participants were post‐menopausal and, we speculate, more likely to have low levels of the activated form of vitamin D due to lower estrogen levels. Therefore, supplementation could have had a greater impact in the elderly female sample due to the relatively lower activated vitamin D levels associated with peri‐ and post‐menopausal changes. Having said that, in our sample, the D+ group had a significantly higher percentage of females, compared to the D− group (D+: 70.5% females, D−: 46.9% females). The higher percentage of females on vitamin D might be explained by the high risk of bone loss, fracture, and osteoporosis among peri‐ and post‐menopausal females and the well‐known protective effects of vitamin D on bone health. Osteoporosis is one of the most common musculoskeletal problems among post‐menopausal females and is more common in females than in males. 32 Estrogen deficiency is one of the key factors contributing to osteoporosis post‐menopause. 33 Several RCTs have demonstrated a positive effect of vitamin D on bone mineral density. 34 The higher risk of osteoporosis in peri‐ and post‐menopausal females compared to males may account for greater vitamin D supplementation rate among older females in our sample. Nonetheless, these findings further contribute to the evidence base highlighting the importance of sex‐specific differences in dementia risk factors. 35 , 36
In both NC and MCI, exposure to vitamin D was associated with significantly lower incidence rate of dementia than no exposure; this association was more robust in NC, which had a 56% reduction in incidence versus MCI, which had a 33% reduction compared to the D− group. Similar findings were observed for each vitamin D formulation. These findings emphasize the importance of interventions early in the disease course, ideally before overt cognitive symptoms. If vitamin D is involved in Aβ clearance, 7 , 8 early supplementation might influence the amyloid cascade at a stage when a greater impact on amyloid and the subsequent production of phosphorylated tau might be observed. In MCI, the disease may have progressed further, with other contributors to progression in play. At this stage, external interventions like vitamin D may be less effective to mitigate dementia relative to earlier in the disease course.
While depression was associated with a 35% greater incidence of dementia, no significant interaction was identified between depression and exposure to vitamin D. The case definition of depression may be a source of variability in the modeling, as the GDS is a self‐report measure with a reference range of only 1 week. 37 This measure may be at risk of poor specificity for depression due to the short reference range resulting in transient or reactive symptoms included as cases. Further, there is a risk of poor sensitivity for self‐reports, possibly due to anosognosia. 38 , 39 Future studies can explore different depression case definitions to determine whether there are depression subgroups more closely linked to neurodegeneration, and more responsive to vitamin D. 40 , 41 , 42 , 43
In our study, while exposure to vitamin D was associated with significantly lower incidence of dementia in both APOE ε4 carriers and non‐carriers, the effect was greater in non‐carriers. Similar findings have been observed in the past, 44 explained by less vitamin D deficiency in APOE ε4 carriers. APOE ε4 carriers may have higher concentrations of circulating vitamin D compared to non‐carriers despite the same intake, due to greater intestinal absorption of dietary vitamin D and lower renal excretion. 45 , 46 With a potentially higher baseline vitamin D level, the APOE ε4 carriers may benefit less from vitamin D supplementation, as demonstrated by our findings. Alternatively, APOE ε4 is the strongest genetic risk factor for AD 47 and vitamin D supplementation may simply not be able to overcome the risk attendant with this allele.
With regard to the different vitamin D formulations, in our study all formulations were associated with lower dementia incidence rates and the HRs did not differ significantly across formulations (calcium–vitamin D, HR = 0.56; cholecalciferol, HR = 0.63; ergocalciferol, HR = 0.61). Past studies on the association of calcium–vitamin D and dementia are limited. One longitudinal study of 4,143 non‐dementia elder females, 2,034 of whom were on calcium carbonate combined with vitamin D and 2,109 on placebo, reported no significant between‐group differences in incident dementia, MCI, or cognitive function. 20 In our study, we had no information on the dosages of calcium and vitamin D taken nor the baseline vitamin D and calcium levels. Future clinical trials with a full account of dosage and baseline levels of vitamin D and calcium, are required to clarify this association. Furthermore, evidence has shown that cholecalciferol may be more effective than ergocalciferol at raising and maintaining serum vitamin D levels. 23 , 24 However, in our study both formulations demonstrated similar effects for lower dementia incidence. More information on dosages and baseline serum levels of each formulation could help clarify this relationship further.
