Summary
Physical activity benefits both fitness and cognition. However, its effect on long-term memory is unclear. In this study, we evaluated the effect of acute and chronic exercise on long-term spatial memory for a new virtual reality task. Participants were immersed in the virtual environment and navigated a wide arena that included target objects. We assessed spatial memory in two conditions (encoded targets separated by a short or long distance) and found that 25 min of cycling after encoding — but not before retrieval — was sufficient to improve the long-term memory retention for the short, but not for the long distance. Furthermore, we found that participants who engaged in regular physical activity showed memory for the short-distance condition whereas controls did not. Thus, physical activity could be a simple way to improve spatial memories.
Subject areas: Biological sciences, Neuroscience, Behavioral neuroscience, Cognitive neuroscience
Graphical abstract

Highlights
-
•
We developed a spatial memory task in a virtual reality environment
-
•
An acute bout of physical activity improved spatial memory consolidation
-
•
Chronic exercise group showed benefits for spatial memory
-
•
Acute physical activity did not affect spatial memory retrieval
Biological sciences; Neuroscience; Behavioral neuroscience; Cognitive neuroscience
Introduction
Physical activity has been shown to benefit both fitness and cognition. In particular, Blondell, Hammersley-Mather, and Veerman1 have shown that regular aerobic exercise protects against cognitive decline. Also, regular aerobic exercise considerably improves performance in tasks that involve attention, processing speed, and executive function.2 However, the relationship between aerobic exercise and memory is not well understood. For example, Voss et al.3 have summarized many findings linking physical activity and memory and concluded that there is a positive effect of aerobic exercise on different types of memory such as visuospatial, story recall, and relational in both cognitively normal middle-aged and older adults. On the other hand, Roig et al.4 concluded in another review that long-term cardiovascular exercise has no significant effect on long-term memory and produces only small improvements in short-term memory. No negative effects of physical activity were found on cognitive functions. Even though most of the above-mentioned studies have shown numerous benefits of aerobic exercise, most of them have evaluated the benefits of chronic physical activity. Several studies have shown that the rate of abandonment of these practices is very high and is owing to various factors mainly related to lack of time.5 Therefore, differentiating the effects of chronic and acute physical activity is highly relevant to clinical and everyday applications.
Other studies have focused on the effect of short bouts of exercise on various cognitive functions. A single session of variable duration of moderate aerobic exercise has been shown to improve numerous executive functions such as attention,6 inhibitory control,7 and cognitive flexibility,8 among other cognitive processes (for a review, see Tomporowski9). In addition, several investigations studied the effect of a single bout of exercise on short-term memory, showing a significant effect of a 10-min walk followed by a 15- to 30-min rest on the recall of words learned immediately after exercise.10 Also, they showed that 30 min of cycling before learning a story improved its short-term retention 30 min later11 compared to a control group. Although these studies improved our understanding of the acute effects of exercise on memory formation, few studies evaluated the impact of physical activity on long-term memory. The main studies in this field found associated improvements in the consolidation of motor,12 emotional,13 and picture-location14 memories. In particular, in those three studies, a single bout of exercise after encoding was enough to improve the different long-term retention 24 h later.
In this study, we focused on studying the effect of exercise on spatial memory. This type of memory, that records information about the environment and the location of objects in it, deteriorates with aging and Alzheimer’s disease,15,16,17 so it is important to study simple ways to improve it. Spatial memory has been evaluated using different tasks in rodents18 and, in the last few decades, several tasks have been developed to study this function in humans. The development of virtual reality environments became particularly useful for spatial cognition research.19,20 Some studies have shown a positive effect of physical activity on this type of memory in rodents and humans.15,21,22,23,24 But again, most of them have focused on investigating chronic physical activity. Therefore, studies evaluating the role of acute doses of physical activity on this type of memory are missing.
In this study, we evaluated the effect of acute and chronic physical activity on the consolidation and retrieval of spatial memory in a novel virtual reality task. It was carried out with the support of a software solution, including a post hoc virtual reality environment (Computer Assisted Virtual Environment – CAVE), which provides a greater sense of immersion than tasks performed with computer screens.25,26 We hypothesize that acute and chronic physical activity may have a beneficial effect on spatial memory.
Results
Effect of exercise on spatial memory consolidation
To investigate the effect of physical activity on different phases of spatial memory processing, we designed a task in a virtual reality environment. The background we used consisted of a fairly uniform landscape with some valleys and mountains (Figure 1A, upper). The task started with a pre-encoding (pre-EN) phase in which participants had to move toward a single flag using a joystick, pick it up and then return to the starting point. Afterward, they had to select between 360 possible flags in a virtual circumference that included the position of the one already picked up. This procedure was repeated up to ten times (see the average number of trials for each group in Table S1). Next, participants performed a control test, in which 2 flags appeared separated by an angle of 60°, and then they had to select between the 360 flags, the one that had appeared previously on their right-hand side. After the pre-encoding stage, the participants started the first encoding phase (EN1). Two flags appeared (consecutively) separated by an angle of either 20° (short-distance condition) or 40° (long-distance condition) and each participant had to pick them up. The condition was randomly assigned to each subject who, from then on, performed consecutive trials of the same condition. During the test phase (TS1, delay 0 h), 360 flags appeared and participants had to select which one was positioned exactly in the middle of the two encoded flags (Figure 1A, upper right picture. Participants were located in the center of the flag circumference. The perspective of the depicted image was chosen to show how the flags were positioned). After half an hour, they performed the second encoding phase (EN2, analogous to the first one, but the new position of the flags was randomly generated within the same circumference), and immediately after, control (short-distance: n = 10, 5 female; long-distance: n = 9, 2 female; sedentary) and chronic (short-distance: n = 10, 2 female; long-distance: n = 9, 3 female; athletes) groups watched a video for 25 min whereas the acute group (short-distance: n = 9, 4 female; long-distance: n = 9, 1 female; sedentary) cycled for 25 min. Memory for EN2 was evaluated 24 h later (TS2, delay 24 h). Lastly, they performed the last encoding (EN3) that was evaluated immediately after (TS3, delay 0 h). Figure 1A shows a schematic of the complete protocol.
