Abstract
Objective
The human acellular vessel (HAV) was evaluated for surgical bypass in a phase II study. The primary results at 24 months after implantation have been reported, and the patients will be evaluated for ≤10 years.
Methods
In the present report, we have described the 6-year results of a prospective, open-label, single-treatment arm, multicenter study. Patients with advanced peripheral artery disease (PAD) requiring above-the-knee femoropopliteal bypass surgery without available autologous graft options had undergone implantation with the HAV, a bioengineered human tissue replacement blood vessel. The patients who completed the 24-month primary portion of the study will be evaluated for ≤10 years after implantation. The present mid-term analysis was performed at the 6-year milestone (72 months) for patients followed up for 24 to 72 months.
Results
HAVs were implanted in 20 patients at three sites in Poland. Seven patients had discontinued the study before completing the 2-year portion of the study: four after graft occlusion had occurred and three who had died of causes deemed unrelated to the conduit, with the HAV reported as functional at their last visit. The primary results at 24 months showed primary, primary assisted, and secondary patency rates of 58%, 58%, and 74%, respectively. One vessel had developed a pseudoaneurysm deemed possibly iatrogenic; no other signs of structural failure were reported. No rejections or infections of the HAV occurred, and no patient had required amputation of the implanted limb. Of the 20 patients, 13 had completed the primary portion of the study; however, 1 patient had died shortly after 24 months. Of the remaining 12 patients, 3 died of causes unrelated to the HAV. One patient had required thrombectomy twice, with secondary patency achieved. No other interventions were recorded between 24 and 72 months. At 72 months, five patients had a patent HAV, including four patients with primary patency. For the entire study population from day 1 to month 72, the overall primary, primary assisted, and secondary patency rate estimated using Kaplan-Meier analysis was 44%, 45%, and 60% respectively, with censoring for death. No patient had experienced rejection or infection of the HAV, and no patient had required amputation of the implanted limb.
Conclusions
The infection-resistant, off-the-shelf HAV could provide a durable alternative conduit in the arterial circuit setting to restore the lower extremity blood supply in patients with PAD, with remodeling into the recipient’s own vessel over time. The HAV is currently being evaluated in seven clinical trials to treat PAD, vascular trauma, and as a hemodialysis access conduit.
Keywords: Arterial reconstruction, Bioengineered blood vessel, Human acellular vessel, Long-term outcomes, Peripheral artery disease
Clinical Relevance
Patients with peripheral artery disease who require surgical revascularization need options when autologous grafts are not available. The human acellular vessel (HAV) has been demonstrated to have characteristics similar to those of autologous vessels in terms of resistance to infection, mechanics, and a very low risk of rejection. Safety and performance were evaluated for ≤6 years after implantation of an HAV in a femoropopliteal position. Overall, the secondary patency rate estimated using the Kaplan-Meier method was 60% at 72 months, with 45% primary patency. No infection or rejection episodes had occurred with the HAV conduits. These data have demonstrated the durability of the HAV and suggest the occurrence of cellular remodeling by the host.
Graphical abstract
Article Highlights.
-
•
Type of Research: A phase II study with long-term follow-up
-
•
Key Findings: Of 20 patients, 5 had maintained human acellular vessel (HAV) patency at 72 months. Overall, no infections or rejections of the HAV had occurred, and no amputations were performed on the extremity involved with the original revascularization.
-
•
Take Home Message: The bioengineered HAV represents a durable option for vascular repair.
Globally, >236 million people aged >25 years are living with peripheral artery disease (PAD).1,2 The estimated prevalence of atherosclerotic PAD in individuals aged >40 years in the United States has ranged from 5.8% to 10.7%,1,3, 4, 5 and the prevalence of PAD has been growing, with an increase of 13.1% in high-income countries between 2000 and 2010.1,2,6,7
Aggressive management of chronic limb ischemia and limb salvage have been associated with improved long-term patient survival and quality of life.8, 9, 10 An unmet need exists for improved solutions to reduce, postpone, or, even, obviate the risk of amputation and to provide long-term efficacy for a broader population of patients living with advanced PAD.
Long-term above-the-knee bypass data have demonstrated excellent primary patency rates using autogenous veins compared with synthetic conduits.6,11 However, the saphenous vein will not be available for a large proportion of patients. A retrospective review of elective femoropopliteal bypass in the Vascular Quality Initiative database from 2003 to 2018 in which the greater saphenous vein or a polytetrafluoroethylene (PTFE) graft had been used showed that 47% of the 7430 bypasses had used the PTFE graft.12,13 These synthetic conduits can pose significant risks of infection and can develop rapid intimal hyperplasia-induced occlusion and acute or subacute thrombosis.14, 15, 16 Xenografts, most often of porcine or bovine origin, and cadaveric cryopreserved allografts, represent other alternatives but have demonstrated high rates of thrombosis, calcification, and aneurysm formation, and the risk of early mechanical failure and foreign body inflammatory responses.17, 18, 19, 20, 21 Vascular conduits that would be immediately available with a low immunogenic potential and with improved long-term patency and clinical outcomes would broaden the options for patients who require arterial repair or replacement.