Despite the benefit of a large sample, there are several limitations of this study that merit consideration. Vitamin D exposure was dichotomized to determine exposure‐associated risk. NACC medication sheets did not record information on exposure history; therefore, potential heterogeneities in exposure duration were not accounted for in the analysis. Neither dosing nor baseline vitamin D levels were available and thus, it is unknown if rates of incident dementia differed based on dosing or vitamin D deficiency. Higher doses or greater intake of vitamin D have been linked to better cognition and lower risk, especially in vitamin D deficiency. 18 , 30 , 48 Future clinical trials should consider dosing of vitamin D supplementation, while paying close attention to baseline serum vitamin D levels. Clarifying exposure duration, dose–response relationships, and the role of vitamin D deficiency will be necessary to inform intervention studies. The primary analysis of the present study combined data from exposure to three vitamin D formulations. Participants with baseline exposure to any of these formulations were considered D+, potentially limiting interpretation. However, sensitivity analyses revealed that each formulation alone was associated with a similar lower dementia risk, consistent with the primary findings. However, our study did not account for exposure to supplementations other than vitamin D, which may have had additional contribution in lowering dementia risk. Considering that sun exposure is the most important natural source of vitamin D, the lack of information on participant‐level exposure to sunlight can be considered another limitation of the present study. The nature of the NACC cohort and possible selection bias toward highly educated White participants may limit the generalizability of the findings. 49 Furthermore, differences in socioeconomic status (SES) across participants may have contributed to differences in exposure to vitamin supplementation. Individuals with higher SES may have been more likely to take vitamin supplements. SES may also be associated with healthy lifestyles, pace of biological aging, and risk of dementia. Studies have shown that lower levels of education and wealth were associated with accelerated biological aging, faster memory decline, 50 and substantially greater risk of dementia. 51 While we included education in our models, the NACC dataset has a dearth of information related to SES and therefore, SES differences could not be accounted for in our study. Future studies using cohorts with more comprehensive data on SES and other social factors would certainly provide valuable information on the associations of SES with exposure to supplementation and risk of dementia. Finally, we have no information about the reason for visiting an ADRC, especially for individuals with NC, or for taking vitamin D supplements. Despite these limitations, our findings implicate vitamin D as a potential agent for dementia prevention and provide additional support for its use in at‐risk individuals for AD dementia.
These findings can also inform future studies of vitamin D supplementation and incident dementia for power analyses, appropriate covariates, and effect modification. Future trials should consider differences in sex, race, sun exposure, and APOE status when recruiting participants. Information on dosage of vitamin D supplementation as well as the baseline levels of vitamin D will be essential to further clarify the efficacy and refine the target population for vitamin D supplementation in preventing AD and dementia.
CONFLICTS OF INTEREST STATEMENT
Maryam Ghahremani: none. Eric E. Smith: none. Hung‐Yu Chen: none. Byron Creese: none. Zahra Goodarzi: none. Zahinoor Ismail: personal fees for consulting/advisory boards for Otsuka/Lundbeck; consulting fees paid to institution by Biogen and Roche. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The NACC database is funded by NIA/NIH grant U24‐AG072122. NACC data are contributed by the NIA‐funded ADRCs: P30‐AG019610 (PI Eric Reiman, MD), P30‐AG013846 (PI Neil Kowall, MD), P50‐AG008702 (PI Scott Small, MD), P50‐AG025688 (PI Allan Levey, MD, PhD), P50‐AG047266 (PI Todd Golde, MD, PhD), P30‐AG010133 (PI Andrew Saykin, PsyD), P50‐AG005146 (PI Marilyn Albert, PhD), P50‐AG005134 (PI Bradley Hyman, MD, PhD), P50‐AG016574 (PI Ronald Petersen, MD, PhD), P50‐AG005138 (PI Mary Sano, PhD), P30‐AG008051 (PI Thomas Wisniewski, MD), P30‐AG013854 (PI Robert Vassar, PhD), P30‐AG008017 (PI Jeffrey Kaye, MD), P30AG010161 (PI David Bennett, MD), P50 AG047‐366 (PI Victor Henderson, MD, MS), P30‐AG010129 (PI Charles DeCarli, MD), P50‐AG016573 (PI Frank LaFerla, PhD), P50‐AG005131 (PI James Brewer, MD, PhD), P50‐AG023501 (PI Bruce Miller, MD), P30‐AG035982 (PI Russell Swerdlow, MD), P30‐AG028383 (PI Linda Van Eldik, PhD), P30‐AG053760 (PI Henry Paulson, MD, PhD), P30‐AG010124 (PI John Trojanowski, MD, PhD), P50‐AG005133 (PI Oscar Lopez, MD), P50‐AG005142 (PI Helena Chui, MD), P30‐AG012300 (PI Roger Rosenberg, MD), P30‐AG049638 (PI Suzanne Craft, PhD), P50‐AG005136 (PI Thomas Grabowski, MD), P50‐AG033514 (PI Sanjay Asthana, MD, FRCP), P50‐AG005681 (PI John Morris, MD), P50‐AG047270 (PI Stephen Strittmatter, MD, PhD). Zahinoor Ismail is by the Canadian Institutes of Health Research (BCA2633). Maryam Ghahremani is supported by the Mathison Centre Postdoctoral Fellowship in Mental Health at the University of Calgary, Canada. This study was supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. We also thank Dr. Mary Ganguli from the University of Pittsburgh for her valuable input on the study.
