Summary
The courtship ritual is a dynamic interplay between males and females. Courtship successfully leading to copulation is determined by the intention of both parties which is conveyed by complex action sequences. In Drosophila, the neural mechanisms controlling the female’s willingness to mate, or sexual receptivity, have only recently become the focus of investigations. Here, we report that pre-mating sexual receptivity in females requires activity within a subset of serotonergic projection neurons (SPNs), which positively regulate courtship success. Of interest, a male-derived sex peptide, SP, which was transferred to females during copulation acted to inhibit the activity of SPN and suppressed receptivity. Downstream of 5-HT, subsets of 5-HT7 receptor neurons played critical roles in SP-induced suppression of sexual receptivity. Together, our study reveals a complex serotonin signaling system in the central brain of Drosophila which manages the female’s desire to mate.
Subject areas: Natural sciences, Neuroscience, Behavioral neuroscience, Molecular neuroscience
Graphical abstract
Highlights
-
•
A pair of serotoninergic neurons (SPN) tunes sexual receptivity in virgin females
-
•
Sex peptide from males inhibits the activity of SPN in mated females
-
•
5-HT7 receptor neurons receive SP signals from SPN
-
•
Silencing 5-HT7 receptor neurons in virgins evokes strong rejection behaviors
Natural sciences; Neuroscience; Behavioral neuroscience; Molecular neuroscience
Introduction
In many animals, males and females participate in discrete and stereotyped action sequences for courtship. Similar sexual dimorphism also exists in Drosophila. The courtship actions of male flies are often more elaborate and frequent than females.1 However, although seemingly more passive, the decision of the female fly is critical for the ritual to reach successful copulation. A voluntary female shows her desire to mate by stopping her movement (pause) and opening her vaginal plate to allow a pursuing male to copulate.2,3,4
The mating decision of a female fly is a response to external stimuli, such as the male’s courtship song, and depends on her internal states, including recent mating experience.5 Recent investigations have led to the identification of the neurons and related circuit mechanisms underlying the sexual receptivity of virgin females, many of which are female-specific. Auditory signals from the male courtship song are integrated via a pair of descending neurons, vpoDNs, to promote female receptivity.4 Two interneuron clusters, Spin-A and Spin-D, likely process the olfaction and gustatory cues during courtship.6 Furthermore, pCd and pC1 neurons, both of which are doublesex-positive, mediate male-specific pheromone and courtship songs in virgin females.7 Additional genes, neural transmitters and neural peptides are shown to be critical for sexual receptivity in pre-mating females. The female mutants of chaste and TRPA lead to decreased and increased courtship success rates, respectively, whereas the responsible neurons remain unidentified.8,9 The neurotransmitter dopamine and neural peptides Drosulfakinin (DSK) have been investigated for their roles in regulating female receptivity.10,11 The residing neurons and corresponding receptors have also been revealed, and DSK neurons are direct targets of pC1 neurons.11 Outside the brain, neurons in the abdominal ganglion and genitalia, particularly the Abd-B neurons, promote pausing in virgin females.2
As in many insect species, successful mating in Drosophila induces a profound effect on subsequent behaviors, especially in females. The post-mating females exhibit diminished sexual receptivity, increased egg-laying, altered motor activity and sleep cycles, as well as elevated appetite.12,13 Both the experience of copulation and the material transfer during the copulation process contribute to the switch of sexual desire in female flies.14,15 A key inducer of the post-mating behavioral switch is a male-derived 36-amino-acid peptide pheromone, sex peptide (SP), which is transferred into the female reproductive tract with the sperm during copulation. SP is stored and then gradually depleted, eventually leading to regain of sexual receptivity of mated females.16 SP binds to the sex peptide receptor (SPR), a G protein-coupled receptor, and silences sex peptide sensory neurons (SPSNs) in the reproductive tract.17,18,19,20 This signal is then propagated sequentially through Mip neurons, and SP abdominal ganglion neurons (SAG) to reach the central brain.21,22 The central pC1 neurons regulating receptivity in virgins are likely the target of the SPSN/SAG pathway.23 Notably, in addition to the female reproductive tract, the SPR is also expressed in the brain, but the role of these SPR neurons in female sexual receptivity is unknown. In addition, octopamine released by the octopaminergic neurons in the abdominal ganglion in females is also a modulator of post-mating behavior.24 Furthermore, central complex neurons were shown to regulate the behavioral switch from initial rejection to acceptance in pre-mating virgin females, which is a process involving dopamine signals.10
Given the complex regulatory processes of courtship decisions, it is of interest to gain further information regarding the neural signaling mediating this behavior. 5-hydroxytryptamine (5-HT, serotonin) participates in a broad range of processes, including reproduction and sexual behaviors, in both invertebrates and vertebrates.25,26,27,28 In Drosophila, 5-HT and its receptors, as well as certain related neurons, were shown to regulate courtship behaviors in males.29,30,31,32 Some evidence suggested that the serotonin system also has a role in the sexual motivation of females. A pharmacological study demonstrated that long-term treatment with SB258719, a 5-HT7 receptor antagonist, adversely affected sexual behaviors in females.29 Recently, 5-HT and two receptors (5-HT1A and 5-HT7) were shown to control receptivity in virgin females.33 As only a subset of serotoninergic neurons, specifically the Trh+fru+ 5-HT-PLP neurons have been indicated in female receptivity,33 how the serotonin system fits into the neural circuits controlling sexual motivation in females requires further investigation.
In this paper, we studied the neural circuit mechanisms of virgin female sexual receptivity surrounding serotoninergic neurons and receptor neurons. We identified subsets of neurons in females that are critical for normal courtship success. One pair of the SPN promotes sexual motivation in virgin females, which in turn is suppressed by the SP as a result of copulation. We further found that the 5-HT7 receptor neurons act downstream to mediate SPN signals to regulate female sexual receptivity.
Results
5-HT neurons are necessary for the sexual receptivity of virgin females
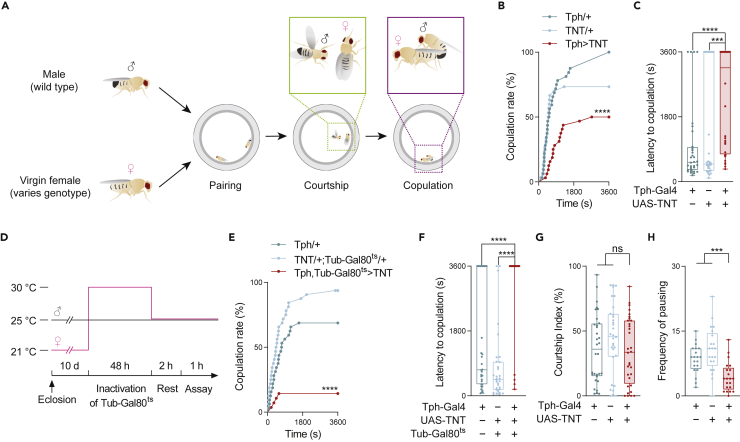
To quantify the courtship behaviors in Drosophila, we paired a virgin female with a wild-type male in a chamber (Figure 1A). Using wild-type males enables us to associate the impact on courtship with the genetic manipulations in females only. To evaluate the functions of serotoninergic neurons in females, we globally silenced serotonin-releasing neurons using a broad driver, Tph-Gal4, to express tetanus toxin light chain (TNT) and quantified subsequent courtship outcomes, including the rate of successful copulation and the latency to copulation.34,35,36
Figure 1.
5-HT neurons regulate sexual receptivity of virgin females
(A) Schema of the experimental procedure used to quantify courtship behavior. A virgin female of a designated genotype was paired with a wild-type male in a courtship chamber. The male initiated the courtship ritual that typically ended with copulation when the male mounted the female.
(B and C) Quantification of behavioral characteristics when a Tph-Gal4>UAS-TNT female was paired with a wild-type male (Canton S). Tph/+indicates Tph-Gal4/+, and TNT/+indicates UAS-TNT/+; both served as genetic controls. Similar acronyms are used in the following figures. (B) Events of successful copulation over time, compared with genetic controls (n = 30–32). (C) Elapsed time before reaching successful copulation (n = 30–32).
(D–F) Quantification of courtship events after silencing 5-HT neurons only in adult females. (D) Schema to silence target neurons only in adult stages with the Tub-Gal80ts system. Before the behavior assay, female flies were treated at 30°C for 2 days to induce the expression of TNT. (E and F) Copulation rate and latency to copulation in Tph-Gal4, Tub-Gal80ts>UAS-TNT females and controls after temperature-shift treatment as shown in (D) (n = 28–32).
(G) Courting attempts of wild-type males toward females of Tph-Gal4>UAS-TNT or control females. (n = 32).
(H) The frequencies of pauses of Tph-Gal4>UAS-TNT females and control females toward the pursuing wild-type males (n = 20-21).
All genotypes and experimental conditions are indicated with the plots. In the box-and-whisker plot, the whiskers mark the minimum and maximum, the box includes the 25th to 75th percentiles, and the line within the box indicates the median of the dataset. The log-rank test was applied to (B) and (E), and the asterisk indicates the statistical analysis of the experimental group and one of two controls that has less significance with the experimental group. The Kruskal–Wallis test was performed for (C) and (F)-(H). ns, not significant (p> 0.05); ∗∗∗p< 0.001; ∗∗∗∗p< 0.0001.
When neurons labeled by Tph-Gal4 were silenced in the virgin females, copulations with a longer latency and approximately half of the pairs failed to copulate by the end of the observation period (Figures 1B, 1C and S1A). We used Tub-GAL80ts to suppress 5-HT neurons only within designated time windows37 (Figure 1D). At the restrictive temperature, which removes the inhibitory effect of GAL80ts on GAL4, virgin females with UAS-TNT; Tph-Gal4, Tub-Gal80ts exhibited lower copulation rates and longer latencies, compared to the genetic controls (Figures 1E and 1F). These results indicated that the 5-HT neurons in virgin females are essential for normal courtship activities leading to successful copulation. Moreover, in the pairs successfully initiating copulation, their copulation duration was not affected by silencing these 5-HT neurons, suggesting that 5-HT neurons in females mainly modulate the process leading to copulation (Figure S1B).
In Drosophila, the overall courtship success is influenced by several interrelated behaviors of females, including actions triggering male sexual behavior, immobilization to allow mounting by the courting male, and repulsive actions to repel males.38,39,40,41 We first investigated whether the decreased copulation rate of Tph>TNT virgin females was because of a low motivation of the males owing to the passive or assertive behaviors of the females. As shown in Figure 1G, when we evaluated the courtship behaviors of wild-type males toward the Tph>TNT females and the genetic controls, there was no significant difference in the courtship index among these groups. This indicated that the reduction in copulation rate after silencing 5-HT neurons in females is unlikely because of the reduction of courtship motivation in males caused by the inhibitory behavioral actions of the females.
