Abstract
Background & Aims
The albumin-bilirubin (ALBI) score is calculated using serum levels of total bilirubin and albumin as a simple method to assess liver function. This study investigated the ability of baseline ALBI score/grade measurements to assess histological stage and disease progression in individuals with primary biliary cholangitis (PBC) in a large Japanese nationwide cohort.
Methods
A total of 8,768 Japanese patients with PBC were enrolled between 1980 and 2016 from 469 institutions, among whom 83% received ursodeoxycholic acid (UDCA) only, 9% received UDCA and bezafibrate, and 8% were given neither drug. Baseline clinical and laboratory parameters were retrospectively retrieved and reviewed from a central database. Associations of ALBI score/grade with histological stage, mortality, and need for liver transplantation (LT) were evaluated using Cox proportional hazards models.
Results
During the median follow-up period of 5.3 years, 1,227 patients died (including 789 from liver-related causes) and 113 underwent LT. ALBI score and ALBI grade were significantly associated with Scheuer’s classification (both p <0.0001). ALBI grade 2 or 3 had significant associations with all-cause mortality or need for LT as well as liver-related mortality or need for LT according to Cox proportional hazards regression analysis (hazard ratio 3.453, 95% CI 2.942–4.052 and hazard ratio 4.242, 95% CI 3.421–5.260, respectively; both p <0.0001). Cumulative LT-free survival rates at 5 years in the ALBI grade 1, 2, and 3 groups were 97.2%, 82.4%, and 38.8%, respectively, while respective non-liver-related survival rates were 98.1%, 86.0%, and 42.0% (both p <0.0001, log-rank test).
Conclusions
This large nationwide study of patients with PBC suggested that baseline measurements of ALBI grade were a simple non-invasive predictor of prognosis in PBC.
Impact and implications
Primary biliary cholangitis (PBC) is an autoimmune liver disease characterized by progressive destruction of intrahepatic bile ducts. This study examined the ability of albumin-bilirubin (ALBI) score/grade to estimate histological findings and disease progression in PBC by means of a large-scale nationwide cohort in Japan. ALBI score/grade were significantly associated with Scheuer’s classification stage. Baseline ALBI grade measurements may be a simple non-invasive predictor of prognosis in PBC.
Keywords: Prognosis, Transplantation, Ursodeoxycholic acid
Abbreviations: ALBI, albumin-bilirubin; ALP, alkaline phosphatase; AMA, anti-mitochondrial autoantibody; AUC, area under the ROC curve; BZF, bezafibrate; HR, hazard ratio; LSM, liver stiffness measurement; LT, liver transplantation; M2BPGi, Mac-2-binding protein glycosylation isomer; PBC, primary biliary cholangitis; pc, corrected p; ROC, receiver-operating characteristic; UDCA, ursodeoxycholic acid; ULN, upper limit of normal
Graphical abstract
Highlights
-
•
In Japan, nationwide surveys on PBC have been conducted every 3 years since 1980.
-
•
The ALBI score was associated with Scheuer’s classification in PBC.
-
•
ALBI grade >2 had significant associations with mortality in this cohort study.
-
•
Baseline measurements of ALBI grade may be a simple predictor of prognosis in PBC.
Introduction
Primary biliary cholangitis (PBC) is an autoimmune liver disease characterized by the presence of highly specific anti-mitochondrial autoantibody (AMA) in the serum and the progressive destruction of intrahepatic bile ducts.1 Although the cause of PBC remains poorly understood, genetic factors including human leukocyte antigen (HLA)[2], [3], [4] and non-HLA allelic variants5,6 have been associated with PBC along with environmental factors.1,7
Ursodeoxycholic acid (UDCA) is a first-line treatment for PBC. The drug ameliorates liver biochemistry, delays histological progression to cirrhosis, and improves liver transplantation (LT)-free survival.[8], [9], [10] As one-third of patients treated with UDCA monotherapy show an incomplete treatment response, a combination of UDCA and bezafibrate (BZF) has been used to improve biochemical responses and long-term prognosis in patients with early-stage PBC.[11], [12], [13] In Europe and the US, obeticholic acid (OCA) is a second-line therapy for patients with an inadequate response or intolerance to UDCA. Although the therapeutic effects of OCA have been described in several reports,[14], [15], [16] the drug has not yet been approved by the national medical insurance program of Japan.
A subgroup of patients with PBC are at a high risk of progressing to cirrhosis and, ultimately, liver failure.1 Simple and reliable non-invasive methods, such as Mac-2 binding protein glycosylation isomer17 and vibration-controlled transient elastography,18 have therefore been developed to monitor disease progression.