Figure 1.
Effect of exercise on spatial memory consolidation
(A–D) Schematic representation of the whole protocol (in the testing phase, upper right picture, participants are located in the center of the circumference). The performances were calculated as the distances to the target (the position of the flag in the middle of the two encoded flags minus the position of the flag selected by each participant in the TS) and are shown as the mean ± SEM for the different groups (CT: control, AC: acute, CH: chronic). Absolute distances are shown for participants who performed the task in (B) the short-distance condition (CT, n = 10; AC, n = 9; CH, n = 10) or (C) the long-distance condition (CT, n = 9; AC, n = 9; CH, n = 9). One-way ANOVA revealed significant differences only in the absolute distances shown for participants who performed the task with (B) the short-distance condition with a 24h-delay ∗p< 0.05. Normalized distances (taking into account the error in each condition) are shown in panel (D). A two-way ANOVA revealed only an interaction effect (p = 0.01) and the multiple comparisons test revealed a significant difference between CT and AC groups in the short-distance condition (∗p = 0.01). For performances during the pre-EN phase, see also Figure S1.
The distinction between sedentary people and athletes was corroborated by participants’ reports of the International Physical Activity Questionnaire (IPAQ) short version. This questionnaire was completed by each subject after the end of TS3. The chronic group (CH) had a significantly higher IPAQ score (meaning more physical activity) compared to the control (CT) and acute (AC) groups, and no differences were observed between the latter two groups (Kruskal-Wallis analysis, short-distance: H = 19.22, p< 0.0001 and long-distance: H = 12.59, p = 0.0018).
During the pre-EN phase, after the second trial, participants were instructed to use spatial strategies to orient themselves in the arena, so that they could use a specific distant feature in the background to locate the flags. Accuracy was measured as the difference between the target and the selected flag positions (distance to target, Figure S1). A repeated-measures ANOVA analysis revealed a significant effect of trials (short-distance, Figure S1A: F(4,32) = 14.64, p< 0.001, η2 = 0.28; long-distance, Figure S1B: F(4,32) = 20.63, p< 0.001, η2 = 0.22) and no differences between groups (short-distance: F(2,16) = 0.03, p = 0.967, η2 = 5.67e-4; long-distance: F(2,16) = 0.06, p = 0.939, η2 = 0.002) or interactions (short-distance: F(8,64) = 1.98, p = 0.064, η2 = 0.09; long-distance: F(8,64) = 0.82, p = 0.591, η2 = 0.04). This indicates that participants improved their performance throughout the trials.
The accuracy in the control test (using a separation between flags of 60°) was similar for all groups (ANOVA test: F(5,50) = 1.61, p = 0.18, η2 = 0.14), indicating that they all began with a uniform ability to use the joystick and a similar spatial orientation in the CAVE.
As mentioned above, each participant was assigned to one of the two conditions [Figure 1A, short- (a = 20°) or long-distance (a = 40°)] and performed the EN1 that was evaluated immediately afterward (delay 0 h). We calculated the absolute value of the difference between the flag positioned in the middle of the two encoded flags (target) and the selected flag. No differences were observed between groups in the two conditions (short-distance: F(2,26) = 0.42, p = 0.66, η2 = 0.03; long-distance: F(2,24) = 0.94, p = 0.41, η2 = 0.07).
To find out whether exercise influenced the consolidation of spatial memories, participants performed a second encoding session (EN2) followed by an intervention (cycling in the acute group or watching a video in the control and chronic groups) that was tested 24 h later (TS2, delay 24 h). The mean distance covered by the participants on the stationary bike was 7.29 ± 0.86 km in the short-distance group and 6.88 ± 0.70 km in the long-distance group (no differences were found between groups, p = 0.39, d = 0.519). We first analyzed each condition separately and found that the absolute distance to the target measured 24 h after learning was significantly different between groups in the short-distance condition (Figure 1B, short-distance: F(2,26) = 4.95, p = 0.015, η2 = 0.28). Post hoc pairwise comparisons showed that only the acute (AC) group selected shorter distances to the target than the control (CT) group (AC vs. CT: p = 0.012, d = 1.43; CH vs. CT: p = 0.474, d = 0.47). This outcome was not observed in the long-distance condition. The physical intervention had no impact on the group that solved the task with a greater separation between flags (Figure 1C, long-distance: F(2,24) = 0.06, p = 0.94, η2 = 0.005). Next, to compare performances between conditions, we normalized the data to the maximum error in each condition if participants remembered one of the two flags. Thus, for the short-distance condition (20°), we normalized the distance to the target by 20 and for the long-distance condition (40°) by 40 (Figure 1D). We performed a two-way ANOVA (group x condition) and found an interaction effect (F(2,50) = 4.54, p = 0.015, η2 = 0.15) and no main effect (group: F(2,50) = 2.26, p = 0.115, η2 = 0.08; condition: F(1,50) = 0.59, p = 0.445, η2 = 0.01). Sidak’s multiple comparisons test revealed a significant effect only in the CT vs. AC comparison in the short-distance condition (t(50) = 3.62, p = 0.01).