Compared with mated females, a receptive virgin female should display fewer rejection behaviors and more immobilizations (pauses) to allow the courting male to initiate copulation.42 Our behavioral analysis showed that compared with the genetic controls, virgin females of Tph>TNT paused less frequently, further suggesting that 5-HT neurons are necessary for sexual receptivity of mature virgin females (Figure 1H).
To investigate whether the neurotransmitter, 5-HT, is also involved in sexual receptivity in females, we reduced levels of 5-HT in 5-HT neurons using RNAi against the rate-limiting enzyme for serotonin synthesis, tryptophan hydroxylase (TRH).43Trh knockdown in females (Tph>TrhRNAi) decreased the copulation rates and increased the copulation latency, compared with the control groups (Figures S1C and S1D). Furthermore, we tested the effect of elevating the serotonin levels in females on the copulation rate. Overexpression of Trh in 5-HT neurons did not change the copulation rate or the latency to copulation (Figures S1E and S1F). These behavioral results suggest that maintaining a normal production level of 5-HT in these neurons is necessary for sexual receptivity in virgins.
Distinct subgroups of 5-HT neurons mediate sexual receptivity
Tph>GFP signals revealed about fifty 5-HT-positive neurons in distinct groups distributed in the brain and ventral nerve cord (VNC) in an adult female Drosophila.31 The heterogeneity of Tph-Gal4 labeled neurons led us to identify the subgroup of 5-HT neurons responsible for the sexual receptivity of females by surveying split-Gal4 lines that label 5-HT neurons.
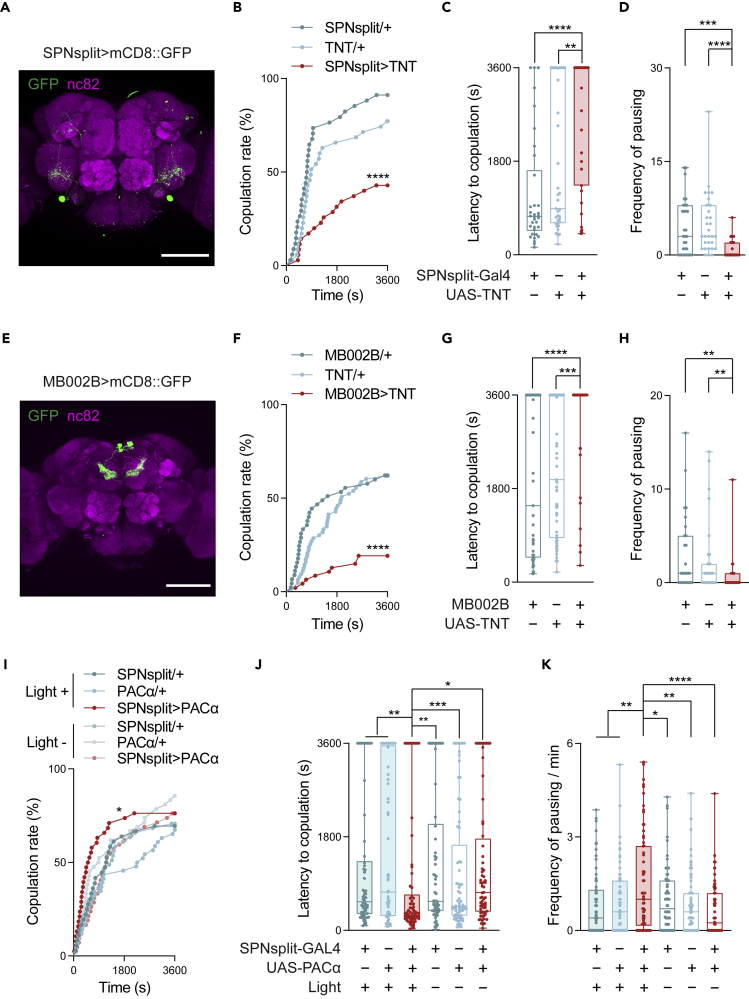
SPN are a pair of serotonin projection neurons located in the gnathal ganglia (GNG) terminating in the central brain.44 As shown in Figures 2A–2C, silencing SPN in females decreased the copulation rate and increased the copulation latency. Similar phenotypes were also observed when SPN were suppressed in females via the expression of inward-rectifier potassium ion channel Kir2.145 (FiguresS2A and S2B). Furthermore, suppressing SPN decreased the frequency of pausing during courtship, suggesting engagement of behaviors inhibitory to copulation (Figure 2D).
Figure 2.
Distinct subgroups of 5-HT neurons mediate sexual receptivity
(A–D) Expression pattern of SPN (A) and the behavioral changes when the SPN were silenced with TNT (B-D). (A) Immunofluorescence of SPN (green) in a female SPNsplit-Gal4>UAS-mCD8::GFP. nc82 (magenta) serves as a counterstaining. (B and C) Quantification of the copulation rate (B) and latency to copulation (C) when SPNsplit-Gal4>UAS-TNT females were paired with wild-type males (n = 34-35). (D) The frequencies of pauses of the SPNsplit-Gal4>UAS-TNT females and control females toward the pursuing wild-type males (n = 30-31).
(E–H) Expression pattern of MB002B neurons (E) and the behavioral changes when the neurons were silenced with TNT (F-H). (E) Immunofluorescence of MB002B neurons (green) in a female of MB002B-Gal4>UAS-mCD8::GFP. nc82 (magenta) serves as a counterstaining. (F and G) Quantification of the copulation rate (F) and latency to copulation (G) when MB002B>UAS-TNT females were paired with wild-type males (n = 45–63). (H) Frequency of immobilization of the MB002B>UAS-TNT females and control females toward the pursuing wild-type males (n = 29–31).
(I–K) Quantification of courtship behaviors after SPN were activated by optogenetics for 80s.
(I and J) Quantification of the copulation rate (I) and latency to copulation (J) when SPNsplit-Gal4>UAS-PACα females were paired with wild-type males (n = 62–73).
(K) The pauses per minute of the SPNsplit-Gal4>UAS-PACα females toward the pursuing wild-type males (n = 62-73).
All genotypes and experimental conditions are indicated with the plots. In (I), for simplicity, only a portion of data points was visualized whereas the overall curve was derived from the entire dataset. In the box-and-whisker plot, the whiskers mark the minimum and maximum, the box includes the 25th to 75th percentiles, and the line within the box indicates the median of the dataset. The log-rank test was applied to (B), (F), and (I). Among the statistical comparisons between the experimental group and controls, only the ones with less significance are shown in (B) and (F). In (I), the statistical comparison was shown between the experimental group (with light) and the no-light control (with the same genotype). The Kruskal–Wallis test was performed for (C)-(D), (G)-(H), and (J)-(K). ∗p< 0.05; ∗∗p< 0.01; ∗∗∗p< 0.001; ∗∗∗∗p< 0.0001. Scale bar in (A) and (E): 100 μm.
We tested another subgroup of 5-HT neurons labeled by a split-Gal4, MB002B. The small cluster of MB002B neurons comprises two types of mushroom body output neurons (MBONs), MBON-β′2mp and MBON-γ5β′2a (Figure 2E). Silencing MB002B neurons in females decreased the copulation rate and increased the copulation latency (Figures 2F and 2G). In addition, silencing MB002B neurons decreased the frequency of pausing during courtship (Figure 2H). Therefore, in addition to SPN, other subgroups of 5-HT neurons are involved in controlling sexual behaviors in females.
Although overexpression of Trh had little influence on female receptivity, we addressed whether 5-HT neurons can boost sexual receptivity in females. We first used Tph-Gal4 to drive UAS-PACα to optogenetically activate the entire population of 5-HT neurons.46 This manipulation in females elevated the copulation rate and decreased the copulation latency, indicating a heightened sexual receptivity (Figures S2C and S2D).
We then investigated whether activating the SPN and MB002B neurons influences sexual receptivity. Similar to activating Tph-Gal4 neurons, optogenetically activating SPN in virgin females increased the copulation rate, decreased the copulation latency, and increased the frequency of pausing during courtship (Figures 2I–2K). Similar activation of SPN on the mated females had no effect on re-mating behavior (Figure S2E). These results demonstrate that the activity of SPN is sufficient to promote sexual receptivity in females. In contrast, activating the MB002B neurons in females decreased the copulation rate (Figures S2F and S2G). Therefore, although both subgroups of 5-HT neurons take part in sexual receptivity in females, they might assume different modulatory roles. Taken together, the active state of 5-HT neurons can bidirectionally regulate sexual receptivity in females.
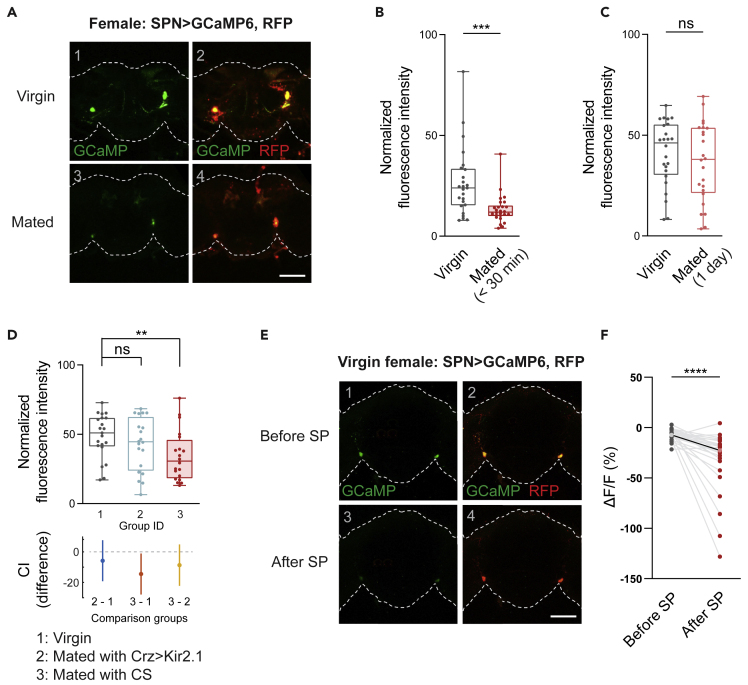
Activity of SPNs mirrors sexual receptivity
The necessary and sufficient role of SPN in female receptivity led us to examine whether their activity corresponds to the receptive states of the females. We used a genetically encoded calcium indicator, GCaMP, to quantify the activities of SPN in females before and after copulation.47 As shown in Figures 3A and 3B, the newly mated females exhibited lower fluorescent signals in their SPN than that in virgins, suggesting the mating process causes a low-active state in the female SPN.
Figure 3.
