Abstract
Amycolatopsis sp. strain HT-6, a poly(tetramethylene succinate) (PTMS)-degrading actinomycete, was observed to degrade poly(tetramethylene carbonate) (PTMC). In a liquid culture with 150 mg of PTMC film, 59% degradation was achieved, but with a low yield of cell growth. On the other hand, PTMS copolymerized with a small amount of PTMC, forming a copolyester carbonate (PEC) that was completely and rapidly degraded with a high yield of cell growth.
Many approaches have been proposed for solving the worldwide problem of plastic waste, such as recycling and using biodegradable plastic materials. Aliphatic polyester and polycarbonate are two typical plastic polymers with a good potential for use as biodegradable plastic, owing to their susceptibilities to lipolytic enzymes and microorganisms distributed widely in nature (1–5, 7, 8). In general, the higher the melting point of a plastic polymer, the lower the biodegradability tends to be (9). Poly(tetramethylene succinate) (PTMS) is an aliphatic polyester with a high melting point (113°C) (Fig. 1a) and is available commercially as BIONOLLE (Showa High-Polymer, Tokyo, Japan). The enzymatic degradability of PTMS was reported to be lower than that of poly(ɛ-caprolactone) (PCL), a low-melting-point (62°C) aliphatic polyester (9). We evaluated the distribution of PTMS-degrading microorganisms in a soil environment and isolated an actinomycete strain (strain HT-6) capable of degrading PTMS (5). Further studies on its taxonomic characteristics and 16S rRNA gene sequence led to the classification of the strain as a member of the genus Amycolatopsis. The strain has been preserved at the patent depository of the National Institute of Bioscience and Human-Technology under the no. FERM P-15023.
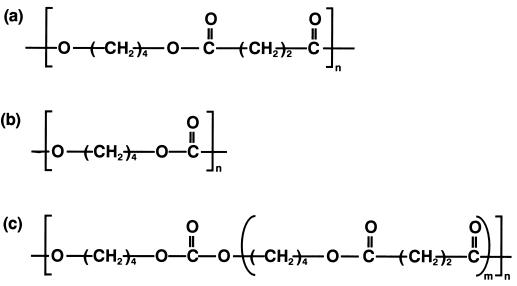
FIG. 1.
Chemical structures of PTMS (a), PTMC (b), and PEC (c).
In addition to the properties of biodegradability and melting point, hydrolysis resistance is also an important factor in biodegradable plastic applications. Aliphatic polycarbonate is known to have greater hydrolysis resistance than aliphatic polyester. However, population counts of poly(ethylene carbonate)- and poly(hexamethylene carbonate)-degrading microorganisms show that degrading microorganisms are found in certain environments (4, 7). Moreover, enzymatic degradation of poly(tetramethylene carbonate) (PTMC) (Fig. 1b) by lipolytic enzymes indicates that even though the enzymes are able to degrade PTMC, the magnitude of PTMC degradation is lower than that of PCL degradation (6). Recently, Mitsubishi Gas Chemical Co. (Yokohama, Japan) has marketed a copolyester carbonate (PEC), namely, poly[oligo(tetramethylene succinate)-co-(tetramethylane carbonate)]. As shown in Fig. 1c, PEC is composed of a polyester part (PTMS) and a polycarbonate part (PTMC). The content of carbonate inside the copolymer is changeable. No study on the degradability of this copolymer has been reported. However, clarification of its properties is needed because the production of this polymer has already been started on an industrial scale. In this study, we evaluated the degradability of PTMC and PEC with Amycolatopsis sp. strain HT-6. At the same time, their hydrolysis resistance was examined and compared with that of other available biodegradable polymers.