We compared male and female performance and did not find significant differences between sexes [two-way ANOVA, short-distance: (control, n_F = 5, n_M = 5; acute, n_F = 4, n_M = 5; chronic, n_F = 2, n_M = 8) F(1,23) = 0.48, p = 0.50, η2 = 0.02; long-distance: (control, n_F = 2, n_M = 7; acute, n_F = 1, n_M = 8; chronic, n_F = 3, n_M = 6) F(1,21) = 0.37, p = 0.55, η2 = 0.02].
Finally, participants carried out an additional encoding (EN3) which was tested immediately (the responses of two participants in the short-distance chronic group were lost). Similar performances were observed for all groups under both conditions (short-distance: F(2,24) = 1.03, p = 0.37, η2 = 0.079; long-distance: F(2,24) = 2.58, p = 0.097, η2 = 0.177).
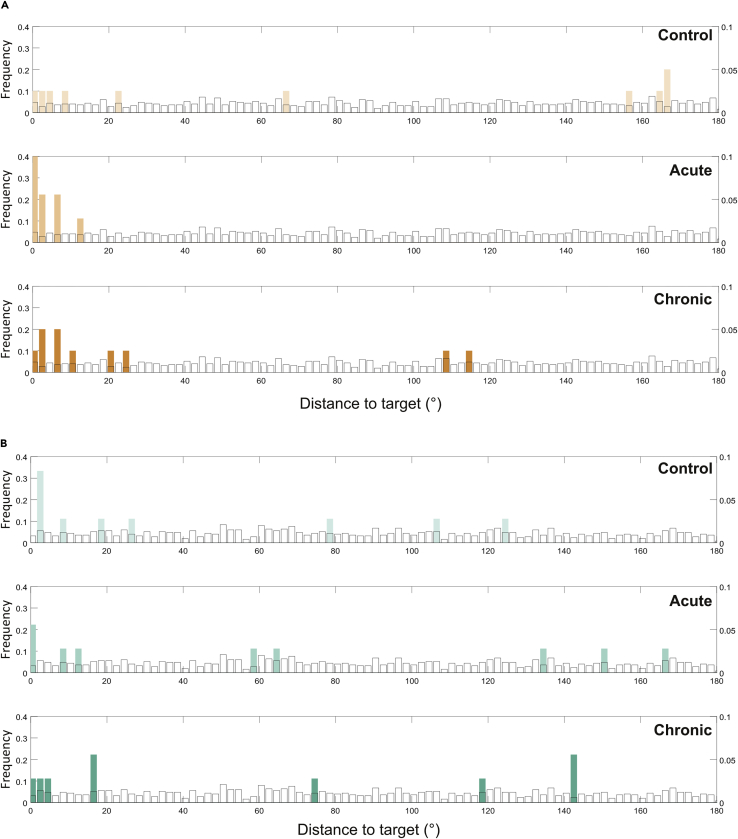
We reasoned that if physical activity promoted long-term memory, both the acute and chronic groups would not select the flag randomly during the long-term memory test (TS2). We analyzed the distributions of the data to determine whether participants were choosing a flag randomly. This would lead to a uniform distribution among all possible positions if they did not remember (i.e. “no memory”) or a distribution of the data around zero if they did. We compared the performance distributions with 1000 uniform distributions randomly generated using a Kolmogorov-Smirnov test and then averaged the pvalue of each iteration. Results indicated that all distributions of delay 0 h were significantly different from the random uniform distribution (p< 0.05). In other words, all groups were able to remember the locations of the flags immediately after EN. In contrast, for the 24 h delay, we observed that all distributions were not significantly different from the uniform distribution, with the exception of the acute and chronic groups in the short-distance condition (Figure 2A, p< 0.01 and p< 0.05, respectively). This may reflect that these groups did remember the position of the flags learned on the previous day. Conversely, the control group in the short-distance condition and all groups in the long-distance one (Figure 2B) showed no memory for the position of the flags (p> 0.05 in all cases). It is worth noting that some participants chose a flag close to the target and some others very far away and a few at an intermediate point, resulting in a uniform distribution.
Figure 2.
Comparison between performance and uniform distributions
We plotted the frequency obtained for each possible angle (i.e., how many participants obtained a particular distance to the target). The orange bars represent the participants who were in the short-distance condition (20°).
(A) and the green bars in the long-distance condition (40°).
(B). Also, we plotted a uniform distribution generated randomly (white bars). The uniform distribution plotted is just representative because the distributions used in the comparison were of the same length as the data. We compared the performance distribution with the uniform distributions in each case by a Kolmogorov-Smirnov test. We obtained significant differences only in the acute and chronic groups in the short-distance condition: p< 0.01 and p< 0.05, respectively.
Replication experiment
We performed a replication study in an independent group of sedentary subjects for the short-distance condition. They completed the pre-EN phase, and all the consecutive ENs and TSs mentioned above using a 20° separation between flags (short-distance condition). The control (CT) and acute (AC) groups consisted of 10 (2 female) and 12 (3 female) participants, respectively. Both groups included only people who reported little or no physical activity. We found no significant differences in their IPAQ scores (Mann-Whitney test, U = 48, p = 0.4418).
As we saw in the main experiment, participants from both groups improved their performance during the pre-EN. A repeated-measures ANOVA analysis revealed a main effect of trials (F(4,36) = 21.08, p< 0.001, η2 = 0.42) and no effect of group (F(1,9) = 3.26, p = 0.104, η2 = 0.04) or interaction (F(4,36) = 0.45, p = 0.772, η2 = 0.01). In addition, all the participants started the main task with a similar performance on the control test (t(20) = 0.66, p = 0.516, d = 0.28).