The HAV is a bioengineered, decellularized human blood vessel available off the shelf that can be used as a replacement conduit using standard surgical techniques. We previously reported the primary results of a phase II clinical trial evaluating the bioengineered human acellular vessel (HAV) as a conduit for vascular repair or replacement in patients with symptomatic PAD at 24 months after implantation (Clinicaltrials.gov identifier, NCT01872208).22 Twenty patients with advanced PAD documented by imaging studies and clinical presentation had undergone bypass surgery with an HAV implanted in the above-the-knee femoropopliteal position. At 2 years postoperatively, the HAVs demonstrated functionality with no signs of rejection by the recipient or structural failure. No patient had required amputation of the implanted limb nor had experienced HAV infection during the 24-month period. Also, histologic examination of HAV tissue samples showed repopulation of the conduit by host cells and vascular remodeling. The long-term follow-up portion of the study is ongoing.
The aim of the present report was to share the results from a mid-term analysis at 72 months of the long-term follow-up portion of the phase II study to add to the scientific evidence for this new bioengineered human tissue product.
Methods
Investigational product: HAV
The HAV is a tubular conduit of human extracellular matrix, measuring 6 mm in diameter and 42 cm in length. It is manufactured in bioreactors that deliver cyclic radial strain to human vascular smooth muscle cells cultured on a degradable polymer scaffold in a controlled environment.23, 24, 25 At the conclusion of the culture, the engineered vascular tissue constructs are decellularized to remove alloreactive targets using a process that preserves the three-dimensional extracellular matrix structure and its protein components, including human collagen types I, III, and IV, fibronectin, and vitronectin, and other matrix molecules.26, 27, 28 Transmission electron micrographs have shown that the collagen fibrils in the HAV mimic the three-dimensional alignment found in native vessels.28 The structural and mechanical properties of the HAV were characterized, and the average wall thickness, suture strength, and burst pressure were similar to, or exceeded, those of native human vessels.29 Histologic and imaging studies have consistently demonstrated that HAVs remodel over time into living vascular tissue after surgical implantation, with colonization by autologous cells and the appearance of neovessels outside of and within the conduit wall.29
HAVs are aseptically packaged and stored refrigerated until required for implantation, offering a bioengineered human tissue replacement blood vessel available off the shelf that can be implanted using standard surgical techniques. Eight clinical studies in the United States, Europe, and Israel are evaluating three clinical indications: the HAV as an arteriovenous access for hemodialysis for patients with end-stage renal disease; as a bypass conduit in PAD; and for repair and reconstruction of traumatic arterial injuries (one study). As of April 10, 2022, 374 patients with end-stage renal disease, 35 with PAD, and 51 with vascular trauma have undergone implantation of a HAV. In addition, a HAV has been implanted in >23 patients as an investigational new drug under the Food and Drug Administration program for expanded access.
Study design
The objectives of the present phase II prospective, open-label, single-treatment-arm, multicenter study were to evaluate the safety and efficacy of HAVs implanted in the above-the-knee femoropopliteal position in patients with PAD who had presented with critical limb ischemia, rest pain, or claudication at a walking distance of ≤200 m. The inclusion criteria required documented femoral artery occlusion of ≥10 cm that was not suitable for endovascular treatment, two below-the-knee vessels that were patent to the ankle with good runoff, and autologous vein grafts that were either not suitable or not feasible for use. The present study was conducted in full conformity with the principles of the Declaration of Helsinki (revised 2008), Good Clinical Practice, and International Council for Harmonization requirements for Good Clinical Practice.
Primary trial portion (previously reported 24-month data)
The primary portion of the present trial had examined the performance of the HAVs during the first 24 months for patency, necessary graft interventions, and clinical outcomes. The 24-month results have been previously reported.22 In brief, 20 patients had been enrolled at three sites between October 2013 and June 2014. Each enrolled patient had received a single HAV that had been implanted using standard surgical techniques. The proximal anastomosis was at the common femoral artery in 17 patients and the proximal superficial femoral artery in 3 patients. The distal anastomosis was at the distal superficial femoral artery in 11 patients and the above-the-knee popliteal artery in 9 patients. Of the 20 patients enrolled, 7 had had a claudication distance of <50 m, with 5 presenting with a distance of ≤20 m, 2 patients had presented with rest pain, and all the patients had had an ankle brachial index (ABI) of ≤0.76. Of the 20 patients, 7 had withdrawn before the end of the 2-year portion of the trial, 4 because of occlusion of the HAV. Three patients had died of causes deemed not related to the HAV or the surgery; the HAV was patent at their last visit for all three patients. The Kaplan-Meier estimates of the HAV patency rates, censoring for deaths, were 58% for primary patency, 58% for primary assisted patency, and 74% for secondary patency at 24 months. Other than a possibly iatrogenic pseudoaneurysm, which was treated by excising the segment of HAV and interposing a segment of ePTFE and which had remained patent for the rest of the study, no structural failures or ruptures had occurred. From a clinical perspective, improvement in the signs and symptoms was apparent, with an increase in the median claudication distance from 50 to 1000 m at 6 weeks. The claudication distance had remained stable for all patients with a patent graft and was associated with a return to normal function and no decrease over time of the median ABI.