The active states of SPN reflect sexual receptivity
(A) Representative images of GCaMP6 signals (green) in SPN of a virgin (A1-2) and a newly mated (A3-4) female. Red signals in (A2) and (A4) show the corresponding cell clusters. The genotype is SPNsplit-Gal4>UAS-GCaMP6, UAS-myr::RFP. The dashed lines indicate the profiles of brains.
(B–D) Normalized fluorescence intensities of GCaMP6 signals in SPN of virgin females (gray) and mated females (red) at different times after mating. (B) Comparison of the age-matched virgins and newly-mated females (n = 25, 26). (C) Comparison of the age-matched virgins and mated females 1 day after mating (n = 24, 24). (D) Comparison of normalized fluorescence intensities of GCaMP6 signals in SPN in three classes of females. The top panel shows the intensity values of three groups (n = 21, 20, 20). Group 1: virgin females (un-mated); Group 2: females mated with Crz-Gal4>UAS-Kir2.1 males; Group 3: females mated with wild-type males (Canton S). The bottom panel shows the confidence intervals for the difference between indicated groups (95% confidence).
(E) Representative images of SPN in virgin females responding to 60 μM SP treatment. (E1-2): before treatment. (E3-4): after SP treatment. Green indicates the GCaMP signals, and red labels the corresponding cell clusters. The genotype is SPNsplit-Gal4>UAS-GCaMP6, UAS-myr::RFP. The dashed lines indicate the profiles of brains.
(F) Change of normalized fluorescence intensity (ΔF/F) of GCaMP6 signals in SPN of virgin females, after SP treatment (n = 26). The light gray and red dots indicate the mean of each dataset.
All genotypes and experimental conditions are indicated with the plots. In the box-and-whisker plot, the whiskers mark the minimum and maximum, the box includes the 25th to 75th percentiles, and the line within the box indicates the median of the dataset. The unpaired t-test was performed for (B)-(C). The Kruskal–Wallis test was performed for (D). The paired t-test was performed for (F). ns, not significant (p> 0.05); ∗∗p< 0.01; ∗∗∗p< 0.001; ∗∗∗∗p< 0.0001. Scale bar in (A) and (E): 100 μm.
The mating process in Drosophila quickly switches the behavioral repertoire in females including suppressed sexual receptivity. However, the mated females gradually revert to the pre-mating behavioral status within a few days.19,48,49 Notably, as shown in Figure 3C, the activity of SPN also returned to the normal level after one day in the females reared alone after mating, suggesting that the mating experience resulted in alterations in the activity of SPN. The correlation of the activity status of SPN with mating experience indicates that these neurons are regulated by the mating experience of females.
The changes in the internal state and behaviors of females are likely triggered by two stimuli in a successful courtship: the sensory experience and the transfer of seminal products (sperm and seminal fluid proteins). The male-derived substances were shown to contribute to the female post-mating effect.17,18,19,20 The multi-modality sensory components of mating are also known to cause a reduction of female receptivity in Drosophila.15 Therefore, we undertook to distinguish whether one or both of the stimuli are involved in the induction of low SPN activity after copulation.
To disassociate the sensory effects of copulation from the reception of male-derived substances, we paired virgin females with non-ejaculatory Crz > Kir2.1 males, which provides a mating experience but avoids the transfer of male materials.15,50 As shown in Figure 3D, compared with wild-type males, copulation with non-ejaculatory males did not significantly decrease the activity of SPN in the female, indicating that the act and sensory experience of copulation does not result in changes in the activity of SPN, strongly pointing to the transfer of male products as the cause of reductions in SPN activity.
Among the male-derived transferred proteins, SP, produced in the male accessory gland, is a key factor regulating post-mating behavioral changes in females.16,51,52,53 In females, the SPR in the SPN is involved in enhancing aversive long-term memory.54 We addressed whether the SP affects the activity of SPN when applied directly to the brain. As shown in Figures 3E and 3F, compared with the control group (Figure S3A), when bath-applied to a whole-brain explant, the SP effectively decreased the activity of SPN. The SP concentration used here (60 μm) is chosen because injection of SP of similar concentrations elicited behavioral effects in females.55,56 We further investigated whether SP inhibit the activity of SPN in females with only experience of courtship. We found that in the brains of females mated with Crz > Kir2.1 males, the activity of SPN was reduced by the treatment of SP (Figures S3B and S3C). These results indicate that the transfer of SP during copulation is responsible for the activity changes in the SPN, which results in reductions in sexual receptivity in the mated females.
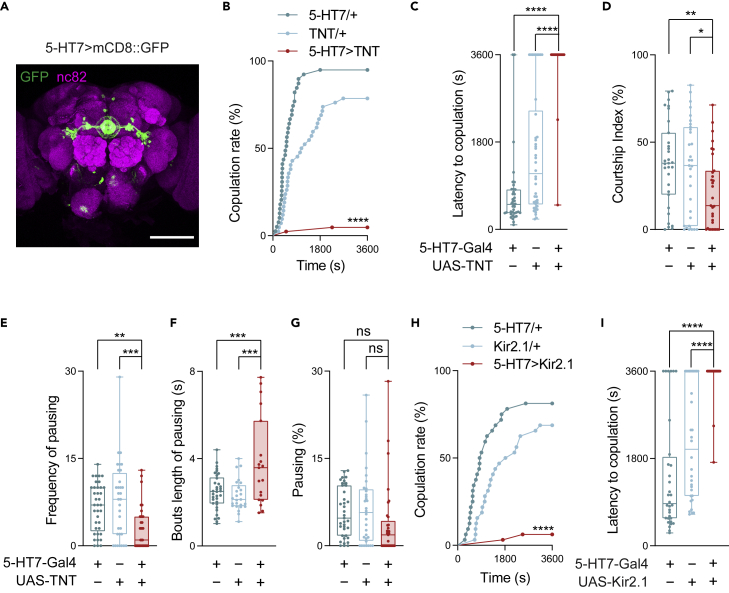
5-HT7 receptor neurons are required for sexual receptivity
After showing the role of 5-HT neurons in female receptivity, we wished to extend our understanding to identify the receptor neurons. With a pilot screen, we found that silencing the 5-HT7 receptor neurons in virgin females gave rise to a minimal copulation rate. The most severe deficiency in sexual receptivity prompted us to focus on the role of 5-HT7 receptor neurons. A previous pharmacological study with SB258719 indicated this receptor subtype is essential for normal sexual behavior in females.29 5-HT7-Gal4 is an enhancer GAL4 driver that strongly labels neurons in the ellipsoid body, including R4d neurons and R3a/R3d neurons.29 5-HT7-Gal4 also weakly labeled neurons in the ventral-lateral protocerebrum (VLP) and the subesophageal zone (SEZ) (Figure 4A).
Figure 4.
5-HT7 receptor neurons are required for sexual receptivity
(A) Immunofluorescence of 5-HT7 neurons (green) in the brain of a 5-HT7-Gal4>UAS-mCD8::GFP female. nc82 (magenta) serves as a counterstaining.
(B–D) Quantification of behavioral characteristics when a 5-HT7-Gal4>UAS-TNT female was paired with a wild-type male (Canton S). (B) Elapsed time before reaching the successful copulation (n = 39-42). (C) The frequency of pauses of 5-HT7-Gal4>UAS-TNT females toward the pursuing wild-type males (n = 39-42). (D) Courting attempts of wild-type males toward 5-HT7-Gal4>UAS-TNT females or control females. (n = 31).
(E–G) Quantification of pausing events of 5-HT7-Gal4>UAS-TNT females when paired with a wild-type male (Canton S). (E) The frequency of pauses of 5-HT7-Gal4>UAS-TNT females and control females toward the pursuing wild-type males (n = 31-32). (F) The duration of pausing bouts (n = 31-32). (G) The proportion of the summed pausing durations to the total time (n = 31-32).
(H and I) Quantification of the courtship events in pairs of a 5-HT7-Gal4>UAS-Kir2.1 female and a wild-type male: copulation rate (H) and latency to copulation (I) (n = 32).
All genotypes and experimental conditions are indicated with the plots. In the box-and-whisker plot, the whiskers mark the minimum and maximum, the box includes the 25th to 75th percentiles, and the line within the box indicates the median of the dataset. The log-rank test was applied to (B) and (H), and among the statistical comparisons between the experimental group and controls, only the ones with less significance are shown. The Kruskal–Wallis test was performed for (C)-(G) and (I). ns, not significant (p ≥ 0.05); ∗p< 0.05; ∗∗p< 0.01; ∗∗∗p< 0.001; ∗∗∗∗p< 0.0001. Scale bars in (A) is 100 μm.
Inhibition of 5-HT7 receptor neurons in females strongly decreased the copulation rate and greatly increased the copulation latency (Figures 4B and 4C). In addition, silencing 5-HT7 receptor neurons in females lowered the courtship intensity of the paired males (Figure 4D). Of interest, compared with controls, 5-HT7>TNT females also paused less frequently for the pursuit males, indicating a reduced motivation for courtship (Figure 4E). Similar behavioral effects were also observed using 5-HT7 > Kir2.1 females (Figures 4H and 4I). We further used a different 5-HT7 driver to cross-validation these results. 5-HT7Gal4 was generated by an end-out knock-out strategy to replace the coding region of 5-HT7.57 The expression of 5-HT7Gal4 is similar to the above 5-HT7-Gal4 (Figure S4A). Silencing the neurons labeled by 5-HT7Gal4 indeed recapitulated the phenotype of a reduced copulation rate and increased latency to copulation (Figures S4B and S4C). These results support the courtship deficits associated with SB258719, and reveal that the 5-HT7 receptor neurons in females participate in promoting sexual receptivity, which in part motivates courtship in males.