Groups showed no significant differences in any of the tasks evaluated with a 0 h delay (TS1: t(20) = 0.97, p = 0.342, d = 0.42; TS3: t(20) = 1.19, p = 0.248, d = 0.51).
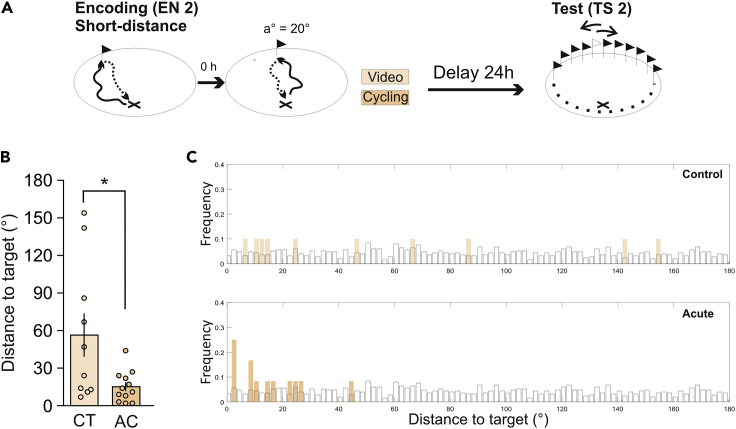
Like in the first experiment, participants in the control group watched a video for 25 min and participants in the acute group cycled for 25 min after EN2, and both groups were tested 24 h later (Figure 3A). The mean distance covered by the participants on the stationary bike was 6.89 ± 1.51 km. We found significant differences between groups (Figure 3B, t(20) = 2.59, p = 0.017, d = 1.11), with the acute group performing better. A post hoc analysis revealed that the power achieved was 0.8.
Figure 3.
Replication experiment
(A) A schematic of the encoding phase followed by the intervention and testing is shown.
(B) Results were obtained from an independent group of participants. All participants performed all trials in the short-distance condition (20°). Performances were calculated as distances to the target (the position of the flag in the middle of the two encoded flags minus the position of the flag selected by each participant in the TS) and are shown as the mean ± SEM for the control (CT, n = 10) and acute (AC, n = 12) groups. The t-test analysis revealed significant differences between groups, ∗p = 0.017.
(C) We plotted the frequency obtained for each possible angle (i.e., data distribution, orange bars) and a uniform distribution generated randomly (white bars). We compared both distributions for each group using a Kolmogorov-Smirnov test. We obtained significant differences in the acute group, p< 0.01, and no difference in the control group, p> 0.05.
Then, we ran the Kolmogorov-Smirnov analysis on the new data, comparing the data distribution of each group with 1000 randomly generated uniform distributions (Figure 3C). We found that the data distribution of the control group was not significantly different from the uniform distribution (p> 0.05) whereas the data distribution of the acute group was (p< 0.01). Therefore, in comparison with the control subjects, participants who cycled (acute group) were able to remember the position of the flags.
Effect of exercise on spatial memory retrieval
Finally, to investigate whether exercise additionally modulated spatial memory retrieval, we recruited a different group of participants to perform a similar protocol in the short-distance condition. Instead of cycling or watching the video immediately after EN2, they spent 25 min cycling on a stationary bike (acute, AC: n = 10, 4 female) or watching a video (control, CT: n = 10, 4 female) just before TS2, when they were asked to remember the position of the flags learned the day before (Figure 4A). No differences were observed between CT and AC groups on either of the two delays: 0 and 24 h (Figure 4B, delay 0 h: t(18) = 0.307, p = 0.762, d = 0.137 and 4C, delay 24 h: t(18) = 0.110, p = 0.914, d = 0.049).
Figure 4.
Effect of exercise on spatial memory retrieval
(A–C) Schematic representation of the encoding and testing phases. Performance was calculated as the distances to the target (the position of the flag in the middle of the two encoded flags minus the position of the flag selected by each participant in the TS) and is shown as the mean ± SEM for control (CT, n = 10) and acute (AC, n = 10) groups. Both groups performed the task in the short-distance condition in both: the delay of 0 h (B) and the delay of 24 h (C). The acute group exercised before retrieval. The t-test did not reveal significant differences in either of the two delays p> 0.05.
Together, these results suggest that performing physical exercise immediately after encoding - but not before retrieval - promotes long-term retention of a spatial memory only when the separation between targets was short (20°-separation compared to 40°-separation).
Discussion
In this work, we developed a task to evaluate spatial memory in humans in an immersive environment and studied the effect of acute and chronic physical activity on both consolidation and retrieval of this type of memory. The results showed that a single bout of acute physical activity after encoding, but not before retrieval, was sufficient to promote the long-term retention of spatial memories of targets located close to each other (short-distance condition). This result was replicated using an independent group of participants. In addition, participants who performed acute exercise (acute group) and those who engaged in regular physical activity (chronic group) selected the position of the flag in a non-random way.