Histologic analyses of HAV samples showed signs of repopulation by host cells and remodeling into a blood vessel with the presence of neomicrovessels within the conduit wall.22 The clinical, ultrasound, and angiographic examinations did not reveal evidence of structural degradation or true aneurysm formation of the HAV over time. The HAVs were well tolerated, and safety was consistent with previous experience.
Follow-up to 72 months
The patients who had completed the primary 24-month period of the study were invited to enroll in the long-term follow-up portion of the trial, with monitoring for ≤10 years after implantation (120 months). The first portion of the follow-up period required annual ascertainment of patient and HAV status by the investigator during routine clinical visits or telephone interviews with the patients or their physician. A questionnaire was used that covered the patient’s status, known patency of the conduit, and any conduit interventions or other vascular procedures on the operated leg. The monitoring protocol was expanded after 48 months of follow-up to require clinic visits (or telephone visits to accommodate the patient and medical circumstances during the COVID-19 [coronavirus disease 2019] pandemic) every 6 months for the focused vascular examination on the limb treated with the HAV, assessment of patient status, including interval history, complications, any HAV interventions or other surgical procedures on the treated limb, and Doppler ultrasound to assess the conduit, unless the HAV had become occluded or had been removed.
Statistical analysis
Data ≤72 months were included in the statistical analysis of the present interim report, including HAV patency, interventions required to maintain or restore patency, ABI, PAD signs and symptoms, and safety events. The time to loss of patency was evaluated using the Kaplan-Meier method, with separate assessments for primary, primary assisted, and secondary patency. Patency was assessed by examination and history for ≤48 months and by Doppler ultrasound and/or questionnaire for ≤72 months. Patients were censored at death, study withdrawal, or loss to follow-up. The other parameters were summarized descriptively.
Results
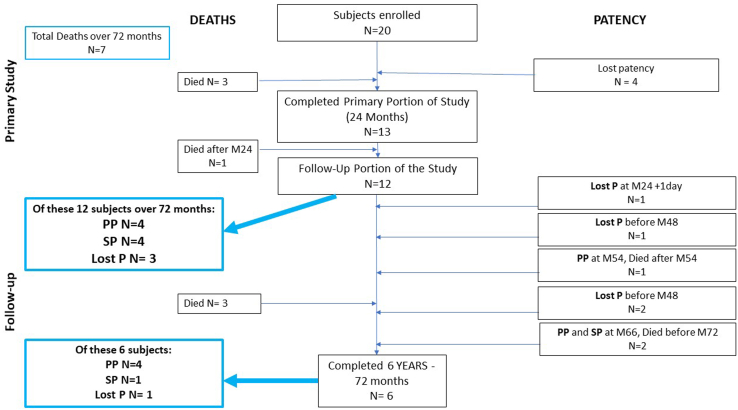
A total of 20 patients were enrolled in the primary study. Of the 20 patients, 13 had completed the 24-month primary portion of the study.22 All the patients had received antithrombotic prophylaxis in conjunction with HAV implantation, consisting of intraoperative heparin and low-molecular-weight heparin daily until fully mobilized and a combination of two antiplatelet agents (aspirin, 75-300 mg; and clopidogrel, 75 mg daily) for long-term treatment after discontinuation of low-molecular-weight heparin. One patient had died of cerebrovascular complications after thrombolysis of the HAV conduit shortly after completing the 24-month period. Of the remaining 12 patients, by the end of the 72-month period, 3 had died, the HAV had lost patency in 4, and the HAV had remained patent without intervention during follow-up in 5 patients (Fig 1). Three patients had died during follow-up, one each of lung cancer, coronary heart disease and ventricular arrhythmia, and metastatic cancer. Their HAV was presumed patent at the time of death according to the latest information on file. One patient had lost patency of the HAV shortly after completing the primary portion of the trial; one patient had retained patency until 36 months; and two patients had retained patency until 48 months. One patient had developed a pseudoaneurysm of iatrogenic nature 12 weeks after implantation of the HAV. The segment of the HAV with the pseudoaneurysm was excised and replaced with an ePTFE segment. This patient was one of the two patients with patency for >4 years but had lost patency at 60 months. At 72 months, 4 of the 12 patients still retained primary patency and 1 retained secondary patency. After 24 months, one patient had required two interventions involving open thrombectomy. For all other patients, no interventions to restore or maintain patency of the HAV were recorded.
Fig 1.
Patient disposition. M, Month; P, patency; PP, primary patency; SP, secondary patency.
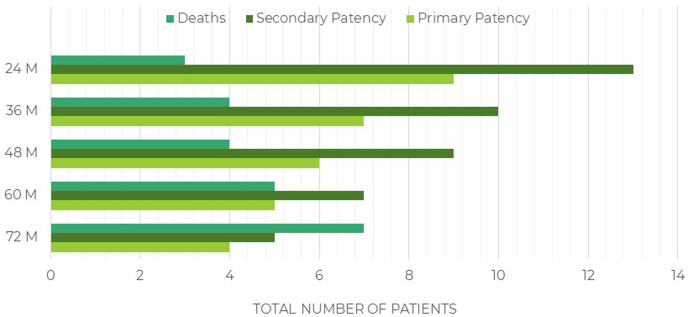
During their individual follow-up periods, 8 of the 12 patients (66%) had retained secondary patency of the HAV implant until either their death or the 72-month milestone. The total number of deaths in the present study was seven at 72 months, for a mortality rate of 35% for this cohort of patients with severe PAD at 6 years of follow-up (Table I; Fig 2).