Recently, a novel method was developed in support of the conventional Child–Pugh grade to assess liver function, called the albumin-bilirubin (ALBI) score/grade, which is calculated using only serum albumin and bilirubin values.19 Although several lines of evidence have demonstrated the usefulness of ALBI score/grade to predict prognosis in patients with PBC,[20], [21], [22] the sample sizes and number of institutions were limited. In Japan, nationwide surveys on PBC have been conducted every 3 years since 1980. The survey database includes information on the background, biochemical laboratory values, histological findings at the time of diagnosis, and prognosis of approximately 8,700 patients registered to date.
The aim of the present study was to validate the ability of ALBI score/grade to estimate histological findings and disease progression in PBC using this large-scale nationwide cohort.
Patients and methods
Patients
Nationwide surveys of patients with PBC have been performed 16 times at intervals of approximately 3 years by the Intractable Hepato-Biliary Diseases Study Group for Research on Measures for Intractable Disease, which is supported by Health Labor Science Research Grants in Japan. A total of 8,768 patients were registered between 1980 and 2016 among 469 institutions. This study was conducted following the principles of the 1975 Declaration of Helsinki. The protocol was reviewed and approved by the Institutional Review Board of Shinshu University School of Medicine (approval number: 4906) and the local institutional review board at each participating institution.
The diagnosis of PBC was based on criteria established by the Intractable Hepato-Biliary Diseases Study Group of Japan.23 Patients who met at least two of the following criteria were diagnosed as having PBC: 1) biochemical evidence of chronic cholestasis; 2) positive serum AMA; and 3) histological features compatible with PBC. In this registry, center type, date of birth and sex, date of diagnosis, presence of pruritus and biochemical test findings (alkaline phosphatase [ALP], total bilirubin, and albumin) at the time of diagnosis, histological stage (Scheuer’s classification), and treatment protocol (UDCA and/or BZF) were recorded as baseline data. Pruritus status in this study was recorded as with/without, not using such semi-quantitative methods as visual analog scales or PBC-40.24 Final follow-up date and outcome at that time (LT, liver-related death, and all-cause death) were noted as well. Longitudinal data on biochemical liver tests, treatment response, and histological stage were unavailable.13 Patients were divided into two groups based on age, <65 and ≥65 years old. Total bilirubin and albumin were categorized as normal or abnormal, ALP as low (≤1.67x upper limit of normal [ULN]) or high (>1.67x ULN), and histological stage as early (1–2) or late (3–4). PBC symptoms were defined as pruritus, overt jaundice, rupture of esophagogastric varices, ascites, and hepatic encephalopathy.25
ALBI calculations
ALBI score was calculated based on a previous study,19 as follows: (log10 bilirubin × 17.1 × 0.66) + (albumin × 10 × -0.085), with bilirubin in mg/dl and albumin in g/dl. ALBI grade cut-off points were as follows: ≤−2.60 (grade 1), >−2.60 to ≤−1.39 (grade 2), and >−1.39 (grade 3).
Statistical analysis
Categorical variables were compared by the chi-squared test. Continuous baseline data were expressed as the median and 95% CI and statistically evaluated using the Mann-Whitney U test. Receiver-operating characteristic (ROC) curves were used to evaluate the prediction accuracy for histological stage. Optimal cut-off values were determined by Youden’s index. Survival without LT and survival without liver-related death or LT were analyzed using a multivariate Cox proportional hazards model. All variables included in the model were obtained at baseline. Multivariate analysis included all significant (p <0.05) variables in the preceding univariate analysis. The Kaplan-Meier method and log-rank testing were used to estimate disease progression. A p value of <0.05 was considered statistically significant after Bonferroni correction for multiple testing. Statistical analyses were performed using StatFlex ver. 7.0.11 (Artech Co., Ltd., Osaka, Japan).
Results
Clinical characteristics of patients
The clinical profile of the cohort is shown in Table 1. Median age at the time of diagnosis was 57 years, with a female predominance (86%). Median follow-up period was 5.3 years (95% CI 0.2–20.1). Of the entire cohort, 29% of patients were symptomatic. We found that 83% of patients received UDCA only, 9% received UDCA+BZF, and 8% received no treatment. Median ALBI score was -2.74 and the percentages of ALBI grades 1, 2, and 3 were 63%, 33%, and 4%, respectively. Scheuer’s stage was I for 35%, II for 23%, III for 10%, and IV for 3% of patients. During the observation period, all-cause death, liver-related death, and LT were recorded in 14%, 9%, and 1% of patients, respectively. Notably, 87% of patients were enrolled by tertiary facilities, suggesting a higher presence of individuals with advanced disease than in general clinics and correspondingly higher mortality rates.
Table 1.