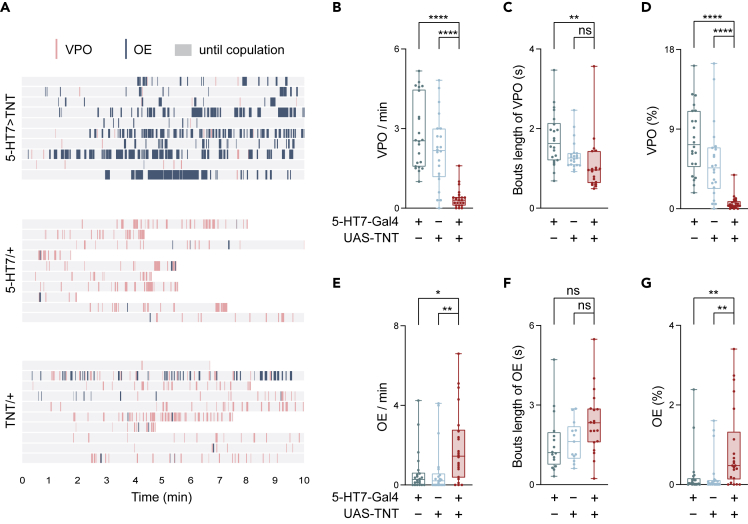
We found it intriguing to understand the very low courtship rate (lower than 5%, Figure 4B) which was reflected as reduced courtship attempts by males (Figure 4D) and decreased frequency of pausing by females (Figure 4E), when 5-HT7 receptor neurons were silenced in females. We further analyzed the duration of each pause and the total pausing duration. Silencing 5-HT7 receptor neurons led to longer pauses in the 5-HT7 females (Figure 4F), but their overall pause durations in the evaluation period exhibited only a small tendency to decrease (Figure 4G). The effect of fewer pauses is probably canceled out partially by the longer duration of each pause, which prompted us to explore the root of lost sexual receptivity in 5-HT7>TNT females. Analyzing the behavioral characteristics of females during their pauses revealed that females with silenced 5-HT7 receptor neurons exhibited abnormal responses to the approaching males in two aspects: vaginal plate opening (VPO) and ovipositor extrusion (OE), which are indicators of the willingness or unwillingness, respectively, of the female to copulate4,58 (Figure 5A). For both VPO and OE behaviors, we calculated the frequency (bouts per min), the bout duration, and the proportion of the total bout durations in the evaluation period (Figures 5B–5G). Compared with the controls, the 5-HT7>TNT virgin females exhibited a much smaller VPO frequency (Figure 5B) and a very short total duration (Figure 5D), suggesting a strongly reduced willingness to copulate when 5-HT7 receptor neurons were silenced. On the other hand, observation of OE in 5-HT7>TNT virgin females revealed a higher frequency of events (Figure 5E) and a longer overall duration than controls (Figure 5G). We observed that a 5-HT7/TNT virgin female even showed OE when a male simply walked by. All of these suggested heightened rejection behaviors toward the paired males. Therefore, on silencing of 5-HT7 receptor neurons, whereas the females still pause when pursued by males, their behaviors counteract promoting copulation.
Figure 5.
5-HT7 receptor neurons tune the sexual receptivity in females
(A) Raster plots illustrating the bouts of OE (dark blue bar) and VPO (pink bar) of females when paired with wild-type males (Canton S). 10 pairs were shown for each genotype. Gray regions indicate the period from the beginning to the onset of copulation.
(B–D) Quantification of vaginal plate opening of 5-HT7-Gal4>UAS-TNT females when paired with a wild-type male (Canton S). (B) Comparison of the frequencies of VPO bouts per minute (n = 22). (C) Comparison of the durations of VPO bouts (n = 22). (D) The proportion of the summed VPO durations to the total time (n = 22).
(E–G) Quantification of ovipositor extrusion of 5-HT7-Gal4>UAS-TNT females when paired with a wild-type male (Canton S).
(E) Comparison of the frequencies of OE bouts per minute (n = 22).
(F) Comparison of the duration of OE bouts (n = 22).
(G) The proportion of the summed OE durations to the total time (n = 22).
All genotypes and experimental conditions are indicated with the plots. In the box-and-whisker plot, the whiskers mark the minimum and maximum, the box includes the 25th to 75th percentiles, and the line within the box indicates the median of the dataset. The Kruskal–Wallis test was performed for (B)-(G). ns, not significant (p ≥ 0.05); ∗p< 0.05; ∗∗p< 0.01; ∗∗∗∗p< 0.0001.
To explore the root of reduced motivation in males, we analyzed the effects of females’ appearance and behaviors on the courtship behaviors of the paired wild-type males using timed events of both flies (Figures S5A and S5B). We first quantified the percentage of males that did not engage in courtship during the first 5 min of pairing. We found no significant differences between the male group paired with 5-HT7>TNT females and that paired with control females, suggesting that silencing the 5-HT7 receptor neurons in females does not impact the “first impression” of the males toward the females (Figure S5C). We then quantified the effects of OE and VPO behaviors of females on the courtship motivation of paired males. The males showed more courtship behaviors after the first OE by 5-HT7>TNT females, suggesting males are less likely affected by an early rejection (Figures S5D and S5E). Notably, the males paired with normal females displayed increased courtship behaviors after the first VPO, indicating a possible encouraging effect of the female VPO (Figures S5G and S5H). Inferring the effects of later events was made difficult by greatly heightened behavior events in both males and females as time passes by (Figures S5A, S5B, S5F, and S5I). Nevertheless, during the process of courting 5-HT7>TNT females, the change in motivation of wild-type males from initially normal to eventfully decreased suggests the motivation of the courting male is suppressed by the rejective responses of the females, among which OE is the most frequent and conspicuous actions of the unwilling females.
The 5-HT7 receptor in Drosophila exhibits substantial homology (approximately 57%) to the human 5-HT7 receptor,59 which is a Gs-coupled receptor that, when activated, leads to elevated neuronal excitability.60 We measured the activity of the 5-HT7 receptor neurons on application of 5-HT to brain explants. As shown in Figures S4D and S4E, applying 1 mM of 5-HT hydrochloride61 to the female brain effectively elevated the GCaMP fluorescence in the 5-HT7 receptor neurons.
5-HT7 receptor neurons mediate SP signals
To explore the connectivity of 5-HT7 receptor neurons, we used a transsynaptic labeling system, trans-Tango, to simultaneously visualize both the primary neurons and their postsynaptic neurons.62 Neurons located postsynaptically to SPN are found in multiple brain regions (Figures S6A and S6B). For example, SPN project to the superior clamp surrounding the MB peduncle (SCL) (Figure S6C) and superior medial protocerebrum (SMP), suggesting an information relay from SPN to MB. Intriguingly, whereas the prominent group of 5-HT7 receptor neurons was the ring neurons of EB (Figure 4A), no signal of the postsynaptic neurons of SPN was observed in the ring neurons region (Figure S6C). We further evaluated the trans-Tango data of 5-HT7 receptor neurons for potential target neurons. Comparing the expression patterns suggested that the SPN and 5-HT7 receptor neurons likely form connections in GNG, saddle/antennal mechanosensory and motor center (SAD/AMMC), SMP and SCL (Figures S6D–S6F). Furthermore, the postsynaptic targets of 5-HT7 receptor neurons were distributed more extensively than 5-HT7 receptor neurons, notably including the ring area itself as well (Figures S6D and S6G), suggesting that the 5-HT signal is further expanded to more brain regions thereby broadcasting the status or change of status of sexual receptivity across the brain.
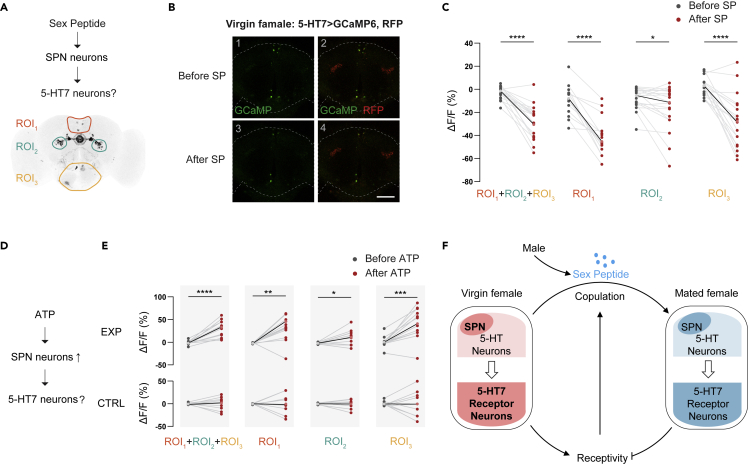
We then addressed whether 5-HT7 receptor neurons function downstream of SPN for SP-induced changes. Similar to the SPN imaging experiments, we monitored the activity changes of the 5-HT7 receptor neurons in brain explants from virgin females when the SP was applied to the solvent. Three brain regions corresponding to locations with harboring dense cell bodies of 5-HT7 receptor neurons were measured simultaneously (ROI1-3, Figure 6A). After the treatment of SP, the fluorescence signals in all regions combined were significantly decreased (Figures 6B and 6C). The postsynaptic analysis of SPN targets in the trans-Tango experiment above suggested that distinctly located subsets of 5-HT7 receptor neurons participate differently in mediating SPN signals. Consistent with this hypothesis, among the three ROIs, SP dramatically reduced the activities of 5-HT7 receptor neurons in ROI1 (SMP regions) and ROI3 (GNG region), but only slightly decreased those in ROI2 (EB regions, Figure 6C).
Figure 6.
Subsets of 5-HT7 receptor neurons function downstream of SPN in response to the treatment of SP
(A) Schema of the experimental design for investigating the functional connection of SPN and 5-HT7 receptor neurons. The SP was delivered to the brain explant of a virgin female, and the activities of 5-HT7 receptor neurons in different brain regions were monitored. Three brain regions were measured, ROI1 (orange), ROI2 (green) and ROI3 (yellow), each corresponding to areas with dense cell bodies of 5-HT7 positive neurons.
(B) Representative images of 5-HT7 receptor neurons responding to 60 μM SP treatment. (B1-2): before SP treatment. (B3-4): after SP treatment. Green indicates the GCaMP signals, and red labels the corresponding 5-HT7 cell clusters. The dashed lines indicate the profiles of brains. The genotype is 5-HT7-Gal4>UAS-GCaMP6, UAS-myr::RFP.
(C) Changes of normalized fluorescence intensities of GCaMP6 signals in 5-HT7 receptor neurons of virgin females after the treatment of SP, in three brain regions, ROI1, ROI2 and ROI3 separately, as well as signals from all three regions combined (ROI1 + ROI2 + ROI3) (n = 16-19). The gray dots indicate ΔF/F (%) before the treatment of SP, and the red dots indicate ΔF/F (%) after SP. The light gray and red dots indicate the mean of each dataset.
(D and E) Visualization of the functional connection of SPN and 5-HT7 receptor neurons by monitoring the activity in 5-HT7 receptor neurons while stimulating SPN in virgin females. (D) Schema of the experimental design. ATP was used to activate SPN expressing P2X2 whereas the activities of 5-HT7 receptor neurons in three ROIs (same as in (A)) were monitored. (E) Changes of normalized fluorescence intensities of GCaMP6 signals in 5-HT7 neurons after activating SPN with ATP (n = 9-12). Top: the experimental flies (EXP, genotype: SPN-AD/UAS-P2X2, LexAop-GCaMP6f; SPN-DBD/5-HT7-LexA); bottom: the genetic controls (CTRL, genotype:+/UAS-P2X2, LexAop-GCaMP6f; +/5-HT7-LexA). The gray dots indicate ΔF/F (%) before the treatment of ATP, and the red dots indicate ΔF/F (%) after ATP. The light gray and red dots indicate the mean of each dataset.