Table I.
Patients stratified by patency and year of follow-up (N = 20)
| Variable | Month, No. |
||||
|---|---|---|---|---|---|
| 24 | 36 | 48 | 60 | 72 | |
| Primary patencya | 9 | 7 | 6 | 5 | 4 |
| Secondary patencyb | 13 | 10 | 9 | 7 | 5 |
| Lost patency | 4 | 6 | 7 | 8 | 8 |
| Death | 3 | 4 | 4 | 5 | 7 |
Functional patency of graft without any intervention to maintain or restore patency; a patient with primary patency was also considered to have secondary patency.
Functional patency with or without preceding successful interventional or surgical procedures to maintain or reestablish patency.
Fig 2.
Total number of patients stratified by patency status and year of follow-up for 6 years.
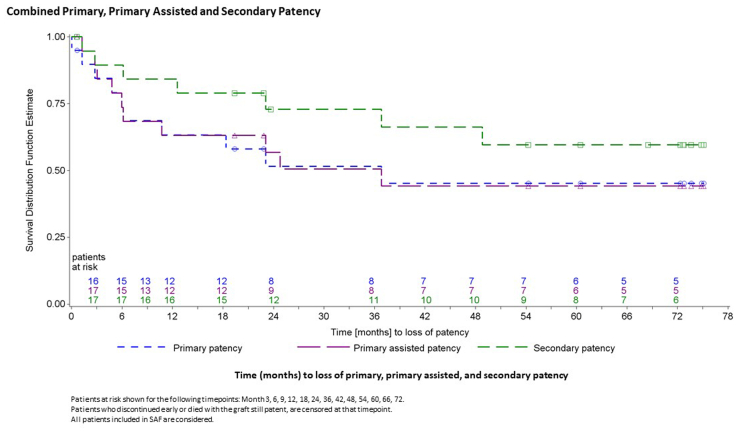
The patency outcomes of the implanted HAV were analyzed using Kaplan-Meier analyses to calculate the conduit patency probabilities, treating patients who had withdrawn, died, or been lost to follow-up as censored at the time of the event if the HAV conduit had been patent at the last evaluation.
In the long-term follow-up group of 12 patients, the primary and primary assisted patency rate at 72 months were both estimated at 58%, and the secondary patency rate was estimated at 75%. Including all patients originally enrolled in the study, the overall secondary patency rate for the study at 72 months was estimated at 60%, with a primary assisted patency rate of 44% and a primary patency rate of 45%. The Kaplan-Meier curves for primary, primary assisted, and secondary patency are shown in Fig 3.
Fig 3.
Survival estimates for whole study population from 0 to 72 months for primary, primary assisted, and secondary patency, with death not counted as an event. SAF, Safety analysis set.
Intermittent claudication distance and ABI
At 24 months, the median claudication distance was 1000 m, with a median ABI of 0.96, and both stayed at that level or higher until 60 months. At 72 months, of the six evaluable patients, the patient with an early occluded HAV reported a claudication distance of 130 m, two patients still had a distance >1000 m, and three patients with a patent HAV were able to walk 700 m (ABI, 0.88), 300 m, and 200 m (ABI, 1.0).
Doppler ultrasound assessments
The mid-HAV diameter was assessed using duplex ultrasound at 48, 54, 60, 66, and 72 months (Table II). The mid-HAV diameter had gradually increased over time, from 5.9 mm to 7.4 mm, but the increase was not clinically significant. We found no tendency toward a progressive narrowing of the mid-section of the HAVs over time. None of the HAVs evaluated had shown any evidence of aneurysm formation.
Table II.
Human acellular vessel (HAV) diameter during long-term follow-up
| Month | Patients, No. | Patients missing, No. | Mid-HAV diameter, mm |
|
|---|---|---|---|---|
| Mean ± SD | Median (range) | |||
| 48 | 7 | 3 | 6.13 ± 0.94 | 5.90 (5.3-7.7) |
| 54 | 7 | 0 | 6.46 ± 0.89 | 6.60 (5.0-7.7) |
| 60 | 5 | 0 | 6.42 ± 0.73 | 6.30 (5.5-7.2) |
| 66 | 5 | 0 | 7.00 ± 0.89 | 7.20 (5.5-7.8) |
| 72 | 2 | 2 | 7.40 ± 0.57 | 7.40 (7.0-7.8) |
SD, Standard deviation.