Characteristics of 8,768 patients with PBC.
| Characteristic | Median (95% CI) or n (%) |
|---|---|
| Age at diagnosis (years) | 57 (49–65) |
| Female | 7,552 (86%) |
| Follow-up period (years) | 5.3 (0.2–20.1) |
| Clinical stage (asymptomatic/symptomatic) | 6,246 (71%)/2,522 (29%) |
| Treatment (UDCA/UDCA + BZF/BZF/none) | 7,238 (83%)/770 (9%)/40 (0%)/720 (8%) |
| ALP ( × ULN) | 1.43 (0.55–4.79) |
| Total bilirubin (mg/dl) | 0.7 (0.4–3.6) |
| Albumin (g/dl) | 4.1 (3.0–4.7) |
| ALBI score | -2.74 (-3.36 to -1.52) |
| ALBI grade (1/2/3) | 5,543 (63%)/2,888 (33%)/337 (4%) |
| Scheuer’s stage (I/II/III/IV/NA) | 3,089 (35%)/2,019 (23%)/866 (10%)/282 (3%)/2,512 (29%) |
| All-cause death/liver-related death/liver transplantation | 1,227 (14%)/789 (9%)/113 (1%) |
ALBI, albumin-bilirubin; ALP, alkaline phosphatase; BZF, bezafibrate; PBC, primary biliary cholangitis; NA, not assessed; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Diagnostic ability of ALBI score/grade for histological stage
Fig. 1 presents box plots of ALBI score values according to Scheuer’s classification stage. Median ALBI score increased with Scheuer’s stage: −2.85 (stage I), −2.70 (stage II), −2.50 (stage III), and −2.03 (stage IV). We detected a significant correlation between ALBI score and disease progression defined by Scheuer’s classification (corrected p [pc] <0.0001). Moreover, significant differences were noted for ALBI score among Scheuer’s classifications (p <0.0001). When disease was stratified as early stage (Scheuer’s stage I and II) or late stage (Scheuer’s stage III and IV), the median ALBI score in the late-stage group was significantly higher than that in the early-stage group (−2.39 vs. −2.81, p <0.0001) (Fig. S1).
Fig. 1.
Distribution of ALBI score by Scheuer’s classification in patients with PBC.
Boxes represent the interquartile range of the data. The lines across the boxes indicate the median value. p values were calculated by the Mann-Whitney U test with Bonferroni correction. ALBI, albumin-bilirubin; PBC, primary biliary cholangitis.
The proportions of patients with different Scheuer’s classification were then stratified by ALBI grade (Fig. 2). Patients with ALBI grade 1 accounted for 76% of patients in stage I, 62% in stage II, 40% in stage III, and 22% in stage IV. The proportion of patients with ALBI grade 1 fell significantly with increasing Scheuer’s stage (p <0.0001). For patients with early-stage or late-stage disease, the proportions with ALBI grade 1 were 70% and 36%, with ALBI grade 2 were 28% and 55%, and with ALBI grade 3 were 1% and 10%, respectively (p <0.0001) (Fig. S2).
Fig. 2.
Proportion of patients with PBC by Scheuer’s classification stratified by ALBI grade.
The proportion of patients with ALBI grade 1 was 76% for Scheuer’s stage I, 62% for stage II, 40% for stage III, and 22% for stage IV. The proportion of patients with ALBI grade 1 fell with increasing stage (p <0.0001). p values were calculated by the chi-squared test. ALBI, albumin-bilirubin; PBC, primary biliary cholangitis.
Since ALBI score is a useful marker to assess histological stage, ROC curve analysis was performed to determine the diagnostic accuracy of ALBI score for each stage of Scheuer’s classification. The calculated area under the ROC curve (AUC), optimal cut-off value, sensitivity, specificity, positive predictive value, and negative predictive value for each stage are listed in Table S1. The AUCs for predicting stage ≥III (late stage) and IV (cirrhosis) were 0.726 and 0.808, respectively, for which the respective optimal cut-off values were -2.59, and -2.35.
Prediction and risk factors associated with all-cause death, liver-related death, and need for LT
As shown in Table S2, 1,340 patients experienced all-cause death or need for LT and 902 patients experienced liver-related mortality or need for LT during follow-up. Patients with liver-related mortality or need for LT were significantly younger than those who survived transplant free (p <0.0001). The frequencies of asymptomatic and ALBI grade 1 were significantly lower for the all-cause mortality or need for LT and the liver-related mortality or need for LT groups, respectively (all p <0.0001). The levels of ALP and total bilirubin were significantly higher in the non-survivor groups (all p <0.0001). ALBI score was significantly worse in the non-survivor groups compared to the LT-free survivor group (both p <0.0001). Among patients who died of any cause, the frequency of females was significantly lower (p = 0.002), but this was not observed for liver-related mortality.