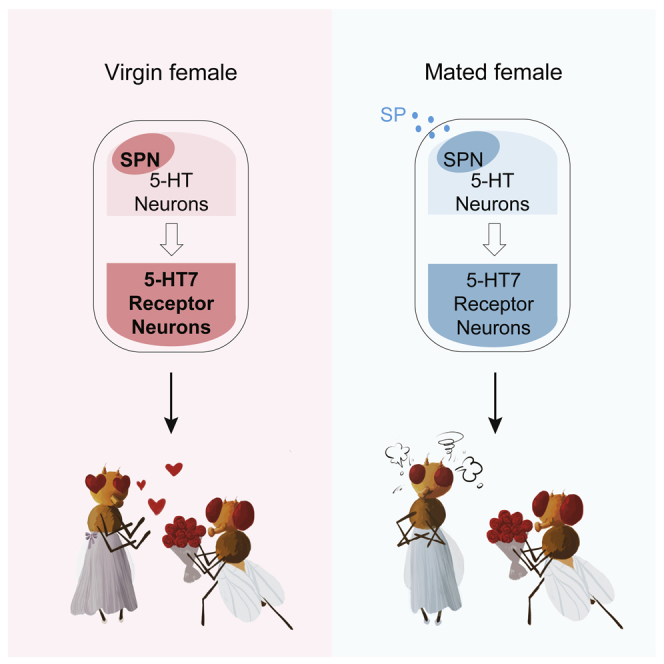
(F) A working model showing the 5-HT system in the central brain modulates female sexual receptivity. Subsets of 5-HT neurons and receptor neurons promote the courtship desire in the brain of a virgin female. After a successful copulation, the mated female receives the male-derived SP that suppresses its 5-HT and 5-HT receptor neurons. The suppression signal subsequently propagates through the downstream circuits via 5-HT7 receptor neurons, leading to a decreased courtship desire and elevated rejection behaviors.
All genotypes and experimental conditions are indicated with the plots. In the box-and-whisker plot, the whiskers mark the minimum and maximum, the box includes the 25th to 75th percentiles, and the line within the box indicates the median of the dataset. The paired t-test was performed for (C), and (E). ∗p< 0.05; ∗∗∗∗p< 0.0001. Scale bar in (B): 100 μm.
We also evaluated the functional relationship between SPN and 5-HT7 receptor neurons by measuring the changes in the activity of 5-HT7 receptor neurons while stimulating SPN (Figure 6D). The application of ATP activated the SPN via the expressed P2X2, an ATP-dependent depolarizing ion channel.63 This also elicited calcium responses in 5-HT7 receptor neurons in all three ROIs, although to different degrees, but not in the genetic controls (Figure 6E). Therefore, the structural connection from trans-Tango experiment and functional analysis from calcium imaging suggest that SPN have structural and functional connections with 5-HT7 receptor neurons in all three regions for SP-triggered signals.
Taken together, our findings depict a dynamic process with negative feedback involving both males and females where SPN/5-HT7 receptor neurons in the central brain promote sexual receptivity in virgins for successful copulation. The SP transferred from males during copulation subsequently suppresses the SPN/5-HT7 pathway to decrease female receptivity (Figure 6F).
Discussion
In this study, we investigated the role of the 5-HT system in modulating willingness to copulate in female Drosophila. We found that 5-HT and 5-HT neurons are essential in virgin females for courtship outcomes leading to copulation. During courtship, females with suppressed 5-HT neurons pause less frequently for the pursuing males than control females. We identified a pair of serotoninergic neurons, SPN, in females which positively regulated sexual receptivity of virgin females. When transferred from a male to a female during copulation, SP leads to a switch of female receptivity, and here, we showed that the activity of SPN in females is repressed by SP. Finally, we provide evidence that distinct 5-HT7 receptor neurons mediate the SP’s effect on sexual receptivity to other brain regions, suggesting a broad and complex neural circuit that tunes sexual receptivity in females according to their sexual experience.
Our finding is consistent with the view that as an ancient neural signaling system, the 5-HT system participates strongly in the neural circuit controlling courtship behaviors in animals. In rodents, serotonin signaling controls sexual preference in both males and females.25,28 In male flies, activating 5-HT neurons suppresses copulation attempts and reduces copulation rate, whereas knocking down Trh in dsx+ neurons or silencing serotoninergic dsx+ neurons inhibits copulation.31,32 In this study, we investigated the sexual drive of virgin females, which is behaviorally expressed as sexual receptivity of virgin females. We found that inhibition of 5-HT neurons reduces sexual receptivity in females, which was reflected by decreased copulation rate, delayed copulation events, and infrequent pauses for the courting males. Taken together, in both males and females, 5-HT neurons participate in modulating the motivation of copulation, despite the sexual dimorphism of specific sexual behaviors.
The sexual drive in females changes dramatically after copulation. Previously, studies identified that experiences of courtship and material transfer during the copulation process contribute to this switch of post-mating sexual desire. Significant progress has been made recently in identifying the neuronal basis of the switch and the post-mating behavior which involves the transfer of the male-derived SP during copulation, together with sperm and seminal fluid. In the abdominal ganglion, SP silenced the excitability of SPSNs, which further modulated neuronal circuits including SPSN-Mip-SAG neurons,22 resulting in reduced female receptivity. Our results suggest an additional regulatory pathway triggered by SP, and that this pathway starts in the brain where SP targets a pair of 5-HT neurons, and that SPN plays an important role in regulating virgin receptivity: activating SPN elevates female receptivity whereas silencing SPN decreases female receptivity. Although multiple neurons have been reported to play regulatory roles in virgin female receptivity,2,4,6,7,10,11,17,18,19,21,22,64 only manipulations in subsets of dsx-positive neurons (including pCd, pC1), DSK neurons, dopamine neurons and their downstream R2/R4m neurons, together with serotoninergic neurons upregulated receptivity in virgin females, suggesting distinct pathways to regulate receptivity in virgin and mated females. Furthermore, material transfer during copulation, rather than the courtship experience itself, renders a decreased activity in SPN. Moreover, SP reduces the activity of SPN. Finally, our imaging results showed that after copulation the activity of SPN recovers within 24 h, faster than expected recovery of receptivity, indicating the critical involvement of other regulatory pathways in suppressing the receptivity of mated females. Together, these results suggest the physiological relevance of SPN in regulating sexual drive in virgins. The relationship of this regulatory pathway to the SPSN/Mip/SAG axis is discussed later.
The large population and distinct clusters of 5-HT neurons in the fly brain suggest that this system mediates diverse, and sometimes even opposite, functions. Two subsets of 5-HT neurons, SPN and MB002B, were indicated here to participate in the sexual drive of females. Another subset of serotoninergic neurons, 5-HT-PLP neurons, was reported recently controlling the receptivity of virgin females as well.33 Therefore, there are at least three subsets of 5-HT neurons regulating the sexual drive of females. Notably among these, the SPN were previously known for different functions. SPN were implicated in processes underlying overcoming the loser effect in defeated males, and in controlling memory consolidation and enhancing long-term memory in females.34,44,54 In this study, we found that SPN promote the sexual desire of virgin females and in turn, are suppressed by successful copulation. We speculate that in a female brain, SPN act as a regulatory point by SP for long-term changes in the mating-related behavioral status. That is, as a part of the switch from a pre-mating brain state to a post-mating brain state, the SP effects on SPN play at least dual roles, affecting sexual desire and memory formation. Furthermore, although the functions of SPN in males and females demonstrate sexual dimorphism, there is a general theme in both genders that SPN neurons are part of circuits exerting long-term, rather than short-term, effects on behavioral states.
Besides SPN, we identify another subgroup of 5-HT neurons, MB002B neurons that are also involved in the sexual receptivity of virgins. Although SPN are located in the GNG, MB002B mainly consists of the mushroom body output neurons, MBON-β′2mp and MBON-γ5β′2a. Although they both regulate sexual receptivity, 5-HT neurons of SPN and MB002B have differential actions. Although silencing either subgroup reduces the sexual receptivity, activating SPNs promotes the sexual receptivity of virgins but activating MB002B neurons inhibits the sexual receptivity of virgins. The differential effects of two 5-HT neuronal groups indicate that distinct subgroups of 5-HT neurons partake in a complicated interaction network to modulate sexual drive in females, which requires further investigations to disentangle. Moreover, the functional relationship of the serotoninergic subgroups (including SPN, MB002B, and 5-HT-PLP neurons), such as whether they are independent, collaborative, or hierarchical, remains to be determined which requires new genetic tools not available yet.
5-HT7 receptor neurons are the key mediator in the circuitry of 5-HT neurons for controlling female sexual receptivity. Silencing the 5-HT7 receptor neurons reduced dramatically the sexual drive in females, which suggests a mechanistic basis for findings with SB258719 pharmacological study showing a role for this receptor in courtship and mating.29 The strong effect of silencing 5-HT7 receptor neurons resulted from an increased frequency of ovipositor extrusions and decreased frequency of vaginal plate opening, both behaviors reflecting a low desire of the females to mate. Both structural and functional data support that subsets of 5-HT7 receptor neurons in GNG, SMP and EB mediate the SP signal from SPN.
Another implication worth noting from our data is the potential involvement of the mushroom bodies in regulating female sexual receptivity. In the brain, multiple types of neurons have been suggested to regulate virgin female receptivity.4,6,7,10,11,64 Except for the R2/R4m and R4d neurons in the ellipsoid body, the locations of other neurons did not seemingly associate sexual receptivity with prominent structures.10 When we used the split-Gal4 approach to attribute the behavioral effects to smaller groups of responsible neurons, we identified a subset of 5-HT neurons (MB002B) that predominately project into particular compartments of the β′ or γ lobe of the mushroom body. Furthermore, the connectivity analysis provides additional evidence. Both SPN and 5-HT7 receptor neurons project into SCL surrounding the MB peduncle, possibly to feed the related information into the mushroom body system. Therefore, when taken together with previous work, both the ellipsoid body and mushroom body, which are two massive structures in the central brain, regulate sexual receptivity in females.
Limitations of the study
The structural and functional experiments suggested that subsets of 5-HT7 receptor neurons in GNG and SMP mediate the SP signal from SPN, whereas the involvement of the 5-HT7-positive neurons in EB is relatively weak. Notably, these 5-HT7-positive EB neurons consist of R4d ring neurons. It was previously reported that the cholinergic R4d neurons, being both GABAA-positive and Dop1R2-positive, normally repress sexual receptivity in virgins.10 The relationship between the two populations of R4d neurons remains to be determined. Nevertheless, being suppressive to sexual receptivity, these GABAA-positive R4d neurons are unlikely to mediate the signals from SPN promoting sexual receptivity. Analysis of the roles of different subtypes of 5-HT7 receptor neurons and their downstream circuits would require new genetic agents to precisely manipulate each population, which is not yet available.