Computed tomography angiography
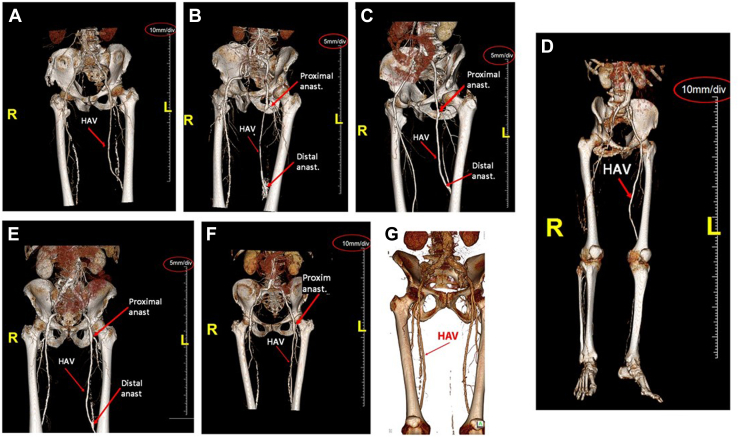
To further determine the mechanical durability of the HAV in the arterial position at long implantation times, computed tomography (CT) angiography was performed (Fig 4). Seven patients with a patent HAV in the femoropopliteal position were evaluated between 50 and 55 months. For all seven patients, the HAVs showed no evidence of aneurysmal dilatation, implying that the HAVs did not experience mechanical breakdown, after multiple years of exposure to the high pressures of the arterial system. At the follow-up of 72 months, no patient had reported rest pain, ischemic ulcers, or amputation of the affected limb.
Fig 4.
Reconstructed three-dimensional computed tomography (CT) angiograms of human acellular vessels (HAVs) at 50 to 55 months for seven patients enrolled in the 6-year follow-up study. A, Angiogram at 55 months after HAV femoropopliteal bypass. B, Angiogram at 51 months after HAV femoropopliteal bypass. C, Angiogram at 51 months after HAV femoropopliteal bypass. D, Angiogram at 50 months after HAV femoropopliteal bypass. E, Angiogram at 51 months after HAV femoropopliteal bypass. F, Angiogram at 51 months after HAV femoropopliteal bypass. G, Angiogram at 51 months after HAV femoropopliteal bypass. Red arrows indicate anastomoses and/or the HAV body; and red ovals indicate scale bars.
At the follow-up of 72 months, no patient had reported rest pain, ischemic ulcers, or amputation of the affected limb. The overall secondary patency rate for the entire study population from day 1 to 72 months was estimated at 60%, with no reports of acute mechanical failure or rupture of the HAV. No additional histologic samples of HAV were obtained between 24 and 72 months.
Discussion
The findings from our analysis have shown the performance of the bioengineered HAVs implanted for the management of PAD for ≤72 months, the longest term follow-up for any engineered tissue used to treat PAD. In this relatively small group of patients, the HAV appeared to be safe for long-term use. No infection, immune rejection, or damage due to exposure to the high-pressure outflow and resistance of arterial circulation was reported in these patients with PAD. Unlike many conduits biologically derived from xenograft or allogeneic sources, the HAV displayed no evidence of aneurysmal degeneration over time in the arterial circulation.18,19 The only case of pseudoaneurysm diagnosed and treated at 12 weeks after implantation of the HAV was associated with an endovascular procedure on day 1. Pseudoaneurysms are known to form in all vascular structures, including native arteries, veins, arterialized vein grafts, and synthetic vascular grafts, with the most common vascular pseudoaneurysm seen in the native common femoral artery after instrumentation during cardiac catheterization.30, 31, 32, 33 A number of endovascular devices have been reported to cause pseudoaneurysms, including wires, catheters, and balloons.34
The overall death rate was 35% (7 of 20 patients within 6 years), consistent with the current literature for patients with PAD, accounting for age, comorbidities, and advanced disease stage.35 No deaths were attributed to the HAV during the study period. Overall, the secondary patency probability rates obtained by Kaplan-Meier estimates, with death censored, was 60%, with an estimate of 45% for primary patency from day 1 (initial bypass surgery) to 6 years (72 months).
The use of the harvested greater saphenous vein has remained the most trusted approach, with an expected secondary patency rate at 5 years of ∼70% to 75% for femoropopliteal above-the-knee bypass surgical revascularization for patients with PAD. However, ≤40% of these patients will lack adequate veins for this use and will require implantation of synthetic grafts.6 Synthetic grafts have demonstrated expected patency rates of 40% to 60% at 5 years in this same population.6 For such patients, the secondary patency rate of 60% at 6 years with the HAV is encouraging, although such results must be confirmed by larger, controlled trials.