According to Cox proportional hazards regression analysis, all-cause mortality or need for LT and liver-related mortality or need for LT were significantly associated with age ≥65 years (hazard ratio [HR] 2.074; 95% CI 1.777–2.421; p <0.0001 and HR 1.268; 95% CI 1.026–1.566; p = 0.028, respectively), being symptomatic (HR 2.721; 95% CI 2.347–3.154; p <0.0001 and HR 3.735; 95% CI 3.084–4.524; p <0.0001, respectively), ALP ≥1.67x ULN (HR 1.577; 95% CI 1.357–1.834; p <0.0001 and HR 1.927; 95% CI 1.577–2.355; p <0.0001, respectively), and ALBI grade ≥2 (HR 3.453; 95% CI 2.942–4.052; p <0.0001 and HR 4.242; 95% CI 3.421–5.260; p <0.0001, respectively) (Table 2, Table 3). Female sex was a protective factor against all-cause mortality or need for LT (HR 1.460; 95% CI 1.211–1.761; p <0.0001), but not against liver-related mortality or need for LT.
Table 2.
Factors associated with all-cause mortality or LT in PBC.
| Factor | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age ≥65 years | 1.780 | 1.577–2.013 | <0.0001 | 2.074 | 1.777–2.421 | <0.0001 |
| Male | 1.524 | 1.319–1.761 | <0.0001 | 1.460 | 1.211–1.761 | <0.0001 |
| Symptomatic | 4.937 | 4.413–5.524 | <0.0001 | 2.721 | 2.347–3.154 | <0.0001 |
| ALP ≥1.67 xULN | 2.281 | 1.976–2.634 | <0.0001 | 1.577 | 1.357–1.834 | <0.0001 |
| Total bilirubin ≥1.0 mg/dl | 4.358 | 3.907–4.862 | <0.0001 | |||
| Albumin <3.9 g/dl | 3.619 | 3.247–4.034 | <0.0001 | |||
| ALBI score ≥-2.518 | 5.127 | 4.587–5.731 | <0.0001 | |||
| ALBI grade ≥2 | 4.633 | 4.131–5.195 | <0.0001 | 3.453 | 2.942–4.052 | <0.0001 |
The Cox proportional hazard model included age, sex, symptomatic status, ALP, and ALBI grade for calculations considering the multicollinearity of albumin, total bilirubin, and ALBI grade, whose condition number was 1.443.
ALBI, albumin-bilirubin; ALP, alkaline phosphatase; HR, hazard ratio; LT, liver transplantation; PBC, primary biliary cholangitis; ULN, upper limit of normal.
Table 3.
Factors associated with liver-related mortality or LT in PBC.
| Factor | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age ≥65 years | 1.181 | 1.003–1.390 | 0.046 | 1.268 | 1.026–1.566 | 0.028 |
| Male | 1.268 | 1.050–1.532 | <0.0001 | |||
| Symptomatic | 7.485 | 6.470–8.660 | <0.0001 | 3.735 | 3.084–4.524 | <0.0001 |
| ALP ≥1.67 xULN | 3.107 | 2.570–3.757 | <0.0001 | 1.927 | 1.577–2.355 | <0.0001 |
| Total bilirubin ≥1.0 mg/dl | 6.702 | 5.820–7.719 | <0.0001 | |||
| Albumin <3.9 g/dl | 4.192 | 3.668–4.790 | <0.0001 | |||
| ALBI score ≥-2.518 | 7.040 | 6.107–8.115 | <0.0001 | |||
| ALBI grade ≥2 | 5.928 | 5.123–6.860 | <0.0001 | 4.242 | 3.421–5.260 | <0.0001 |
The Cox proportional hazard model included age, sex, symptomatic status, ALP, and ALBI grade for calculations considering the multicollinearity of albumin, total bilirubin, and ALBI grade, whose condition number was 1.440.
ALBI, albumin-bilirubin; ALP, alkaline phosphatase; HR, hazard ratio; LT, liver transplantation; PBC, primary biliary cholangitis; ULN, upper limit of normal.
Cumulative incidences of survival without LT and survival free of liver-related death or LT
The cumulative survival rates without LT and free of liver-related death or LT at 5 years in the ALBI grade 1, 2, and 3 groups were 97.2%, 82.4%, and 38.8%, and 98.1%, 86.0%, and 42.0%, respectively (Fig. 3). Kaplan-Meier testing by ALBI grade yielded three distinct curves (pc <0.0001, log-rank test). Moreover, significant differences were found in group comparisons (ALBI grade 1 vs. grade 2, ALBI grade 1 vs. grade 3, and ALBI grade 2 vs. grade 3, all pc <0.0001, log-rank test).
Fig. 3.