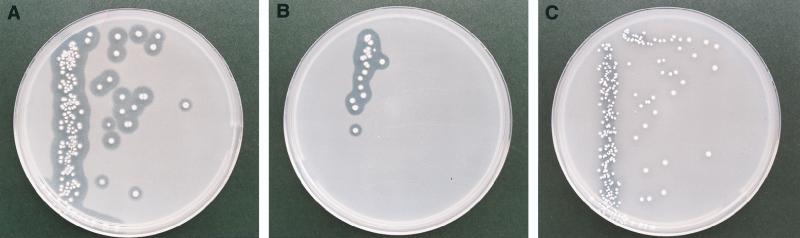
A clear-zone method was used for testing the degrading ability of Amycolatopsis sp. strain HT-6. Agar plates containing basal medium with an emulsion of 0.1% (wt/vol) PTMS (BIONOLLE #1020; Showa High-Polymer), PTMC (prepared by polycondensation of PTMC oligomer obtained from Asahi Chemical Industry Co.), and PEC with a carbonate content of 17.1 mol% (Mitsubishi Gas Chemical Co.) were prepared by the method previously reported (3, 5). After incubation at 30°C for 7 days, clear zones were formed on all tested plates (Fig. 2). Members of our group previously reported that the strain can form clear zones on PCL and poly(β-hydroxybutyrate) plates (5). This suggests that the degrading agent secreted by the strain shows a wide range of substrate specificity, degrading not only ester bonds but also carbonate bonds. In order to measure the degrading and assimilating abilities of the strain, tests of liquid cultures with films of PTMS, PTMC, and PEC with carbonate contents of 5.8, 11.0, 14.8, and 17.1 mol% were then carried out. The average thickness of each film was 50 μm. Prior to the preparation of these cultures, a seed culture was prepared by culturing the strain in 100 ml of basal medium emulsified with 100 mg of PEC (17.1 mol%) on a rotary shaker (180 rpm) at 30°C for 6 days. Ten milliliters of culture broth was then inoculated into 100 ml of basal medium containing 150 mg of each film, followed by shaking for 7 days. Residual film was recovered from the culture broth by three extractions with 100 ml of chloroform. The extract was then dried at 80°C in a vacuum overnight. The cells were collected by centrifugation at 4,500 × g for 10 min and dried at 105°C for 16 h. Experiments were done in triplicate, and the mean value for each set of experiments was determined and used for comparisons. As shown in Table 1, about 70% of the PTMS film was degraded and 32 mg (dry weight) of cells was produced. In the case of PTMC, about 59% of the film was degraded but only 9 mg (dry weight) of cells was formed, which was very close to the amount formed in the cell control, i.e., 6 mg. On the other hand, the PEC film with the low carbonate content of 5.8 mol% was degraded about 68%, i.e., almost the same as that for PTMS degradation, whereas PEC films with carbonate contents of 11.0, 14.8, and 17.1 mol% were degraded completely. The melting points of the PEC films were above 105°C, which is between those of conventional plastic polymers, i.e., polypropylene and polyethylene. It is thought that introducing carbonate into PTMS caused disorder in the crystal structure, thus lowering its melting point and increasing its susceptibility to enzymatic and microbial attacks.
FIG. 2.
Colonies of Amycolatopsis sp. strain HT-6 and clear zones formed on PTMS (A), PEC (17.1 mol% carbonate) (B), and PTMC (C) plates.
TABLE 1.
Properties of PTMS, PTMC, and PEC films in liquid cultures of Amycolatopsis sp. strain HT-6
| Film | Carbonate content (mol%) | Melting point (°C) | Degree of degradation (%) | Growth yield (mg [dry wt] of cells) |
|---|---|---|---|---|
| Controla | 6.2 ± 1.3 | |||
| PTMS | 0.0 | 114 | 70.2 ± 2.1 | 31.6 ± 1.6 |
| PTMC | 100.0 | 65 | 59.4 ± 1.7 | 9.4 ± 1.5 |
| PEC S50-197 | 5.8 | 110 | 67.5 ± 2.3 | 32.7 ± 1.0 |
| PEC S50-16 | 11.0 | 107 | 97.0 ± 0.9 | 38.3 ± 1.8 |
| PEC S50-17 | 14.8 | 104 | 98.6 ± 0.5 | 36.4 ± 1.0 |
| PEC S50-25 | 17.1 | 101 | 98.1 ± 0.6 | 33.2 ± 1.3 |
Control was a culture without film addition.