Ghahremani M, Smith EE, Chen H‐Y, Creese B, Goodarzi Z, Ismail Z. Vitamin D supplementation and incident dementia: Effects of sex, APOE, and baseline cognitive status. Alzheimer's Dement. 2023;15:e12404. 10.1002/dad2.12404
REFERENCES
- 1. Gauthier S, Rosa‐Neto P, Morais JA, Webster C. World Alzheimer Report 2021: Journey Through the Diagnosis of Dementia. Alzheimer's Disease International; 2021; [Google Scholar]
- 2. Gauthier S, Albert M, Fox N, et al. Why has therapy development for dementia failed in the last two decades? Alzheimers Dement. 2016;12(1):60‐64. 10.1016/j.jalz.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 3. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montero‐Odasso M, Ismail Z, Livingston G. One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case “for” and “against”. Alzheimers Res Ther. 2020;12(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cashman KD, Dowling KG, Skrabakova Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033‐1044. 10.3945/ajcn.115.120873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sizar O, Khare S, Goyal A, Bansal P, Givler A. Vitamin D Deficiency. StatPearls; 2021. [PubMed] [Google Scholar]
- 7. Guo YX, He LY, Zhang M, Wang F, Liu F, Peng WX. 1,25‐Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Abeta1‐40 brain‐to‐blood efflux and peripheral uptake transport. Neuroscience. 2016;322:28‐38. 10.1016/j.neuroscience.2016.01.041 [DOI] [PubMed] [Google Scholar]
- 8. Mizwicki MT, Menegaz D, Zhang J, et al. Genomic and nongenomic signaling induced by 1alpha,25(OH)2‐vitamin D3 promotes the recovery of amyloid‐beta phagocytosis by Alzheimer's disease macrophages. J Alzheimers Dis. 2012;29(1):51‐62. 10.3233/JAD-2012-110560 [DOI] [PubMed] [Google Scholar]
- 9. Lin CI, Chang YC, Kao NJ, Lee WJ, Cross TW, Lin SH. 1,25(OH)2D3 alleviates Abeta(25‐35)‐induced tau hyperphosphorylation, excessive reactive oxygen species, and apoptosis through interplay with glial cell line‐derived neurotrophic factor signaling in SH‐SY5Y cells. Int J Mol Sci. 2020;21(12):4215. 10.3390/ijms21124215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta‐analysis. Nutr J. 2015;14:76. 10.1186/s12937-015-0063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schietzel S, Fischer K, Brugger P, et al. Effect of 2000 IU compared with 800 IU vitamin D on cognitive performance among adults age 60 years and older: a randomized controlled trial. Am J Clin Nutr. 2019;110(1):246‐253. 10.1093/ajcn/nqz081 [DOI] [PubMed] [Google Scholar]
- 12. Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F. Effects of vitamin D supplementation on cognitive function and blood Abeta‐related biomarkers in older adults with Alzheimer's disease: a randomised, double‐blind, placebo‐controlled trial. J Neurol Neurosurg Psychiatry. 2019;90(12):1347‐1352. 10.1136/jnnp-2018-320199 [DOI] [PubMed] [Google Scholar]
- 13. Beauchet O, Cooper‐Brown LA, Allali G. Vitamin D supplementation and cognition in adults: a systematic review of randomized controlled trials. CNS Drugs. 2021;35(12):1249‐1264. 10.1007/s40263-021-00876-z [DOI] [PubMed] [Google Scholar]
- 14. Du Y, Liang F, Zhang L, Liu J, Dou H. Vitamin D supplement for prevention of Alzheimer's disease: a systematic review and meta‐analysis. Am J Ther. 2021;28(6):e638‐e648. 10.1097/MJT.0000000000001302 [DOI] [PubMed] [Google Scholar]
- 15. DeDea L. Understanding vitamin D formulations. JAAPA. 2013;26(10):10,12. 10.1097/01.JAA.0000435258.26357.4c [DOI] [PubMed] [Google Scholar]