Cumulative LT-free survival rates of patients with PBC according to ALBI grade.
Cumulative incidences of (A) all-cause mortality or LT and (B) liver-related mortality or LT (corrected p <0.0001 and corrected p <0.0001, respectively, log-rank test). ALBI, albumin-bilirubin; LT, liver transplantation; PBC, primary biliary cholangitis.
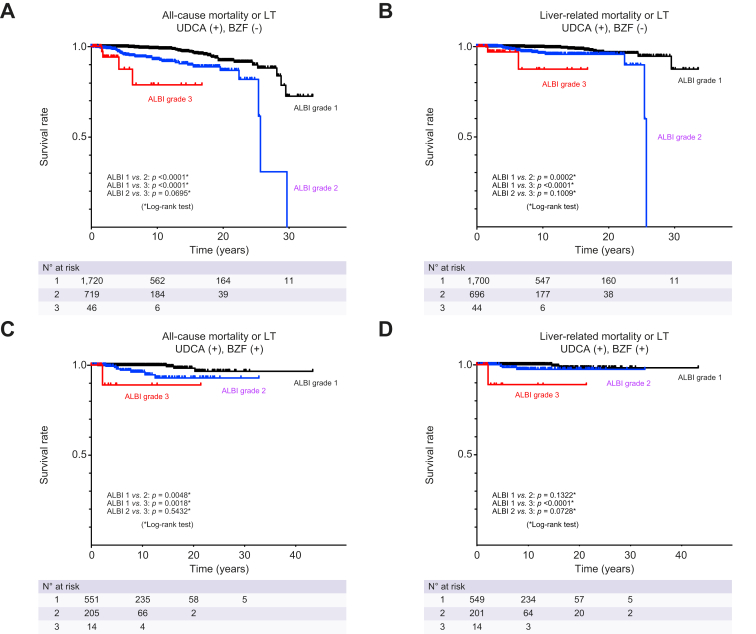
Recent studies have shown that both UDCA monotherapy and UDCA+BZF combination therapy improve PBC prognosis. Therefore, two groups consisting of patients receiving UDCA monotherapy and those receiving BZF in addition to UDCA were created to examine whether ALBI grade could predict survival. Patients who received BZF only were excluded due to insufficient numbers. Patients receiving UDCA+BZF combination therapy exhibited a more favorable prognosis than those receiving UDCA alone (p = 0.003) (Fig. S3), which confirmed the results of Tanaka et al.13 Among patients receiving UDCA monotherapy, ALBI grade 1 was associated with significantly better survival without LT and survival free of liver-related death or LT compared with ALBI grade 2 and ALBI grade 3, respectively (both p <0.001) (Fig. 4). We observed a non-significant difference between ALBI grade 2 and 3 for all-cause mortality or LT (p = 0.0695) and for liver-related mortality or LT (p = 0.1009). In addition, among patients receiving UDCA+BZF, those with ALBI grade 1 had significantly increased rates of LT-free survival than those with ALBI grade 2 and 3 (both p <0.005). A significant difference in liver-related mortality or LT was also seen between ALBI grade 1 and 3 (p <0.0001). Lastly, the cumulative survival rates without LT and free of liver-related death or LT were assessed by log-rank testing, revealing significant differences based on ALBI grade, as well as variables including age, sex, clinical stage, and ALP level (all p <0.05) (Fig. S4–7).
Fig. 4.
Cumulative LT-free survival rates of patients with PBC according to ALBI grade and treatment.
Cumulative incidences of (A) all-cause mortality or LT and (B) liver-related mortality or LT in patients treated with UDCA only. Cumulative incidences of (C) all-cause mortality or LT and (D) liver-related mortality or LT in patients treated with UDCA and BZF. p values were calculated by the log-rank test. ALBI, albumin-bilirubin; BZF, bezafibrate; LT, liver transplantation; PBC, primary biliary cholangitis; UDCA, ursodeoxycholic acid.
Discussion
The present study confirmed that ALBI score/grade could predict PBC progression, which was in agreement with previous smaller cohorts.[20], [21], [22] To our knowledge, this is the largest multicenter cohort study to date evaluating the efficacy of ALBI score/grade in Japanese patients with PBC. We identified two important clinical characteristics: 1) ALBI score/grade may be useful in estimating pathological stage according to Scheuer’s classification, and 2) ALBI grade may be useful in predicting the prognosis of patients with PBC.