Patient-specific factors such as the location of the anastomoses, degree and extent of atherosclerosis, comorbidities, and anatomic characteristics are known to significantly alter and decrease patency. The ever-present risk of infection of synthetic grafts has remained a challenge. Although readily available, the variable short- and long-term patency rates, poorly matched mechanical compliance, and risk of infection have also limited the use of synthetic grafts (eg, ePTFE, Dacron) for this population of patients, although the lack of veins means that 40% of PAD patients in the United States will receive synthetic bypass grafts.36 Newer approaches with coating the lumen of the synthetic grafts with heparin or other exploratory agents such as micro-RNAs to decrease intimal hyperplasia,37,38 VEGF-R2 (vascular endothelial growth factor receptor 2),39 angiogenic micro-RNAs to stimulate endothelial cell growth,40 resorbable polymers to promote endothelialization,41 or seeding with autologous endothelial cells42 have not yet demonstrated significant benefits. The overall weighted average primary patency from six selected peer-reviewed studies for heparin-coated synthetic grafts was 60% at 6 years after above-the-knee bypass.43 Also, a study of autologous endothelialized grafts used for femoropopliteal bypass in patients with severe PAD reported a patency of 76% at 5 years, comparable to that of the native saphenous veins in their study.42
In our small sample of patients, the patient and anatomic characteristics did not seem to correlate with the time to loss of HAV patency. The use of alternative cryopreserved allogenic tissue (ie, cryovein or cryoartery) has been limited in patients with critical limb ischemia, especially in an infected field, mainly because of the known poor outcomes for durability, thrombosis, and mechanical degradation.6,19,44 The diameter of the HAV is 6 mm; however, Klingelhoefer et al45 showed a significant difference in favor of larger diameter grafts (8-mm vs 6-mm grafts) in terms of patency and limb salvage for above-the-knee bypass. Xenografts composed of animal-derived crosslinked blood vessels have even more limitations owing to the progressive biodegradation with aneurysmal degeneration and mechanical failure.17,18,20,46 In the present study, HAVs demonstrated continued durability in a high-pressure circulation and functioned acceptably in a patient population with a high rate of cardiovascular risk factors (ie, advanced age, hypertension, diabetes, established atherosclerosis). Additionally, no reports or clinical signs were present of any clinical immunologic response to the HAV implant with long-term use.
The data from the CT angiograms performed at ∼4.5 years after surgery showed no evidence of the HAVs either narrowing or dilating significantly and no focal mechanical defects. Although our dataset was small, the CT angiography data support the finding that the HAV will be mechanically stable over time in the arterial circulation.
The study limitations were the small sample size from which statistical inferences could be difficult and that not all surveillance assessments occurred during in-person clinic visits. However, these data have provided initial evidence that the HAV can be safe and effective over time in patients with lower extremity atherosclerotic disease. To the best of our knowledge, the present study is the longest term follow-up of any implanted engineered tissue used to treat PAD and has shown the overall potential of bioengineered human tissues in the long-term treatment of vascular disease.
Another limitation was that the patients who lost HAV patency before the 2-year period were not included in the long-term follow-up. Furthermore, and in contrast to other studies of the HAV used for vascular dialysis access, no biopsies were available after 2 years from this PAD patient cohort. Although this likely reflected the low rate of surgical reintervention on the HAV, the lack of histologic samples meant that the cellular repopulation in these patients could not be assessed. Finally, the limited life expectancy of patients with late-stage PAD limited our overall ability to gather long-term functional information for this population.
Overall, these initial findings are encouraging for the long-term function of the HAV and support further prospective and long-term studies of the HAV as a conduit to repair or replace vessels and offer a surgical alternative to patients with PAD.
Conclusions
The overall results of these extended long-term follow-up data suggest that the HAV remains safe and well tolerated in the long-term revascularization of serious PAD. The HAV withstood long-term use without reported mechanical failures in a high-pressure, high-outflow resistance arterial circuit. The HAV is available off the shelf, with a unique regenerative capacity and “immune quietness,” and could offer an alternative conduit for arterial repair and reconstruction, especially for patients lacking an autologous saphenous vein. The long-term follow-up portion of our study is continuing. We will be monitoring patients for ≤10 years after implantation of the HAV to confirm its performance durability and reliability in the long term.
Author Contributions
Conception and design: PG, MG, MI, AK, JL, HP, SP, RS, WT, JT, WW, NZ, TZ, LN
Analysis and interpretation: JL, HP, WT, LN
Data collection: PG, MG, MI, AK, JL, HP, SP, RS, WT, JT, WW, NZ, TZ, LN
Writing the article: JL, LN
Critical revision of the article: PG, MG, MI, AK, JL, HP, SP, RS, WT, JT, WW, NZ, TZ, LN
Final approval of the article: PG, MG, MI, AK, JL, HP, SP, RS, WT, JT, WW, NZ, TZ, LN
Statistical analysis: LN
Obtained funding: Not applicable
Overall responsibility: LN
Acknowledgments
The authors would like to acknowledge Mauricio Berdugo, Shawn Gage, Emmanuelle Hugentobler, Tom Kozma, Alan Kypson, Ryan McKee, Carlos Martin, Luigi Pascarella, Alison Pilgrim, and Kimberly Vandermeer for their help with our report.
Footnotes
Clinical Trail Registration: NCT01872208.