- 16. Jorde R, Kubiak J, Svartberg J, et al. Vitamin D supplementation has no effect on cognitive performance after four months in mid‐aged and older subjects. J Neurol Sci. 2019;396:165‐171. 10.1016/j.jns.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 17. Hu J, Jia J, Zhang Y, Miao R, Huo X, Ma F. Effects of vitamin D3 supplementation on cognition and blood lipids: a 12‐month randomised, double‐blind, placebo‐controlled trial. J Neurol Neurosurg Psychiatry. 2018;89(12):1341‐1347. 10.1136/jnnp-2018-318594 [DOI] [PubMed] [Google Scholar]
- 18. Pettersen JA. Does high dose vitamin D supplementation enhance cognition? A randomized trial in healthy adults. Exp Gerontol. 2017;90:90‐97. 10.1016/j.exger.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 19. Owusu JE, Islam S, Katumuluwa SS, et al. Cognition and Vitamin D in older African‐American Women‐ Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J Am Geriatr Soc. 2019;67(1):81‐86. 10.1111/jgs.15607 [DOI] [PubMed] [Google Scholar]
- 20. Rossom RC, Espeland MA, Manson JE, et al. Calcium and vitamin D supplementation and cognitive impairment in the women's health initiative. J Am Geriatr Soc. 2012;60(12):2197‐1205. 10.1111/jgs.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomized controlled trial of high‐dose vitamin D2 followed by intranasal insulin in Alzheimer's disease. J Alzheimers Dis. 2011;26(3):477‐484. 10.3233/JAD-2011-110149 [DOI] [PubMed] [Google Scholar]
- 22. Przybelski R, Agrawal S, Krueger D, Engelke JA, Walbrun F, Binkley N. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int. 2008;19(11):1621‐1628. 10.1007/s00198-008-0619-x [DOI] [PubMed] [Google Scholar]
- 23. Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25‐hydroxyvitamin D status: a systematic review and meta‐analysis. Am J Clin Nutr. 2012;95(6):1357‐1364. 10.3945/ajcn.111.031070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84(4):694‐697. 10.1093/ajcn/84.4.694 [DOI] [PubMed] [Google Scholar]
- 25. Norman AW. Intestinal calcium absorption: a vitamin D‐hormone‐mediated adaptive response. Am J Clin Nutr. 1990;51(2):290‐300. 10.1093/ajcn/51.2.290 [DOI] [PubMed] [Google Scholar]
- 26. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's disease centers’ uniform data set (UDS): The neuropsychological test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21(3):249‐258. [DOI] [PubMed] [Google Scholar]
- 28. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210‐216. [DOI] [PubMed] [Google Scholar]
- 29. Annweiler C, Montero‐Odasso M, Llewellyn DJ, Richard‐Devantoy S, Duque G, Beauchet O. Meta‐analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimers Dis. 2013;37(1):147‐171. 10.3233/JAD-130452 [DOI] [PubMed] [Google Scholar]
- 30. Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of alzheimer's disease: a 7‐year follow‐up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205‐1211. 10.1093/gerona/gls107 [DOI] [PubMed] [Google Scholar]
- 31. Buchanan JR, Santen R, Cauffman S, Cavaliere A, Greer RB, Demers LM. The effect of endogenous estrogen fluctuation on metabolism of 25‐hydroxyvitamin D. Calcif Tissue Int. 1986;39(3):139‐144. 10.1007/BF02555109 [DOI] [PubMed] [Google Scholar]
- 32. Group ECW. Perimenopausal risk factors and future health. Hum Reprod Update. 2011;17(5):706‐717. 10.1093/humupd/dmr020 [DOI] [PubMed] [Google Scholar]
- 33. Gambacciani M, Spinetti A, de Simone L, et al. The relative contributions of menopause and aging to postmenopausal vertebral osteopenia. J Clin Endocrinol Metab. 1993;77(5):1148‐1151. 10.1210/jcem.77.5.8077305 [DOI] [PubMed] [Google Scholar]
- 34. Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25(4):585‐591. 10.1016/j.beem.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 35. Guan DX, Rockwood K, Smith EE, Ismail Z. Sex moderates the association between frailty and mild behavioral impairment. J Prev Alzheimers Dis. 2022;9(4):692‐700.. 10.14283/jpad.2022.61 [DOI] [PubMed] [Google Scholar]