The ALBI score has been studied in 181 patients with biopsy-proven PBC to date, including only three patients with Scheuer’s stage IV.21 Since histological findings were available for over 7,000 patients in this cohort as well as 1,184 patients with late-stage disease, there was a sufficient number of cases to clarify the relationship between ALBI score and histopathology. Our results showed that ALBI score/grade correlated significantly with histological stage and between early and late disease stages. It is clinically important to identify late-stage disease or cirrhosis at diagnosis using non-invasive tests, since the level of fibrosis is a key parameter for risk stratification in PBC. In this study, the diagnostic accuracy of ALBI for cirrhosis was 80.1% (Table S1). Elsewhere, the accuracy of liver stiffness measurement (LSM) by vibration-controlled transient elastography was 93.6%18 and that of the serum biomarker M2BPGi was 74.8%,17 which indicated that diagnostic ability was highest for LSM. As the ALBI score had a high negative predictive value of 98.2% (Table S1), an ALBI value of -2.38 or less may enable the prognostication of patients without cirrhosis. Murillo et al. reported advanced histological fibrosis as an independent predictor of treatment failure and survival despite a biochemical response to treatment in patients with PBC.26 Thus, the prompt identification of clinically relevant fibrosis at diagnosis will enable earlier consideration of treatment escalation. Since Scheuer’s classification was not designed to directly assess liver fibrosis, it was difficult to pinpoint the utility of ALBI score/grade for the determination of liver fibrosis. Another pathological classification by Nakanuma et al.27 might be more suitable to estimate liver fibrosis, but we were unable to adopt it as most patients were enrolled before its development. Fujita et al. assessed ALBI score in 382 Japanese patients with chronic hepatitis C29. Median ALBI scores in patients with F4 were -1.823 in their study and -2.03 in our own. Although similar, these findings may not be directly comparable due to possible differences in ALBI values for hepatitis C infection and PBC, the latter of which is a cholestatic disease.
Gennaro et al. identified that serum albumin and bilirubin levels were the most significant prognostic variables for survival in cirrhosis in a review of 118 studies.29 Accordingly, ALBI grade is calculated using those parameters. There have been several reports on the efficacy of ALBI grade to predict the clinical outcome of chronic liver disease. Hiraoka et al. observed the usefulness of ALBI grade for prognostic evaluation in 2,584 Japanese patients with hepatocellular carcinoma.30 Several other studies have supported the clinical utility of ALBI score in estimating the prognosis of chronic hepatitis C,28 chronic hepatitis B,31 and PBC.20,21 In particular, ALBI score showed the highest AUC values for predicting overall survival and incidence of LT within 2 years after the start of observation in a Japanese PBC cohort of 409 patients.22 The present study validated the efficacy of ALBI grade to predict survival in patients with PBC using a large-scale nationwide database with a median follow-up period of over 5 years. Another strength of this investigation was a relatively high number of cases with advanced disease, including 866 patients at Scheuer’s stage III and 282 patients at stage IV, along with the outcomes of all-cause death in 1,227 patients (14%), liver-related death in 789 patients (9%), and LT in 113 patients (1%). Supported by a larger cohort power, our results showed that all-cause mortality or need for LT and liver-related mortality or need for LT had significant associations with ALBI grade. While patients with ALBI grade 3 tended to exhibit a more rapid progression to death, those with ALBI grade 1 showed a lower mortality risk. Moreover, ALBI grade remained a good predictive marker when patients were stratified by age, symptomatic status, or ALP level, as well as in patients receiving UDCA monotherapy or the combination of BZF and UDCA.
This study had several limitations mainly due to its retrospective nature. First, there was a lack of several biochemical parameters required to calculate the FIB-4 index and APRI. Second, LSM reportedly has very high diagnostic accuracy for cirrhosis,18 but had not been developed at the start of the present study. We have already begun an investigation of LSM and PBC pathogenesis in another Japanese cohort. Third, although risk scores that take into account treatment responsiveness, such as the UK-PBC risk score,32 GLOBE score,33 and Ehime score,34 are often used to predict the prognosis of patients with PBC, our national survey database did not contain laboratory data after the start of treatment, and so we were unable to estimate prognosis based on treatment responsiveness. Therefore, it is difficult to make definitive conclusions on the clinical usefulness of the ALBI score on treatment effect. On the other hand, if prognosis predicted by ALBI score before treatment is judged to be poor, a second line can be added from treatment onset to provide combined therapy. This is a future issue to consider as it is possible to improve long-term prognosis by modifying the initial therapeutic regimen based on pre-treatment ALBI results. Lastly, many reported symptoms in this study were extracted as subjective descriptions from the medical records of each facility, without specific judgement criteria.
In conclusion, this large nationwide study of patients with PBC showed that baseline measurements of ALBI score/grade might serve as a simple non-invasive predictor of histology and prognosis in PBC.
Financial support
This work was supported by JSPS KAKENHI Grant Number 20K08282 and the MHLW Research Program on Intractable Hepatobiliary Disease, grant number JPMH20FC1023.