Although there is consensus that physical exercise benefits memory, it is not clear the type of exercise, its duration, timing, and memory phases affected by it. Moreover, most of the effects related to memory enhancement have been found in typical laboratory memory tasks, making it difficult to predict whether everyday memories would be improved in the same way. Our results demonstrated that 25 min of exercise improved the consolidation of learned information within a virtual reality immersive environment only when the position of the learned targets was close to one another, but not when they were more distant. Moreover, exercise right before retrieval did not have a significant effect. These results suggest the existence of specific time windows for exercise effectiveness (after encoding and not before retrieval). Previous studies have demonstrated that a single bout of physical activity immediately after learning was also able to improve the long-term retention of motor12 and emotional13 memories. Furthermore, van Dogen et al.14 have shown that a single bout of physical activity immediately after learning had no effect on picture-location memory but did have an effect when it was given 4 h after learning. Therefore, the time window of exercise effectiveness could differ for the different types of memories studied.27 However, further studies are needed to continue to understand in depth the role of exercise interventions on different types and phases of memory. In addition, we observed that the distribution of performances for the chronic group differed significantly from a uniform distribution, indicating an improvement in memory also for participants who perform physical activity regularly. We can conclude that both acute and chronic exercise showed memory benefits for spatial memory as measured by this virtual reality task. However, it is worth pointing out that the benefits of physical activity in this study appeared to be more effective for the acute than for the chronic group.
Some studies have found a positive effect of chronic physical activity on several spatial memory tasks in both rodents and humans.28,29 In addition, those studies associated the improvements in cognition with increased brain-derived neurotrophic factors (BDNF) levels in rodents’ brains28 or human serum.29 However, studies evaluating the role of acute physical activity on long-term spatial memories are still lacking. Here, we found that acute physical activity improved long-term retention of spatial memory only when the separation between flags during encoding was 20° (short-distance condition). We thought of two possible and opposing explanations for this result. It has been shown that humans combined information from multiple landmarks to locate a target, especially when landmarks were separated by a short distance.30,31 Therefore, remembering and integrating shorter distances may become easier. In this sense, the physical activity intervention could be improving the ability to integrate spatial information and memory consolidation of a simpler task. However, we cannot demonstrate this with our data because when comparing normalized performance in both conditions, we found no significant differences between control groups. On the other hand, the short-distance condition may be more difficult to integrate because the positions of the flags are very similar and, therefore, we cannot differentiate one from the other. In this scenario, physical activity could be enhancing the ability to “separate patterns” as demonstrated previously.32 It is possible that, in the future, neural data is useful to determine whether short- and long-distance targets are processed in different ways.
There are numerous studies where differences were found between men and women in terms of spatial strategies or cognition, both in real33 and virtual reality environments.34 However, in this work, we found no differences between the sexes reported by the subjects. Although recent work supports our results,35 this result could also be because of the small sample size.
The task developed here allowed the participant to interact with and navigate through a virtual reality environment. The participants could visualize the target in a spatial context and then be able to recall its position relative to the background, in a more naturalistic manner. In addition, we have recently demonstrated that to correctly solve our pre-encoding task within the CAVE, the participants needed to use spatial strategies.20 The virtual reality device allowed the exact reproduction of the conditions between each participant, without depending on the exploration of environmental or climatological factors. Previous studies have shown that spatial information can be reliably transferred between real and virtual reality environments when the virtual reality environment was modeled from the real35; and, that, in some cases, the brain areas involved in both scans are analogous.36,37,38 It is worth noting that there is also some evidence in the reverse direction. For example, it has been shown that rodents’ place cells play a different role in the real and virtual worlds.39 Therefore, developing tasks that allow evaluating different cognitive abilities in virtual environments provide valuable tools that allow greater precision when navigating spatial contexts.
Altogether, our findings provide evidence for the efficacy of exercise interventions as a valuable tool for increasing declarative memory consolidation in humans, specifically when the encoding phase presents spatially close locations. The low cost, healthy and practical nature of exercise makes it ideal for interventions in educational and clinical settings. We believe, our results could inspire future applications of exercise to boost long-term episodic memory in various populations.
Limitations of the study
The present study has a few limitations. First, we did not collect neurophysiological correlates with associate the behavioral results with exercise-induced neuroplastic changes nor collect physiological data related to the physical intervention itself. However, we standardized the exercise intervention simulating the situation a person might encounter at home when using a stationary bike, and still found improvements in memory. Second, we did not assess the effect of the physical intervention on short-term memory to differentiate it from an effect specifically on memory consolidation. Third, we did not measure regular caffeine consumption to assess possible withdrawal effects. Four, the sample size of the main experiment was small. However, we were able to replicate the main result with an adequate sample size. Fifth, we had fewer women than men and, therefore, results comparing performance between genders have no sufficient statistical power. Last, because participants used a joystick control to move around in the CAVE, they lacked vestibular and proprioceptive cues and this may have affected the sense of presence in the virtual environment.40
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Task Code | This paper | https://doi.org/10.17605/OSF.IO/JBFPN |
| Software and algorithms | ||
| JASP | JASP | https://jasp-stats.org/ |
| Matlab | Mathworks | www.mathworks.com/products/matlab.html |
| Prism | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Fabricio Ballarini (fballarini@itba.edu.ar).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Participants
Ninety-eight healthy young adults (30 women) participated in this study. Of the total number of participants, 19 were athletes (5 women), recruited from the athletic group of the Universidad del Centro de la Provincia deBuenos Aires who reported performing physical activity at least three times a week. The remaining 79 participants belonged to the sedentary group, who declared not to have done physical exercise over the last year. The mean measured body mass index (BMI) of the sample was (24.78 ± 4.92) kg/m2 (no differences were found among groups). All participants had normal or corrected-to-normal vision and normal color vision. The inclusion criteria required that all participants were between 18 and 35 years old (24.00 ± 4.44), did not consume psychotropic drugs, were native Spanish speakers, and did not report neurological or neuropsychiatric diseases. All subjects were required to refrain from consumption of caffeine during the encoding and testing day. For more information on subjects’ demographics, see Table S1.