- 36. Gosselin P, Guan D, Chen H‐Y, et al. The relationship between hearing and mild behavioral impairment and the influence of sex: a study of older adults without dementia from the COMPASS‐ND Study. J Alzheimers Dis Rep. 2022;6(1):57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ismail Z, Gatchel J, Bateman DR, et al. Affective and emotional dysregulation as pre‐dementia risk markers: exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int Psychogeriatr. 2018;30:185‐196. 10.1017/S1041610217001880 [DOI] [PubMed] [Google Scholar]
- 38. Verhülsdonk S, Quack R, Höft B, Lange‐Asschenfeldt C, Supprian T. Anosognosia and depression in patients with Alzheimer's dementia. Arch Gerontol Geriatr. 2013;57(3):282‐287. [DOI] [PubMed] [Google Scholar]
- 39. Ismail Z, Elbayoumi H, Fischer CE, et al. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta‐analysis. JAMA Psychiatry. 2017;74(1):58‐67. 10.1001/jamapsychiatry.2016.3162 [DOI] [PubMed] [Google Scholar]
- 40. Ismail Z, Aguera‐Ortiz L, Brodaty H, et al. The Mild Behavioral Impairment Checklist (MBI‐C): a rating scale for neuropsychiatric symptoms in pre‐dementia populations. J Alzheimers Dis. 2017;56(3):929‐938. 10.3233/JAD-160979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimer's & Dementia. 2016;12(2):195‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miao R, Chen HY, Gill S, et al. Plasma beta‐amyloid in mild behavioural impairment ‐ neuropsychiatric symptoms on the Alzheimer's continuum. J Geriatr Psychiatry Neurol. 2022;35(3):434‐441. 10.1177/08919887211016068 [DOI] [PubMed] [Google Scholar]
- 43. Hu S, Patten S, Charlton A, et al. Validating the mild behavioral impairment checklist in a cognitive clinic: comparisons with the neuropsychiatric inventory questionnaire. J Geriatr Psychiatry Neurol. 2022:8919887221093353. 10.1177/08919887221093353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dursun E, Alaylioglu M, Bilgic B, et al. Vitamin D deficiency might pose a greater risk for ApoEvarepsilon4 non‐carrier Alzheimer's disease patients. Neurol Sci. 2016;37(10):1633‐1643. 10.1007/s10072-016-2647-1 [DOI] [PubMed] [Google Scholar]
- 45. Egert S, Rimbach G, Huebbe P. ApoE genotype: from geographic distribution to function and responsiveness to dietary factors. Proc Nutr Soc. 2012;71(3):410‐424. 10.1017/S0029665112000249 [DOI] [PubMed] [Google Scholar]
- 46. Huebbe P, Nebel A, Siegert S, et al. APOE epsilon4 is associated with higher vitamin D levels in targeted replacement mice and humans. FASEB J. 2011;25(9):3262‐3270. 10.1096/fj.11-180935 [DOI] [PubMed] [Google Scholar]
- 47. Harold D, Abraham R, Hollingworth P, et al. Genome‐wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41(10):1088‐1093. 10.1038/ng.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pettersen JA. Vitamin D and executive functioning: are higher levels better? J Clin Exp Neuropsychol. 2016;38(4):467‐477. 10.1080/13803395.2015.1125452 [DOI] [PubMed] [Google Scholar]
- 49. Babulal GM, Zhu Y, Roe CM, et al. The complex relationship between depression and progression to incident cognitive impairment across race and ethnicity. Alzheimers Dement. 2022;18(12):2593‐2602. 10.1002/alz.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Avila‐Rieger J, Turney IC, Vonk JMJ, et al. Socioeconomic status, biological aging, and memory in a diverse national sample of older us men and women. Neurology. 2022;99(19):e2114‐e2124. 10.1212/WNL.0000000000201032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang AY, Hu HY, Ou YN, et al. Socioeconomic status and risks of cognitive impairment and dementia: a systematic review and meta‐analysis of 39 prospective studies. J Prev Alzheimers Dis. 2023;10(1):83‐94. doi: 10.14283/jpad.2022.81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