Whether other 5-HT receptors or 5-HT receptor neurons are involved downstream of SPN to regulate female receptivity is still open. It was reported that 5-HT1A and 5-HT7 receptors, but not the other three - 5-HT1B, 5-HT2A and 5-HT2B, are required for female receptivity in virgins.33 Whether 5-HT1A receptor or 5-HT1A receptor neurons are involved in SP/SPN signaling is not known. Although suppressing the 5-HT7 receptor neurons in females leads to an almost complete loss of receptivity, this does not exclude the involvement of other 5-HT receptor neurons. Our calcium imaging results suggest a functional hierarchy between SPN and 5-HT7 receptor neurons, and trans-Tango results indicate a possible direct connection between SPN and 5-HT7 receptor neurons. A direct connection between them still awaits further evidence. If SPN and 5-HT7 receptor neurons are not directly connected, additional neurons are needed to directly receive the serotoninergic signal from SPN to relay it further downstream. The 5-HT1A neurons would be a suitable candidate for serving as the “in-between” neurons. However, in contrast with the 5-HT7 receptor, the 5-HT1A receptor is inhibitory. Hence, to achieve an overall excitatory effect from SPN to 5-HT7 receptor neurons, this scenario calls for additional neurons or even a cascade of neurons acting downstream of 5-HT1A receptor neurons. Another possibility is the existence of “in-between” neurons is subsets of 5-HT7 receptor neurons that themselves are also serotoninergic. Moreover, besides the hierarchical relationship discussed above, 5-HT1A neurons could act parallel to the 5-HT7 receptor neurons and make a probably minor contribution to regulating sexual receptivity.
SP regulates female receptivity through both the SPN/5-HT7 pathway in the central and the afferent SPSN/Mip/SAG pathway in the peripheral. Besides the different sites of action, the mating phases the two pathways act on seem to be different as well. However, they could be part of a larger neural circuit controlling female receptivity, instead of merely two redundant pathways with enhanced robustness. In mated females, activating Mip and SAG neurons overturns the suppression of courtship receptivity, but activating SPN could not. This could be interpreted that the SPSN/Mip/SAG pathway plays a dominant role in the post-mating phase over the SPN/5-HT7 pathway, hence leading to the question of the relationship between these two pathways, particularly whether SPN/5-HT7 is under direct regulation of the SPSN/Mip/SAG pathway. One particular set of central neurons, pC1, might hold a key to the answer. The pC1 neurons receive SP signals through SPSN/Mip/SAG axis and further affect post-mating behaviors including female receptivity.4 In particular, activating pC1 neurons promotes courtship receptivity in both virgin and mated females. It is possible that the apparent dominance of SPSN/Mip/SAG over SPN/5-HT7 in the post-mating phase is carried through by pC1 neurons. Further evidence is required to ascertain the relationship between pC1 neurons and SPN. Nevertheless, it was shown that the effect of SP on long-term memory in post-mating females is mediated by SPN, rather than SPSN neurons;54 therefore, the SPSN/Mip/SAG pathway and SPN/5-HT7 pathway at least act independently in regulating some post-mating behaviors.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-nc82 (mouse) | DSHB | CAT# nc82; RRID:AB_2314866 |
| Rhodamine (TRITC) Conjugated Goat anti-Mouse IgG (H+L) | ZSGB-BIO | CAT# ZF 0313; RRID:AB_2571577 |
| Rhodamine (TRITC) Conjugated Goat anti-Rabbit IgG (H+L) | ZSGB-BIO | CAT# ZF 0316; RRID:AB_2728778 |
| HA-Tag (rabbit) | Cell Signaling | CAT# 3724; RRID:AB_1549585 |
| Chemicals, peptides, and recombinant proteins | ||
| Serotonin hydrochloride | Alfa | CAT# B21263 |
| Sex peptide | Sangon Biotech | MW: 4348.03 Lot No.: P22185-20210207 |
| Adenosine 5′-triphosphate disodium salt hydrate | Sigma | CAT# A2383-5G |
| NaCl | Sinopharm Chemical Reagent Co.,Ltd | CAT# 10019308 |
| KCl | XiLONG SCIENTIFIC | CAS# 7447-40-7 |
| CaCl2 | Sinopharm Chemical Reagent Co.,Ltd | CAT# 10005861 |
| MgCl2.6H2O | Sinopharm Chemical Reagent Co.,Ltd | CAT# 10012892 |
| NaHCO3 | Sinopharm Chemical Reagent Co.,Ltd | CAT# 10018992 |
| NaH2PO4.2H2O | XiLONG SCIENTIFIC | CAS# 13472-35-0 |
| Trehalose | Sigma-Aldrich | CAT# T9531-10G |
| Sucrose | Sigma-Aldrich | CAT# V900116-500G |
| HEPES | Sigma-Aldrich | CAT# V900477-100G |
| Experimental models: Organisms/strains | ||
| Drosophila: Wildtype Canton S | Li Liu | RRID: DGRC_ 105666 |
| Drosophila: Tph-Gal4 | Yi Rao | Park et al., 200635 |
| Drosophila: UAS-PACα | Benjamin Kottler and Martin Schwarzel | Bellmann et al., 201065 |
| Drosophila: UAS-mCD8::GFP | Bloomington Drosophila Stock Center | RRID: BDSC_32185 |
| Drosophila: Tub-Gal80ts | Yan Li and Aike Guo | McGuire et al., 200437 |
| Drosophila: Crz-Gal4 | Bloomington Drosophila Stock Center | RRID: BDSC_51976 |
| Drosophila: UAS-Trh | Bloomington Drosophila Stock Center | RRID: BDSC_27638 |
| Drosophila: UAS-Trh-RNAi | Julie H. Simpson | Albin et al., 201543 |
| Drosophila: UAS-TNTE | Yan Li and Aike Guo | Sweeney et al., 199536 |
| Drosophila: UAS-Kir2.1 | Li Liu | Baines et al., 200145 |
| Drosophila: 5-HT7Gal4 | Yi Rao | Qian et al., 201757 |
| Drosophila: 5-HT7-LexA | Yi Rao | Deng et al., 201966 |
| Drosophila: 5-HT7-Gal4 | Charles D Nichols | Becnel et al., 201129 |
| Drosophila: UAS-Denmark | Yan Li and Aike Guo | RRID: BDSC_33061 |
| Drosophila: trans-Tango | Bloomington Drosophila Stock Center | RRID: BDSC_77124 |
| Drosophila: 20XUAS-IVS-GCaMP6m (attP40) | Bloomington Drosophila Stock Center | RRID: BDSC_42748 |
| Drosophila: SPN split-Gal4 | Thomas Preat | Scheunemann et al., 201844 |
| Drosophila: MB002B | Bloomington Drosophila Stock Center | RRID: BDSC_68305 |
| Drosophila: LexAop-GCaMP6f, UAS-P2X2 | Fang Guo and Chang Liu | N/A |
| Software and algorithms | ||
| Prism9 | GraphPad Software | URL: http://www.graphpad.com/;RRID:SCR_002798 |
| MATLAB R2018a | MathWorks | URL: http://www.mathworks.com/products/matlab/; RRID:SCR_001622 |
| Fiji | NIH | URL: https://fiji.sc/;RRID: SCR_002285 |
| Adobe Illustrator | Adobe | URL: https://www.adobe.com/;RRID:SCR_010279 |
| Deposited data | ||
| Raw and analyzed data | This paper; Mendeley Data | Mendeley Data: https://doi.org/10.17632/9y236v53m4.1 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yan Zhu (zhuyan@ibp.ac.cn).
Materials availability
This study did not generate new unique reagents. All key resources are listed in the key resources table. Further information and requests for resources and reagents should be directed to the lead contact.
Experimental model and subject details
Fly stocks and genetics
Flies were usually reared at 25°C and 60% humidity in a 12:12hr light:dark regimen (light on at 08:00) unless otherwise indicated. The flies used for the optogenetic experiments were reared in the dark. The standard fly media included water (1000 mL), cornmeal (77.7 g), yeast (32.1 g), agar (8 g), calcium chloride (0.726 g), sucrose (31.62 g), glucose (63.2 g), potassium sorbate (2 g), and methyl p-hydroxybenzoate (1.5 g). Flies for CsChrimson experiments were raised without all-trans-retinal.
Newly emerged virgin females were collected within 6 h after eclosion and reared individually in a 2-mL Eppendorf tube containing 0.5 mL of food. To measure re-mating behavior, females were mated 48 hours prior to the test. Wild-type males (Canton S, CS, 6-8 d old) were used in all the experiments to serve as the mating partners for female flies (6-8 d old). The CS males were collected within 8 h after eclosion and reared in groups of 50 with sufficient food. All behavioral assays were carried out at 25°C and 60% humidity between 15:00 and 19:00.
The details of the fly stocks are listed in the key resources table. Brief descriptions of three lines are provided below. Canton S was obtained from the Kyoto Stock Center (stock number: 105666). The SPNsplit-Gal4 flies (obtained from T. Preat) included two transgenes: one inserted into the attp40 site, containing an activation domain fused with a regulatory sequence from VT026326, and another inserted into the attp2 site, containing a DNA-binding domain fused with a regulatory sequence from VT057280.44 The fly of LexAop-GCaMP6f, UAS-P2X2 was generated by F. Guo through genetic recombination.
Method details
Courtship assay
The protocol for measuring courtship was adapted from previous work with some modifications.34 The circular courtship chamber had a radius of 7 mm and a height of 3.5 mm. Agarose was placed in the chamber to maintain the humidity. During the behavior test, a female and a male were loaded into a courtship chamber by gentle aspiration without anesthesia. Fly behaviors were recorded at 25 frames per second for at least 1 hour.
The copulation rate represents the percentage of the pairs successfully reaching copulation out of all pairs tested. Latency to copulation is the time elapsed from the introduction of flies to the successful copulation. To simplify quantification, when no copulation occurred throughout the 60 min period, the copulation latency was assigned as 3600 s. The copulation duration was measured as the duration (seconds) from the onset of copulation to the finish of copulation. The courtship index was measured as the percentage of time in a 5 min observation period that the tester male spent courting the target females, including tapping, following, wing vibration, and attempted copulation, except in Figures S5D–S5I. The frequency of pausing was measured as how many times in the first 5 min after introduction the virgins stopped walking when pursued by males. Typically, the pairs had not reached copulation yet within this period, except for the pairs containing Tph-Gal4/UAS-PACα females. For the pairs containing Tph-Gal4/UAS-PACα females (Figure 2K), we used the number of pauses per minute (the total number of pauses divided by the period, which is either the elapsed time before copulation or 5 min, whichever comes first), instead of the frequency of pausing.
For quantifying ovipositor extrusion (OE) and vaginal plate opening (VPO), high-resolution videos (3840 x 2160) were recorded at 60 fps and visually inspected post hoc. OE was identified from a tube-like ovipositor extension. VPO was identified based on the opening of the vaginal plates and the absence of visible extension of the tube-like ovipositor. The time period for evaluation is either the elapsed time before copulation or 10 min, whichever comes first.