Authors’ contributions
Conceptualization: YY, TU. Data curation: JH, TN. Formal analysis: YY. Investigation: YY, TU. Resources: YY, TU, TK, SJ, AK, AT. Supervision: AK, AT. Visualization: YY, TU. Writing – original draft: YY, TU. All coauthors were involved in the process of reviewing the drafts at various stages, giving their expertise-based suggestions and critical comments to produce the final version of the manuscript. The final version of the manuscript has been approved by all co-authors.
Data availability statement
The dataset generated during this study is available from the corresponding author upon reasonable request.
Conflict of interest
Dr. A. Komori reports receiving consultant fees from Kowa Company and Kaken Pharmaceutical Co. Dr. A. Tanaka reports receiving consultant fees from GlaxoSmithKline and Kowa Company. The remaining authors have nothing to disclose regarding industry funding or other conflicts of interest with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
The authors thank Trevor Ralph for his editorial assistance.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100662.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Selmi C., Bowlus C.L., Gershwin M.E., Coppel R.L. Primary biliary cirrhosis. Lancet. 2011;377:1600–1609. doi: 10.1016/S0140-6736(10)61965-4. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson P.T., Baragiotta A., Heneghan M.A., Floreani A., Venturi C., Underhill J.A., et al. HLA class II alleles, genotypes, haplotypes, and amino acids in primary biliary cirrhosis: a large-scale study. Hepatology. 2006;44:667–674. doi: 10.1002/hep.21316. [DOI] [PubMed] [Google Scholar]
- 3.Invernizzi P., Selmi C., Poli F., Frison S., Floreani A., Alvaro D., et al. Human leukocyte antigen polymorphisms in Italian primary biliary cirrhosis: a multicenter study of 664 patients and 1992 healthy controls. Hepatology. 2008;48:1906–1912. doi: 10.1002/hep.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umemura T., Joshita S., Ichijo T., Yoshizawa K., Katsuyama Y., Tanaka E., et al. Human leukocyte antigen class II molecules confer both susceptibility and progression in Japanese patients with primary biliary cirrhosis. Hepatology. 2012;55:506–511. doi: 10.1002/hep.24705. [DOI] [PubMed] [Google Scholar]
- 5.Joshita S., Umemura T., Yoshizawa K., Katsuyama Y., Tanaka E., Nakamura M., et al. Association analysis of cytotoxic T-lymphocyte antigen 4 gene polymorphisms with primary biliary cirrhosis in Japanese patients. J Hepatol. 2010;53:537–541. doi: 10.1016/j.jhep.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura M., Nishida N., Kawashima M., Aiba Y., Tanaka A., Yasunami M., et al. Genome-wide association study identifies TNFSF15 and POU2AF1 as susceptibility loci for primary biliary cirrhosis in the Japanese population. Am J Hum Genet. 2012;91:721–728. doi: 10.1016/j.ajhg.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershwin M.E., Selmi C., Worman H.J., Gold E.B., Watnik M., Utts J., et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poupon R.E., Balkau B., Eschwege E., Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 9.Poupon R.E., Poupon R., Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N Engl J Med. 1994;330:1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 10.Angulo P., Batts K.P., Therneau T.M., Jorgensen R.A., Dickson E.R., Lindor K.D., et al. Long-term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology. 1999;29:644–647. doi: 10.1002/hep.510290301. [DOI] [PubMed] [Google Scholar]
- 11.Corpechot C., Chazouilleres O., Rousseau A., Le Gruyer A., Habersetzer F., Mathurin P., et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378:2171–2181. doi: 10.1056/NEJMoa1714519. [DOI] [PubMed] [Google Scholar]
- 12.Honda A., Tanaka A., Kaneko T., Komori A., Abe M., Inao M., et al. Bezafibrate improves GLOBE and UK-PBC scores and long-term outcomes in patients with primary biliary cholangitis. Hepatology. 2019;70:2035–2046. doi: 10.1002/hep.30552. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka A., Hirohara J., Nakano T., Matsumoto K., Chazouilleres O., Takikawa H., et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2021;75:565–571. doi: 10.1016/j.jhep.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Nevens F., Andreone P., Mazzella G., Strasser S.I., Bowlus C., Invernizzi P., et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 15.D'Amato D., De Vincentis A., Malinverno F., Vigano M., Alvaro D., Pompili M., et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep. 2021;3 doi: 10.1016/j.jhepr.2021.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murillo Perez C.F., Fisher H., Hiu S., Kareithi D., Adekunle F., Mayne T., et al. Greater transplant-free survival in patients receiving obeticholic acid for primary biliary cholangitis in a clinical trial setting compared to real-world external controls. Gastroenterology. 2022;163:1630–1642. doi: 10.1053/j.gastro.2022.08.054. [DOI] [PubMed] [Google Scholar]