The estimated sample size for the main experiment (ANOVA) to detect a size effect f = 0.3 and power = 0.8 was 37 per group. The estimated sample size for the replication experiment (t-test) to detect a size effect d = 1.1 and power = 0.8 was 11 per group.
This study was reviewed and approved by the Ethical Committee Instituto deTisioneumonología “Prof. Dr. Raúl Vaccarezza”. Written informed consent to participate in this study was provided by the participants.
Method details
Behavioral spatial memory task
We designed a spatial memory task within a virtual reality environment (Computer Assisted Virtual Environment - CAVE-). The protocol consisted of three stages: pre-encoding, encoding, and testing.
During the pre-encoding (pre-EN, Figure 1A) phase,20 the participant was located in the center of the arena with a background consisting of a fairly uniform landscape with some valleys and mountains. At first, a flag appeared in one of the 360 possible random positions within an imaginary circumference of 20 m radius. The subject walked through the virtual arena with the joystick, picked up the flag, and then returned to the mark in the center. Once he/she arrived, a total of 360 flags appeared in a circumference and the subject was asked to select the exact flag position that was previously collected, using a cursor. Immediately after, the placemark changed its position and the participant walked to the new starting point. At that moment, the second trial began with the appearance of a new flag, and the procedure was repeated. The number of trials per participant was variable. Trials were finalized after i) the participant selected the correct distal flag three consecutive times or ii) the procedure was repeated ten times, whichever came first. For each trial, we calculated the distance between the selected and target flags with our software solution.
During the encoding (EN, Figure 1A) phase, the participant was located once again in the center of the arena and a new flag of a different color appeared. The subject picked it up and returned to the starting point. Immediately after, a second - identical - flag appeared in a new position separated from the previous flag position by an angle of a = 20° (short-distance condition) or a = 40° (long-distance condition) within the same circumference. This phase was finalized once the participant collected it and returned to the initial mark.
During the testing (TS, Figure 1A) phase, 360 flags appeared, on the same circumference, and participants had to select the flag positioned exactly in the middle of the two flags collected in the encoding phase.
The protocol performed by the participants started with the pre-EN phase, during which the participant got used to the virtual reality environment and generated a spatial map of the arena. Following this, all participants performed an EN with a spacing angle between flags of 60° and were asked to select the flag that had appeared on the right-handed side (control test). The goal of this test was to assess the accuracy of solving the task before starting the relevant tests. Afterward, participants were randomly assigned to one of the following conditions: 20° separation between flags (short-distance condition) and 40° separation between flags (long-distance condition). All subsequent encoding phases were performed at the same angle. Following the control test, participants carried out the first encoding (EN1), and immediately after it, the first test TS1 (delay 0 h). After completing TS1, they watched a video (to avoid any interference between trials) for half an hour and began the second encoding (EN2). Once finished EN2, sedentary participants were randomly assigned to the control or acute group while the athletes formed the chronic group. Therefore, the control group was made up of sedentary people who watched a video about bicycle races for 25 minutes (similar to previous studies13,41). The acute group was also made up of sedentary people who, instead of watching a video, cycled for 25 minutes. Finally, the chronic group was made up of athletes who had reported performing physical activity at least three times a week and watched the same video as the control group. Afterward, the first day ended and participants returned 24 hours later to perform the second test (TS2) corresponding to the learning of the previous day (delay 24 h). Lastly, they carried out the last encoding and test (EN3 and TS3, respectively) to assess the specificity of the physical intervention. Before leaving the laboratory, participants filled in a short version of the International Physical Activity Questionnaire (IPAQ), which was used as a measure of self-reported physical activity. Participants were asked to report the amount of walking, and the number of times they had performed moderate and vigorous activities over the previous seven days.
With an independent group of sedentary subjects, we replicated the main experiment. To do this, subjects were randomly assigned either to the control or acute group and performed the same protocol as in the main experiment only by using the short-distance condition.
With an independent group of sedentary subjects, we evaluated the effect of the physical activity intervention on the retrieval of this memory only by using the short-distance condition. To do this, the subjects performed the same protocol but instead of separating the experimental groups after EN2, they watched the video on bicycle races (control) or cycled (acute) just before TS2 (Figure 4A).
Computer-assisted virtual environment - CAVE
As a virtual reality device, we used a CAVE. CAVE is a room-sized cube in which the walls and floor are projection screens generating the feeling of immersion for the user.42 Four to six projectors are used to display virtual reality environments on the walls and floor. Each of the projectors is connected to a dedicated PC responsible for generating the image. In the CAVE, the subject can move, giving the feeling of total immersion26 and creating the illusion of having completely unlimited space.
We used the Rubika CAVE, which was developed by members of the PLADEMA Institute (Argentina). It consists of a three-meter cube-shaped steel structure. Its inner walls are covered with special fabrics to allow the rear projection. The projectors used to display the images onto the walls have a 1280x800 resolution, while the one projecting the floor image has a 1980x1080 resolution. Furthermore, four PCs hosted the applications in charge of managing each projection including the effects of the user’s actions.
A joystick allows users to move inside the CAVE and interact in different ways within the application. The participant can move forward, backward, or sideways and can also rotate in the spot using the joystick.
Physical activity
A 25 min cycling task served as the acute exercise intervention carried out on a stationary bike. It started with a 5 min warm-up at the first resistance provided by the bike, after which the resistance augmented at the second level. Participants were asked to maintain the speed constant between 15 - 17 km/h, whichever was most comfortable for them.