Optogenetic stimulation
The protocol of optogenetic stimulation was similar to the previously reported.34 The courtship chambers were topped with a transparent glass sheet for illumination and observation. A 460-nm blue light source (Denjoy DY400-4) was placed 5 mm above the courtship chambers to illuminate the flies inside for 80 s before starting video recording. The light intensity measured with a spectrometer (CCS200/M, Thorlabs) at the site of illumination was 122 mW/cm2.
Immunohistochemistry
The immunohistochemistry protocol was adapted from previous work with some modifications.67 Dissection of intact brains of adult male flies was performed in cold phosphate-buffered saline and fixed in 4% fresh paraformaldehyde solution for 3 h on ice. The tissues were then washed with 0.5% Triton X-100 in 1× phosphate-buffered saline (PBT) three times (15 min each), blocked for 30 min with PBT containing 10% normal goat serum (PNT) at room temperature, and incubated with a primary antibody in a blocking buffer for 24hat 4°C. After washing with PBT three times, the tissues were incubated with a secondary antibody in PNT for 48h at 4°C. The nc82 signals served as counterstaining unless otherwise indicated.
The above protocol was also used for trans-Tango imaging. The flies for trans-Tango analysis were raised at 18°C for 20 to 25 days before dissection as suggested.62
Functional fluorescence imaging
Previously established methods for calcium imaging were used with minor modifications.68 Adult hemolymph-like saline (AHL) consisting of 108 mM NaCl, 5 mM KCl, 2 mM CaCl2, 8.2 mM MgCl2, 4 mM NaHCO3, 1 mM NaH2PO4-H2O, 5 mM trehalose, 10 mM sucrose, and 5 mM HEPES (pH 7.5) was used to bathe the brains for dissection and imaging. Flies were briefly anesthetized on ice, and the brains were quickly dissected into AHL at room temperature. An O-ring (inner diameter of 10 mm) was glued to a glass slide to form a small reservoir to hold a brain in AHL for imaging.
To measure the levels of activity in SPNs before and after copulation, a SPNsplit-Gal4>UAS-GCaMP6, UAS-myr::RFP virgin was paired with a CS male or a Crz-Gal4>UAS-Kir2.1 male in a courtship chamber for 60-120 min. The brains of the courted females were dissected and imaged in vitro. We used SPNsplit-Gal4>UAS-GCaMP6, UAS-myr::RFP virgins placed individually in the courtship chamber for 60-120 min as the virgin controls.
To measure the effects of the SP on the activity of SPN and 5-HT7 receptor neurons, whole-brain explants of SPNsplit-Gal4>UAS-GCaMP6, UAS-myr::RFP or 5-HT7-Gal4>UAS-GCaMP6, UAS-myr::RFP virgins were dissected and imaged in vitro. The synthetic SP (dissolved in AHL, MW: 4348.03, synthesized by Sangon Biotech) was gently injected into saline solutions to reach a final concentration of 60 μM.55,56 The control group received AHL only.
To measure the activity of 5-HT7 receptor neurons while activating SPN, whole-brain explants of SPN-AD/UAS-P2X2, LexAop-GCaMP6f; SPN-DBD/5-HT7-LexA virgins were dissected and imaged in vitro. ATP (2.5 mM, dissolved in AHL) was delivered into saline solutions to activate SPN. The genotype of the genetic control is+/UAS-P2X2, LexAop-GCaMP6f;+/5-HT7-LexA virgins.
To measure the changes in the activity of 5-HT7 receptor neurons when stimulated by 5-HT, whole-brain explants of 5-HT7-Gal4>UAS-GCaMP6, UAS-myr::RFP females were positioned on circular coverslips (5-mm diameter) and placed in a recording chamber containing AHL. Serotonin hydrochloride (1 mM, dissolved in AHL) was gently delivered by a pipette into the chamber. The control group received AHL only.
Calcium imaging was performed using an SP8 confocal microscope (Leica, Wetzlar, Germany) with a 40× water immersion objective. All settings were kept constant between the experimental conditions. Images were taken in 2.0-μm steps and acquired at 512 × 512 pixels. Fluorescence signals were recorded at ∼0.7 Hz. Images were later processed and quantified with Fiji.
GCaMP fluorescence was measured with excitation at 488 nm. The ROI was defined based on the RFP signal highlighting the cell body region. To represent the quantified GCaMP signal of each ROI, the signal intensity of green fluorescent protein was normalized to the RFP signal: F = the average of green signals/the average of red signals. The normalized fluorescence value for each time point was defined as Ft. The change of normalized fluorescence intensity is calculated as: ΔF/F (%) = (Ft−F0)/F0 × 100, where F0 corresponds to the average value of the 5 frames before drug application. In related experiments, the “before” is ΔF/F at the onset of treatments, and the corresponding “after” is ΔF/F at the time of peak response. After pilot experiments, we chose t = 80 s to evaluate the response of SPN to SP, and t = 180 s for the response of 5-HT7 receptor neurons to 5-HT. In protocols measuring the response of 5-HT7 receptor neurons to SP, t = 60 s was chosen for ROI1, and t = 180 s for ROI2, ROI3 and overall ROIs. And when measuring the response of 5-HT7 receptor neurons after activating SPN, t = 96 s was chosen. All imaging and analyses were performed blinded to the experimental conditions.
Quantification and statistical analysis
Statistical analysis
Statistical analysis was performed using Prism 9 (GraphPad Software, La Jolla, CA, USA). All experiments were performed in parallel with both the experimental and control groups. For the copulation rate, the log-rank test was applied. For the change of fluorescence intensity (ΔF/F) before and after drug treatments, the paired t-test was applied. Gray dots indicate data points in the group of before treatments, and red dots indicate data points in the group of after treatments, and light gray and red dots indicate the mean of each dataset. In addition, when comparing two groups, the Student’s t-test was applied for normally distributed data, while the Kruskal–Wallis test was performed for non-normally distributed data. When comparing multiple groups, one-way ANOVA was applied for normally distributed data, while the Mann-Whitney test was performed for non-normally distributed data. When data points in a dataset are plotted in a box-and-whisker plot, the whiskers mark the minimum and maximum of the dataset, the box include data from the 25th to 75th percentiles, and the line within indicates the median. The statistical tests used and sample sizes are indicated in the figure legends. Statistical significance was determined with 95% confidence (p < 0.05). ns, not significant (p ≥ 0.05); ∗p< 0.05; ∗∗p< 0.01; ∗∗∗p< 0.001; ∗∗∗∗p< 0.0001.
Acknowledgments
We thank all members of the Zhu Laboratory for their beneficial discussions. We thank Dr. Chuan Zhou and Baoxu Ma (Chinese Academy of Sciences, CAS) for discussing pre-publication results and their efforts in coordinating publication. We thank Dr. Li Liu and Dr. Yan Li for their insightful discussion. We thank Ms. Meiyan Huang for technical assistance and Dr. Shan Gao for help with the software in behavioral analyses. We thank Fanchen Kong for help with graphical design. We thank Dr. Aike Guo, Dr. Yan Li, Dr. Chang Liu and Dr. Li Liu (CAS), Dr. Yi Rao (Peking University), Dr. Fang Guo (Zhejiang University), Dr. Benjamin Kottler (Queensland Brain Institute), Dr. Martin Schwärzel (University of Berlin), Dr. Julie H. Simpson (University of California, Santa Barbara), Dr. Charles D. Nichols (Louisiana State University), Dr. Thomas Preat (Paris Sciences et Lettres University), as well as the Bloomington Drosophila Stock Center for providing fly strains. This work was supported by NSFC grant (32071007, 9163210042), the Beijing Advanced Discipline Fund, the Key Research Program of Frontier Sciences of CAS (QYZDY-SSW-SMC015), CAS Interdisciplinary Innovation Team, and Bill and Melinda Gates Foundation (OPP1119434) to Y.Z., and NSFC 31771173 to Y.J.S..
Author contributions
S.W.H. and Y.Z. conceived this project; S.W.H. and Y.T.Y. performed all the behavioral tests; Y.T.Y., X.N.L., and Y.J.S. performed the expression analysis; S.W.H., Y.T.Y., and P.H. analyzed the data; K.A.K. and Y.Z. supervised the project; and S.W.H., Y.T.Y., K.A.K., and Y.Z. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: February 2, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106123.
Supplemental information
Data and code availability
-
•
All data have been deposited at Mendeley Data, and are publicly available as of the date of publication. DOI is listed in the key resources table.