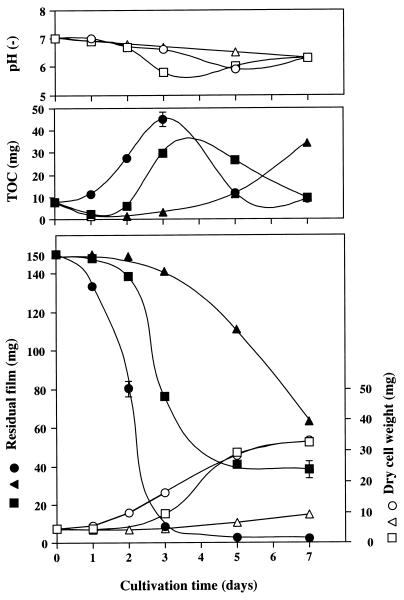
Time courses of PTMS, PTMC, and PEC (17.1 mol% carbonate) film degradations by the strain are shown in Fig. 3. PTMS degradation began at 2 days of culture, and about 40 mg of film remained at 7 days of culture. During the culture, water-soluble total organic carbon (TOC), which was measured with a TOC-5000 analyzer (Shimadzu Corp.), accumulated temporarily, but it then decreased as it was assimilated by the cells. About 35 mg (dry weight) of cells was formed. In a previous study, members of our group identified 1,4-butanediol, 4-hydroxy-n-butyrate, and succinic acid as PTMS degradation products and confirmed that these products could be assimilated by the cells (5). In the case of PTMC degradation, it seems that degradation occurred very slowly; only 83 mg of PTMC film was degraded at 7 days of culture. There was no decrease in TOC accumulation thereafter and only a small amount of cell growth was formed. This result suggests that PTMC degradation products were hardly assimilated by the strain. Suyama and Tokiwa reported that PTMC degradation by lipoprotein lipase from Pseudomonas spp. produced final products such as 1,4-butanediol, carbon dioxide, and di(4-hydroxybutyl) carbonate (6). The degradation of PEC film proceeded rapidly and completely, i.e., more than 95% of the PEC film was degraded in only 3 days. The amount of TOC peaked due to the accumulation of water-soluble degradation products and then decreased, resulting in the formation of about 35 mg (dry weight) of cells.
FIG. 3.
Time courses of PTMS, PTMC, and PEC degradation by Amycolatopsis sp. strain HT-6. ■ and □, PTMS; ▴ and ▵, PTMC; ● and ○, PEC (17.1 mol% carbonate).
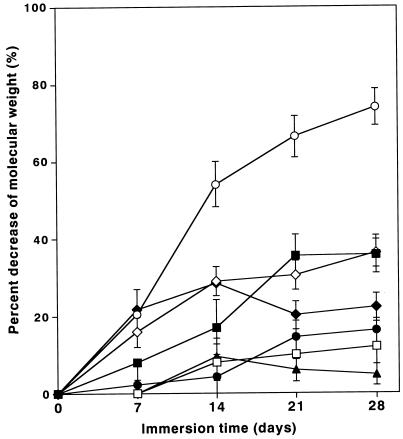
Polymers having carbonate bonds are known to be resistant to hydrolysis. The hydrolysis resistances of PTMC and PEC were examined. Other commercially available biodegradable plastic materials were used for comparison, namely, PCL (TONE; Union Carbide Co.), poly(lactic acid) (PLA) (Lacty; Shimadzu Co.), and poly[(tetramethylene succinate)-co-(tetramethylene adipate)] (PTMS/A) (BIONOLLE #3020; Showa High-Polymer). An amount of 0.5 g of each plastic film (an average of 50 μm in thickness) was immersed in 20 ml of 0.1 M phosphate buffer solution (pH 7.0), followed by shaking in an incubator at 58°C for 28 days. The decrease in molecular weight during immersion was monitored. Molecular weight was measured by gel permeation chromatography (HLC-8020 chromatograph; Tosoh Co.) with a column system of TSK gel 4000 Hxl, 3000 Hxl, 2000 Hxl, and 1000 Hxl. The initial molecular weights of PCL, PTMS, PTMS/A, PTMC, PEC (S50-16), PEC (S50-25), and PLA were 5.8 × 104, 5.8 × 104, 6.4 × 104, 3.7 × 104, 7.7 × 104, 9.0 × 104, and 10.8 × 104, respectively. As shown in Fig. 4, PTMC was the most stable, followed by PCL; their molecular weights decreased only slightly even after 28 days of immersion. The molecular weights of PTMS and PTMS/A dropped from their initial molecular weights by 70%, while PLA was easily hydrolyzed. On the other hand, the decrease in the molecular weight of PEC was less than those of PTMS and PTMS/A, suggesting that PEC is more resistant to hydrolysis.