Quantification and statistical analysis
Details of statistical analysis are provided within the relevant figure legends and the results section. Data are presented as the distance to the target (°) calculated with our software solution as the absolute value of the position of the central flag (in the middle of the two learned flags) minus the position of the flag selected by the participant. The results were presented as the mean ± SEM. When the data did not follow a normal distribution, it was transformed using the natural logarithmic function and then analyzed using the one-way or two-way analysis of variance (ANOVA). In cases of significant differences, post hoc analyses were made with the Tukey test when in one or both factors, the p-value was significant. In the replication experiment, we analyzed the results using a t-test. A result was considered significant when p< 0.05.
The data of the performance distributions were compared with 1000 uniform distributions randomly generated using a Kolmogorov-Smirnov test and then we averaged the p-value obtained in all iterations. All data were analyzed using GraphPad, Matlab, and JASP software.
Acknowledgments
We thank Dr. Jorge Medina for his useful comments on the manuscript and Dr. Timothy Bussey for the helpful discussion of the task. This work was supported by research grants from the National Agency of Scientific and Technological Promotion of Argentina (ANPCyT) to P.B. (PICT 2012-1119, PICT 2015-0110 and PIP 0564).
Author contributions
D.R.B., M.M., F.B., and P.B. designed research; D.R.B., M.F.R., and M.V.C. performed research; D.R.B., M.F.R., F.B., and P.B. analyzed data; M.F.R., M.V.C., and C.G.B. developed VR environments; D.R.B., F.B., and P.B. wrote the paper.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: February 10, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106176.
Contributor Information
Pedro Bekinschtein, Email: pbekinschtein@favaloro.edu.ar.
Fabricio Ballarini, Email: fballarini@itba.edu.ar.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at Open Science Foundation and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Blondell S.J., Hammersley-Mather R., Veerman J.L. Does physical activity prevent cognitive decline and dementia?: a systematic review and meta-analysis of longitudinal studies. BMC Publ. Health. 2014;14:510–512. doi: 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith P.J., Blumenthal J.A., Hoffman B.M., Cooper H., Strauman T.A., Welsh-Bohmer K., Browndyke J.N., Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom. Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voss M.W., Soto C., Yoo S., Sodoma M., Vivar C., van Praag H. Exercise and hippocampal memory systems. Trends Cognit. Sci. 2019;23:318–333. doi: 10.1016/j.tics.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roig M., Nordbrandt S., Geertsen S.S., Nielsen J.B. The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neurosci. Biobehav. Rev. 2013;37:1645–1666. doi: 10.1016/j.neubiorev.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-López M., Granero-Gallegos A., Baena-Extremera A. The abandonment of an active lifestyle within university students: reasons for abandonment and expectations of re-engagement. Psychol. Belg. 2011;51:155–175. [Google Scholar]
- 6.Tsai C.L., Chen F.C., Pan C.Y., Wang C.H., Huang T.H., Chen T.C. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology. 2014;41:121–131. doi: 10.1016/j.psyneuen.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Crush E.A., Loprinzi P.D. Dose-response effects of exercise duration and recovery on cognitive functioning. Percept. Mot. Skills. 2017;124(6):1164–1193. doi: 10.1177/0031512517726920. [DOI] [PubMed] [Google Scholar]
- 8.Tsai C.L., Pan C.Y., Chen F.C., Wang C.H., Chou F.Y. Effects of acute aerobic exercise on a task-switching protocol and brain-derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Exp. Physiol. 2016;101:836–850. doi: 10.1113/EP085682. [DOI] [PubMed] [Google Scholar]
- 9.Tomporowski P.D. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 10.Potter D., Keeling D. Effects of moderate exercise and circadian rhythms on human memory. J. Sport Exerc. Psychol. 2005;27:117–125. [Google Scholar]
- 11.Labban J.D., Etnier J.L. Effects of acute exercise on long-term memory. Res. Q. Exerc. Sport. 2011;82:712–721. doi: 10.1080/02701367.2011.10599808. [DOI] [PubMed] [Google Scholar]
- 12.Roig M., Skriver K., Lundbye-Jensen J., Kiens B., Nielsen J.B. A single bout of exercise improves motor memory. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jentsch V.L., Wolf O.T. Acute physical exercise promotes the consolidation of emotional material. Neurobiol. Learn. Mem. 2020;173 doi: 10.1016/j.nlm.2020.107252. [DOI] [PubMed] [Google Scholar]
- 14.van Dongen E.V., Kersten I.H.P., Wagner I.C., Morris R.G.M., Fernández G. Physical exercise performed four hours after learning improves memory retention and increases hippocampal pattern similarity during retrieval. Curr. Biol. 2016;26:1722–1727. doi: 10.1016/j.cub.2016.04.071. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Horcajo R., Llamas-Alonso J., Cimadevilla J.M. Practice of aerobic sports is associated with better spatial memory in adults and older men. Exp. Aging Res. 2015;41:193–203. doi: 10.1080/0361073X.2015.1001656. [DOI] [PubMed] [Google Scholar]
- 16.Moodley K., Minati L., Contarino V., Prioni S., Wood R., Cooper R., D’Incerti L., Tagliavini F., Chan D. Diagnostic differentiation of mild cognitive impairment due to Alzheimer's disease using a hippocampus-dependent test of spatial memory. Hippocampus. 2015;25:939–951. doi: 10.1002/hipo.22417. [DOI] [PubMed] [Google Scholar]