-
•
This paper does not generate original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Greenspan R.J., Ferveur J.F. Courtship in Drosophila. Annu. Rev. Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 2.Bussell J.J., Yapici N., Zhang S.X., Dickson B.J., Vosshall L.B. Abdominal-B neurons control Drosophila virgin female receptivity. Curr. Biol. 2014;24:1584–1595. doi: 10.1016/j.cub.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall J.C. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 4.Wang K., Wang F., Forknall N., Yang T., Patrick C., Parekh R., Dickson B.J. Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature. 2021;589:577–581. doi: 10.1038/s41586-020-2972-7. [DOI] [PubMed] [Google Scholar]
- 5.Dickson B.J. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai A., Koganezawa M., Yasunaga K.I., Emoto K., Yamamoto D. Select interneuron clusters determine female sexual receptivity in Drosophila. Nat. Commun. 2013;4:1825. doi: 10.1038/ncomms2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou C., Pan Y., Robinett C.C., Meissner G.W., Baker B.S. Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron. 2014;83:149–163. doi: 10.1016/j.neuron.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Juni N., Yamamoto D. Genetic analysis of chaste, a new mutation of Drosophila melanogaster characterized by extremely low female sexual receptivity. J. Neurogenet. 2009;23:329–340. doi: 10.1080/01677060802471601. [DOI] [PubMed] [Google Scholar]
- 9.Sakai T., Kasuya J., Kitamoto T., Aigaki T. The Drosophila TRPA channel, Painless, regulates sexual receptivity in virgin females. Genes Brain Behav. 2009;8:546–557. doi: 10.1111/j.1601-183X.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishimoto H., Kamikouchi A. A feedforward circuit regulates action selection of pre-mating courtship behavior in female Drosophila. Curr. Biol. 2020;30:396–407.e4. doi: 10.1016/j.cub.2019.11.065. [DOI] [PubMed] [Google Scholar]
- 11.Wang T., Jing B., Deng B., Shi K., Li J., Ma B., Wu F., Zhou C. Drosulfakinin signaling modulates female sexual receptivity in Drosophila. Elife. 2022;11:e76025. doi: 10.7554/eLife.76025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaac R.E., Li C., Leedale A.E., Shirras A.D. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc. Biol. Sci. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.M., Su C.Y., Wang J.W. Neuromodulation of innate behaviors in Drosophila. Annu. Rev. Neurosci. 2017;40:327–348. doi: 10.1146/annurev-neuro-072116-031558. [DOI] [PubMed] [Google Scholar]
- 14.Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 15.Shao L., Chung P., Wong A., Siwanowicz I., Kent C.F., Long X., Heberlein U. A neural circuit encoding the experience of copulation in female Drosophila. Neuron. 2019;102:1025–1036.e6. doi: 10.1016/j.neuron.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Liu H., Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Häsemeyer M., Yapici N., Heberlein U., Dickson B.J. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Rezával C., Pavlou H.J., Dornan A.J., Chan Y.B., Kravitz E.A., Goodwin S.F. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol. 2012;22:1155–1165. doi: 10.1016/j.cub.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C.H., Rumpf S., Xiang Y., Gordon M.D., Song W., Jan L.Y., Jan Y.N. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yapici N., Kim Y.J., Ribeiro C., Dickson B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 21.Feng K., Palfreyman M.T., Häsemeyer M., Talsma A., Dickson B.J. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron. 2014;83:135–148. doi: 10.1016/j.neuron.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Jang Y.H., Chae H.S., Kim Y.J. Female-specific myoinhibitory peptide neurons regulate mating receptivity in Drosophila melanogaster. Nat. Commun. 2017;8:1630. doi: 10.1038/s41467-017-01794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F., Wang K., Forknall N., Patrick C., Yang T., Parekh R., Bock D., Dickson B.J. Neural circuitry linking mating and egg laying in Drosophila females. Nature. 2020;579:101–105. doi: 10.1038/s41586-020-2055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezával C., Nojima T., Neville M.C., Lin A.C., Goodwin S.F. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 2014;24:725–730. doi: 10.1016/j.cub.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Jiang Y., Si Y., Kim J.Y., Chen Z.F., Rao Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011;472:95–99. doi: 10.1038/nature09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Techniq. 1999;45:106–121. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Rillich J., Rillich B., Stevenson P.A. Differential modulation of courtship behavior and subsequent aggression by octopamine, dopamine and serotonin in male crickets. Horm. Behav. 2019;114:104542. doi: 10.1016/j.yhbeh.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S., Liu Y., Rao Y. Serotonin signaling in the brain of adult female mice is required for sexual preference. Proc. Natl. Acad. Sci. USA. 2013;110:9968–9973. doi: 10.1073/pnas.1220712110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becnel J., Johnson O., Luo J., Nässel D.R., Nichols C.D. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS One. 2011;6:e20800. doi: 10.1371/journal.pone.0020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo J., Lushchak O.V., Goergen P., Williams M.J., Nässel D.R. Drosophila insulin-producing cells are differentially modulated by serotonin and octopamine receptors and affect social behavior. PLoS One. 2014;9:e99732. doi: 10.1371/journal.pone.0099732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pooryasin A., Fiala A. Identified serotonin-releasing neurons induce behavioral quiescence and suppress mating in Drosophila. J. Neurosci. 2015;35:12792–12812. doi: 10.1523/Jneurosci.1638-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmazer Y.B., Koganezawa M., Sato K., Xu J., Yamamoto D. Serotonergic neuronal death and concomitant serotonin deficiency curb copulation ability of Drosophila platonic mutants. Nat. Commun. 2016;7:13792. doi: 10.1038/ncomms13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma B., Wang R., Liu Y., Deng B., Wang T., Wu F., Zhou C. Serotonin signaling modulates sexual receptivity of virgin female Drosophila. Neurosci. Bull. 2022;38:1277–1291. doi: 10.1007/s12264-022-00908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu S.W., Yang Y.T., Sun Y., Zhan Y.P., Zhu Y. Serotonin signals overcome loser mentality in Drosophila. iScience. 2020;23:101651. doi: 10.1016/j.isci.2020.101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J., Lee S.B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J.M., Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 36.Sweeney S.T., Broadie K., Keane J., Niemann H., Okane C.J. Targeted expression of tetanus toxin light-chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 37.McGuire S.E., Mao Z., Davis R.L. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 38.Beach F.A. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm. Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 39.Grillet M., Dartevelle L., Ferveur J.F. A Drosophila male pheromone affects female sexual receptivity. Proc. Biol. Sci. 2006;273:315–323. doi: 10.1098/rspb.2005.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebreton S., Trona F., Borrero-Echeverry F., Bilz F., Grabe V., Becher P.G., Carlsson M.A., Nässel D.R., Hansson B.S., Sachse S., Witzgall P. Feeding regulates sex pheromone attraction and courtship in Drosophila females. Sci. Rep. 2015;5:13132. doi: 10.1038/srep13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neckameyer W.S. Dopamine modulates female sexual receptivity in Drosophila melanogaster. J. Neurogenet. 1998;12:101–114. doi: 10.3109/01677069809167259. [DOI] [PubMed] [Google Scholar]
- 42.Lasbleiz C., Ferveur J.F., Everaerts C. Courtship behaviour of Drosophila melanogaster revisited. Anim. Behav. 2006;72:1001–1012. doi: 10.1016/j.anbehav.2006.01.027. [DOI] [Google Scholar]
- 43.Albin S.D., Kaun K.R., Knapp J.M., Chung P., Heberlein U., Simpson J.H. A subset of serotonergic neurons evokes hunger in adult Drosophila. Curr. Biol. 2015;25:2435–2440. doi: 10.1016/j.cub.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Scheunemann L., Plaçais P.Y., Dromard Y., Schwärzel M., Preat T. Dunce phosphodiesterase acts as a checkpoint for Drosophila long-term memory in a pair of serotonergic neurons. Neuron. 2018;98:350–365.e5. doi: 10.1016/j.neuron.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baines R.A., Uhler J.P., Thompson A., Sweeney S.T., Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 2001;21:1523–1531. doi: 10.1523/Jneurosci.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schröder-Lang S., Schwärzel M., Seifert R., Strünker T., Kateriya S., Looser J., Watanabe M., Kaupp U.B., Hegemann P., Nagel G. Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods. 2007;4:39–42. doi: 10.1038/Nmeth975. [DOI] [PubMed] [Google Scholar]
- 47.Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning A. A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature. 1962;194:252–253. doi: 10.1038/194252a0. [DOI] [Google Scholar]
- 49.Manning A. The control of sexual receptivity in female Drosophila. Anim. Behav. 1967;15:239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- 50.Tayler T.D., Pacheco D.A., Hergarden A.C., Murthy M., Anderson D.J. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:20697–20702. doi: 10.1073/pnas.1218246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aigaki T., Fleischmann I., Chen P.S., Kubli E. Ectopic expression of sex peptide alters reproductive-behavior of female drosophila-melanogaster. Neuron. 1991;7:557–563. doi: 10.1016/0896-6273(91)90368-A. [DOI] [PubMed] [Google Scholar]
- 52.Chapman T., Bangham J., Vinti G., Seifried B., Lung O., Wolfner M.F., Smith H.K., Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen P.S., Stummzollinger E., Aigaki T., Balmer J., Bienz M., Böhlen P. A male accessory-gland peptide that regulates reproductive-behavior of female Drosophila-melanogaster. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- 54.Scheunemann L., Lampin-Saint-Amaux A., Schor J., Preat T. A sperm peptide enhances long-term memory in female Drosophila. Sci. Adv. 2019;5:eaax3432. doi: 10.1126/sciadv.aax3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ottiger M., Soller M., Stocker R.F., Kubli E. Binding sites of Drosophila melanogaster sex peptide pheromones. J. Neurobiol. 2000;44:57–71. [PubMed] [Google Scholar]
- 56.Soller M., Bownes M., Kubli E. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. Eur. J. Biochem. 1997;243:732–738. doi: 10.1111/j.1432-1033.1997.00732.x. [DOI] [PubMed] [Google Scholar]
- 57.Qian Y., Cao Y., Deng B., Yang G., Li J., Xu R., Zhang D., Huang J., Rao Y. Sleep homeostasis regulated by 5HT2b receptor in a small subset of neurons in the dorsal fan-shaped body of drosophila. Elife. 2017;6:e26519. doi: 10.7554/eLife.26519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F., Wang K., Forknall N., Parekh R., Dickson B.J. Circuit and behavioral mechanisms of sexual rejection by Drosophila females. Curr. Biol. 2020;30:3749–3760.e3. doi: 10.1016/j.cub.2020.07.083. [DOI] [PubMed] [Google Scholar]
- 59.Tierney A.J. Structure and function of invertebrate 5-HT receptors: a review. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2001;128:791–804. doi: 10.1016/S1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 60.Nichols D.E., Nichols C.D. Serotonin receptors. Chem. Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 61.Haynes P.R., Christmann B.L., Griffith L.C. A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster. Elife. 2015;4:e03868. doi: 10.7554/eLife.03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talay M., Richman E.B., Snell N.J., Hartmann G.G., Fisher J.D., Sorkaç A., Santoyo J.F., Chou-Freed C., Nair N., Johnson M., et al. Transsynaptic mapping of second-order taste neurons in flies by trans-tango. Neuron. 2017;96:783–795.e4. doi: 10.1016/j.neuron.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao Z., Macara A.M., Lelito K.R., Minosyan T.Y., Shafer O.T. Analysis of functional neuronal connectivity in the Drosophila brain. J. Neurophysiol. 2012;108:684–696. doi: 10.1152/jn.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mezzera C., Brotas M., Gaspar M., Pavlou H.J., Goodwin S.F., Vasconcelos M.L. Ovipositor extrusion promotes the transition from courtship to copulation and signals female acceptance in Drosophila melanogaster. Curr. Biol. 2020;30:3736–3748.e5. doi: 10.1016/j.cub.2020.06.071. [DOI] [PubMed] [Google Scholar]
- 65.Bellmann D., Richardt A., Freyberger R., Nuwal N., Schwärzel M., Fiala A., Störtkuhl K.F. Optogenetically induced olfactory stimulation in Drosophila larvae reveals the neuronal basis of odor-aversion behavior. Front. Behav. Neurosci. 2010;4:27. doi: 10.3389/fnbeh.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng B., Li Q., Liu X., Cao Y., Li B., Qian Y., Xu R., Mao R., Zhou E., Zhang W., et al. Chemoconnectomics: mapping chemical transmission in Drosophila. Neuron. 2019;101:876–893.e4. doi: 10.1016/j.neuron.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 67.Zhan Y.P., Liu L., Zhu Y. Taotie neurons regulate appetite in Drosophila. Nat. Commun. 2016;7:13633. doi: 10.1038/ncomms13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen D., Sitaraman D., Chen N., Jin X., Han C., Chen J., Sun M., Baker B.S., Nitabach M.N., Pan Y. Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat. Commun. 2017;8:154. doi: 10.1038/s41467-017-00087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data have been deposited at Mendeley Data, and are publicly available as of the date of publication. DOI is listed in the key resources table.
-
•
This paper does not generate original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.