Author conflict of interest: J.H.L., H.L.P., W.T., and L.E.N. own stock or stock options in Humacyte. P.G., M.G., M.I., A.K., S.P., R.S., J.T., W.W., N.Z., and T.Z. have no conflicts of interest.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., et al. Heart Disease and Stroke Statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145:153–639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes F.G., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Allison M.A., Ho E., Denenberg J.O., Langer R.D., Newman A.B., Fabsitz R.R., et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Nehler M.R., Duval S., Diao L., Annex B.H., Hiatt W.R., Rogers K., et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–695.e2. doi: 10.1016/j.jvs.2014.03.290. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita K., Sang Y., Ning H., Ballew S.H., Chow E.K., Grams M.E., et al. Lifetime risk of lower-extremity peripheral artery disease defined by ankle-brachial index in the United States. J Am Heart Assoc. 2019;8:e012177. doi: 10.1161/JAHA.119.012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vartanian S.M., Conte M.S. Surgical intervention for peripheral arterial disease. Circ Res. 2015;116:1614–1628. doi: 10.1161/CIRCRESAHA.116.303504. [DOI] [PubMed] [Google Scholar]
- 7.Vemulapalli S., Dolor R.J., Hasselblad V., Subherwal S., Schmit K.M., Heidenfelder B.L., et al. Comparative effectiveness of medical therapy, supervised exercise, and revascularization for patients with intermittent claudication: a network meta-analysis. Clin Cardiol. 2015;38:378–386. doi: 10.1002/clc.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen L.N., Moneta G.L., Conte M.S., Bandyk D.F., Clowes A.W., Seely B.L. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg. 2006;44:977–984. doi: 10.1016/j.jvs.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones W.S., Patel M.R., Dai D., Vemulapalli S., Subherwal S., Stafford J., et al. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. Am Heart J. 2013;165:809–815.e1. doi: 10.1016/j.ahj.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Geiss L.S., Li Y., Hora I., Albright A., Rolka D., Gregg E.W. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care. 2019;42:50–54. doi: 10.2337/dc18-1380. [DOI] [PubMed] [Google Scholar]
- 11.Almasri J., Adusumalli J., Asi N., Lakis S., Alsawas M., Prokop L.J., et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg. 2018;68:624–633. doi: 10.1016/j.jvs.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 12.Cronenwett J.L., Kraiss L.W., Cambria R.P. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–1537. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Humbarger O., Siracuse J.J., Rybin D., Stone D.H., Goodney P.P., Schermerhorn M.L., et al. Broad variation in prosthetic conduit use for femoral-popliteal bypass is not justified on the basis of contemporary outcomes favoring autologous great saphenous vein. J Vasc Surg. 2019;70:1514–1523.e2. doi: 10.1016/j.jvs.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Ambler G.K., Twine C.P. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. 2018;2:CD001487. doi: 10.1002/14651858.CD001487.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schild A.F., Perez E., Gillaspie E., Seaver C., Livingstone J., Thibonnier A. Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. J Vasc Access. 2008;9:231–235. [PubMed] [Google Scholar]
- 16.Harish A., Allon M. Arteriovenous graft infection: a comparison of thigh and upper extremity grafts. Clin J Am Soc Nephrol. 2011;6:1739–1743. doi: 10.2215/CJN.00490111. [DOI] [PubMed] [Google Scholar]
- 17.Lindsey P., Echeverria A., Cheung M., Kfoury E., Bechara C.F., Lin P.H. Lower extremity bypass using bovine carotid artery graft (Artegraft): an analysis of 124 cases with long-term results. World J Surg. 2018;42:295–301. doi: 10.1007/s00268-017-4161-x. [DOI] [PubMed] [Google Scholar]
- 18.Dobrilovic N., Soukas P., Sadiq I., Goldstein L., Raman J. Early complications of biologic extracellular matrix patch after use for femoral artery repair. J Vasc Surg. 2017;65:705–710. doi: 10.1016/j.jvs.2016.07.131. [DOI] [PubMed] [Google Scholar]
- 19.Hartranft C.A., Noland S., Kulwicki A., Holden C.R., Hartranft T. Cryopreserved saphenous vein graft in infrainguinal bypass. J Vasc Surg. 2014;60:1291–1296. doi: 10.1016/j.jvs.2014.05.092. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter J.P., Tomaszewski J.E. Human saphenous vein allograft bypass grafts: immune response. J Vasc Surg. 1998;27:492–499. doi: 10.1016/s0741-5214(98)70323-4. [DOI] [PubMed] [Google Scholar]
- 21.Albert B., Elena H., Nicole W., Süleyman E., Ralph K., Richard K., et al. Neointimal hyperplasia in allogeneic and autologous venous grafts is not different in nature. Histochem Cell Biol. 2015;144:59–66. doi: 10.1007/s00418-015-1317-3. [DOI] [PubMed] [Google Scholar]
- 22.Gutowski P., Gage S.M., Guziewicz M., Ilzecki M., Kazimierczak A., Kirkton R.D., et al. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg. 2020;72:1247–1258. doi: 10.1016/j.jvs.2019.11.056. [DOI] [PubMed] [Google Scholar]
- 23.Lawson J.H., Glickman M.H., Ilzecki M., Jakimowicz T., Jaroszynski A., Peden E.K., et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016;387:2026–2034. doi: 10.1016/S0140-6736(16)00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl S.L.M., Koh J., Prabhakar V., Niklason L.E. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–666. [PubMed] [Google Scholar]