- 17.Castegnaro A., Howett D., Li A., Harding E., Chan D., Burgess N., King J. Assessing mild cognitive impairment using object-location memory in immersive virtual environments. Hippocampus. 2022;32:660–678. doi: 10.1002/hipo.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris R.G. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12:239–260. [Google Scholar]
- 19.Wiener J.M., Carroll D., Moeller S., Bibi I., Ivanova D., Allen P., Wolbers T. A novel virtual-reality-based route-learning test suite: assessing the effects of cognitive aging on navigation. Behav. Res. Methods. 2020;52:630–640. doi: 10.3758/s13428-019-01264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez M.F., Ramirez Butavand D., Cifuentes M.V., Bekinschtein P., Ballarini F., García Bauza C. A virtual reality platform for memory evaluation: assessing effects of spatial strategies. Behav. Res. Methods. 2021;54:1–13. doi: 10.3758/s13428-021-01758-4. [DOI] [PubMed] [Google Scholar]
- 21.Ang E.T., Dawe G.S., Wong P.T.H., Moochhala S., Ng Y.K. Alterations in spatial learning and memory after forced exercise. Brain Res. 2006;1113:186–193. doi: 10.1016/j.brainres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Van der Borght K., Havekes R., Bos T., Eggen B.J.L., Van der Zee E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav. Neurosci. 2007;121:324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- 23.Cassilhas R.C., Lee K.S., Fernandes J., Oliveira M.G.M., Tufik S., Meeusen R., De Mello M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Zeev T., Hirsh T., Weiss I., Gornstein M., Okun E. The effects of high-intensity functional training (HIFT) on spatial learning, visual pattern separation and attention span in adolescents. Front. Behav. Neurosci. 2020;14:577390. doi: 10.3389/fnbeh.2020.577390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Vives M.V., Slater M. From presence to consciousness through virtual reality. Nat. Rev. Neurosci. 2005;6:332–339. doi: 10.1038/nrn1651. [DOI] [PubMed] [Google Scholar]
- 26.Riva G., Wiederhold B.K., Mantovani F. Neuroscience of virtual reality: from virtual exposure to embodied medicine. Cyberpsychol., Behav. Soc. Netw. 2019;22:82–96. doi: 10.1089/cyber.2017.29099.gri. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roig M., Thomas R., Mang C.S., Snow N.J., Ostadan F., Boyd L.A., Lundbye-Jensen J. Time-dependent effects of cardiovascular exercise on memory. Exerc. Sport Sci. Rev. 2016;44:81–88. doi: 10.1249/JES.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 28.Berchtold N.C., Castello N., Cotman C.W. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M., et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Y., McMillan N., Madan C.R., Spetch M.L., Mou W. Cue integration in spatial search for jointly learned landmarks but not for separately learned landmarks. J. Exp. Psychol. Learn. Mem. Cogn. 2017;43:1857–1871. doi: 10.1037/xlm0000416. [DOI] [PubMed] [Google Scholar]
- 31.Cheng K., Shettleworth S.J., Huttenlocher J., Rieser J.J. Bayesian integration of spatial information. Psychol. Bull. 2007;133:625–637. doi: 10.1037/0033-2909.133.4.625. [DOI] [PubMed] [Google Scholar]
- 32.Suwabe K., Hyodo K., Byun K., Ochi G., Fukuie T., Shimizu T., Kato M., Yassa M.A., Soya H. Aerobic fitness associates with mnemonic discrimination as a mediator of physical activity effects: evidence for memory flexibility in young adults. Sci. Rep. 2017;7:5140. doi: 10.1038/s41598-017-04850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malinowski J.C., Gillespie W.T. Individual differences in performance on a large-scale, real-world wayfinding task. J. Environ. Psychol. 2001;21:73–82. [Google Scholar]
- 34.Astur R.S., Ortiz M.L., Sutherland R.J. A characterization of performance by men and women in a virtual Morris water task:: a large and reliable sex difference. Behav. Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 35.Clemenson G.D., Wang L., Mao Z., Stark S.M., Stark C.E.L. Exploring the spatial relationships between real and virtual experiences: what transfers and what doesn't. Front. Virtual Real. 2020;1 doi: 10.3389/frvir.2020.572122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekstrom A.D., Kahana M.J., Caplan J.B., Fields T.A., Isham E.A., Newman E.L., Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 37.Harvey C.D., Collman F., Dombeck D.A., Tank D.W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs J., Weidemann C.T., Miller J.F., Solway A., Burke J.F., Wei X.X., Suthana N., Sperling M.R., Sharan A.D., Fried I., Kahana M.J. Direct recordings of grid-like neuronal activity in human spatial navigation. Nat. Neurosci. 2013;16:1188–1190. doi: 10.1038/nn.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravassard P., Kees A., Willers B., Ho D., Aharoni D.A., Cushman J., Aghajan Z.M., Mehta M.R. Multisensory control of hippocampal spatiotemporal selectivity. Science. 2013;340:1342–1346. doi: 10.1126/science.1232655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usoh M., Arthur K., Whitton M.C., Bastos R., Steed A., Slater M., Brooks F.P., Jr. Proceedings of the 26th annual conference on Computer graphics and interactive techniques. 1999, July. Walking> walking-in-place> flying, in virtual environments; pp. 359–364. [Google Scholar]
- 41.Coles K., Tomporowski P.D. Effects of acute exercise on executive processing, short-term and long-term memory. J. Sports Sci. 2008;26:333–344. doi: 10.1080/02640410701591417. [DOI] [PubMed] [Google Scholar]
- 42.Cruz-Neira C., Sandin D.J., DeFanti T.A., Kenyon R.V., Hart J.C. The CAVE: audio visual experience automatic virtual environment. Commun. ACM. 1992;35:64–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at Open Science Foundation and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.