FIG. 4.
Molecular weight decreases of various biodegradable polymer films during immersion in phosphate buffer (pH 7.0) at 58°C. ■, PTMS; ▴, PTMC; ⧫, PEC (11.0 mol% carbonate); ●, PEC (17.1 mol% carbonate); ◊, PTMS/A; ○, PLA; □, PCL.
The use of biodegradable plastic with properties similar to those of conventional plastic but with high biodegradability is expected. PEC showed hydrolysis resistance between those of PTMC and PTMS. On the other hand, the microbial degradability of PEC was confirmed to be higher than those of both of its constituents. PEC is favorable for blending with starch when a process under high moisture conditions is necessary for fluidizing or gelatinizing the starch in an extruder.
Acknowledgments
We thank Haruo Nishida for advice and valuable information.
REFERENCES
- 1.Abramowicz D A, Keese C R. Enzymatic transesterification of carbonates in water restricted environments. Biotechnol Bioeng. 1989;33:149–156. doi: 10.1002/bit.260330203. [DOI] [PubMed] [Google Scholar]
- 2.Mochizuki M, Mukai K, Yamada K, Ichise N, Murase S, Iwaya Y. Structural effects upon enzymatic hydrolysis of poly(butylene succinate-co-ethylene succinate)s. Macromolecules. 1997;30:7403–7407. [Google Scholar]
- 3.Nishida H, Tokiwa Y. Distribution of poly(β-hydroxybutyrate) and poly(ɛ-caprolactone) aerobic degrading microorganisms in different environments. J Environ Polym Degrad. 1993;1:227–233. [Google Scholar]
- 4.Nishida H, Tokiwa Y. Confirmation of anaerobic poly(2-oxepanone) degrading microorganisms in environments. Chem Lett. 1994;1994:1293–1296. [Google Scholar]
- 5.Pranamuda H, Tokiwa Y, Tanaka H. Microbial degradation of an aliphatic polyester with a high melting point, poly(tetramethylene succinate) Appl Environ Microbiol. 1995;61:1828–1832. doi: 10.1128/aem.61.5.1828-1832.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suyama T, Tokiwa Y. Enzymatic degradation of an aliphatic polycarbonate, poly(tetramethylene carbonate) Enzyme Microb Technol. 1997;20:122–126. [Google Scholar]
- 7.Suyama T, Hosoya H, Tokiwa Y. Bacterial isolates degrading aliphatic polycarbonates. FEMS Microbiol Lett. 1998;161:255–261. doi: 10.1111/j.1574-6968.1998.tb12956.x. [DOI] [PubMed] [Google Scholar]
- 8.Takanashi M, Nomura Y, Yoshida Y, Inoue S. Functional polycarbonate by copolymerization of carbon dioxide and epoxide: synthesis and hydrolysis. Makromol Chem. 1982;183:2085–2092. [Google Scholar]
- 9.Tokiwa Y, Suzuki T, Takeda K. Hydrolysis of polyesters by Rhizopus arrhizus lipase. Agric Biol Chem. 1986;50:1323–1325. [Google Scholar]