- 25.Dahl S.L.M., Kypson A.P., Lawson J.H., Blum J.L., Strader J.T., Li Y., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 26.Niklason L.E., Gao J., Abbott W.M., Hirschi K.K., Houser S., Marini R., et al. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 27.Poh M., Boyer M., Solan A., Dahl S.L., Pedrotty D., Banik S.S., et al. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 28.Niklason L.E., Abbott W., Gao J., Klagges B., Hirschi K.K., Ulubayram K., et al. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 29.Kirkton R.D., Santiago-Maysonet M., Lawson J.H., Tente W.E., Dahl S.L.M., Niklason L.E., et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019;11:eaau6934. doi: 10.1126/scitranslmed.aau6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts S.R., Main D., Pinkerton J. Surgical therapy of femoral artery pseudoaneurysm after angiography. Am J Surg. 1987;154:676–680. doi: 10.1016/0002-9610(87)90242-x. [DOI] [PubMed] [Google Scholar]
- 31.Skillman J.J., Kim D., Baim D.S. Vascular complications of percutaneous femoral cardiac interventions. Arch Surg. 1988;123:1207–1212. doi: 10.1001/archsurg.1988.01400340033006. [DOI] [PubMed] [Google Scholar]
- 32.McMillan I., Murie J.A. Vascular injury following cardiac catheterization. Br J Surg. 1984;71:832–835. doi: 10.1002/bjs.1800711108. [DOI] [PubMed] [Google Scholar]
- 33.Kresowik T.F., Khoury M.D., Miller B.V., Winniford M.D., Shamma A.R., Sharp W.J., et al. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J Vasc Surg. 1991;13:328–333. discussion: 333-5. [PubMed] [Google Scholar]
- 34.Ferretti G.R., Thony F., Link K.M., Durand M., Wollschläger K., Blin D., et al. False aneurysm of the pulmonary artery induced by a Swan-Ganz catheter: clinical presentation and radiologic management. AJR Am J Roentgenol. 1996;167:941–945. doi: 10.2214/ajr.167.4.8819388. [DOI] [PubMed] [Google Scholar]
- 35.Caro J., Migliaccio-Walle K., Ishak K.J., Poroskorovsky I. The morbidity and mortality following a diagnosis of peripheral artery disease: long-term follow-up of a large database. BMC Cardiovasc Disord. 2005;5:14. doi: 10.1186/1471-2261-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rychlik I.J., Davey P., Murphy J., O’Donnell M.E. A meta-analysis to compare Dacron versus polytetrafluoroethylene grafts for above-knee femoropopliteal artery bypass. J Vasc Surg. 2014;60:506–515. doi: 10.1016/j.jvs.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 37.Naegeli K.M., Kural M.H., Li Y., Wang J., Hugentobler E.A., Niklason L.E. Bioengineering human tissues and the future of vascular replacement. Circ Res. 2022;13:109–126. doi: 10.1161/CIRCRESAHA.121.319984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen M., Zhi D., Wang L., Cui C., Huang Z., Zhao Y., et al. Local delivery of dual microRNAs in trilayered electrospun grafts for vascular regeneration. ACS Appl Mater Interfaces. 2020;12:6863–6875. doi: 10.1021/acsami.9b19452. [DOI] [PubMed] [Google Scholar]
- 39.Hytönen J.P., Leppänen O., Taavitsainen J., Korpisalo P., Laidinen S., Alitalo K., et al. Improved endothelialization of small-diameter ePTFE vascular grafts through growth factor therapy. Vasc Biol. 2019;1:1–9. doi: 10.1530/VB-18-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding M.H., Lozoya E.G., Rico R.N., Chew S.A. The role of angiogenesis inducing microRNAs in vascular tissue engineering. Tissue Eng Part A. 2020;26:1283–1302. doi: 10.1089/ten.TEA.2020.0170. [DOI] [PubMed] [Google Scholar]
- 41.Bastijanic J.M., Marchant R.E., Kligman F., Allemang M.T., Lakin R.O., Kendrick D., et al. In vivo evaluation of biomimetic fluorosurfactant polymer-coated expanded polytetrafluoroethylene vascular grafts in a porcine carotid artery bypass model. J Vasc Surg. 2016;63:1620–1630.e4. doi: 10.1016/j.jvs.2015.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deutsch M., Meinhart J., Zilla P., Howanietz N., Gorlitzer M., Froeschl A., et al. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009;49:352–362. doi: 10.1016/j.jvs.2008.08.101. discussion: 362. [DOI] [PubMed] [Google Scholar]
- 43.W.L. Gore & Associates GORE® PROPATEN® vascular graft above-knee bypass primary patency. https://www.goremedical.com/products/propaten/above-knee-data Available at:
- 44.Desai M., Hamilton G. In: Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists. Fitridge R., Thompson M., editors. University of Adelaide Press; 2011. Graft materials past and future; pp. 621–651. [PubMed] [Google Scholar]
- 45.Klingelhoefer E., Bergert H., Kersting S., Ludwig S., Weiss N., Schönleben F., et al. Predictive factors for better bypass patency and limb salvage after prosthetic above-knee bypass reconstruction. J Vasc Surg. 2016;64:380–388.e1. doi: 10.1016/j.jvs.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 46.Kelley T.M., Jr., Kashem M., Wang H., McCarthy J., Carroll N.D., Moser G.W., et al. Anterior leaflet augmentation with CorMatrix porcine extracellular matrix in twenty-five patients: unexpected patch failures and histologic analysis. Ann Thorac Surg. 2017;103:114–120. doi: 10.1016/j.athoracsur.2016.05.090. [DOI] [PubMed] [Google Scholar]