- 17.Umemura T., Joshita S., Sekiguchi T., Usami Y., Shibata S., Kimura T., et al. Serum wisteria floribunda agglutinin-positive mac-2-binding protein level predicts liver fibrosis and prognosis in primary biliary cirrhosis. Am J Gastroenterol. 2015;110:857–864. doi: 10.1038/ajg.2015.118. [DOI] [PubMed] [Google Scholar]
- 18.Cristoferi L., Calvaruso V., Overi D., Viganò M., Rigamonti C., Degasperi E., et al. Accuracy of transient elastography in assessing fibrosis at diagnosis in naïve patients with primary biliary cholangitis: a dual cut-off approach. Hepatology. 2021;74:1496–1508. doi: 10.1002/hep.31810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson P.J., Berhane S., Kagebayashi C., Satomura S., Teng M., Reeves H.L., et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan A.W., Chan R.C., Wong G.L., Wong V.W., Choi P.C., Chan H.L., et al. New simple prognostic score for primary biliary cirrhosis: albumin-bilirubin score. J Gastroenterol Hepatol. 2015;30:1391–1396. doi: 10.1111/jgh.12938. [DOI] [PubMed] [Google Scholar]
- 21.Fujita K., Nomura T., Morishita A., Shi T., Oura K., Tani J., et al. Prediction of transplant-free survival through albumin-bilirubin score in primary biliary cholangitis. J Clin Med. 2019;8 doi: 10.3390/jcm8081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T., Ishigami M., Morooka H., Yamamoto K., Imai N., Ishizu Y., et al. The albumin-bilirubin score as a predictor of outcomes in Japanese patients with PBC: an analysis using time-dependent ROC. Sci Rep. 2020;10 doi: 10.1038/s41598-020-74732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidelines for the management of primary biliary cirrhosis: the intractable hepatobiliary disease study group supported by the ministry of health, labour and welfare of Japan. Hepatol Res. 2014;44(Suppl S1):71–90. doi: 10.1111/hepr.12270. [DOI] [PubMed] [Google Scholar]
- 24.Jacoby A., Rannard A., Buck D., Bhala N., Newton J.L., James O.F., et al. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut. 2005;54:1622–1629. doi: 10.1136/gut.2005.065862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince M.I., Chetwynd A., Craig W.L., Metcalf J.V., James O.F. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865–870. doi: 10.1136/gut.2003.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murillo Perez C.F., Hirschfield G.M., Corpechot C., Floreani A., Mayo M.J., van der Meer A., et al. Fibrosis stage is an independent predictor of outcome in primary biliary cholangitis despite biochemical treatment response. Aliment Pharmacol Ther. 2019;50:1127–1136. doi: 10.1111/apt.15533. [DOI] [PubMed] [Google Scholar]
- 27.Nakanuma Y., Zen Y., Harada K., Sasaki M., Nonomura A., Uehara T., et al. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: interobserver agreement. Pathol Int. 2010;60:167–174. doi: 10.1111/j.1440-1827.2009.02500.x. [DOI] [PubMed] [Google Scholar]
- 28.Fujita K., Oura K., Yoneyama H., Shi T., Takuma K., Nakahara M., et al. Albumin-bilirubin score indicates liver fibrosis staging and prognosis in patients with chronic hepatitis C. Hepatol Res. 2019;49:731–742. doi: 10.1111/hepr.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Hiraoka A., Kumada T., Michitaka K., Toyoda H., Tada T., Ueki H., et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1031–1036. doi: 10.1111/jgh.13250. [DOI] [PubMed] [Google Scholar]
- 31.Chen R.C., Cai Y.J., Wu J.M., Wang X.D., Song M., Wang Y.Q., et al. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis B-related cirrhosis. J Viral Hepat. 2017;24:238–245. doi: 10.1111/jvh.12638. [DOI] [PubMed] [Google Scholar]
- 32.Carbone M., Sharp S.J., Flack S., Paximadas D., Spiess K., Adgey C., et al. The UK-PBC risk scores: derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology. 2016;63:930–950. doi: 10.1002/hep.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammers W.J., Hirschfield G.M., Corpechot C., Nevens F., Lindor K.D., Janssen H.L., et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804–1812 e1804. doi: 10.1053/j.gastro.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 34.Azemoto N., Abe M., Murata Y., Hiasa Y., Hamada M., Matsuura B., et al. Early biochemical response to ursodeoxycholic acid predicts symptom development in patients with asymptomatic primary biliary cirrhosis. J Gastroenterol. 2009;44:630–634. doi: 10.1007/s00535-009-0051-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated during this study is available from the corresponding author upon reasonable request.